Abstract
Sulfonylureas, a commonly-used class of medication used to treat type 2 diabetes, have been associated with an increased risk of cardiovascular disease. Their effects on QT interval duration and related electrocardiographic phenotypes are potential mechanisms for this adverse effect. In eleven ethnically diverse cohorts that included 71 857 European, African American, and Hispanic/Latino ancestry individuals with repeated measures of medication use and electrocardiogram (ECG) measurements, we conducted a pharmacogenomic genome-wide association study of sulfonylurea use and three ECG phenotypes: QT, JT, and QRS intervals. In ancestry-specific meta-analyses, 8 novel pharmacogenomic loci met the threshold for genome-wide significance (P < 5 x 10−8), and a pharmacokinetic variant in CYP2C9 (rs1057910) that has been associated with sulfonylurea-related treatment effects and other adverse drug reactions in previous studies was replicated. Additional research is needed to replicate the novel findings and to understand their biological basis.
INTRODUCTION
Sulfonylureas are the oldest class of oral glucose-lowering therapy used to treat type 2 diabetes, and despite the emergence of several new classes of diabetes drugs in recent years,1 sulfonylureas remain the most widely prescribed oral therapy after metformin.2 Since the University Group Diabetes Program trial found that the first-generation sulfonylurea chlorpropamide increased the risk of cardiovascular mortality over 40 years ago,3 there have been concerns about the cardiovascular safety of sulfonylureas. Several studies since then have found that treatment with sulfonylureas is associated with an increased risk of cardiovascular events and mortality compared with other glucose-lowering drugs.4, 5
As one potential mechanism of cardiovascular toxicity, sulfonylureas can prolong the QT interval,6, 7 a marker of cardiac repolarization that is associated with fatal arrhythmias and sudden cardiac death.8–12 Indeed, QT prolongation has been one of the most common safety issues leading to drug withdrawals from the market.13, 14 Since 2005, the Food and Drug Administration has required clinical studies to evaluate whether a new drug prolongs the QT interval greater than 5 millisecond (ms) prior to regulatory approval.15
Variation in the QT interval is heritable,16, 17 and large scale genome-wide association (GWA) studies have identified at least 35 genetic loci associated with this trait, which collectively explain about 10% of inter-individual variation in the QT interval.18 Pharmacogenomic studies of sulfonylurea use and the QT interval may help to unravel the biologic mechanisms underlying the cardiovascular toxicity of sulfonylureas. However, previous pharmacogenomic studies of the glucose-lowering or adverse effects of sulfonylureas have been small and focused on candidate genes,19–22 and most findings have not replicated.23, 24 In the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium Pharmacogenomics Working Group, a previous GWA study of sulfonylurea-QT interactions that included approximately 30 000 European ancestry individuals with cross-sectional measures of drug use and the QT interval did not identify any pharmacogenomic loci at genome-wide levels of significance.25
To increase our power to identify novel pharmacogenomic loci for sulfonylureas, we extended this effort to include several additional diverse-ancestry cohorts with a high prevalence of sulfonylurea use. Additionally, we incorporated repeated measures of drug exposure and phenotype with novel analytic methods.26 Because genetic variants can have different effects on the two components of the QT interval27 -- the JT interval, which measures primarily repolarization, and the QRS interval, which measures primarily conduction and depolarization -- we also extended our analyses to include them.
METHODS
Study Population and Overview
Eleven cohorts participated in this meta-analysis from the CHARGE28 Pharmacogenomics Working Group: Age, Gene/Environment Susceptibility – Reykjavik Study (AGES); Atherosclerosis Risk in Communities (ARIC) Study; Cardiovascular Health Study (CHS); Health, Aging, and Body Composition (Health ABC); Hispanic Community Health Study/Study of Latinos (HCHS/SOL); Jackson Heart Study (JHS); Multi-Ethnic Study of Atherosclerosis (MESA); Netherlands Epidemiology of Obesity (NEO) Study; Prospective Study of Pravastatin in the Elderly at Risk (PROSPER); Rotterdam Study cohorts 1 and 2; and the Women’s Health Initiative (WHI) (Supplementary Text). Cohorts contributed results from European ancestry (EA), African American (AA), and/or Hispanic/Latino ancestry (HA) populations. All cohorts had at least one study visit with an assessment of medication use and a resting 12-lead electrocardiogram (ECG); AGES, ARIC, CHS, the Rotterdam Study, MESA, and WHI had multiple study visits with these assessments and contributed repeated measures. Each cohort followed a pre-specified analysis protocol, and findings from within-cohort analyses were combined in three sets of ancestry-specific meta-analyses (EA, AA, HA) for three ECG phenotypes (QT, JT, and QRS intervals), for a total of nine primary analyses. All available cohorts were included in this single discovery effort, rather than a two-stage design with discovery and replication, to improve our power to identify significant pharmacogenomic interactions.29, 30 This study was approved by the institutional review board of each cohort.
Inclusion and Exclusion Criteria
Participants with genome-wide genotype data and with ECG measurements and medication assessments at the same study visits were eligible. The following exclusion criteria were applied: poor ECG quality; atrial fibrillation; second or third degree atrioventricular heart block; QRS interval > 120 ms; a paced rhythm; history of heart failure; pacemaker implantation; pregnancy; and ancestry other than European, African American, or Hispanic/Latino. For studies with repeated measures, exclusion criteria were applied for each visit-specific observation.
Drug Exposure Assessment
Sulfonylurea drugs are listed in Supplemental Table 1. Sulfonylurea use was assessed through medication inventories conducted at study visits, or using information from a pharmacy database for the Rotterdam Study (Supplemental Table 2). Some cohorts assessed medication use on the day of the study visit, while others assessed medication use within an interval of time prior to the study visit, typically 2 weeks. For cohorts with repeated measures, the number of participants exposed to sulfonylureas (Nexposed) was the sum of the estimated number of independent observations at which each participant was exposed, calculated from the following equation:
where the summand is the product of the estimated number of independent observations and the proportion of observations at which a participant was exposed,31 with ni being the number of observations for participant i, ρ̂ an estimate of the pairwise visit-to-visit correlation in outcome within participants from a generalized estimating equation (GEE)-exchangeable model that does not contain genetic data, and #{Eit = 1} the number of observations for which participant i was exposed.26
Phenotype Measurement
QT and QRS intervals were recorded from resting, supine or semi-recumbent, standard 12-lead ECGs (Supplemental Table 2). Across all cohorts, comparable procedures were used for preparing participants, placing electrodes, recording, transmitting, processing, and controlling the quality of ECGs. Cohorts used Marquette MAC 5000, MAC 1200, or MAC PC (GE Healthcare, Milwaukee, Wisconsin, USA), Burdick Eclips 850i (Cardiac Science, Manchester, UK), or ACTA (EASOTE, Florence, Italy) machines. Recordings were processed using Marquette 12SL, MEANS, or University of Glasgow software. The JT interval was calculated by the formula: JT = QT – QRS.
Genotyping and Imputation
All cohorts performed genome-wide genotyping with either Affymetrix (Santa Clara, CA, USA) or Illumina (San Diego, CA, USA) arrays, and used similar quality control thresholds for excluding samples and single nucleotide polymorphisms (SNPs) (Supplemental Table 3). Sex mismatches, duplicate samples, and first-degree relatives (except in HCHS/SOL and JHS) were excluded. DNA samples and SNPs with call rates less than 90–98%, depending on the cohort, were excluded. Within each cohort, SNPs with minor allele frequencies (MAF) less than 1% or that failed Hardy-Weinberg equilibrium were excluded.
Genotypes were imputed using ancestry-specific HapMap2,32–34 HapMap3, 1000 Genomes Phase 1, or 1000 Genomes Phase 3 reference panels (Supplemental Table 3).35, 36 Genotypes imputed from build 37 of the human genome were lifted over to build 3637, 38 to enable comparisons between imputation platforms, and all results were restricted to SNPs present in HapMap2.
Statistical Analysis
GWA analyses were performed by each cohort separately, and ancestry-specific results for each ECG phenotype were combined with meta-analysis. Within each cohort, for approximately 2.5 million genotyped or imputed autosomal SNPs, sulfonylurea-SNP interactions were estimated with an additive genetic model using mixed effects models, GEE, or linear regression with robust standard errors. The analytic model varied based on the study design and the availability of longitudinal data (Supplemental Table 4). All analyses were adjusted for age, sex, study site or region, principal components of genetic ancestry, visit-specific RR interval (inversely related to heart rate), and visit-specific use of QT prolonging medications. The QT-prolonging effect of medications was categorized as definite, possible, or conditional, according to the University of Arizona Center for Education and Research on Therapeutics (UAZ CERT) system of classification, and adjusted for as binary variables for each category (presence of any versus none).39 HCHS/SOL incorporated estimates of relatedness into all analyses. Cohort-specific results were corrected for genomic inflation.
Previous simulations demonstrated that models using robust standard errors underestimate the variance of coefficient estimates for SNPs with low MAFs.26 To account for this, corrected standard errors were calculated using a t distribution as the reference distribution. Cohort and SNP-specific degrees of freedom (df) for the t distribution were estimated primarily using Satterthwaite’s method.40 For cohorts unable to implement Satterthwaite’s method, an approximate df was calculated as two times the cohort- and SNP-specific product of the SNP imputation quality (0–1), MAF (0.00–0.50), and Nexposed. Standard errors were then corrected by assuming a normal reference distribution that yielded the t distribution-based P values from the coefficient estimates. Furthermore, because simulations demonstrated that corrected standard errors were unstable when minor allele counts among the exposed were low, an approximate df filter of 10 was applied to cohort-specific results across all SNPs.
Primary analyses
For each ECG phenotype and for each ancestral population, SNP-by-treatment interaction coefficients and corrected standard errors were combined with inverse-variance weighted meta-analysis using METAL.41 SNPs had to meet quality control criteria and pass the df filter in at least two studies to be included. The threshold for statistical significance was P < 5x10−8, which has been used in other GWA studies of correlated phenotypes.42, 43 For each locus with multiple SNPs meeting the threshold for statistical significance, a lead SNP with the lowest P value was identified. Significant loci and loci at suggestive levels of statistical significance (P < 10−6) were annotated using information from several genomics and bioinformatics databases. RefSeq genes within 500 kb of lead SNPs were identified from the UCSC Genome Browser.44 The NHGRI-EBI GWAS Catalog was queried for other traits associated with lead SNPs in GWA studies.45 HaploReg (Broad Institute) was queried to identify missense coding variants in linkage disequilibrium (LD) (R2 < 0.8) with lead SNPs.46 Cis-expression quantitative trait loci (cis-eQTLs) in LD with lead SNPs were identified from several gene expression databases, including ScanDB and the Broad Institute GTEx Portal, that include samples from multiple cell lines and tissue sites, including whole blood, leukocytes, subcutaneous adipose, skeletal muscle, lung, skin, fibroblasts, arterial wall, and left ventricular and atrial heart tissue.47
Secondary analyses
All ancestry-specific summary results were combined in a trans-ethnic inverse-variance weighted meta-analysis using METAL. Because effects may be heterogeneous across different racial/ethnic populations,48, 49 we conducted additional trans-ethnic analyses using the Bayesian MANTRA method, with a genome-wide significance threshold of log10(Bayes Factor [BF]) > 6.50
Previous candidate gene pharmacogenetic studies have identified several pharmacokinetic and pharmacodynamic loci for sulfonylurea-associated glucose-lowering effects and hypoglycemia.19–23, 51–54 Also, large-scale GWA studies have identified 35 replicated genetic loci for QT interval main effects.18 For these candidate SNPs, the P value threshold for statistical significance was 0.05 divided by the total number of tests conducted across all ECG phenotypes and populations: 0.05 / 158 = 3.2 x 10−4.
For the QT interval, we also assessed for enrichment of candidate SNP-by-treatment interactions with a high probability of being functional for cardiac conduction and repolarization phenotypes. SNPs that fell within 50 kb of transcripts that are preferentially expressed in the left ventricle were identified using the GTEx database (839 transcripts). SNPs in these gene regions were filtered to those falling within DNAse I hypersensitivity, H3K4me3 or CTCF chip-seq peaks assayed in human cardiomyocytes from the NIH Roadmap Epigenomics Consortium (http://www.roadmapepigenomics.org). Additionally, SNPs that were eQTLs in left ventricle tissue (P < 1 x 10−10) were selected.55, 56 All variants were pruned using ancestry-matched LD patterns from the 1000 Genomes project at a level of R2 > 0.5,57 resulting in 9 004, 8 424 and 5 437 candidate SNPs for EA, AA and HA analyses respectively. The P value threshold for statistical significance for these candidate SNP analyses was 0.05 divided by the total number of SNPs selected (P < 5.6 x 10−6 for EA, P < 5.9 x 10−6 for AA, and P < 5.6 x 10−6 for HA). The selection of candidate SNPs was validated by evaluating enrichment for low P value variants using main-effect SNP associations from the QT Interval-International GWAS Consortium.58
RESULTS
Characteristics of the 11 cohorts and 21 ancestry-specific analysis populations are listed in Table 1. There were 45 002 EA participants (Nexposed 2 095 [4.7%]), 11 731 AA participants (Nexposed 1 167 [9.9%]), and 15 124 HA participants (Nexposed 794 [5.2%]), for a total of 71 857 (Nexposed 4 056 [5.6%]). Mean durations of ECG intervals ranged from 397 to 414 ms for QT, 300 to 325 ms for JT, and 85 to 98 ms for QRS. The correlation between traits was evaluated among EA and AA participants of CHS: QRS and JT were highly correlated (R2 > 0.5), while QRS was not correlated with either QRS or JT (R2 < 0.1).
Table 1.
Characteristics of study populations
| Cohort | N | Nexposed (%) | Age, y (SD) | Female, N (%) | QT interval, ms (SD) | JT interval, ms (SD) | QRS interval, ms (SD) |
|---|---|---|---|---|---|---|---|
| European Ancestry | |||||||
| AGES | 2 587 | 64 (2.5) | 75 (4.7) | 925 (64) | 406 (34) | 316 (33) | 90 (10) |
| ARIC | 8 597 | 379 (4.4) | 54 (5.7) | 4 453 (53) | 399 (29) | 308 (29) | 91 (10) |
| CHS | 3 055 | 280 (9.2) | 72 (5.3) | 1 880 (63) | 414 (32) | 321 (30) | 88 (10) |
| Health ABC | 1 441 | 81 (5.6) | 74 (2.8) | 714 (49) | 414 (32) | 324 (32) | 90 (11) |
| MESA | 2 256 | 71 (3.1) | 62 (10.1) | 1 156 (52) | 412 (29) | 320 (29) | 93 (9) |
| NEO | 5 366 | 94 (1.8) | 56 (5.9) | 2 521 (47) | 406 (29) | 313 (29) | 93 (10) |
| PROSPER | 4 555 | 243 (5.3) | 75 (3.3) | 2 445 (47) | 414 (36) | 320 (35) | 94 (11) |
| Rotterdam 1 | 4 805 | 216 (4.5) | 69 (8.6) | 2 891 (60) | 397 (29) | 300 (28) | 97 (11) |
| Rotterdam 2 | 1 889 | 84 (4.4) | 65 (7.6) | 1 070 (57) | 403 (28) | 305 (28) | 98 (11) |
| WHI GARNET | 3 943 | 304 (7.7) | 66 (6.8) | 3 642 (100) | 400 (32) | 314 (31) | 86 (9) |
| WHI MOPMAP | 1 324 | 36 (2.7) | 63 (6.6) | 1 224 (100) | 402 (30) | 316 (30) | 86 (8) |
| WHIMS | 5 184 | 243 (4.7) | 69 (6.0) | 4 811 (100) | 401 (30) | 315 (30) | 86 (9) |
| Total | 45 002 | 2 095 (4.7) | |||||
| African American | |||||||
| ARIC | 2 191 | 213 (9.7) | 53 (5.8) | 1 322 (62) | 400 (33) | 310 (32) | 90 (10) |
| CHS | 707 | 141 (20.0) | 73 (5.6) | 447 (65) | 409 (35) | 317 (36) | 88 (11) |
| Health ABC | 1 020 | 111 (10.9) | 73 (2.9) | 588 (58) | 411 (35) | 322 (34) | 88 (11) |
| JHS | 2 122 | 117 (5.5) | 50 (11.8) | 1 244 (61) | 410 (30) | 319 (30) | 92 (1) |
| MESA | 1 464 | 135 (9.2) | 62 (10.0) | 796 (54) | 410 (32) | 319 (31) | 91 (10) |
| WHI SHARe | 4 227 | 450 (10.6) | 61 (6.8) | 3 860 (100) | 401 (34) | 316 (33) | 85 (9) |
| Total | 11 731 | 1 167 (9.9) | |||||
| Hispanic/Latino | |||||||
| HCHS/SOL | 12 024 | 518 (4.3) | 46 (13.8) | 7 155 (60) | 416 (28) | 325 (29) | 91 (10) |
| MESA | 1 316 | 134 (10.2) | 61 (10.3) | 681 (52) | 409 (30) | 318 (30) | 91 (10) |
| WHI SHARe | 1 784 | 142 (7.9) | 60 (6.4) | 1 627 (100) | 402 (30) | 316 (30) | 86 (9) |
| Total | 15 124 | 794 (5.2) | |||||
| Total, all ancestries | 71 857 | 4 056 (5.6) | |||||
. ms = milliseconds, SD = standard deviation, y = years. Study abbreviations: AGES = Age, Gene/Environment Susceptibility – Reykjavik Study, ARIC = Atherosclerosis Risk in Communities Study, CHS = Cardiovascular Health Study, Health ABC = Health, Aging, and Body Composition Study, HCHS/SOL = Hispanic Community Health Study/Study of Latinos, JHS = Jackson Heart Study, MESA = Multi-Ethnic Study of Atherosclerosis, NEO = Netherlands Epidemiology of Obesity, PROSPER = Prospective Study of Pravastatin in the Elderly at Risk, Rotterdam 1 = first cohort of the Rotterdam Study, Rotterdam 2 = second cohort of the Rotterdam study, WHI GARNET = Women’s Health Initiative Genome-wide Association Research Network into Effects of Treatment, WHI MOPMAP = Women’s Health Initiative Modification of Particulate Matter-Mediated Arrhythmogenesis in Populations, WHI SHARe = Women’s Health Initiative SNP Health Association Resource, WHIMS = Women’s Health Initiative Memory Study.
Primary analysis results
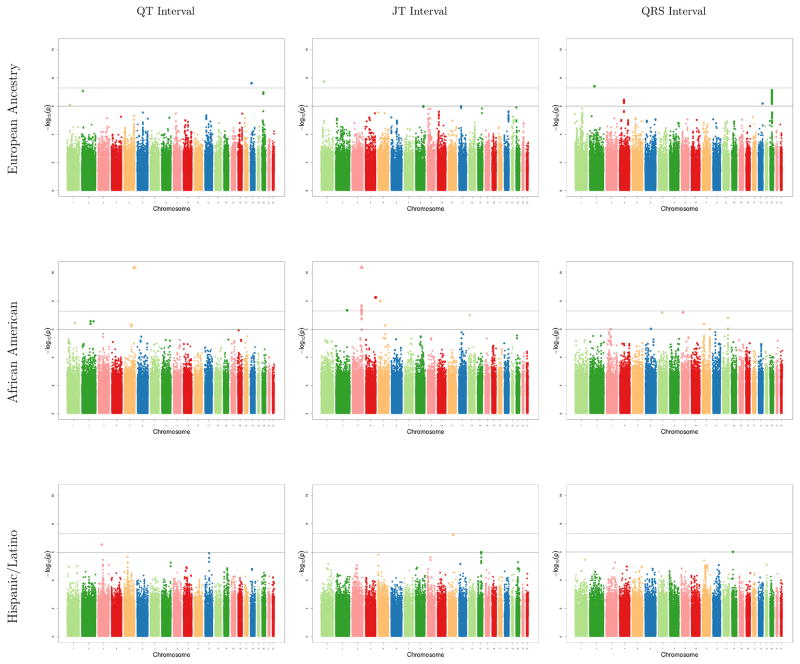
Sulfonylurea-SNP interaction results from cohort-specific GWA analyses were well-calibrated: genomic inflation factors for ancestry-specific meta-analyzed results ranged from to 0.97 to 1.04 (Supplemental Table 5). A total of 31 sulfonylurea-SNP interaction associations met the genome-wide threshold for significance, comprising 8 unique loci (Figure, Table 2). Each locus was significant for only one of the three ECG phenotypes (2 QT, 5 JT, 1 QRS) and in only one racial/ethnic population (3 EA, 5 AA). Absolute values for effect sizes ranged from 4 to 16 ms. All loci were intergenic and none had substantial LD with coding variants. Supplemental Table 6 lists the SNP-phenotype associations for the 8 significant loci in each ancestry-specific meta-analysis; none reached even nominal levels of significance in the other populations (P < 0.05).
Figure.
Manhattan plots from each ancestry specific meta-analysis (row) for sulfonylurea-SNP interaction associations with each ECG phenotype (column). The dashed line is the genome-wide threshold for significance (P < 5 x 10−8). The solid line is the threshold for suggestive associations (P < 10−6). SNPs with P values < 10−10, outside of the range of the Y axis, are denoted by triangles.
Table 2.
Summary of significant sulfonylurea-SNP interaction associations with QT, JT, and QRS intervals from ancestry-specific GWAS meta-analyses (P < 5 x 10−8)
| Lead SNP | Chr:position (hg19) | Nearest gene | Race | Studies | Min/alt alleles | MAF | Effect | SE | P | Function | Other GWAS | Coding | eQTL (P<5x10−8) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QT interval | |||||||||||||
| rs9966832 | 18:23405188 | SS18 | EA | 3 | G/A | 0.03 | −10.4 | 1.9 | 2.3E-08 | Intergenic | Periodontitis66 | ||
| rs830233 | 5:165403746 | AA | 4 | A/G | 0.05 | −16.3 | 2.3 | 2.5E-12 | Intergenic | ||||
| JT interval | |||||||||||||
| rs1890262 | 1:62114402 | TM2D1,NFIA | EA | 2 | A/G | 0.03 | 14.9 | 2.6 | 1.8E-08 | Intergenic | |||
| rs12468579 | 2:191832264 | GLS,STAT1 | AA | 6 | G/A | 0.49 | 4.1 | 0.8 | 4.5E-08 | Intergenic | GLS60–63, MFSD660 | ||
| rs1478173 | 3:162276405 | AA | 2 | C/A | 0.03 | −15.0 | 2.1 | 1.0E-12 | Intergenic | ||||
| rs17281245 | 4:182635289 | TENM3 | AA | 5 | C/T | 0.06 | 8.8 | 1.5 | 5.4E-09 | Intergenic | |||
| rs7713675 | 5:28750307 | LSP1P3 | AA | 4 | C/T | 0.05 | −12.2 | 2.1 | 9.8E-09 | Intergenic | |||
| QRS interval | |||||||||||||
| rs7595140 | 2:71551621 | ZNF638,PAIP2B | EA | 4 | G/C | 0.03 | −5.7 | 1.0 | 3.8E-08 | Intergenic | |||
EA = European ancestry, AA = African American, HA = Hispanic/Latino ancestry, MAF = minor allele frequency, SE = standard error. Studies = number of cohorts contributing to ancestry-specific analysis. Other GWAS = phenotypes associated with lead SNP (P < 5 x 10−8) in other genome-wide association studies. Coding = lead SNP in linkage disequilibrium (r2 > 0.8) with a protein coding variant. eQTL = transcripts associated with SNPs in linkage disequilibrium (r2 > 0.8) with lead SNP.
The TM2D1-NFIA locus (rs1890262) on chromosome 1 was approximately 200 kb away from a locus associated with QRS interval main effects; NFIA encodes a transcription factor of unknown significance for cardiac tissue development.59 A locus on chromosome 2 (rs12468579) was 2 kb away from GLS and was also identified as a cis-eQTL for GLS and MFSD6 transcripts in blood, lung, and prostate;60–63 GLS encodes glutaminase, which catalyzes the production of glutamine, the most abundant excitatory neurotransmitter in the central nervous system.64 The chromosome 3 locus (rs1478173) was approximately 115 kb away from a locus for coronary artery disease.65 The only locus associated with another trait (periodontitis) in a previous GWA study was rs9966832 near SS18 on chromosome 18.66
Among the 37 suggestive associations (P value < 10−6 but > 5 x 10−8) (Supplemental Table 7), 15 (41%) were intronic, one was a missense variant, three were in LD (r2 > 0.8) with missense variants, and five were cis-eQTLs in multiple tissues. Several of the sub-threshold loci were located in or near genes that might be relevant to cardiac conduction, repolarization, or arrhythmogenesis. For example, rs6035275 is an intronic SNP in SLC24A3, a potassium-dependent sodium/calcium ion exchanger that plays a role in calcium homeostasis,67 and rs624896 is located 24 kb away from KCNN2, a voltage-independent calcium-activated potassium channel that helps to regulate neuronal electrical conduction.68
Secondary analysis results
Trans-ethnic fixed effects meta-analyses and MANTRA analyses did not identify any additional loci (results not shown). Among the candidate SNPs, only one was significantly associated with an ECG phenotype when multiple comparisons were accounted for (Table 3). This SNP, rs1057910 (Ile359Leu), is a loss of function variant that defines the *3 haplotype of CYP2C9, a highly polymorphic cytochrome P450 (CYP) enzyme that metabolizes 15–20% of all known drugs that undergo phase I oxidative metabolism.69 For the sulfonylurea-SNP interaction, the minor allele of rs1057910 was associated with a 7.6 ms (standard error [SE] 2.1 ms) decrease in the QT interval (P = 2.3 x 10−4) in HA cohorts (MAF 0.05), but not in EA cohorts (MAF 0.07). This SNP did not meet filtering criteria for meta-analysis in the AA cohorts. The more common functional variant (rs1799853) that defines the *2 haplotype of CYP2C9 (MAF 0.13 in EA, 0.09 in HA) was also evaluated, but it was not significantly associated with any of the ECG phenotypes.
Table 3.
Results for pharmacokinetic, pharmacodynamic, and QT main effect candidate SNPs.
| SNP | Chr | Gene | P values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| QT | JT | QRS | |||||||||
| EA | AA | HA | EA | AA | HA | EA | AA | HA | |||
| Pharmacokinetic | |||||||||||
| rs105791019 | 10 | CYP2C9 | 0.42 | 2.3E-4 | 0.06 | 0.55 | 0.38 | 4.1E-3 | |||
| rs179985319 | 10 | CYP2C9 | 0.99 | 0.33 | 0.81 | 0.25 | 0.75 | 0.62 | |||
| Pharmacodynamic | |||||||||||
| rs1049435551 | 1 | NOS1AP | 0.27 | 0.51 | 0.89 | 0.87 | 0.88 | 0.62 | 0.37 | 0.07 | 0.74 |
| rs790314652, 53 | 10 | TCF7L2 | 0.30 | 0.94 | 0.70 | 0.70 | 0.44 | 0.24 | 0.51 | 0.89 | 0.79 |
| rs1225537252, 53 | 10 | TCF7L2 | 0.39 | 0.12 | 0.71 | 0.77 | 0.22 | 0.50 | 0.51 | 0.04 | 0.86 |
| rs521523, 54 | 11 | KCNJ11 | 0.93 | 0.83 | 0.57 | 0.16 | 0.01 | 0.84 | 0.33 | 0.40 | 0.76 |
| rs75711021 | 11 | ABCC8 | 1.00 | 0.68 | 0.47 | 0.08 | 2.5E-3 | 0.60 | 0.24 | 0.15 | 0.66 |
| QT main effect18 | |||||||||||
| rs2298632 | 1 | TCEA3 | 0.29 | 0.88 | 0.20 | 0.78 | 0.89 | 0.78 | 0.58 | 0.87 | 0.75 |
| rs846111 | 1 | RNF207 | 1.00 | 0.88 | 0.79 | 0.82 | 0.34 | 0.84 | 0.64 | 0.67 | 0.91 |
| rs10919070 | 1 | ATP1B1 | 0.91 | 0.40 | 0.25 | 0.90 | 0.48 | 0.35 | |||
| rs12143842 | 1 | NOS1AP | 0.44 | 0.88 | 0.75 | 0.67 | 0.29 | 0.52 | 0.90 | 0.49 | 0.97 |
| rs295140 | 2 | SPATS2L | 0.12 | 0.54 | 0.88 | 0.12 | 0.42 | 0.29 | 0.67 | 0.83 | 0.67 |
| rs938291 | 2 | SP3 | 0.79 | 0.41 | 0.07 | 0.41 | 0.10 | 0.83 | 0.75 | 0.58 | 0.65 |
| rs7561149 | 2 | TTN-CCDC141 | 0.85 | 0.72 | 0.96 | 0.84 | 0.41 | 0.44 | 0.43 | 0.69 | 0.49 |
| rs12997023 | 2 | SLC8A1 | 0.29 | 0.51 | 0.61 | 0.23 | 0.50 | 0.15 | 0.77 | 0.44 | 0.22 |
| rs6793245 | 3 | SCN5A-SCN10A | 0.95 | 0.48 | 0.55 | 0.17 | 0.57 | 0.85 | 0.80 | 0.65 | 0.94 |
| rs17784882 | 3 | C3ORF75 | 0.16 | 0.26 | 0.31 | 0.55 | 0.91 | 0.32 | 0.12 | 0.40 | 0.57 |
| rs3857067 | 4 | SMARCAD1 | 0.82 | 0.18 | 0.46 | 0.76 | 0.32 | 0.81 | 0.33 | 0.78 | 0.41 |
| rs2363719 | 4 | SLC4A4 | 0.23 | 0.72 | 0.05 | 0.89 | 0.95 | 0.51 | 0.27 | 0.84 | 0.28 |
| rs10040989 | 5 | GFRA3 | 0.93 | 0.70 | 0.12 | 0.14 | 0.12 | 0.39 | 0.35 | 0.82 | 0.09 |
| rs7765828 | 6 | GMPR | 0.63 | 0.44 | 0.23 | 0.37 | 0.19 | 0.05 | 0.99 | 0.03 | 0.40 |
| rs11153730 | 6 | SLC35F1-PLN | 0.84 | 0.67 | 0.27 | 0.24 | 0.52 | 0.70 | 0.45 | 0.16 | 0.37 |
| rs9920 | 7 | CAV1 | 0.36 | 0.01 | 0.52 | 0.64 | 0.08 | 0.85 | |||
| rs2072413 | 7 | KCNH2 | 0.30 | 0.88 | 0.75 | 0.27 | 0.38 | 0.77 | 0.82 | 0.70 | 0.95 |
| rs1961102 | 8 | AZIN1 | 0.33 | 0.22 | 0.18 | 0.30 | 1.00 | 0.96 | 0.44 | 0.51 | 0.19 |
| rs11779860 | 8 | LAPTM4B | 0.74 | 0.74 | 0.08 | 0.14 | 0.46 | 0.65 | 0.23 | 0.82 | 0.16 |
| rs16936870 | 8 | NCOA2 | 0.08 | 0.11 | 0.96 | 0.24 | 0.82 | 0.16 | 0.02 | 0.19 | 0.54 |
| rs174583 | 10 | FEN1-FADS2 | 0.87 | 0.26 | 0.98 | 0.98 | 0.57 | 0.16 | 0.98 | 0.35 | 0.48 |
| rs2485376 | 10 | GBF1 | 0.86 | 0.50 | 0.51 | 0.03 | 0.41 | 0.07 | 0.13 | 0.73 | 0.79 |
| rs7122937 | 11 | KCNQ1 | 0.25 | 0.31 | 0.11 | 0.20 | 0.15 | 0.38 | 0.12 | 0.54 | 0.29 |
| rs3026445 | 12 | ATP2A2 | 0.94 | 0.29 | 0.42 | 0.23 | 0.81 | 0.89 | 0.33 | 0.28 | 0.50 |
| rs728926 | 13 | KLF12 | 0.30 | 0.29 | 0.50 | 0.46 | 0.70 | 0.20 | 0.75 | 0.21 | 0.16 |
| rs2273905 | 14 | ANKRD9 | 0.38 | 0.31 | 0.16 | 0.71 | 0.66 | 0.50 | 0.21 | 0.13 | 0.09 |
| rs3105593 | 15 | USP50-TPRM7 | 0.71 | 0.89 | 0.44 | 0.73 | 0.91 | 0.41 | 0.80 | 0.35 | 0.29 |
| rs735951 | 16 | LITAF | 0.34 | 0.08 | 0.52 | 0.28 | 0.43 | 0.23 | 0.59 | 0.10 | 0.92 |
| rs1052536 | 17 | LIG3 | 0.58 | 0.70 | 0.77 | 0.65 | 0.67 | 0.39 | 0.65 | 0.40 | 0.70 |
| rs246185 | 16 | MKL2 | 0.11 | 0.99 | 0.31 | 0.81 | 0.71 | 0.54 | 0.32 | 0.73 | 0.28 |
| rs246196 | 16 | CNOT1 | 0.38 | 0.96 | 0.35 | 0.74 | 0.97 | 0.91 | 0.19 | 0.60 | 0.39 |
| rs1296720 | 16 | CREBBP | 0.73 | 0.32 | 0.33 | 0.29 | 0.29 | 0.36 | 0.14 | ||
| rs1396515 | 17 | KCNJ2 | 0.76 | 0.98 | 0.78 | 0.41 | 0.19 | 0.64 | 0.72 | 0.69 | 0.64 |
| rs9892651 | 17 | PRKCA | 0.49 | 0.54 | 0.29 | 0.44 | 0.38 | 0.98 | 0.24 | 0.94 | 0.37 |
| rs1805128 | 21 | KCNE1 | 0.69 | 0.48 | 0.36 | ||||||
EA = European ancestry, AA = African American, HA = Hispanic/Latino ancestry. With Bonferroni correction for 158 tests, the threshold for statistical significance was 3.1 x 10−4. Significant associations are bolded.
Selecting additional candidate SNPs based on bioinformatic analysis of annotation from cardiac gene expression and regulatory marks active in cardiomyocytes did not identify additional loci. While these variants were enriched for signals among main-effects QT analyses (Supplemental Figure 1), none met our statistical significance threshold for sulfonylurea-SNP interactions with the QT, JT or QRS intervals (Supplemental Figure 2).
DISCUSSION
In this study, we identified eight novel loci for sulfonylurea-genetic interactions with the QT, JT, and QRS intervals. For seven of these pharmacogenomic associations, the effect size was > 5ms, the threshold for regulatory concern established by the FDA. Compared to our previous effort, which included 869 sulfonylurea users among approximately 30 000 EA participants and failed to identify any genome-wide significant loci, this effort included over 4 000 sulfonylurea users among over 70 000 participants from diverse ancestries. Broadening the racial/ethnic composition of the study population and extending our investigation to related ECG phenotypes improved our ability to identify pharmacogenomic loci; most were identified in AA populations and for the JT interval.
Some of the novel pharmacogenomic loci discovered in our study were near (but not in LD with) loci for related traits, such as the NFIA locus for QRS interval main effects59 and a locus on chromosome 3 for coronary artery disease.65 None of the eight loci were near genes that have a clear role in cardiac conduction or repolarization, and even with the use of several bioinformatics resources, the biologic mechanism that would explain these drug-gene interactions are unknown. Among the loci that did not meet the genome-wide threshold for statistical significance but had a P value < 10−6, several were located in or near potassium ion channels or ion exchanger genes involved in electrical conduction. Without rigorous statistical evidence to support these sub-threshold associations, however, their validity is uncertain and replication is needed.
We also assessed candidate SNPs involved in the pharmacokinetics and pharmacodynamics of sulfonylureas and SNPs associated with the QT interval in main effects GWA analyses. Among these SNPs, only a well-known functional variant in CYP2C9 was identified as a pharmacogenomic locus in our study, and among HA participants only. Variant rs1057910 (CYP2C9*3) reduces the catalytic activity of CYP2C9, the main CYP isoenzyme involved in the metabolism of sulfonylureas,69, 70 and this variant has been associated with severe skin reactions from phenytoin use71 and warfarin-related hemorrhage.72, 73 Allele frequencies for rs1057910 were similar among HA and EA participants in our study, which has also been reported elsewhere.69, 74 To our knowledge, only one previous study has identified CYP2C9 as a pharmacogenomic locus in a HA population; 75 among 122 male Puerto Rican patients on warfarin therapy, functional variants in CYP2C9 and VKORC1 were associated with lower warfarin dose requirements and a higher risk of warfarin adverse effects.76 Other studies, conducted primarily in EA populations, have evaluated the impact of CYP2C9 functional variants on sulfonylurea-related treatment response and adverse effects. In one study, the presence of either the CYP2C9*2 or the CYP2C9*3 haplotype was associated an increased reduction in hemoglobin A1c and an increased probability of achieving adequate glycemic control,19 and in another study these variants were associated with an increased risk of hypoglycemia among elderly persons.77
In our study, the variant rs1057910 was associated with a shorter QT interval among HA participants. This was a surprising finding, because reduced function variants in CYP2C9 decrease the clearance of sulfonylureas,70 which would be expected to prolong the QT interval. A short QT interval, which can be hereditary or acquired, has been associated with cardiac arrhythmias and an increased risk of death.78–80 Various drugs can also shorten the QT interval, and whether drug-induced shortening of the QT interval causes cardiac arrhythmias is an area of debate.81 Although many pharmacogenomic findings for diabetes drugs23, 24 and for other types of drug therapies82, 83 have failed to replicate in the past, there is now a growing body of evidence that rs1057910 may be a genuine pharmacogenomic locus for sulfonylureas. Whether this variant contributes to the increased cardiovascular risk associated with sulfonylureas in a subset of the population is uncertain.
Strengths of our study include repeated high-quality phenotype measurements recorded from ECGs conducted at study visits, a large sample size, and the inclusion of diverse ancestry populations. There were also several limitations. With the exception of the two cohorts from the Rotterdam Study, medication use was assessed with the inventory method,84 and some participants classified as sulfonylurea users may have failed to take the medication on the day of the study visit. However, changes in diabetes medications typically occur over a period of months or years rather than weeks, and this type of misclassification would bias associations toward the null, decreasing power to identify pharmacogenomic associations. By the same rationale, this type of misclassification is expected to decrease rather than increase the chance of false positive findings.
Because all available analysis populations from the CHARGE consortium were included in a single-stage discovery analysis, which is a more powerful approach than a two-stage approach that includes separate discovery and validation samples,29, 30 there was no opportunity to assess the validity of our findings through replication in independent study populations. The increasing availability of electronic health data and the decreasing cost of genotyping has led to the emergence of a new model for genomic discovery research: biobanks that link genetic data on tens or even hundreds of thousands of individuals with prescription records and other electronic health data to create large data repositories. Some biobank studies, such as the UK Biobank85, have conducted ECGs as a part of study visits, while others86 may have access to ECGs obtained through clinical care. Although the large sample sizes in these biobank studies may be attractive for pharmacogenomics research, results from ECGs and other clinical tests that are conducted during the course of clinical care may be related to the indication for conducting the test, which can result in confounding and false positive associations.
In conclusion, we have identified several novel loci for sulfonylurea-related changes in various ECG phenotypes in a large multi-site pharmacogenomics study conducted within the CHARGE consortium. Although these findings may explain some of the cardiovascular risk associated with sulfonylureas for some individuals, replication in independent study populations is necessary and further work is needed to determine the genetic and biologic mechanisms of these drug-gene interactions.
Supplementary Material
Acknowledgments
Age, Gene/Environment Susceptibility – Reykjavik Study (AGES): This study has been funded by NIH contracts N01-AG-1-2100 and 271201200022C, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). The study is approved by the Icelandic National Bioethics Committee, VSN: 00-063. The researchers are indebted to the participants for their willingness to participate in the study.
Atherosclerosis Risk in Communities (ARIC): The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung and Blood Institute Contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C and HHSN268201100012C), R01HL087641, R01HL59367 and R01HL086694; National Human Genome Research Institute Contract U01HG004402; and National Institutes of Health Contract HHSN268200625226C. We thank the staff and participants of the ARIC study for their important contributions. Infrastructure was partly supported by Grant No. UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research.
Cardiovascular Health Study (CHS): This CHS research was supported by NHLBI contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086; and NHLBI grants U01HL080295, R01HL087652, R01HL105756, R01HL103612, R01HL120393, and R01HL085251with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The provision of genotyping data was supported in part by the National Center for Advancing Translational Sciences, CTSI grant UL1TR000124, and the National Institute of Diabetes and Digestive and Kidney Disease Diabetes Research Center (DRC) grant DK063491 to the Southern California Diabetes Endocrinology Research Center. NS was supported by R01HL116747 and RO1HL111089. JSF was supported by K08HL116640.
Health, Aging, and Body Composition (Health ABC): This research was supported by NIA Contracts N01AG62101, N01AG62103 and N01AG62106. The genome-wide association study was funded by NIA Grant 1R01AG032098-01A1 to Wake Forest University Health Sciences and genotyping services were provided by the Center for Inherited Disease Research (CIDR). CIDR is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University, Contract No. HHSN268200782096C. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
Hispanic Community Health Study/Study of Latinos (HCHS/SOL): We thank the participants and staff of the HCHS/SOL study for their contributions to this study. The baseline examination of HCHS/SOL was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices contributed to the first phase of HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research (NIDCR), National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, NIH Institution-Office of Dietary Supplements. The Genetic Analysis Center at University of Washington was supported by NHLBI and NIDCR contracts (HHSN268201300005C AM03 and MOD03). Genotyping efforts were supported by NHLBI HSN 26220/20054C, NCATS CTSI grant UL1TR000124, and NIDDK Diabetes Research Center (DRC) grant DK063491.
Jackson Heart Study (JHS): We thank the Jackson Heart Study (JHS) participants and staff for their contributions to this work. The JHS is supported by contracts HHSN268201300046C, HHSN268201300047C, HSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities.
Multi-Ethnic Study of Atherosclerosis (MESA): MESA and MESA SNP Health Association Resource (SHARe) are conducted and supported by the National Heart, Lung and Blood Institute (NHLBI) in collaboration with MESA investigators. Support is provided by grants and contracts N01 HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169 and RR-024156. Additional funding was supported in part by the Clinical Translational Science Institute grant UL1RR033176 and is now at the National Center for Advancing Translational Sciences, CTSI grant UL1TR000124. We also thank the other investigators, the staff and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Netherlands Epidemiology of Obesity (NEO): The authors of the NEO study thank all individuals who participated in the Netherlands Epidemiology in Obesity study, all participating general practitioners for inviting eligible participants and all research nurses for collection of the data. We thank the NEO study group, Pat van Beelen, Petra Noordijk and Ingeborg de Jonge for the coordination, lab and data management of the NEO study. The genotyping in the NEO study was supported by the Centre National de Génotypage (Paris, France), headed by Jean-Francois Deleuze. The NEO study is supported by the participating Departments, the Division and the Board of Directors of the Leiden University Medical Center, and by the Leiden University, Research Profile Area Vascular and Regenerative Medicine. Dennis Mook-Kanamori is supported by Dutch Science Organization (ZonMW-VENI Grant 916.14.023).
Prospective Study of Pravastatin in the Elderly at Risk (PROSPER): The PROSPER study was supported by an investigator initiated grant obtained from Bristol-Myers Squibb. Professor Dr J W Jukema is an Established Clinical Investigator of the Netherlands Heart Foundation (Grant No. 2001 D 032). Support for genotyping was provided by the seventh framework program of the European commission (Grant No. 223004) and by the Netherlands Genomics Initiative (Netherlands Consortium for Healthy Aging Grant 050-060-810).
Rotterdam Study (RS): The RS is supported by the Erasmus Medical Center and Erasmus University Rotterdam; The Netherlands Organization for Scientific Research; The Netherlands Organization for Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly; The Netherlands Heart Foundation; the Ministry of Education, Culture and Science; the Ministry of Health Welfare and Sports; the European Commission; and the Municipality of Rotterdam. Support for genotyping was provided by The Netherlands Organization for Scientific Research (NWO) (175.010.2005.011, 911.03.012) and Research Institute for Diseases in the Elderly (RIDE). This study was supported by The Netherlands Genomics Initiative (NGI)/Netherlands Organization for Scientific Research (NWO) Project No. 050-060-810. This collaborative effort was supported by an award from the National Heart, Lung and Blood Institute (R01-HL-103612, PI BMP).
Women’s Health Initiative Clinical Trial (WHI CT): The Women’s Health Initiative clinical trials were funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. All contributors to WHI science are listed @ https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf. ELB was supported in part by a grant from the National Cancer Institute (5T32CA009001). WHI GARNET: Within the Genomics and Randomized Trials Network, a GWAS of Hormone Treatment and CVD and Metabolic Outcomes in the WHI was funded by the National Human Genome Research Institute, National Institutes of Health, U.S. Department of Health and Human Services through cooperative agreement U01HG005152 (Reiner). All contributors to GARNET science are listed @ https://www.garnetstudy.org/Home. WHI MOPMAP: The Modification of PM-Mediated Arrhythmogenesis in Populations was funded by the National Institute of Environmental Health Sciences, National Institutes of Health, U.S. Department of Health and Human Services through grant R01ES017794 (Whitsel). WHI SHARe: The SNP Health Association Resource project was funded by the National Heart, Lung and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contract N02HL64278 (Kooperberg). WHI WHIMS: The Women’s Health Initiative Memory Study (WHIMS+) Genome-Wide Association Study was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contract HHSN268201100046C (Anderson).
Footnotes
CONFLICTS OF INTEREST
BMP serves on the DSMB of a clinical trial of a device funded by the manufacturer (Zoll LifeCor) and on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson.
REFERENCES CITED
- 1.Nathan DM. Diabetes: Advances in Diagnosis and Treatment. JAMA. 2015;314(10):1052–1062. doi: 10.1001/jama.2015.9536. [DOI] [PubMed] [Google Scholar]
- 2.Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the u.s 2003–2012. Diabetes Care. 2014;37(5):1367–1374. doi: 10.2337/dc13-2289. [DOI] [PubMed] [Google Scholar]
- 3.Meinert CL, Knatterud GL, Prout TE, Klimt CR. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes. 1970;19(Suppl):789–830. [PubMed] [Google Scholar]
- 4.Monami M, Genovese S, Mannucci E. Cardiovascular safety of sulfonylureas: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2013;15(10):938–953. doi: 10.1111/dom.12116. [DOI] [PubMed] [Google Scholar]
- 5.Simpson SH, Lee J, Choi S, Vandermeer B, Abdelmoneim AS, Featherstone TR. Mortality risk among sulfonylureas: a systematic review and network meta-analysis. Lancet Diabetes Endocrinol. 2015;3(1):43–51. doi: 10.1016/S2213-8587(14)70213-X. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda T. QT prolongation in type 2 diabetes mellitus treated with glibenclamide. Diabete Metab. 1994;20(6):565–567. [PubMed] [Google Scholar]
- 7.Najeed SA, Khan IA, Molnar J, Somberg JC. Differential effect of glyburide (glibenclamide) and metformin on QT dispersion: a potential adenosine triphosphate sensitive K+ channel effect. Am J Cardiol. 2002;90(10):1103–1106. doi: 10.1016/s0002-9149(02)02776-5. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz PJ, Wolf S. QT interval prolongation as predictor of sudden death in patients with myocardial infarction. Circulation. 1978;57(6):1074–1077. doi: 10.1161/01.cir.57.6.1074. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Post WS, Blasco-Colmenares E, Dalal D, Tomaselli GF, Guallar E. Electrocardiographic QT interval and mortality: a meta-analysis. Epidemiology. 2011;22(5):660–670. doi: 10.1097/EDE.0b013e318225768b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Post WS, Dalal D, Blasco-Colmenares E, Tomaselli GF, Guallar E. QT-interval duration and mortality rate: results from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2011;171(19):1727–1733. doi: 10.1001/archinternmed.2011.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow E, Bernjak A, Williams S, Fawdry RA, Hibbert S, Freeman J, et al. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes. 2014;63(5):1738–1747. doi: 10.2337/db13-0468. [DOI] [PubMed] [Google Scholar]
- 12.Heller S, Darpo B, Mitchell MI, Linnebjerg H, Leishman DJ, Mehrotra N, et al. Considerations for assessing the potential effects of antidiabetes drugs on cardiac ventricular repolarization: A report from the Cardiac Safety Research Consortium. Am Heart J. 2015;170(1):23–35. doi: 10.1016/j.ahj.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH. Timing of new black box warnings and withdrawals for prescription medications. JAMA : the journal of the American Medical Association. 2002;287(17):2215–2220. doi: 10.1001/jama.287.17.2215. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi ZP, Seoane-Vazquez E, Rodriguez-Monguio R, Stevenson KB, Szeinbach SL. Market withdrawal of new molecular entities approved in the United States from 1980 to 2009. Pharmacoepidemiol Drug Saf. 2011;20(7):772–777. doi: 10.1002/pds.2155. [DOI] [PubMed] [Google Scholar]
- 15.Shah RR. Drugs, QTc interval prolongation and final ICH E14 guideline : an important milestone with challenges ahead. Drug Saf. 2005;28(11):1009–1028. doi: 10.2165/00002018-200528110-00003. [DOI] [PubMed] [Google Scholar]
- 16.Hanson B, Tuna N, Bouchard T, Heston L, Eckert E, Lykken D, et al. Genetic factors in the electrocardiogram and heart rate of twins reared apart and together. Am J Cardiol. 1989;63(9):606–609. doi: 10.1016/0002-9149(89)90907-7. [DOI] [PubMed] [Google Scholar]
- 17.Newton-Cheh C, Larson MG, Corey DC, Benjamin EJ, Herbert AG, Levy D, et al. QT interval is a heritable quantitative trait with evidence of linkage to chromosome 3 in a genome-wide linkage analysis: The Framingham Heart Study. Heart Rhythm. 2005;2(3):277–284. doi: 10.1016/j.hrthm.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Arking DE, Pulit SL, Crotti L, van der Harst P, Munroe PB, Koopmann TT, et al. Genetic association study of QT interval highlights role for calcium signaling pathways in myocardial repolarization. Nat Genet. 2014;46(8):826–836. doi: 10.1038/ng.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou K, Donnelly L, Burch L, Tavendale R, Doney AS, Leese G, et al. Loss-of-function CYP2C9 variants improve therapeutic response to sulfonylureas in type 2 diabetes: a Go-DARTS study. Clin Pharmacol Ther. 2010;87(1):52–56. doi: 10.1038/clpt.2009.176. [DOI] [PubMed] [Google Scholar]
- 20.Holstein A, Plaschke A, Ptak M, Egberts EH, El-Din J, Brockmoller J, et al. Association between CYP2C9 slow metabolizer genotypes and severe hypoglycaemia on medication with sulphonylurea hypoglycaemic agents. Br J Clin Pharmacol. 2005;60(1):103–106. doi: 10.1111/j.1365-2125.2005.02379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng Y, Mao G, Ren X, Xing H, Tang G, Li Q, et al. Ser1369Ala variant in sulfonylurea receptor gene ABCC8 is associated with antidiabetic efficacy of gliclazide in Chinese type 2 diabetic patients. Diabetes Care. 2008;31(10):1939–1944. doi: 10.2337/dc07-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Javorsky M, Klimcakova L, Schroner Z, Zidzik J, Babjakova E, Fabianova M, et al. KCNJ11 gene E23K variant and therapeutic response to sulfonylureas. Eur J Intern Med. 2012;23(3):245–249. doi: 10.1016/j.ejim.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Sesti G, Laratta E, Cardellini M, Andreozzi F, Del Guerra S, Irace C, et al. The E23K variant of KCNJ11 encoding the pancreatic beta-cell adenosine 5′-triphosphate-sensitive potassium channel subunit Kir6. 2 is associated with an increased risk of secondary failure to sulfonylurea in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006;91(6):2334–2339. doi: 10.1210/jc.2005-2323. [DOI] [PubMed] [Google Scholar]
- 24.Cho HJ, Lee SY, Kim YG, Oh SY, Kim JW, Huh W, et al. Effect of genetic polymorphisms on the pharmacokinetics and efficacy of glimepiride in a Korean population. Clin Chim Acta. 2011;412(19–20):1831–1834. doi: 10.1016/j.cca.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Avery CL, Sitlani CM, Arking DE, Arnett DK, Bis JC, Boerwinkle E, et al. Drug-gene interactions and the search for missing heritability: a cross-sectional pharmacogenomics study of the QT interval. Pharmacogenomics J. 2014;14(1):6–13. doi: 10.1038/tpj.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sitlani CM, Rice KM, Lumley T, McKnight B, Cupples LA, Avery CL, et al. Generalized estimating equations for genome-wide association studies using longitudinal phenotype data. Stat Med. 2015;34(1):118–130. doi: 10.1002/sim.6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akylbekova EL, Payne JP, Newton-Cheh C, May WL, Fox ER, Wilson JG, et al. Gene-environment interaction between SCN5A-1103Y and hypokalemia influences QT interval prolongation in African Americans: the Jackson Heart Study. Am Heart J. 2014;167(1):116–122. e111. doi: 10.1016/j.ahj.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Psaty BM, O’Donnell CJ, Gudnason V, Lunetta KL, Folsom AR, Rotter JI, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: Design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circulation Cardiovascular genetics. 2009;2(1):73–80. doi: 10.1161/CIRCGENETICS.108.829747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skol AD, Scott LJ, Abecasis GR, Boehnke M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet. 2006;38(2):209–213. doi: 10.1038/ng1706. [DOI] [PubMed] [Google Scholar]
- 30.Thomas DC, Casey G, Conti DV, Haile RW, Lewinger JP, Stram DO. Methodological Issues in Multistage Genome-wide Association Studies. Stat Sci. 2009;24(4):414–429. doi: 10.1214/09-sts288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157(4):364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 32.International HapMap Consortium. The International HapMap Project. Nature. 2003;426(6968):789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 33.International HapMap Consortium. A haplotype map of the human genome. Nature. 2005;437(7063):1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.International HapMap Consortium. Altshuler DM, Gibbs RA, Peltonen L, Altshuler DM, Gibbs RA, et al. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467(7311):52–58. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The 1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature. 2010;467(7319):1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The 1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422):56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The Human Genome Browser at UCSC. Genome Research. 2002;12(6):996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.UCSC Human Genome Browser Lift Genome Annotations.
- 39.Arizona Center for Education and Research on Therapeutics QTDrugs Lists. [Accessed November 17, 2014]; https://www.crediblemeds.org/
- 40.Satterthwaite FE. An approximate distribution of estimates of variance components. Biometrics. 1946;2(6):110–114. [PubMed] [Google Scholar]
- 41.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganesh SK, Zakai NA, van Rooij FJ, Soranzo N, Smith AV, Nalls MA, et al. Multiple loci influence erythrocyte phenotypes in the CHARGE Consortium. Nat Genet. 2009;41(11):1191–1198. doi: 10.1038/ng.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nalls MA, Couper DJ, Tanaka T, van Rooij FJ, Chen MH, Smith AV, et al. Multiple loci are associated with white blood cell phenotypes. PLoS Genet. 2011;7(6):e1002113. doi: 10.1371/journal.pgen.1002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The human genome browser at UCSC. Genome Res. 2002;12(6):996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42(Database issue):D1001–1006. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40(Database issue):D930–934. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X, Gierman HJ, Levy D, Plump A, Dobrin R, Goring HH, et al. Synthesis of 53 tissue and cell line expression QTL datasets reveals master eQTLs. BMC Genomics. 2014;15:532. doi: 10.1186/1471-2164-15-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramos E, Doumatey A, Elkahloun AG, Shriner D, Huang H, Chen G, et al. Pharmacogenomics, ancestry and clinical decision making for global populations. The pharmacogenomics journal. 2013 doi: 10.1038/tpj.2013.24. [DOI] [PubMed] [Google Scholar]
- 49.Thomas D. Gene–environment-wide association studies: emerging approaches. Nature reviews Genetics. 2010;11(4):259–272. doi: 10.1038/nrg2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris AP. Transethnic meta-analysis of genomewide association studies. Genetic epidemiology. 2011;35(8):809–822. doi: 10.1002/gepi.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Becker ML, Aarnoudse AJ, Newton-Cheh C, Hofman A, Witteman JC, Uitterlinden AG, et al. Common variation in the NOS1AP gene is associated with reduced glucose-lowering effect and with increased mortality in users of sulfonylurea. Pharmacogenet Genomics. 2008;18(7):591–597. doi: 10.1097/FPC.0b013e328300e8c5. [DOI] [PubMed] [Google Scholar]
- 52.Pearson ER, Donnelly LA, Kimber C, Whitley A, Doney AS, McCarthy MI, et al. Variation in TCF7L2 influences therapeutic response to sulfonylureas: a GoDARTs study. Diabetes. 2007;56(8):2178–2182. doi: 10.2337/db07-0440. [DOI] [PubMed] [Google Scholar]
- 53.Holstein A, Hahn M, Korner A, Stumvoll M, Kovacs P. TCF7L2 and therapeutic response to sulfonylureas in patients with type 2 diabetes. BMC Med Genet. 2011;12:30. doi: 10.1186/1471-2350-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holstein A, Hahn M, Stumvoll M, Kovacs P. The E23K variant of KCNJ11 and the risk for severe sulfonylurea-induced hypoglycemia in patients with type 2 diabetes. Horm Metab Res. 2009;41(5):387–390. doi: 10.1055/s-0029-1192019. [DOI] [PubMed] [Google Scholar]
- 55.Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348(6235):648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koopmann TT, Adriaens ME, Moerland PD, Marsman RF, Westerveld ML, Lal S, et al. Genome-wide identification of expression quantitative trait loci (eQTLs) in human heart. PLoS One. 2014;9(5):e97380. doi: 10.1371/journal.pone.0097380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422):56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arking DE, Pulit SL, Crotti L, van der Harst P, Munroe PB, Koopmann TT, et al. Genetic association study of QT interval highlights role for calcium signaling pathways in myocardial repolarization. Nat Genet. 2014;46(8):826–836. doi: 10.1038/ng.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sotoodehnia N, Isaacs A, de Bakker PI, Dorr M, Newton-Cheh C, Nolte IM, et al. Common variants in 22 loci are associated with QRS duration and cardiac ventricular conduction. Nat Genet. 2010;42(12):1068–1076. doi: 10.1038/ng.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kirsten H, Al-Hasani H, Holdt L, Gross A, Beutner F, Krohn K, et al. Dissecting the genetics of the human transcriptome identifies novel trait-related trans-eQTLs and corroborates the regulatory relevance of non-protein coding locidagger. Hum Mol Genet. 2015;24(16):4746–4763. doi: 10.1093/hmg/ddv194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hao K, Bosse Y, Nickle DC, Pare PD, Postma DS, Laviolette M, et al. Lung eQTLs to help reveal the molecular underpinnings of asthma. PLoS Genet. 2012;8(11):e1003029. doi: 10.1371/journal.pgen.1003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Westra HJ, Peters MJ, Esko T, Yaghootkar H, Schurmann C, Kettunen J, et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013;45(10):1238–1243. doi: 10.1038/ng.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larson NB, McDonnell S, French AJ, Fogarty Z, Cheville J, Middha S, et al. Comprehensively evaluating cis-regulatory variation in the human prostate transcriptome by using gene-level allele-specific expression. American journal of human genetics. 2015;96(6):869–882. doi: 10.1016/j.ajhg.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olalla L, Gutierrez A, Campos JA, Khan ZU, Alonso FJ, Segura JA, et al. Nuclear localization of L-type glutaminase in mammalian brain. J Biol Chem. 2002;277(41):38939–38944. doi: 10.1074/jbc.C200373200. [DOI] [PubMed] [Google Scholar]
- 65.Slavin TP, Feng T, Schnell A, Zhu X, Elston RC. Two-marker association tests yield new disease associations for coronary artery disease and hypertension. Hum Genet. 2011;130(6):725–733. doi: 10.1007/s00439-011-1009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teumer A, Holtfreter B, Volker U, Petersmann A, Nauck M, Biffar R, et al. Genome-wide association study of chronic periodontitis in a general German population. J Clin Periodontol. 2013;40(11):977–985. doi: 10.1111/jcpe.12154. [DOI] [PubMed] [Google Scholar]
- 67.Kraev A, Quednau BD, Leach S, Li XF, Dong H, Winkfein R, et al. Molecular cloning of a third member of the potassium-dependent sodium-calcium exchanger gene family, NCKX3. J Biol Chem. 2001;276(25):23161–23172. doi: 10.1074/jbc.M102314200. [DOI] [PubMed] [Google Scholar]
- 68.Schumacher MA, Rivard AF, Bachinger HP, Adelman JP. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 2001;410(6832):1120–1124. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- 69.Van Booven D, Marsh S, McLeod H, Carrillo MW, Sangkuhl K, Klein TE, et al. Cytochrome P450 2C9-CYP2C9. Pharmacogenet Genomics. 2010;20(4):277–281. doi: 10.1097/FPC.0b013e3283349e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tornio A, Niemi M, Neuvonen PJ, Backman JT. Drug interactions with oral antidiabetic agents: pharmacokinetic mechanisms and clinical implications. Trends Pharmacol Sci. 2012;33(6):312–322. doi: 10.1016/j.tips.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 71.Chung WH, Chang WC, Lee YS, Wu YY, Yang CH, Ho HC, et al. Genetic variants associated with phenytoin-related severe cutaneous adverse reactions. JAMA. 2014;312(5):525–534. doi: 10.1001/jama.2014.7859. [DOI] [PubMed] [Google Scholar]
- 72.Jorgensen AL, FitzGerald RJ, Oyee J, Pirmohamed M, Williamson PR. Influence of CYP2C9 and VKORC1 on patient response to warfarin: a systematic review and meta-analysis. PLoS One. 2012;7(8):e44064. doi: 10.1371/journal.pone.0044064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang J, Chen Y, Li X, Wei X, Chen X, Zhang L, et al. Influence of CYP2C9 and VKORC1 genotypes on the risk of hemorrhagic complications in warfarin-treated patients: a systematic review and meta-analysis. Int J Cardiol. 2013;168(4):4234–4243. doi: 10.1016/j.ijcard.2013.07.151. [DOI] [PubMed] [Google Scholar]
- 74.Saldana-Cruz AM, Leon-Moreno LC, Sanchez-Corona J, Marquez-de Santiago DA, Mendoza-Carrera F, Castro-Martinez XH, et al. CYP2C9 and CYP2C19 Allele and Haplotype Distributions in Four Mestizo Populations from Western Mexico: An Interethnic Comparative Study. Genet Test Mol Biomarkers. 2016 doi: 10.1089/gtmb.2016.0115. [DOI] [PubMed] [Google Scholar]
- 75.Claudio-Campos K, Duconge J, Cadilla CL, Ruano G. Pharmacogenetics of drug-metabolizing enzymes in US Hispanics. Drug Metab Pers Ther. 2015;30(2):87–105. doi: 10.1515/dmdi-2014-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Valentin II, Rivera G, Nieves-Plaza M, Cruz I, Renta JY, Cadilla CL, et al. Pharmacogenetic association study of warfarin safety endpoints in Puerto Ricans. P R Health Sci J. 2014;33(3):97–104. [PMC free article] [PubMed] [Google Scholar]
- 77.Klen J, Dolzan V, Janez A. CYP2C9, KCNJ11 and ABCC8 polymorphisms and the response to sulphonylurea treatment in type 2 diabetes patients. Eur J Clin Pharmacol. 2014;70(4):421–428. doi: 10.1007/s00228-014-1641-x. [DOI] [PubMed] [Google Scholar]
- 78.Gaita F, Giustetto C, Bianchi F, Wolpert C, Schimpf R, Riccardi R, et al. Short QT Syndrome: a familial cause of sudden death. Circulation. 2003;108(8):965–970. doi: 10.1161/01.CIR.0000085071.28695.C4. [DOI] [PubMed] [Google Scholar]
- 79.Wolpert C, Schimpf R, Veltmann C, Giustetto C, Gaita F, Borggrefe M. Clinical characteristics and treatment of short QT syndrome. Expert review of cardiovascular therapy. 2005;3(4):611–617. doi: 10.1586/14779072.3.4.611. [DOI] [PubMed] [Google Scholar]
- 80.Iribarren C, Round AD, Peng JA, Lu M, Klatsky AL, Zaroff JG, et al. Short QT in a cohort of 1. 7 million persons: prevalence, correlates, and prognosis. Ann Noninvasive Electrocardiol. 2014;19(5):490–500. doi: 10.1111/anec.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holbrook M, Malik M, Shah RR, Valentin JP. Drug induced shortening of the QT/QTc interval: an emerging safety issue warranting further modelling and evaluation in drug research and development? J Pharmacol Toxicol Methods. 2009;59(1):21–28. doi: 10.1016/j.vascn.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 82.Ioannidis JP, Tarone R, McLaughlin JK. The false-positive to false–negative ratio in epidemiologic studies. Epidemiology. 2011;22(4):450–456. doi: 10.1097/EDE.0b013e31821b506e. [DOI] [PubMed] [Google Scholar]
- 83.Aslibekyan S, Claas SA, Arnett DK. To replicate or not to replicate: the case of pharmacogenetic studies: Establishing validity of pharmacogenomic findings: from replication to triangulation. Circ Cardiovasc Genet. 2013;6(4):409–412. doi: 10.1161/CIRCGENETICS.112.000010. discussion 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol. 1992;45(6):683–692. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 85.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gaziano JM, Concato J, Brophy M, Fiore L, Pyarajan S, Breeling J, et al. Million Veteran Program: A mega-biobank to study genetic influences on health and disease. J Clin Epidemiol. 2016;70:214–223. doi: 10.1016/j.jclinepi.2015.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.