Abstract
The purpose of this study was to conduct a randomized test of clinic and home-based incentives plus parent training for adolescent problem alcohol use. Adolescents (N=75) with alcohol misuse, with or without other substance misuse were enrolled. All youth received individual Motivational Enhancement Therapy/Cognitive Behavior Therapy and weekly urine drug testing. The experimental condition (EXP) included Abstinence Incentives (ABI; clinic-based incentives for abstinence from all substances) plus weekly behavioral parent training that included a parent-delivered, abstinence-based, substance monitoring contract. The comparison condition (CONTROL) included Attendance Incentives (ATTI). All adolescents met DSM-IV criteria for alcohol abuse or dependence or reported recent binge drinking, and 77% (N=58) met criteria for a cannabis use disorder or had recent cannabis use at baseline. Alcohol and cannabis use outcomes were compared across treatment conditions. A similar percentage of youth maintained complete alcohol abstinence across the 36-week follow up in both conditions. However, among youth not entirely abstinent from alcohol, EXP resulted in a lower percentage of days using alcohol during the 36 weeks after the end of treatment than CONTROL. Among youth who also used cannabis at baseline, results showed similar benefits of EXP on cannabis use days. Combined individual and family based treatment, plus abstinence based incentives can reduce substance use days during and after treatment over and above individual evidence-based psychosocial treatment plus attendance incentives. Future research should focus on identifying cost-effective components and incentive levels and delivery via technology to facilitate dissemination.
Keywords: Adolescent, alcohol abuse, cannabis, incentives, parent training
Alcohol use among teens exceeds use of any other substance, with nearly 23% of 8th to 12th graders reporting some alcohol use in the past month, and about 20% of 12th graders reporting binge drinking (≥5 drinks) at least once in the past two weeks (Johnston, O’Malley, Miech, Bachman, & Schulenberg, 2015). In the United States, 13% of all youth admissions to substance use treatment report alcohol as the primary substance (SAMHSA, 2014).
Overall, well-specified types of stand-alone, individual, group, family, and integrated approaches demonstrate efficacy for treating adolescent substance use (Hogue, Henderson, Ozechowski, & Robbins, 2014). Abstinence Based Incentives (ABI; often referred to as contingency management) has shown efficacy across multiple types of adult substance use disorders including alcohol (Lussier, Heil, Mongeon, Badger, & Higgins, 2006; Prendergast, Podus, Finney, Greenwell, & Roll, 2006). Fewer studies have tested ABI with adolescents, but several have shown positive outcomes on cannabis use (Godley et al., 2014; Henggeler, McCart, Cunningham, & Chapman, 2012; Stewart, Felleman, & Arger, 2015). Only Godley et al. reported alcohol use outcomes separately from cannabis outcomes, with reductions observed in both alcohol use days and heavy alcohol use.
We have developed an adolescent intervention model (Stanger, Budney, Kamon, & Thostensen, 2009; Stanger, Ryan, Scherer, Norton, & Budney, 2015) that utilizes clinic-based ABI plus home-based ABI that teaches parents to use rewards and consequences contingent on substance testing results. In addition, adolescents receive individual therapy (Motivational Enhancement Therapy/Cognitive Behavior Therapy: MET/CBT) (Sampl & Kadden, 2001; Webb, Scudder, Kaminer, & Kadden, 2001) and parents receive a comprehensive parent training (PT) curriculum (Dishion & Kavanagh, 2003). Results of two trials targeting adolescents with cannabis misuse (and excluding youth with alcohol dependence) showed positive effects of this intervention with and without the full PT curriculum (Stanger et al., 2009; Stanger et al., 2015).
The current study sought to replicate and extend these results to youth selected based on their alcohol misuse by comparing ABI plus PT (EXP) with evidence-based counseling plus attendance incentives (CONTROL). For brevity, this report focuses on the complete 36-week post treatment period. We hypothesized that youth receiving EXP would report both fewer alcohol and cannabis use days because EXP targeted abstinence from all substances.
Methods
Participants
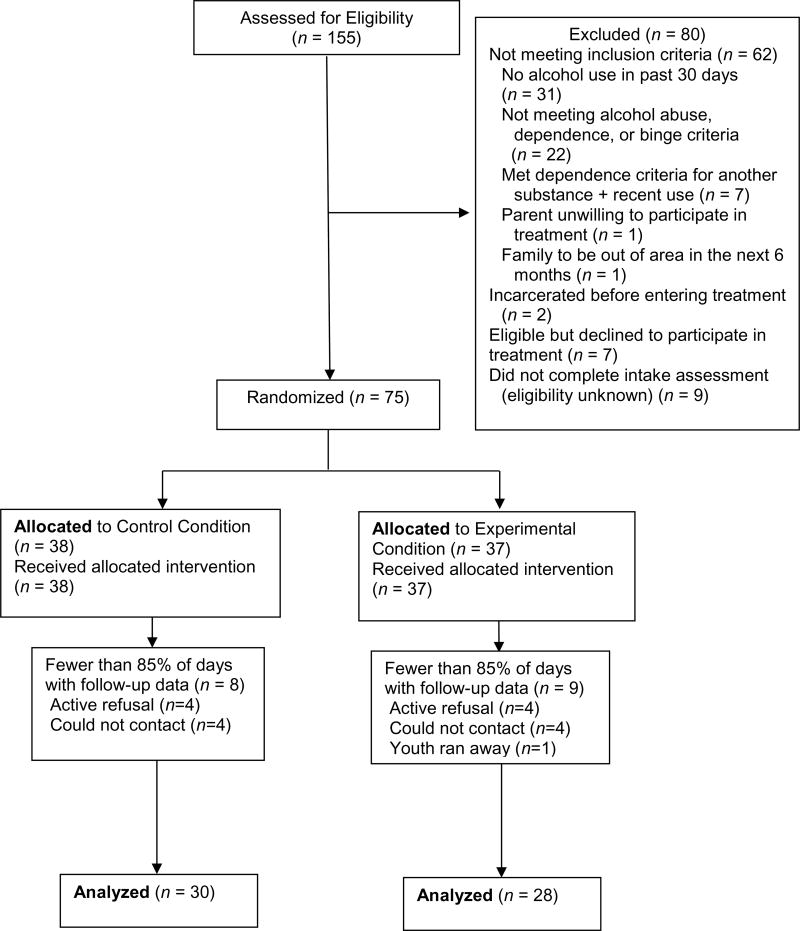
The Institutional Review Board of the University of Arkansas for Medical Sciences approved the study. Families were referred by schools, the justice system, therapists, physicians, or parents. Inclusion criteria were: 1) age 12–18 years; 2) reported use of alcohol during the prior 30 days or an alcohol positive urine test; 3) met criteria for alcohol abuse or dependence, or reported one or more binge episode (≥ 5 drinks) in the past 90 days; 4) living with a parent/guardian who agreed to participate; and 5) planned to be in the area for at least the next 6 months. Youth were excluded if they had a past 6-month DSM diagnosis of dependence on a substance other than cannabis, alcohol, or tobacco and had used that substance in the past 30 days. Informed consent was obtained from the parent(s); assent (consent if 18) was obtained from the adolescent. Minimum likelihood allocation (Aickin, 1982) was used to randomly assign participants (N=75; see Figure 1) while balancing across conditions on: alcohol dependence, gender, cannabis use (use in past 30 days or cannabis positive specimen), ≤10th grade, conduct problems (T-score ≥64 on the externalizing scale of the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001), and ethnicity (minority). Youth were enrolled between December 2007 and October 2011 and follow-up assessments were completed by June 2012.
Figure 1.
Consort diagram.
Measures
The Vermont Structured Diagnostic Interview (VSDI; psychometric information available in Hudziak, Copeland, Stanger, & Wadsworth, 2004) was used to assess past 6-month DSM-IV substance use disorders (Stanger et al., 2009; Stanger et al., 2015). Past 12-week frequency of substance use was assessed using the Time-Line Follow Back (TLFB; Sobell, Sobell, Litten, & Allen, 1992) at 12, 24, and 36 weeks after the end of treatment by research assistants not blinded to condition (due to staffing and budgetary constraints). The percentage of days of alcohol and cannabis use during the 36-week follow up was calculated as the number of reported days of use divided by the number of days for which data were provided. Incomplete data (fewer than 85% of possible days) were coded as missing. Primary parents completed the CBCL (Achenbach & Rescorla, 2001) and the Alabama Parenting Questionnaire (APQ; Frick, 1991; Wells et al., 2000). Analyses used T-scores from the CBCL Externalizing scale and mean item scores for APQ Positive Involvement, Ineffective Discipline, and Deficient Monitoring.
Intervention Conditions
All youth received individual MET/CBT (Sampl & Kadden, 2001; Webb et al., 2001). Once-weekly urine testing and alcohol breath tests were performed during treatment. Observed urine specimens were tested via onsite immunoassay drug testing (MCG 240: Thermo Scientific, Fremont, CA) for alcohol, cannabis, cocaine, opioids, benzodiazepines, amphetamines, and methamphetamines. Note that for alcohol, ethyl glucuronide (EtG) was the metabolite of ethanol targeted for analysis, which can be detected in urine up to 80 hours after ingestion of alcohol (Wurst, Kempter, Seidl, & Alt, 1999; Wurst, Skipper, & Weinmann, 2003). Invalid specimens (creatinine below 30 mg/dl) required a replacement specimen within 24 hours.
Control Condition (CONTROL)
Youth received attendance-based incentives (ATTI) to equalize participation across conditions (Stanger et al., 2009; 2015). ATTI was administered using the fishbowl method (Petry, Martin, Cooney, & Kranzler, 2000). One pull was earned the first week, increasing by 1 per week for each consecutive visit and provision of a valid specimen, up to a maximum of 5 pulls per week (maximum 60 pulls/~$146). Failure to attend or provide a valid specimen reset pulls to 1, however, after 3 consecutive weeks of providing valid specimens, pulls were reset to the prior maximum. The fishbowl contained tickets for good job (no prize; n=250); small ($1.50 prizes; n=209), medium ($20 gift cards; n=40), or large prizes ($100 gift cards; n=1). Parents attended the first session and were contacted weekly to report on youth substance use and receive substance testing results. Parents were not instructed on how to respond to the test results.
Experimental Condition (EXP)
Youth received a clinic-delivered abstinence-based fishbowl program and a home-based incentives and consequences program. Abstinence was defined as a negative urinalysis for all substances, plus negative parent and adolescent reports of use. Youth received 10 pulls for the first week of abstinence, increasing by 2 pulls for each week of consecutive abstinence up to 20 pulls (maximum 250 pulls; ~$607). Substance use reset the pulls to 10. After 3 weeks of consecutive abstinence, pulls reset to the prior maximum.
The home-based program instructed parents to develop a Substance Monitoring Contract (SMC) that specified weekly positive and negative consequences for abstinence or use (manual available in Kamon, Budney, & Stanger, 2005). Parents received .02 saliva alcohol tests to use at home. Parents earned fishbowl pulls for session attendance, implementation of the SMC, and administering breath tests (single/two parent maximum=83/111 pulls; ~$200/$270). Parents also received additional parent training (PT) using Adolescent Transitions (Dishion & Kavanagh, 2003), an evidence-based program targeting concerns in addition to substance use.
Continuing Care Components
After 14 weeks, families were offered an additional 12 weeks of urine testing to facilitate parental monitoring. CONTROL youth earned 1 fishbowl pull for attending and providing a specimen, increasing by 1 pull up to 5 pulls for each consecutive specimen provided (maximum 50 pulls; ~$120). EXP youth earned 5 pulls for abstinence, increasing by 1 for each consecutive week of abstinence up to 10 (maximum 105 pulls; ~$255). EXP parents could schedule six additional sessions to review the SMC and parenting strategies.
Therapists, treatment integrity and fidelity
Four female clinicians (one master’s, three postdoctoral) served as therapists. All sessions were videotaped. Adherence to Adolescent Transitions was assessed using the Fidelity of Implementation system (Forgatch, Patterson, & DeGarmo, 2005) for parenting interventions. Doctoral level raters rated two randomly selected sessions for each EXP family (95% of families had ≥1 rated session). Approximately 50% of those sessions were rated by two raters with ≥80% agreement. Mean fidelity score for overall quality was 5.18 (SD=1.19) on a 9-point scale, indicating scores in the “acceptable” range, which is comparable to other published reports (Hukkelberg & Ogden, 2013) and our prior work (Stanger et al., 2015).
Adherence to MET/CBT was assessed using the Yale Adherence Competence Scale (YACS; Carroll et al., 2000) rating frequency/extensiveness and competence on 7-point scales. Raters were three bachelor level staff trained to ≥80% agreement with doctoral level staff. Fifty percent of participants were randomly selected and each had one MET and CBT session rated. The mean frequency/extensiveness ratings were: MET=3.76 (SD = 1.93); CBT=2.04 (SD=1.29). Skill level ratings were: MET=4.67 (SD = .77); CBT=3.00 (SD = .92). Ratings were comparable to other published reports (Gibbons et al., 2010) and our prior study (Stanger et al., 2015).
Statistical Methods
Zero-inflated Poisson (ZIP) models compared alcohol and cannabis use between conditions during the 36 weeks after the end of treatment (Atkins, Baldwin, Zheng, Gallop, & Neighbors, 2013). ZIP models were used because days of substance use after treatment were highly skewed, i.e., ~38% of youth had 0% of days used alcohol and cannabis. Cannabis use outcomes were tested only among youth meeting criteria for a cannabis use disorder or reporting cannabis use or testing positive for cannabis at baseline (N=58). Seventeen (23% of 75) and 15 (26% of 58 participants had data on fewer days than necessary to calculate percentage of days used alcohol and cannabis, respectively. Mixed models compared intake vs. 9-month scores for parenting and externalizing. All models were fit adjusted for variables that differed significantly between conditions at baseline using SAS version 9.4.
Results
Sample Characteristics
Table 1 shows demographic and substance use comparisons at intake. Overall, the sample was mostly male (75%) and mostly white (81%) with a mean age of 16.1 (SD=1.2). About half (53%) met criteria for an alcohol use disorder (the remainder reported binge episodes), and 75% met criteria for a cannabis use disorder. Treatment conditions differed on two variables; CONTROL participants had higher socioeconomic status and were more likely to meet criteria for cannabis dependence. These variables were controlled in all analyses.
Table 1.
Sample characteristics for each treatment condition
| EXP (n = 37) | CONTROL (n = 38) | X2 or t | p-value | |
|---|---|---|---|---|
|
| ||||
| N/(%) or M (SD) | N/(%) or M (SD) | |||
| Male | 27 (73.0%) | 29 (76.3%) | .11 | .74 |
| Race | .42 | .52 | ||
| Minoritya | 8 (21.6%) | 6 (15.8%) | ||
| White | 29 (78.4%) | 32 (84.2%) | ||
| Mean SESb | 6.2 (2.2) | 7.2 (1.6) | 2.36 | .02 |
| Mean Age | 16.1 (1.2) | 16.2 (1.2) | .17 | .86 |
| Two- parent participation | 22 (59.5%) | 22 (57.9%) | .02 | .89 |
| Female primary parent | 31 (83.8%) | 32 (84.2%) | .003 | .96 |
| Tobacco user | 30 (81.1%) | 30 (79.0%) | .05 | .82 |
| Mean intake proportion of days used alcohol in past 30 | .12 (.16) | .12 (.14) | .06 | .95 |
| Mean drinks per drinking day | 5.7 (4.9) | 6.4 (4.5) | .65 | .52 |
| Intake cannabis positive specimen | 13 (35.1%) | 13 (34.2%) | .01 | .93 |
| Mean intake proportion of days used cannabis in past 30 | .28 (.35) | .27 (.34) | −.07 | .95 |
| DSM Substance Use Disordersc | ||||
| Alcohol | .17 | .92 | ||
| Dependence | 7 (18.9%) | 7 (19.4%) | ||
| Abuse only | 12 (32.4%) | 14 (36.8%) | ||
| None | 18 (48.7%) | 17 (44.7%) | ||
| Cannabis | 8.05 | .02 | ||
| Dependence | 10 (27.0%) | 19 (50.0%) | ||
| Abuse only | 18 (48.6%) | 7 (18.4%) | ||
| None | 9 (24.3%) | 12 (31.6%) | ||
| DSM Mental Health Disorders | ||||
| Parent Reportd | ||||
| ODD+/or CD | 14 (37.8%) | 16 (42.1%) | .14 | .71 |
| ADHD | 17 (46.0%) | 14 (36.8%) | .64 | .42 |
| Major Depression +/or GAD | 8 (21.6%) | 8 (21.1%) | .004 | .95 |
| Youth Reportc | ||||
| ODD+/or CD | 11 (29.7%) | 11 (29.0%) | .01 | .94 |
| ADHD | 8 (21.6%) | 11 (29.0%) | .53 | .47 |
| Major Depression +/or GAD | 8 (21.6%) | 10 (26.3%) | .22 | .63 |
| Parenting Measuresd | ||||
| Positive Involvement | 3.7 (0.5) | 3.5 (0.5) | 1.79 | 0.08 |
| Deficient monitoring | 2.2 (0.6) | 2.4 (0.6) | 1.56 | 0.12 |
| Negative discipline | 2.0 (0.5) | 2.1 (0.5) | 1.01 | 0.32 |
| Externalizing t-scored | 62.8 (10.7) | 64.6 (10.7) | 0.72 | 0.47 |
Note: EXP=Experimental Condition; CONTROL=Control Condition; SES=Socioeconomic Status; DSM=Diagnostic and Statistical Manual; ODD=Oppositional Defiant Disorder; CD=Conduct Disorder; ADHD=Attention-Deficit/Hyperactivity Disorder; GAD=Generalized Anxiety Disorder.
Minority represents youth who identified as Black, Hispanic or More than One Race.
Hollingshead (1975) 9-step occupation scale;
Based on youth interview;
Based on primary parent interview or questionnaires
Retention, Participation, and Incentive Earnings
Table 2 shows retention, participation and incentive earnings for each condition. Retention was high across conditions, with >85% attending during the last treatment week. Follow-up participation rates ranged from 75–80%. Comparisons of those with and without ≥85% of non-missing TLFB days during the 36-week follow-up showed no significant baseline differences across conditions on demographic, substance use or psychopathology variables (data not shown). CONTROL youth earnings were >90% of the potential maximum indicating high participation. EXP youth earnings were about 55% of the maximum. EXP parents implemented the SMC on average 8.5 of the 11 weeks the contract was active, and administered about 3 saliva alcohol tests per week on average. Both CONTROL and EXP teens attended less than half of the continuing care visits. EXP parents attended ∼1 continuing care session, on average.
Table 2.
Participation, retention and earnings for each treatment condition.
| Participation, Retention, and Earnings Outcomes | EXP (n = 37) | CONTROL (n = 38) | X2 or t | p-value |
|---|---|---|---|---|
|
| ||||
| N/(%) or M (SD) | N/(%) or M (SD) | |||
| % attending during week 14 | 32 (86.5%) | 34 (89.5%) | .16 | .69 |
| Mean sessions attended | 12.4 (2.9) | 12.6 (2.3) | .29 | .78 |
| % follow up participation | ||||
| 12 weeks after treatment end | 31 (83.8%) | 31 (81.6%) | .06 | .80 |
| 24 weeks after treatment end | 28 (75.7%) | 27 (71.0%) | .21 | .65 |
| 36 weeks after treatment end | 30 (81.1%) | 30 (78.9%) | .05 | .82 |
| Mean maintenance urine tests attended of 12 | 5.2 (4.6) | 5.3 (4.2) | .13 | .90 |
| Mean teen incentive earnings | $337.32 (231.98) | $136.58 (66.54) | ||
| Mean parent incentive earnings (one-parent family) | $184.44 (62.79) | N/A | ||
| Mean parent incentive earnings (two-parent family) | $250.54 (114.50) | N/A | ||
| Mean sessions ≥1 parent attended of 14 | 12.4 (3.0) | N/A | ||
| Mean number of weeks (of 11 possible) parent(s) enforced consequences | 8.5 (3.0) | N/A | ||
| Mean number of weeks (of 14) parent(s) used ≥1 alcohol saliva test | 9.9 (3.3) | N/A | ||
| Mean total number alcohol saliva tests used per family | 44.9 (25.3) | N/A | ||
| Mean maintenance parent sessions attended of 6 | .9 (1.3) | N/A | ||
Note: EXP=Experimental Condition; CONTROL=Control Condition
Substance Use
Differences in percentage days of alcohol and cannabis use (among baseline cannabis users) were tested with ZIP models covering the period between end of treatment and the 36 week follow-up (Table 3). For both alcohol and cannabis, the likelihood of reporting complete abstinence did not differ between conditions. However, among those who did not completely abstain, the mean percentages of days used alcohol and cannabis, were significantly lower for EXP vs. CONTROL (see Table 3 for test statistics). Similar results were obtained when restricting analyses to participants with substance use data on at least 25% of days.
Table 3.
Zero-inflated Poisson models of percent days used alcohol and cannabis across the 36-week follow-up period.
| Substance Use Outcomes | Alcohol Use | Cannabis Use | ||
|---|---|---|---|---|
| EXP | CONTROL | EXP | CONTROL | |
| N | 28 | 30 | 21 | 22 |
| N/(%) or M (SD) | N/(%) or M (SD) | N/(%) or M (SD) | N/(%) or M (SD) | |
| N/% with >1% days use | 19 (67.9%) | 19 (63.3%) | 15 (71.0%) | 11 (50.0%) |
| Mean (SD) % Days use if days ≥1% | 8.3% (9.6%) | 12.0% (11.2%) | 26.6% (29.9%) | 36.5% (37.6%) |
|
Days Used Odds Ratio for ≥ 1% vs. 0% ZIP model test |
Odds Ratio (95% CI)=1.21 (.38,3.85) X2(2)=.11, p=.74 |
Odds Ratio (95% CI)=2.67 (0.69,10.36) X2(2)=2.02, p=.15 |
||
|
% Days Used Ratio of Means ZIP model test |
Ratio of Means (95% CI)=.74 (.59,.92) X2(2)=7.41, p=.007 |
Ratio of Means (95% CI)=.63 (.53,.41) X2(2)=30.24, p=<.0001 |
||
Note: EXP=Experimental Condition; CONTROL=Control Condition; CI= Confidence Interval; ZIP=Zero Inflated Poisson. Analyses included SES and Cannabis Dependence as covariates.
Parenting and Externalizing Psychopathology
Table 4 shows pre-post results for the three parenting scales and CBCL externalizing symptoms. Externalizing scores declined significantly from intake to the 9 month follow up. There were no significant changes in the parenting outcomes. There were also no significant effects of treatment condition, or interactions between treatment condition and time.
Table 4.
Mixed models of parenting and externalizing following treatment (intake vs. 36-Week follow-up).
| Model Terms | Positive Involvement | Deficient Monitoring | Ineffective Discipline | Externalizing t-score | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | |
| Intercept | 3.55 (.24) | <.0001 | 1.72 (.27) | <.0001 | 1.98 (.23) | <.0001 | 53.25 (5.17) | <.0001 |
| Time (36-week vs. Intake) | 0.069 (.010) | .48 | −0.013 (.11) | .91 | −.15 (.08) | .054 | −.12 (2.1) | <.0001 |
| Treatment Condition (EXP vs. CONTROL) | .19 (.12) | .13 | −.13 (.13) | .36 | −.10 (.11) | .37 | −.23 (2.1) | .93 |
| Time (36-week vs. Intake) * Treatment Condition (EXP vs. CONTROL) |
−0.12 (.14) | .40 | −.11 (.16) | .49 | −.17 (.11) | .13 | 5.4 (3.0) | .08 |
Analyses included SES and Cannabis Dependence as covariates.
Discussion
Across both EXP and CONTROL conditions, a similar large percentage of youth showed complete abstinence from alcohol and cannabis during the 36 week follow up period. However, among youth who were not entirely abstinent, those receiving EXP showed a lower percentage of alcohol and cannabis use days during follow up than those who received CONTROL. These results are similar in magnitude (d~.30) to our prior results for cannabis use, with levels of baseline use similar to those observed here (Stanger et al., 2009; Stanger et al., 2015) and those of others (e.g., Henggeler et al., 2012) in showing benefits of ABI. However, the use of ZIP models revealed a novel pattern of intervention effects, with treatment condition effects observed only among youth who used substances after treatment and not among those with complete abstinence. Use of analytic models such as ZIP is important in cases of significant skew and clustering at either or both ends of the distribution, i.e., complete abstinence and no abstinence (Atkins et al., 2013).
One novel intervention component was the use of urine EtG testing for alcohol. Although the 80-hour detection window using this method was too short to reliably detect all alcohol use and confirm complete abstinence with a schedule of once per week testing, it is a much longer detection window than that associated with breath or saliva testing (Wurst et al., 2003). In our study, 25% of all instances of alcohol use were detected using ETG test and not by self- or parent-report. It is also possible that ETG testing served as a deterrent for some youth, and may have increased self-report of alcohol use. Another novel component, parent use of home saliva breath tests, also may have served as a deterrent of use.
The finding of no benefit of ABI on either parenting or conduct problems (despite ABI including a comprehensive parent training intervention focused on conduct problems) is consistent with results of our prior three condition trial for adolescent cannabis users (Stanger et al., 2015) conducted concurrently with this study. In that trial, there was no added benefit on any outcome for the full parent training intervention, including parenting or youth externalizing behavior measures, above and beyond the positive effects of ABI + the SMC. This may have been due to a floor effect; many youth and parents had normal range conduct problems and parenting at baseline.
Some studies have not supported the efficacy of clinic-delivered ABI (Kaminer, Burleson, Burke, & Litt, 2014; Killeen, McRae-Clark, Waldrop, Upadhyaya, & Brady, 2012). Those interventions used significantly lower magnitude incentives (<1/2 the value used in the current study) and/or did not teach parents to use ABI at home. A previous study that did observe positive results with ABI (Henggeler et al., 2012) used lower incentives than the current study (maximum $150), but also had parents implement a home-based contract, suggesting that parent-delivered ABI may be an important active component. The importance of parent involvement is also supported by a prior study showing reduced cannabis use in a brief intervention that included parent sessions (Winters, Fahnhorst, Botzet, Lee, & Lalone, 2012). The inability to separate the impact of clinic vs. home-based ABI and of parent training is a limitation of the current study, and independent replication would also strengthen confidence in these findings.
Other study limitations include the small sample size of youth and significant missing data, treatment at an academic medical center, and research staff not blind to treatment condition. The sample was also predominantly male and white suggesting limits to the generalizability of these results. Parent participation was required, but it should be noted that parental refusal to participate was an uncommon occurrence. Fidelity to both MET/CBT and PT were moderate, despite intensive supervision by two doctoral level expert clinicians, potentially limiting the efficacy of the counseling interventions and highlighting the time and effort required to train clinicians to implement these interventions with high fidelity. Of note, there are evidence-based models to disseminate incentive-based interventions for adolescent substance use suggesting they are cost effective, can be integrated with other treatment models, and are readily adopted by a variety of providers (e.g., substance use or mental health services, and juvenile drug courts) (e.g., Henggeler, Chapman, Rowland, Sheidow, & Cunningham, 2013; McCart, Henggeler, Chapman, & Cunningham, 2012). Further, there is evidence from adult studies that computer-assisted MET/CBT integrated with ABI for treating cannabis use disorder produces comparable outcomes to therapist-delivered MET/CBT at a lower cost (Budney et al., 2015). Technology-delivered interventions hold much promise for cost-effectively delivering complex interventions.
In addition, the benefit of attendance incentives relative to MET/CBT alone was not tested; however, there is some evidence that although attendance incentives increase attendance, they do not significantly improve abstinence outcomes (Carroll et al., 2012). Finally, the ABI intervention reflects a particular operationalization of incentives in terms of the target (abstinence from all substances), use of weekly testing and incentives, and the magnitude of available incentives. Varying these dimensions might lead to better or worse outcomes and higher or lower costs.
Overall, the results suggest that integrating ABI plus family based intervention for substance use with MET/CBT results in lower levels of both alcohol and cannabis compared with MET/CBT plus ATTI, but only among youth who do not maintain complete abstinence after treatment. The relatively large number of youth who maintained complete abstinence in both treatments suggests that future research should seek to identify characteristics of youth likely to respond to less intensive interventions (e.g., those with less frequent/lower quantity use or without substantial comorbid psychopathology). The results also highlight the importance of using analytic methods that capitalize on skewed distribution of substance use variables.
Acknowledgments
This work was supported by NIH Grants R01AA016917, R01DA015186, UL1TR001086, and P30DA029926.
Footnotes
Partial results were also presented at the College on Problems of Drug Dependence annual meeting in 2011.
References
- Achenbach TM, Rescorla LA. Manual for ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- Aickin M. A program for balancing the allocation of subjects in a clinical trial. Computers and Biomedical Research. 1982;15:519–524. doi: 10.1016/0010-4809(82)90014-3. [DOI] [PubMed] [Google Scholar]
- Atkins DC, Baldwin SA, Zheng C, Gallop RJ, Neighbors C. A tutorial on count regression and zero-altered count models for longitudinal substance use data. Psychology of Addictive Behaviors. 2013;27(1):166–177. doi: 10.1037/a0029508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Stanger C, Tilford JM, Scherer EB, Brown PC, Li Z, Walker DD. Computer-assisted behavioral therapy and contingency management for cannabis use disorder. Psychol Addict Behav. 2015;29(3):501–511. doi: 10.1037/adb0000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Corvino J, Nuro KF, Nich C, Sifry RL, Frankforter TL, … Rounsaville BJ. Yale Adherence and Competence Scale (YACS) Guidelines. Yale University Psychotherapy Development Center; 2000. [Google Scholar]
- Carroll KM, Nich C, Lapaglia DM, Peters EN, Easton CJ, Petry NM. Combining cognitive behavioral therapy and contingency management to enhance their effects in treating cannabis dependence: less can be more, more or less. Addiction. 2012;107(9):1650–1659. doi: 10.1111/j.1360-0443.2012.03877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishion TJ, Kavanagh K. Intervening in Adolescent Problem Behavior: A Family-Centered Approach. New York, NY: Guilford Press; 2003. [Google Scholar]
- Forgatch MS, Patterson GR, DeGarmo DS. Evaluating fidelity: Predictive validity for a measure of competent adherence to the Oregon model of parent management training. Behavior Therapy. 2005;36:3–13. doi: 10.1016/S0005-7894(05)80049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ. The Alabama Parenting Questionnaire. Birmingham, AL: University of Alabama; 1991. [Google Scholar]
- Gibbons CJ, Nich C, Steinberg K, Roffman RA, Corvino J, Babor TF, Carroll KM. Treatment process, alliance and outcome in brief versus extended treatments for marijuana dependence. Addiction. 2010;105(10):1799–1808. doi: 10.1111/j.1360-0443.2010.03047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godley MD, Godley SH, Dennis ML, Funk RR, Pasetti LL, Petry NM. A randomized trial of assertive continuing care and contingency management for adolescents with substance use disorders. Journal of Consulting and Clinical Psychology. 2014;82(1):40–51. doi: 10.1037/a0035264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henggeler SW, Chapman JE, Rowland MD, Sheidow AJ, Cunningham PB. Evaluating training methods for transporting contingency management to therapists. J Subst Abuse Treat. 2013;45(5):466–474. doi: 10.1016/j.jsat.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henggeler SW, McCart MR, Cunningham PB, Chapman JE. Enhancing the effectiveness of juvenile drug courts by integrating evidence-based principles. Journal of Consulting and Clinical Psychology. 2012;80(2):264–275. doi: 10.1037/a0027147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogue A, Henderson CE, Ozechowski TJ, Robbins MS. Evidence base on outpatient behavioral treatments for adolescent substance use: updates and recommendations 2007–2013. Journal of Clinical Child & Adolescent Psychology. 2014;43(5):695–720. doi: 10.1080/15374416.2014.915550. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Department of Sociology, Yale University; New Haven, CT: 1975. [Google Scholar]
- Hudziak JJ, Copeland W, Stanger C, Wadsworth M. Screening for DSM-IV externalizing disorders with the Child Behavior Checklist: A receiver-operating characteristic analysis. The Journal of Child Psychology and Psychiatry. 2004;45(7):1299–1307. doi: 10.1111/j.1469-7610.2004.00314.x. [DOI] [PubMed] [Google Scholar]
- Hukkelberg SS, Ogden T. Working alliance and treatment fidelity as predictors of externalizing problem behaviors in parent management training. Journal of Consulting and Clinical Psychology. 2013;81(6):1010–1020. doi: 10.1037/a0033825. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2014: Overview, key findings on adolescent drug use. 2015 Retrieved from Institute on Social Research, University of Michigan website: http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2014.pdf.
- Kaminer Y, Burleson JA, Burke R, Litt MD. The efficacy of contingency management for adolescent cannabis use disorder: a controlled study. Substance Abuse. 2014;35(4):391–398. doi: 10.1080/08897077.2014.933724. [DOI] [PubMed] [Google Scholar]
- Kamon J, Budney A, Stanger C. A contingency management intervention for adolescent marijuana abuse and conduct problems. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(6):513–521. doi: 10.1097/01.chi.0000159949.82759.64. [DOI] [PubMed] [Google Scholar]
- Killeen TK, McRae-Clark AL, Waldrop AE, Upadhyaya H, Brady KT. Contingency management in community programs treating adolescent substance abuse: A feasibility study. Journal of Child and Adolescent Psychiatric Nursing. 2012;25(1):33–41. doi: 10.1111/j.1744-6171.2011.00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. doi: 10.1111/j.1360-0443.2006.01311.x. ADD1311 [pii] [DOI] [PubMed] [Google Scholar]
- McCart MR, Henggeler SW, Chapman JE, Cunningham PB. System-level effects of integrating a promising treatment into juvenile drug courts. J Subst Abuse Treat. 2012;43(2):231–243. doi: 10.1016/j.jsat.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: Contingency management for treatment of alcohol dependence. Journal of Consulting and Clinical Psychology. 2000;68:250–257. doi: 10.1037/0022-006X.68.2.250. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101(11):1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Treatment Episode Data Set (TEDS): 2002–2012. National Admissions to Substance Abuse Treatment Services. 2014 (BHSIS Series S-71, HHS Publication No. (SMA) 14-4850). Retrieved from Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality website: http://www.samhsa.gov/data/sites/default/files/TEDS2012N_Web.pdf.
- Sampl S, Kadden R. Motivational Enhancement Therapy and Cognitive Behavioral Therapy for Adolescent Cannabis Users: 5 Sessions. Vol. 1. Rockville, MD: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2001. [Google Scholar]
- Sobell LC, Sobell MB, Litten R, Allen J. Timeline follow-back: A technique for assessing self-reported alcohol consumption. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Stanger C, Budney AJ, Kamon JL, Thostensen J. A randomized trial of contingency management for adolescent marijuana abuse and dependence. Drug and Alcohol Dependence. 2009;105(3):240–247. doi: 10.1016/j.drugalcdep.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Ryan SR, Scherer EA, Norton GE, Budney AJ. Clinic- and home-based contingency management plus parent training for adolescent cannabis use disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2015;54(6):445–453. e442. doi: 10.1016/j.jaac.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DG, Felleman BI, Arger CA. Effectiveness of Motivational Incentives for Adolescent Marijuana Users in a School-Based Intervention. Journal of Substance Abuse Treatment. 2015;58:43–50. doi: 10.1016/j.jsat.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Webb C, Scudder M, Kaminer Y, Kadden R. The Motivational Enhancement Therapy and Cognitive Behavioral Therapy for Adolescent Cannabis Users. Rockville, MD: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2001. [Google Scholar]
- Wells KC, Epstein JN, Hinshaw SP, Conners CK, Klaric J, Abikoff HB, … Wigal T. Parenting and family stress treatment outcomes in Attention Deficit Hyperactivity Disorder (ADHD): An empirical analysis in the MTA study. Journal of Abnormal Child Psychology. 2000;28:543–553. doi: 10.1023/a:1005131131159. [DOI] [PubMed] [Google Scholar]
- Winters KC, Fahnhorst T, Botzet A, Lee S, Lalone B. Brief intervention for drug-abusing adolescents in a school setting: outcomes and mediating factors. Journal of Substance Abuse Treatment. 2012;42(3):279–288. doi: 10.1016/j.jsat.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurst FM, Kempter C, Seidl S, Alt A. Ethyl glucuronide--a marker of alcohol consumption and a relapse marker with clinical and forensic implications. Alcohol and Alcoholism. 1999;34(1):71–77. doi: 10.1093/alcalc/34.1.71. [DOI] [PubMed] [Google Scholar]
- Wurst FM, Skipper GE, Weinmann W. Ethyl glucuronide--the direct ethanol metabolite on the threshold from science to routine use. Addiction. 2003;98(s2):51–61. doi: 10.1046/j.1359-6357.2003.00588.x. [DOI] [PubMed] [Google Scholar]