Abstract
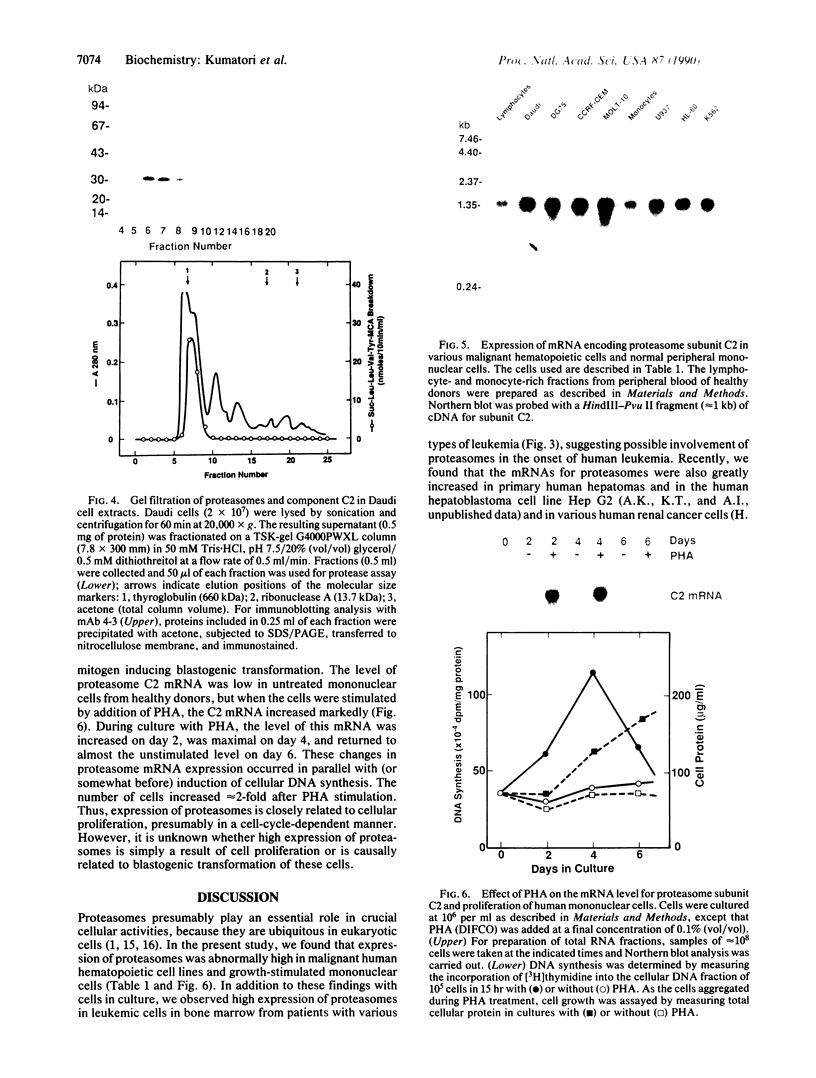
Proteasomes are eukaryotic ring-shaped or cylindrical particles with multicatalytic protease activities. To clarify the involvement of proteasomes in tumorigenesis of human blood cells, we compared their expression in human hematopoietic malignant tumor cells with that in normal peripheral blood mononuclear cells. Immunohistochemical staining showed considerably increased concentrations of proteasomes in leukemic cells from the bone marrow of patients with various types of leukemia and the predominant localization of these proteasomes in the nuclei. Moreover, enzyme immunoassay and Northern blot analysis indicated that the concentrations of proteasomes and their mRNA levels were consistently much higher in a variety of malignant human hematopoietic cell lines than in resting peripheral lymphocytes and monocytes from healthy adults. Proteasome expression was also greatly increased in normal blood mononuclear cells during blastogenic transformation induced by phytohemagglutinin; their expression increased in parallel with induction of DNA synthesis and returned to the basal level with progress of the cell cycle. Thus, abnormally high expression of proteasomes may play an important role in transformation and proliferation of blood cells and in specific functions of hematopoietic tumor cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhayat O., Grossi de Sa F., Infante A. A. Sea urchin prosome: characterization and changes during development. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1595–1599. doi: 10.1073/pnas.84.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo A. P., Tanaka K., Goldberg A. L., Welch W. J. Identity of the 19S 'prosome' particle with the large multifunctional protease complex of mammalian cells (the proteasome). Nature. 1988 Jan 14;331(6152):192–194. doi: 10.1038/331192a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Castaño J. G., Ornberg R., Koster J. G., Tobian J. A., Zasloff M. Eukaryotic pre-tRNA 5' processing nuclease: copurification with a complex cylindrical particle. Cell. 1986 Aug 1;46(3):377–385. doi: 10.1016/0092-8674(86)90658-6. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Domae N., Harmon F. R., Busch R. K., Spohn W., Subrahmanyam C. S., Busch H. Donut-shaped "miniparticles" in nuclei of human and rat cells. Life Sci. 1982 Feb 1;30(5):469–477. doi: 10.1016/0024-3205(82)90464-7. [DOI] [PubMed] [Google Scholar]
- Driscoll J., Goldberg A. L. The proteasome (multicatalytic protease) is a component of the 1500-kDa proteolytic complex which degrades ubiquitin-conjugated proteins. J Biol Chem. 1990 Mar 25;265(9):4789–4792. [PubMed] [Google Scholar]
- Eytan E., Ganoth D., Armon T., Hershko A. ATP-dependent incorporation of 20S protease into the 26S complex that degrades proteins conjugated to ubiquitin. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7751–7755. doi: 10.1073/pnas.86.20.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenburg P. E., Haass C., Kloetzel P. M., Niedel B., Kopp F., Kuehn L., Dahlmann B. Drosophila small cytoplasmic 19S ribonucleoprotein is homologous to the rat multicatalytic proteinase. Nature. 1988 Jan 14;331(6152):190–192. doi: 10.1038/331190a0. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Tanaka K., Kumatori A., Shin S., Yoshimura T., Ichihara A., Tokunaga F., Aruga R., Iwanaga S., Kakizuka A. Molecular cloning of cDNA for proteasomes (multicatalytic proteinase complexes) from rat liver: primary structure of the largest component (C2). Biochemistry. 1989 Sep 5;28(18):7332–7340. doi: 10.1021/bi00444a028. [DOI] [PubMed] [Google Scholar]
- Gautier J., Pal J. K., Grossi de Sa M. F., Beetschen J. C., Scherrer K. Differential cytolocalization of prosomes in axolotl during oogenesis and meiotic maturation. J Cell Sci. 1988 Aug;90(Pt 4):543–553. doi: 10.1242/jcs.90.4.543. [DOI] [PubMed] [Google Scholar]
- Grainger J. L., Winkler M. M. The sea urchin multicatalytic protease: purification, biochemical analysis, subcellular distribution, and relationship to snRNPs. J Cell Biol. 1989 Aug;109(2):675–683. doi: 10.1083/jcb.109.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi de Sa M. F., Martins de Sa C., Harper F., Coux O., Akhayat O., Pal J. K., Florentin Y., Scherrer K. Cytolocalization of prosomes as a function of differentiation. J Cell Sci. 1988 Feb;89(Pt 2):151–165. doi: 10.1242/jcs.89.2.151. [DOI] [PubMed] [Google Scholar]
- Haass C., Pesold-Hurt B., Multhaup G., Beyreuther K., Kloetzel P. M. The PROS-35 gene encodes the 35 kd protein subunit of Drosophila melanogaster proteasome. EMBO J. 1989 Aug;8(8):2373–2379. doi: 10.1002/j.1460-2075.1989.tb08366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hügle B., Kleinschmidt J. A., Franke W. W. The 22 S cylinder particles of Xenopus laevis. II. Immunological characterization and localization of their proteins in tissues and cultured cells. Eur J Cell Biol. 1983 Nov;32(1):157–163. [PubMed] [Google Scholar]
- Kleinschmidt J. A., Escher C., Wolf D. H. Proteinase yscE of yeast shows homology with the 20 S cylinder particles of Xenopus laevis. FEBS Lett. 1988 Oct 24;239(1):35–40. doi: 10.1016/0014-5793(88)80540-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Eisenman R. N. c-myc and c-myb protein degradation: effect of metabolic inhibitors and heat shock. Mol Cell Biol. 1988 Jun;8(6):2504–2512. doi: 10.1128/mcb.8.6.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews W., Driscoll J., Tanaka K., Ichihara A., Goldberg A. L. Involvement of the proteasome in various degradative processes in mammalian cells. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2597–2601. doi: 10.1073/pnas.86.8.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M. J., Croall D. E., DeMartino G. N. ATP-stimulated proteolysis in soluble extracts of BHK 21/C13 cells. Evidence for multiple pathways and a role for an enzyme related to the high-molecular-weight protease, macropain. Arch Biochem Biophys. 1988 Apr;262(1):273–285. doi: 10.1016/0003-9861(88)90189-0. [DOI] [PubMed] [Google Scholar]
- Moscatelli D., Rifkin D. B. Membrane and matrix localization of proteinases: a common theme in tumor cell invasion and angiogenesis. Biochim Biophys Acta. 1988 Aug 3;948(1):67–85. doi: 10.1016/0304-419x(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Pal J. K., Gounon P., Grossi de Sa M. F., Scherrer K. Presence and distribution of specific prosome antigens change as a function of embryonic development and tissue-type differentiation in Pleurodeles waltl. J Cell Sci. 1988 Aug;90(Pt 4):555–567. doi: 10.1242/jcs.90.4.555. [DOI] [PubMed] [Google Scholar]
- Rivett A. J. The multicatalytic proteinase of mammalian cells. Arch Biochem Biophys. 1989 Jan;268(1):1–8. doi: 10.1016/0003-9861(89)90558-4. [DOI] [PubMed] [Google Scholar]
- Sone S., Utsugi T., Nii A., Ogura T. Effects of human alveolar macrophages on the induction of lymphokine (IL 2)-activated killer cells. J Immunol. 1987 Jul 1;139(1):29–34. [PubMed] [Google Scholar]
- Tanaka K., Fujiwara T., Kumatori A., Shin S., Yoshimura T., Ichihara A., Tokunaga F., Aruga R., Iwanaga S., Kakizuka A. Molecular cloning of cDNA for proteasomes from rat liver: primary structure of component C3 with a possible tyrosine phosphorylation site. Biochemistry. 1990 Apr 17;29(15):3777–3785. doi: 10.1021/bi00467a026. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Ichihara A. Involvement of proteasomes (multicatalytic proteinase) in ATP-dependent proteolysis in rat reticulocyte extracts. FEBS Lett. 1988 Aug 15;236(1):159–162. doi: 10.1016/0014-5793(88)80306-5. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Ii K., Ichihara A., Waxman L., Goldberg A. L. A high molecular weight protease in the cytosol of rat liver. I. Purification, enzymological properties, and tissue distribution. J Biol Chem. 1986 Nov 15;261(32):15197–15203. [PubMed] [Google Scholar]
- Tanaka K., Kumatori A., Ii K., Ichihara A. Direct evidence for nuclear and cytoplasmic colocalization of proteasomes (multiprotease complexes) in liver. J Cell Physiol. 1989 Apr;139(1):34–41. doi: 10.1002/jcp.1041390107. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Yoshimura T., Ichihara A., Ikai A., Nishigai M., Morimoto Y., Sato M., Tanaka N., Katsube Y., Kameyama K. Molecular organization of a high molecular weight multi-protease complex from rat liver. J Mol Biol. 1988 Oct 20;203(4):985–996. doi: 10.1016/0022-2836(88)90123-4. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Yoshimura T., Ichihara A., Kameyama K., Takagi T. A high molecular weight protease in the cytosol of rat liver. II. Properties of the purified enzyme. J Biol Chem. 1986 Nov 15;261(32):15204–15207. [PubMed] [Google Scholar]
- Tanaka K., Yoshimura T., Kumatori A., Ichihara A., Ikai A., Nishigai M., Kameyama K., Takagi T. Proteasomes (multi-protease complexes) as 20 S ring-shaped particles in a variety of eukaryotic cells. J Biol Chem. 1988 Nov 5;263(31):16209–16217. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R. L., Gutowski J. K., Katz M., Goldfarb R. H., Cohen S. Induction of DNA synthesis in isolated nuclei by cytoplasmic factors: inhibition by protease inhibitors. Proc Natl Acad Sci U S A. 1987 Jan;84(1):241–245. doi: 10.1073/pnas.84.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]