Abstract
Attention-deficit/hyperactivity disorder (ADHD) is characterized by deficits in impulse control across a range of behaviors, from simple actions to those involving complex decision-making (e.g., preference for smaller-sooner versus larger later rewards). This study investigated whether changes in motor response control with increased cognitive load and motivational contingencies are associated with decision-making in the form of delay discounting among 8-12 year old children with and without ADHD. Children with ADHD (n = 26; 8 girls) and typically developing controls (n = 40; 11 girls) completed a standard go/no-go (GNG) task, a GNG task with motivational contingencies, a GNG task with increased cognitive load, and two measures of delay discounting: a real-time task in which the delays and immediately consumable rewards are experienced in real-time, and a classic task involving choices about money at longer delays. Children with ADHD, particularly girls, exhibited greater delay discounting than controls during the real-time discounting task, whereas diagnostic groups did not significantly differ on the classic discounting task. The effect of cognitive load on response control was uniquely associated with greater discounting on the real-time task for children with ADHD, but not for control children. The effect of motivational contingencies on response control was not significantly associated with delay discounting for either diagnostic group. The findings from this study help to inform our understanding of the factors that influence deficient self-control in ADHD, suggesting that impairments in cognitive control may contribute to greater delay discounting in ADHD.
Keywords: ADHD, delay discounting, motivation, cognition, response control, cognitive load
Attention-deficit/hyperactivity disorder (ADHD) is the most common psychiatric disorder of childhood, affecting approximately 4-12% of school-age children (American Psychiatric Association, 2013; Getahun et al., 2013). Individuals with ADHD present with developmentally inappropriate levels of inattention, hyperactivity, and impulsivity, which can be associated with detrimental functional impairment both academically and socially (American Psychiatric Association, 2013). ADHD is characterized by deficits in impulse control across a range of behaviors, from simple actions (see review by Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005; e.g., Wodka et al., 2007) to those involving complex decision-making (e.g., preference for smaller-sooner versus larger-later rewards) (see meta-analysis by Patros, Alderson, Kasper, et al., 2015; e.g., Rosch & Mostofsky, 2016). Early etiological models posited that a central core deficit in response inhibition underlies the executive function deficits and behavioral symptoms characteristic of ADHD (Barkley, 1997), while others have emphasized the role of atypical motivation, reinforcement sensitivity, and steeper delay discounting (Johansen, Aase, Meyer, & Sagvolden, 2002; Luman, Tripp, & Scheres, 2010; Sagvolden, Johansen, Aase, & Russell, 2005), and the interaction between cognitive deficits and atypical motivation (Castellanos, Sonuga-Barke, Milham, & Tannock, 2006; Sonuga-Barke, 2005).
Impulsive decision-making, defined as a preference for smaller-sooner rewards over larger-delayed rewards, is prominent in multifactorial models of ADHD (Sonuga-Barke, 2002, 2003) and etiological theories emphasizing altered reinforcement sensitivity (see review by Luman et al., 2010). In the ADHD literature, impulsive decision-making is typically assessed with delay of gratification tasks, involving repeated choices between a fixed smaller-sooner reward and a fixed larger-later reward with the dependent variable as the number of choices made for the immediate or delayed option. Fewer studies have used delay discounting tasks, designed to identify the point along a continuum of delays at which the value of immediate and delayed rewards is roughly the same through the use of varying delays and reward amounts. A recent meta-analysis reported comparable effect sizes have been obtained from delay of gratification (g=.47) and delay discounting (g=.50) studies of individuals with ADHD (Patros, Alderson, Kasper, et al., 2015). However, there have been inconsistent findings across studies, likely due in part to variability in characteristics of the task. Specifically, delay discounting is influenced by reward magnitude (Myerson & Green, 1995), delay length, type of reward (e.g., monetary versus non-monetary) (Chapman & Elstein, 1995; Demurie, Roeyers, Baeyens, & Sonuga-Barke, 2013; Friedel, DeHart, Madden, & Odum, 2014; Killeen, 2015), and whether the rewards are immediately consumable (e.g., food versus money) (Forzano, Michels, Sorama, Etopio, & English, 2014). We recently found ADHD-associated increases in delay discounting to be specific to girls and specific to a novel real-time discounting task involving immediately consumable rewards, but not a classic monetary discounting task, suggesting that inconsistent findings across studies may also be due to task differences or individual characteristics such as sex (Rosch & Mostofsky, 2016). Thus, the current study examined delay discounting with two tasks: a commonly used classic discounting task involving monetary rewards and longer delays to permit comparisons to the existing literature and a novel real-time discounting task involving immediately consumable rewards (playing a preferred game) delivered at shorter delays, which may provide a more valid assessment of decision-making in children.
Delay discounting is often discussed as reflecting atypical motivation or response to reward in the ADHD literature, but there is strong evidence from the computational and cognitive neuroscience literatures that delay discounting involves the interaction of neural mechanisms underlying reward processing and cognitive control. Specifically, basal-ganglia-frontal circuits are believed to contribute to reward-based decision-making (Frank & Claus, 2006; Frank, Scheres, & Sherman, 2007), including valuation circuits in ventromedial prefrontal cortex and striatum and choice circuits in lateral prefrontal cortex and parietal cortex (Kable & Glimcher, 2009). The extent to which an individual engages in delay discounting may reflect interindividual variability in the neural mechanisms underlying delay discounting, including reward valuation, cognitive control, and prospection (see review by Peters & Buchel, 2011). Despite the growing evidence that delay discounting involves multiple interacting brain systems related to self-referential, executive, and reinforcement processes (Sonuga-Barke, Cortese, Fairchild, & Stringaris, 2015), the mechanisms of delay discounting in ADHD are poorly understood. The prevailing models suggest that the behavioral preference for immediate rewards observed in individuals with ADHD may be related to an atypical response to reward, deficient cognitive control, or both. Evidence of distinct neuropsychological profiles within ADHD (van Hulst, de Zeeuw, & Durston, 2015) suggests that greater delay discounting often observed in individuals with ADHD may be due to executive dysfunction (Nigg, Willcutt, Doyle, & Sonuga-Barke, 2005; Willcutt et al., 2005), such that diminished cognitive control leads to difficulty delaying gratification, or an atypical response to reinforcement (Luman, Oosterlaan, & Sergeant, 2005; Luman et al., 2010) resulting in differential valuation of immediate and delayed rewards.
One approach to improving our understanding of delay discounting in relation to ADHD is to examine how it relates to other forms of impulse control implicated in ADHD, such as control of rapid responses rather than more deliberative decision-making processes (Patros, Alderson, Kasper, et al., 2015). Among adults with ADHD, weaker response control assessed during a stop signal task was strongly correlated with greater delay discounting (Crunelle, Veltman, van Emmerik-van Oortmerssen, Booij, & van den Brink, 2013). Among children with ADHD, Solanto et al. (2001) found that delay of gratification was uncorrelated with response control (during a stop signal task) among children with and without ADHD, whereas other studies have found significant relationships between delay of gratification, working memory, and response control (Karalunas & Huang-Pollock, 2011; Patros, Alderson, Lea, et al., 2015; Sjowall, Roth, Lindqvist, & Thorell, 2013; Sonuga-Barke, Bitsakou, & Thompson, 2010). None of these studies involving children with ADHD examined the relationship between response control and delay discounting, although similar relationships may be expected.
Consistent with the multi-pathway conceptualization of ADHD, there has been growing interest in the impact of cognitive and motivational factors on response control. For instance, some studies have demonstrated that increased cognitive load impacts response control in children with and without ADHD (Vaurio, Simmonds, & Mostofsky, 2009; Wodka et al., 2007). We recently expanded upon these initial findings, which were conducted in primarily male ADHD samples, to include a large sample of boys and girls with ADHD to evaluate potential sex differences in response control (Seymour, Mostofsky, & Rosch, 2016). The results of this study suggest that increasing cognitive load differentially impacted response control for girls, but not boys, with ADHD compared to typically developing control children. Specifically, girls with ADHD demonstrated poorer response control only under conditions of greater cognitive load, whereas response control was impaired among boys with ADHD regardless of cognitive load (Seymour et al., 2016).
There is also a large literature demonstrating improvement in cognitive task performance with motivational contingencies (i.e., reinforcement and punishment) (e.g., Bubnik, Hawk, Pelham, Waxmonsky, & Rosch, 2015; see review by Luman et al., 2005; e.g., Shiels et al., 2008; Strand et al., 2012), including measures of response control (Rosch et al., 2016). Another recent study from our laboratory expanded upon this work by including a large sample of boys and girls to address potential ADHD-related sex differences in the effect of motivational contingencies on cognitive task performance (Rosch, Dirlikov, & Mostofsky, 2015). This study showed that boys with ADHD showed similar improvements in response control with motivational contingencies as did control boys and girls, whereas girls with ADHD did not show this improvement (Rosch et al., 2015).
Altogether, these studies suggest that individuals with ADHD tend to display greater delay discounting and that motivational contingencies and cognitive load differentially impact response control in children with ADHD. Furthermore, recent evidence suggests that girls and boys with ADHD differ in their degree of delay discounting and the extent to which motivational contingencies and cognitive load impact response control. Examination of the relationship between increased delay discounting and changes in response control with motivational contingencies (Rosch et al., 2015) and cognitive load (Seymour et al., 2016) may elucidate the cognitive and motivational processes underlying delay discounting in ADHD (Rosch & Mostofsky, 2016) and inform our understanding of ADHD-related sex differences.
This study investigated whether changes in response control with cognitive load and motivational contingencies demonstrated in our previous studies are associated with delay discounting among children with and without ADHD. We included participants from our previous studies who had completed multiple go/no-go (GNG) tasks (standard, complex, and motivational; Rosch et al., 2015; Seymour et al., 2016), as well as the real-time and classic delay discounting tasks (Rosch & Mostofsky, 2016) in order to examine whether and how the separate findings of (a) poorer response control with increased cognitive load (Seymour et al., 2016), (b) diminished benefits of motivational contingencies (Rosch et al., 2015), and (c) greater delay discounting in children with ADHD (Rosch & Mostofsky, 2016), are related. This study is novel in its integration of previous findings from our previous study examining delay discounting (Rosch & Mostofsky, 2016) with the same tasks included in the current study and several prior studies characterizing response control using the various go/no-go tasks examined here (Rosch et al., 2015; Seymour et al., 2016; Vaurio et al., 2009; Wodka et al., 2007) to improve our understanding how cognitive and motivational effects on response control relate to delay discounting in children with ADHD.
Our specific hypotheses are as follows: (1) Poorer response control with increasing cognitive load will be associated with greater delay discounting, (2) a lack of improvement in response control with performance-based motivational contingencies will be associated with greater delay discounting, and (3) these relationships will be specific to children with ADHD, although we plan to examine them in control children as well.
Methods
Participants
Analyses were conducted on a sample of 66 8-12 year-old children including 26 children with ADHD combined-type (ADHD-C; 8 girls) and 40 control children (11 girls). For the current analyses, we restricted inclusion to children with the combined subtype of ADHD given the theoretical and empirical emphasis on hyperactive/impulsive symptoms in relation to delay discounting and atypical motivation (Sagvolden et al., 2005; Scheres & Hamaker, 2010; Scheres, Lee, & Sumiya, 2008).1 Participants were primarily recruited through local schools, with additional resources including community-wide advertisement, volunteer organizations, medical institutions, and word of mouth. This study was approved by the Johns Hopkins Institutional Review Board and all data was obtained in compliance with their regulations. After complete description of the study to the participants, written informed consent was obtained from a parent/guardian and assent was obtained from the child.
An initial screening was conducted through a telephone interview with a parent. Children with a history of intellectual disability, learning disability, seizures, traumatic brain injury or other neurological illnesses were excluded from participation. Intellectual ability was assessed using the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler, 2003) and participants with FSIQ scores below 80 were excluded. In addition to inquiring about a history of a learning disability, children were also administered the Word Reading subtest from the Wechsler Individual Achievement Test, Second Edition (WIAT-II; Wechsler, 2002) to further screen for a reading disorder and were excluded if their Word Reading scores fell below a standard score of 85.
Diagnostic status was established through administration of the Diagnostic Interview for Children and Adolescents, Fourth Edition (DICA-IV; Reich, Welner, & Herjanic, 1997),2 which follows DSM-IV criteria for all disorders (American Psychiatric Association, 1994). Children meeting criteria for diagnosis of conduct, mood, generalized anxiety, separation anxiety or obsessive–compulsive disorders on DICA-IV interview were excluded. A comorbid diagnosis of oppositional defiant disorder (ODD) was permitted to obtain a more representative sample given the high co-occurrence of ODD and ADHD (e.g., rates between 30-50%; Jensen et al., 2001) as often done in studies of children with ADHD (e.g., Coghill, Seth, & Matthews, 2013; Gupta & Kar, 2009; Marco et al., 2009; Scheres et al., 2006; Sjowall et al., 2013; Solanto et al., 2007; Sonuga-Barke et al., 2010; Wilson, Mitchell, Musser, Schmitt, & Nigg, 2011). We chose not to permit comorbid internalizing psychopathology such as anxiety and depression to maintain a sample with externalizing psychopathology, which is broadly associated with greater delay discounting (Bobova, Finn, Rickert, & Lucas, 2009). Parents and teachers (when available) also completed the Conners’ Parent and Teacher Rating Scales-Revised Long Version or the Conners-3 (CPRS and CTRS; Conners, 2002, 2008) and the ADHD Rating Scale-IV, home and school versions (ADHD-RS; DuPaul, Power, Anastopoulos, & Reid, 1998). Although teacher report was not always available, we obtained information from the parent about the child’s symptoms and functioning at school during the diagnostic interview.
An ADHD diagnosis was established based on the following criteria: (1) T-score of 65 or higher on the ADHD Inattentive or Hyperactive/Impulsive scales on the CPRS or CTRS, when available, or a raw score of 2 or 3 on at least 6/9 items on the Inattentive or Hyperactivity/Impulsivity scales of the ADHD-RS and (2) an ADHD diagnosis on the DICA-IV. This information was then reviewed and the diagnosis was confirmed by a child neurologist (S.H.M.) based on DSM-IV criteria. Children taking psychotropic medications other than stimulants were excluded from participation and all children taking stimulants were asked to withhold their medication the day of and day prior to the laboratory visit, as in prior work including children on long-acting stimulants (Rosch et al., 2015; Rosch & Mostofsky, 2016; Seymour et al., 2016). Fifteen participants within the ADHD group (58%) were regularly taking a stimulant medication, 10 of which were taking long-acting formulations.
Inclusion in the control group required scores below clinical cutoffs on the parent and teacher (when available) rating scales (CPRS, CTRS, and ADHD-RS). Control participants could not meet diagnostic criteria for any psychiatric disorder based on DICA-IV nor could they have history of neurological disorder, learning disability, or be taking any psychotropic medication. They were also required to have an FSIQ of at least 80 on the WISC-IV and a score of at least 85 on the WIAT-II word reading subtest. Children included in the control group also could not have an immediate family member diagnosed with ADHD. Participants were enrolled in at least one of several ongoing studies in our lab. These studies involve at least two full days of testing (7 hours each day) on a large battery of tasks and neuroimaging measures. Given the overlap in task batteries across studies, participants were often enrolled in additional studies, sometimes returning for a third visit, permitting additional data collection for certain tasks. The sample examined in this study largely overlapped with three prior publications comparing the performance of girls and boys with ADHD to same-sex control children on delay discounting (88% of the current sample also included in Rosch & Mostofsky, 2016), the effects of cognitive load on GNG performance (29% included in Seymour et al., 2016), and the effects of motivational contingencies on GNG performance (84% included in Rosch et al., 2015).
Procedures
The current analyses included participants who completed two delay discounting tasks and three computer-based GNG paradigms: a standard GNG, a complex GNG with increased cognitive load, and a motivational GNG with performance-based motivational contingencies (Mostofsky et al., 2003; Shiels Rosch, Dirlikov, & Mostofsky, 2013; Vaurio et al., 2009; Wodka et al., 2007). The standard GNG task was always administered before delay discounting tasks and other GNG tasks. Complex GNG, motivational GNG, and delay discounting tasks were not formally counterbalanced, however, there was variability in the order of administration in this sample depending on the random assignment of participants to the different studies. All participants completed the delay discounting tasks on the same day and the vast majority completed the emotional and complex GNG tasks on the same day as the discounting tasks (88% and 86%, respectively) whereas the simple GNG task was completed on a different testing day for all but four participants (two per diagnostic group).
Classic Discounting Task
Participants completed a computer-based delay discounting task involving 91 choices between a varying amount of money now ($0-$10.50 in $0.50 increments) or $10.00 after a varying delay (1, 7, 30, or 90 days) (Rosch & Mostofsky, 2016; Wilson et al., 2011). Participants were instructed to indicate whether they preferred the immediate or delayed option using a computer mouse. They were also told that some of the choices were real and they would actually receive the amount of money at the specified delay that they chose for some of the items in the form of gift cards or prizes (two choices semi-randomly selected). This task took 10-15 minutes to complete.
Real-Time Discounting Task
The real-time delay discounting task involved immediately consumable rewards and variable reward and delay amounts (Rosch & Mostofsky, 2016). During this task, participants made nine choices between getting to play a preferred game for a shorter amount of time (either 15, 30, or 45 seconds) immediately or for a fixed longer amount of time (60 seconds) after waiting (either 25, 50, or 100 seconds). Once the participant made a choice, they experienced the delays and rewards associated with that choice in real-time prior to making their next choice. The trial duration was held constant at 160 seconds (i.e., the length of the longest possible trial when the child chooses to wait 100 seconds to play for 60 seconds) regardless of the child’s choice by imposing an adjusting post-reward delay. Participants could bring their own game and were offered several game options (handheld video game, coloring, Legos, etc.) to maximize the rewarding value for each individual. Their preferred game was placed in a clear box in front of them when they made their choices and while waiting to play. This task involved two practice choices, during which participants experienced both the immediate and delayed options, followed by nine test choices and took 40 minutes to complete. The immediate reward values were presented in ascending order within each delay and the order of the delays was fully counterbalanced across subjects.
Standard GNG task
Task stimuli consisted of green spaceships for go trials and red spaceships for no-go trials (20% of trials) presented for 300 ms with an interstimulus interval of 2000 ms, during which a fixation cross was present on-screen. Participants were instructed to push the spacebar with their index finger as quickly as possible in response to green spaceships only. The use of familiar stimulus-response associations (green for go; red for no-go) minimized the perceptual and cognitive demands of the tests. Go and no-go trials appeared in pseudorandom order with the restrictions that there were never fewer than three go trials before a no-go cue and never more than two no-go trials in a row. There were 11 practice trials followed by 240 experimental trials presented in a pseudorandom order. Responses and reaction times (RT) were recorded for the entire trial duration. The task duration was 8 minutes, 19 seconds. This task was typically administered on the first day of testing.
Complex GNG task
The trial structure of the complex GNG task was nearly identical to that of the standard GNG task but the cognitive load varied. The stimuli were identical to those in the standard GNG task, consisting of red or green spaceships presented for 300 ms, followed by a blank screen for 2000 ms. Children were instructed to push the button as quickly as possible in response to a green spaceship and in response to a red spaceship preceded by an even number of green spaceships. They were to refrain from responding to red spaceships preceded by an odd number of green spaceships. There were five practice trials to demonstrate an even sequence, six practice trials to demonstrate an odd sequence, and 11 practice trials with each type of sequence. The task consisted of 207 experimental trials including 163 green go cues; 21 red go cues (i.e., preceded by an even number of green spaceships) and 23 red no-go cues (i.e., preceded by an odd number of green spaceships). Responses and reaction times (RT) were recorded for the entire trial duration. The total time of this task was 7 min, 56 s. This task was typically administered on the second day of testing.
Motivational GNG task
Participants also completed a task similar to the standard GNG task with the addition of immediate trial-by-trial feedback paired with monetary gain and loss (i.e., reinforcement and punishment). The stimuli were identical to those in the standard GNG task, consisting of red or green spaceships presented for 300 ms, followed by a blank screen for 1000 ms, the presentation of visual feedback for 1700 ms, and another blank screen for 500 ms. Responses were recorded during the entire trial length. Contingencies were structured to reinforce fast, accurate responses to go stimuli and to punish failures to inhibit responses to no-go stimuli (commission errors). For correct go responses that were faster than an individualized response deadline (mean + 1 standard deviation of go RT during the standard GNG task), participants earned 10 cents and feedback consisting of three yellow happy faces and picture of a dime was presented. For responses to no-go stimuli (i.e., commission errors), participants lost 50 cents and feedback consisting of three purple frowning faces and 50 cents crossed out with a red X was presented. The total time of this task was 13 min and it was typically administered on the second day of testing. Participants received the money they earned during the task in a check mailed upon completion of the study.
Data Reduction
The primary dependent variable derived from each discounting task was the area under the curve (AUC) calculated based on the indifference point for each delay. For the classic discounting task, the indifference point was defined as midway between the smallest value of the immediate alternative consistently accepted and the largest value consistently rejected for each delay (Wilson et al., 2011). For the real-time discounting task, the indifference point was defined as the lowest immediate value selected for each delay. These indifference points were used to determine the AUC (Myerson, Green, & Warusawitharana, 2001), a common approach to analyzing discounting data (e.g., Reynolds, Penfold, & Patak, 2008; Rosch & Mostofsky, 2016; Scheres, Tontsch, Thoeny, & Sumiya, 2014; Shiels et al., 2009) that eliminates some of the problems associated with measures assuming a hyperbolic function. Smaller AUC values indicate greater delay discounting and greater impulsivity. The AUC was calculated in excel (Reed, Kaplan, & Brewer, 2012).
Analysis of GNG performance focused on the difference in the commission error rate (proportion of no-go trials on which participants responded; ΔCom) for no-go stimuli during the complex and motivational GNG tasks relative to the standard GNG task. Specifically, a difference score was calculated for each subject for the effect of cognitive load on commission error rate (CogLoadΔCom = complex GNG commission error rate − standard GNG commission error rate), such that a higher value represents a greater increase in errors with greater cognitive load. A similar score was calculated for the effect of motivation on commission error rate (MotivationΔCom = standard GNG commission error rate − motivational GNG commission error rate), such that a higher value represents a greater reduction in errors in the context of motivational contingencies.
Four dependent variables were obtained from the five tasks completed by all participants: classic discounting AUC, real-time discounting AUC, CogLoadΔCom, and MotivationΔCom.
Data Analysis
Data analysis was accomplished using SPSS Statistics Version 23 (IBM, Chicago). The analyses conducted in this study examine correlations between delay discounting and GNG task performance, expanding upon findings of diagnostic group differences reported in published manuscripts on these exact delay discounting (Rosch & Mostofsky, 2016) and GNG tasks (Rosch et al., 2015; Seymour et al., 2016) in larger samples. Although diagnostic group differences are not the focus of the current analyses, results of the univariate analysis of covariance (ANCOVA) for each of the dependent variables described above (classic discounting AUC, real-time discounting AUC, CogLoadΔCom, and MotivationΔCom) with the between-subjects factors of diagnosis (ADHD vs. control) and sex (girls vs. boys) are presented to provide a context for examining task associations. The WISC-IV general ability index (GAI), a measure of intellectual reasoning ability, was included as a covariate for all analyses examining diagnostic group differences given the evidence for associations among intelligence and delay discounting (Shamosh & Gray, 2008). We chose to use the GAI as a covariate in the analyses rather than the full-scale intelligence quotient (FSIQ) because FSIQ is influenced by difficulties in working memory and processing speed, which are often present in children with ADHD. In contrast, GAI is based on verbal and perceptual reasoning abilities and may therefore be a more appropriate measure of broad intellectual ability in children with ADHD. For significant and trend-level findings for tests of diagnostic group differences, Cohen’s d is reported as a measure of effect size generally interpreted as d = 0.2, 0.5, and 0.8, indicating a small, medium, and large effect, respectively (Cohen, 1988).
Next, bivariate correlations were conducted in the overall sample examining associations among each of the task dependent variables and GAI. Partial correlations controlling for GAI were then conducted among the task dependent variables in the overall sample and within diagnostic group. Fisher’s r-to-z transformation was used to test whether the correlations differed between diagnostic groups based on a two-tailed test. Correlations of interest were followed-up with hierarchical linear regressions to clarify the relationship between changes in response control with increased cognitive load and motivational contingencies and delay discounting among children with and without ADHD.
Results
Sample Characteristics
Demographic information for the sample is provided in Table 1, along with inferential statistics regarding diagnostic group differences and sex differences within the ADHD sample. The sample was drawn from largely middle class socioeconomic status (Hollingshead, 1975) and was 65% caucasian. Diagnostic groups did not significantly differ in several important demographics including age, sex, ethnicity, SES, WISC-IV FSIQ or GAI.
Table 1.
Demographic and clinical characteristics of study participants.
| Control (n = 40) | ADHD (n = 26) | Group Comparisons | |
|---|---|---|---|
|
| |||
| Mean (SD) | Mean (SD) | p-value | |
| Age (years) | 10.0 (1.1) | 9.7 (1.1) | .271 |
| Sex (boys: girls) | 29:11 | 18:8 | .774 |
| Ethnicity (% caucasian) | 60.0% | 69.2% | .310 |
| SES | 53.2 (9.3) | 53.6 (9.1) | .888 |
| WISC-IV FSIQ | 115.1 (12.3) | 109.6 (12.5) | .086 |
| WISC-IV GAI | 118.0 (13.3) | 112.5 (13.2) | .105 |
| WISC-IV VCI | 119.2 (13.0) | 114.3 (12.9) | .142 |
| WISC-IV PRI | 111.1 (12.9) | 108.5 (13.4) | .428 |
| WISC-IV WMI | 111.2 (15.0) | 104.1 (13.6) | .057 |
| WISC-IV PSI | 101.1 (13.9) | 98.3 (13.4) | .432 |
| ADHD-RS Inatt Raw | 3.2 (2.9) | 18.6 (4.5) | <.001 |
| ADHD-RS HypImp Raw | 2.4 (2.3) | 15.0 (7.1) | <.001 |
| CPRS Inatt T | 44.7 (6.0) | 73.3 (9.6) | <.001 |
| CPRS HypImp T | 46.2 (5.6) | 72.9 (15.3) | <.001 |
| Comorbid ODD % | n/a | 38.5% | n/a |
| Stimulant medication % | n/a | 57.7% | n/a |
Notes: Control = Typically Developing Controls; ADHD = attention-deficit/hyperactivity disorder; SES = socioeconomic status from Hollingshead total score; WISC-IV FSIQ = Wechsler Intelligence Scale for Children Fourth Edition (WISC-IV) Full Scale Intelligence Quotient; WISC-IV GAI = General Ability Index; WISC-IV VCI = Verbal Comprehension Index; WISC-IV PRI = Perceptual Reasoning Index; WISC-IV WMI = Working Memory Index; WISC-IV PSI = Processing Speed Index; ADHD-RS Inatt Raw = DuPaul Parent Inattentive raw score (range 0-27); ADHD-RS HypImp Raw = DuPaul Parent Hyperactive/Impulsive raw score (range 0-27); CPRS Inatt T = Conners Parent Rating Scale-Revised or Conners-3 Parent Inattentive Index T-score; CPRS HypImp T = Conners; Hyperactive/Impulsive Index T-score; ODD = Oppositional Defiant Disorder.
Diagnostic group differences
Examination of univariate tests for the effect of diagnostic group revealed a significant effect for real-time discounting AUC, F(1, 61) = 6.5, p = .013, d=0.65, such that children with ADHD showed greater delay discounting (less AUC) on the real-time discounting task than did controls. This effect was qualified by a Diagnosis × Sex interaction, F(1, 61) = 4.0, p = .050, d = 0.51, with greater discounting among girls with ADHD compared to control girls (p = .009), but not among boys with ADHD compared to control boys (p = .582). There were no significant effects of diagnosis or Diagnosis × Sex interactions for the remaining dependent variables (see Table 2).
Table 2.
Univariate analysis of covariance testing for effects of diagnosis and interactions with sex on task measures.
| Control Boys (n = 29) | Control Girls (n = 11) | ADHD Boys (n = 18) | ADHD Girls (n = 8) | Diagnosis | Diagnosis x Sex | |
|---|---|---|---|---|---|---|
|
| ||||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | p (d) | p (d) | |
| Real-time Discounting AUC | .58(.16) | .60(.15) | .55(.15) | .41(.05) | .013(.65) | .050(.51) |
| Classic Discounting AUC | .47(.32) | .36(.27) | .41(.29) | .35(.15) | .841(.05) | .588(.14) |
| CogLoadΔCom | .03(.20) | -.04(.21) | .01(.25) | .06(.16) | .440(.20) | .978(.01) |
| MotivationΔCom | .13(.18) | .14(.16) | .20(.21) | .21(.23) | .154(.37) | .309(.26) |
Notes: The WISC-IV General Ability Index (GAI) was included as a covariate in the model. Control = typically developing controls; ADHD = attention-deficit/hyperactivity disorder; AUC = area under the curve; CogLoadΔCom = complex go/no-go commission error rate – standard go/no-go commission error rate (more positive number indicates greater increase in commission errors with greater cognitive load); MotivationΔCom = standard go/no-go commission error rate – motivational go/no-go commission error rate (more positive number indicates greater reduction in commission errors with motivational contingencies). d = Cohen’s d effect size estimate.
Correlations
Examination of bivariate zero-order Pearson correlations (see Table 3) indicated that in the overall sample (n = 66), GAI was significantly positively correlated with classic discounting AUC, r(64) = .446, p < .001, such that children with higher GAI scores displayed less discounting. Discounting on the classic and real-time tasks was also positively correlated r(64) = .285, p = .020. In addition, cognitive and motivational effects on commission error rate were significantly negatively correlated, r(64) = -.572, p < .001, indicating that children with less of an effect of motivational contingencies on their performance displayed a greater effect of cognitive load on their performance.
Table 3.
Task correlations among children with ADHD and typically developing controls.
| Full Sample (n = 66) | Classic discounting AUC | Real-time discounting AUC | MotivationΔCom | CogLoadΔCom | GAI |
|---|---|---|---|---|---|
| Classic discounting AUC | 1 | .285* [.05, .49] | .070 [-.17, .31] | .112 [-.13, .34] | .446*** [.23, .62] |
| Real-time discounting AUC | .247* [.01, .46] | 1 | -.121 [-.35, .12] | -.094 [-.33, .15] | .150 [-.10, .38] |
| MotivationΔCom | .049 [-.20, .29] | -.132 [-.36, .11] | 1 | -.572*** [-.72, -.38] | .060 [-.18, .30] |
| CogLoadΔCom | .083 [-.16, .32] | -.109 [-.34, .14] | -.580*** [-.72, -.39] | 1 | .084 [-.16, .32] |
|
| |||||
| ADHD (n = 26) | Classic discounting AUC | Real-time discounting AUC | MotivationΔCom | CogLoadΔCom | GAI |
|
| |||||
| Classic discounting AUC | 1 | .318 [-.08, .63] | .231 [-.17, .57] | -.032 [-.41, .36] | .384 [-.01, .67] |
| Real-time discounting AUC | .251 [-.15, .58] | 1 | .037 [-.36, .42] | -.352 [-.65, .04] | .244 [-.16, .58] |
| MotivationΔCom | .295 [-.10, .61] | .065 [-.33, .44] | 1 | -.796* [-.90, -.59] | -.103 [-.47, .30] |
| CogLoadΔCom | -.215 [-.56, .19] | -.502* [-.74, -.14] | -.497* [-.74, -.14] | 1 | .391* [.01, .68] |
|
| |||||
| Control (n = 40) | Classic discounting AUC | Real-time discounting AUC | MotivationΔCom | CogLoadΔCom | GAI |
|
| |||||
| Classic discounting AUC | 1 | .248 [-.07, .52] | -.001 [-.31, .31] | .202 [-.12, .48] | .469** [.19, .68] |
| Real-time discounting AUC | .271 [-.04, .54] | 1 | -.166 [.45, .15] | .083 [-.23, .38] | .020 [-.29, .33] |
| MotivationΔCom | -.142 [-.43, .18] | -.177 [-.46, .14] | 1 | -.667*** [-.81, -.45] | .258 [-.06, .53] |
| CogLoadΔCom | .290 [-.02, .55] | .086 [-.23, .39] | -.665** [-.84, -.45] | 1 | -.113 [-.41, .21] |
Notes: Values reported in the shaded cells are the zero-order Pearson correlation coefficients [95% confidence intervals] without any covariates whereas partial Pearson correlation coefficients with WISC-IV General Ability Index (GAI) as a covariate [and 95% confidence intervals] are reported in the white cells; ADHD = attention-deficit/hyperactivity disorder; Control = typically developing controls; AUC = Area Under the Curve (more AUC = less discounting); MotivationΔCom = standard go/no-go commission error rate – motivational go/no-go commission error rate (more positive number indicates greater reduction in commission errors with motivational contingencies); CogLoadΔCom = complex go/no-go commission error rate – standard go/no-go commission error rate (more positive number indicates greater increase in commission errors with greater cognitive load).
p<.05;
p<=.01;
p<.001
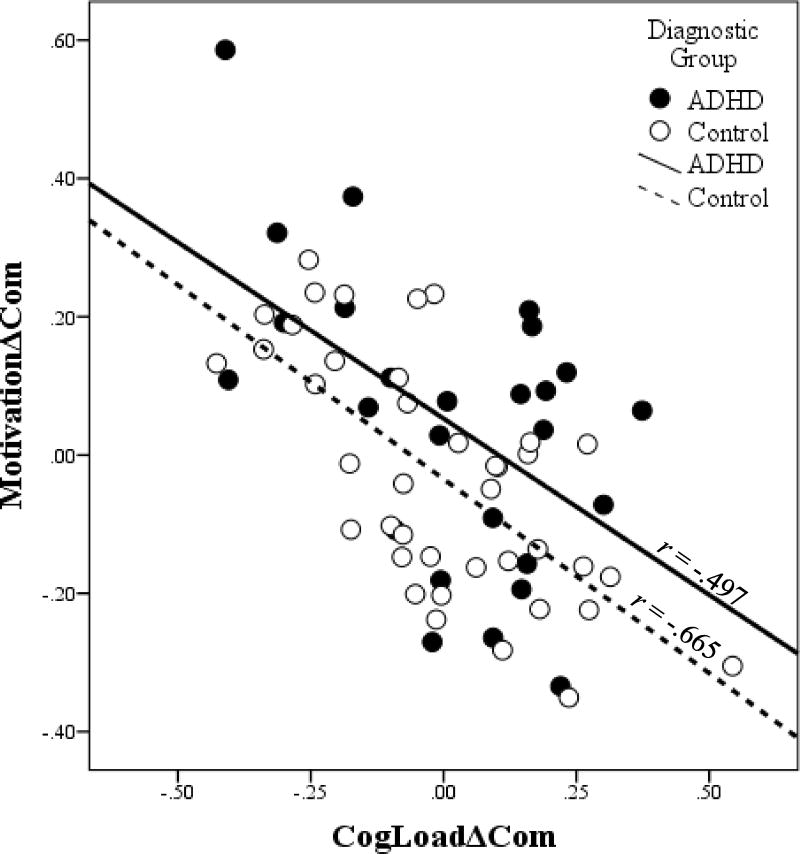
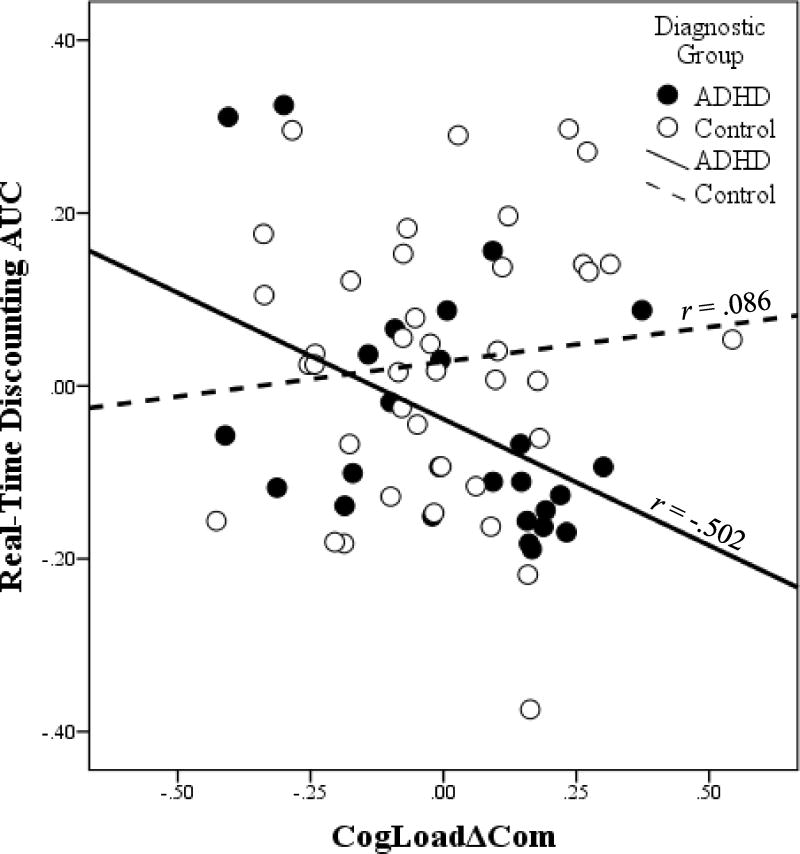
Given the relationships between GAI and delay discounting and GNG task performance, partial correlations among discounting and GNG task performance controlling for GAI were conducted (see Table 3). Within each diagnostic group, strong negative partial correlations were observed between CogLoadΔCom and MotivationΔCom (ADHD: r(23) = -.497, p <.001; control: r(37) = -.665, p <.001; Figure 1), similar to the findings in the full sample without covarying for GAI. Furthermore, CogLoadΔCom was significantly negatively partially correlated with real-time discounting AUC among children with ADHD, r(23) = -.502, p = .011, such that children with ADHD who displayed a greater increase in commission errors with greater cognitive load also showed greater delay discounting (i.e., less AUC) on the real-time task (Figure 2). This relationship was not significant among controls, r(37) = .086, p = .603, with a reliably greater partial correlation in the ADHD group (p = .01). In addition, MotivationΔCom was not significantly partially correlated with real-time discounting AUC among children with ADHD, r(23) = .065, p = .758, a significantly smaller partial correlation (p = .02) than reported above for CogLoadΔCom and real-time discounting. None of the remaining partial correlations were significant (see Table 3).
Figure 1.
The partial correlation between the effect of cognitive load (CogLoadΔCom) and motivational contingencies (MotivationΔCom) on commission error rate during the Go/No-Go tasks controlling for intellectual reasoning ability (WISC-IV general ability index (GAI)) within the attention-deficit/hyperactivity disorder (ADHD) and typically developing control groups (Control). The unstandardized residuals for each variable regressed on GAI are plotted here. Children who showed a greater effect of cognitive load on response control (CogLoadΔCom) also showed less improvement in response control with motivational contingencies (MotivationΔCom) within the ADHD group (r(23) = -.497, p = .011), and the Control group (r(37) = -.665, p <.001).
Figure 2.
The partial correlation between delay discounting as measured by area under the curve (AUC) on the real-time discounting task and the effect of cognitive load on commission error rate controlling for intellectual areasoning ability (WISC-IV general ability index (GAI)) within the attention-deficit/hyperactivity disorder (ADHD) and control (Control) groups. The unstandardized residuals for each variable regressed on GAI are plotted. Children with ADHD who showed a greater increase in commission errors with greater cognitive load also showed more delay discounting (r(23) = -.502, p = .011); no significant relationship was observed in the Control group (r(37) = .086, p = .603).
Regressions
We ran separate hierarchical linear regression models within each diagnostic group to examine whether CogLoadΔCom uniquely related to real-time discounting after accounting for MotivationΔCom. In Model 1, we included GAI and CogLoadΔCom as predictors to determine whether each variable was uniquely associated with real-time discounting after accounting for the other variable. In Model 2, MotivationΔCom was added to the regression model to determine whether CogLoadΔCom significantly predicted real-time discounting after accounting for MotivationΔCom, which is highly correlated with CogLoadΔCom, r(64) = -.572, in addition to GAI. Within the ADHD group, the results of Model 1 suggest that GAI and CogLoadΔCom uniquely predicted real-time discounting, (ps = .027 and .011, respectively). As shown in Model 2, both GAI and CogLoadΔCom remained significant predictors (ps = .020 and .006, respectively), after accounting for MotivationΔCom, which was not a significant predictor of real-time discounting (p = .247) (Table 4). Consistent with the partial correlation results described above, there were no significant relationships among real-time discounting AUC and CogLoadΔCom and MotivationΔCom among controls (Table 4).
Table 4.
Linear regression models examining the relationship between the effects of motivation and cognitive load on commission error rate during go/no-go tasks and real-time delay discounting within diagnostic group.
| Real-time Discounting AUC | |||||
|---|---|---|---|---|---|
|
| |||||
| Model 1 | Model 2 | ||||
|
|
|||||
| Variable | β | 95% CI | β | 95% CI | |
| ADHD | Constant | -0.044 | [-.53, .45] | -0.040 | [-0.53, 0.45] |
| WISC-IV GAI | 0.005* | [.001, .009] | 0.005* | [0.001, 0.01] | |
| CogLoadΔCom | -0.348* | [-.61, -.09] | -0.432** | [-0.73, -0.14] | |
| MotivationΔCom | -0.165 | [-0.45, 0.12] | |||
| R2 | 0.30 | 0.34 | |||
| F | 4.8* | 3.8* | |||
| ΔR2 | 0.04 | ||||
| ΔF | 1.4 | ||||
|
| |||||
| Control | Constant | 0.544* | [.09, 1.0] | 0.515* | [0.05, 0.98] |
| WISC-IV GAI | 0.000 | [-.003, .004] | 0.001 | [-0.003, 0.005] | |
| CogLoadΔCom | 0.064 | [-.18, .31] | -0.042 | [-0.38, 0.29] | |
| MotivationΔCom | -0.194 | [-0.60, 0.21] | |||
| R2 | 0.008 | 0.034 | |||
| F | 0.15 | 0.42 | |||
| ΔR2 | 0.03 | ||||
| ΔF | 0.96 | ||||
Notes: n = 66 (ADHD n = 26, TD n = 40). Control = typically developing controls; ADHD = attention-deficit/hyperactivity disorder; β = Unstandardized regression coefficient; CI = confidence interval; WISC-IV GAI = Wechsler Intelligence Scale for Children Fourth Edition (WISC-IV) General Ability Index; AUC = Area Under the Curve (more AUC = less discounting); MotivationΔCom = standard go/no-go commission error rate – motivational go/no-go commission error rate (more positive number indicates greater reduction in commission errors with motivational contingencies); CogLoadΔCom = complex go/no-go commission error rate – standard go/no-go commission error rate (more positive number indicates greater increase in commission errors with greater cognitive load).
p<.05,
p<.01
In one final set of regressions, we examined whether CogLoadΔCom accounts for diagnostic group differences in real-time discounting. In Model 1, we included GAI and diagnostic group as predictors of real-time discounting. In Model 2, CogLoadΔCom was added to determine whether diagnostic group predicted real-time discounting above and beyond CogLoadΔCom. Model 1 results suggest that diagnostic group marginally predicted real-time discounting, β=-.036, p=.067. This effect persisted after accounting for CogLoadΔCom, β=-.035, p=.074, and CogLoadΔCom did not approach statistical significance, β=-.071, p=.431.
Discussion
Multi-pathway models of ADHD emphasize the interaction between systems involved in cognitive control and motivation (Castellanos et al., 2006). Deficient cognitive control or atypical motivation may contribute to greater delay discounting often observed in ADHD (Peters & Buchel, 2011; Sonuga-Barke et al., 2015). The purpose of the present study was to directly investigate the relationship between the effects of cognitive load and motivation on response control and delay discounting. Our hypotheses stemmed from findings of previous studies wherein a pattern of differential impairment emerged for girls with ADHD. Specifically, girls with ADHD show impaired response control only under conditions of increased cognitive load (Seymour et al., 2016) suggesting weaker cognitive control, as well as diminished improvement in response control when motivational contingencies were introduced suggesting atypical reward sensitivity (Rosch et al., 2015), and greater delay discounting (Rosch & Mostofsky, 2016). In this study we examined the relationship between these findings to elucidate the cognitive and motivational processes underlying delay discounting in ADHD and contribute empirical support to models emphasizing the interplay between different neural systems involving cognition and motivation in delay discounting (e.g., Peters & Buchel, 2011).
Our findings suggest that children with ADHD exhibited greater delay discounting than controls on the real-time discounting task involving immediately consumable rewards. In regard to response control, within and across diagnostic groups, children who experienced poorer response control with increased cognitive load also demonstrated less improvement in response control with motivational contingencies. Furthermore, a greater effect of cognitive load on response control was a unique predictor (after accounting for GAI and the motivational effect on response control) of greater delay discounting on the real-time task for ADHD, but not control children. In contrast, the motivational effect on response control was not significantly associated with delay discounting. This pattern of results suggests that delay discounting in ADHD may be partially accounted for by poor cognitive control rather than atypical motivation. However, diagnostic group differences in discounting remained after accounting for cognitive control, suggesting that other factors are likely at play and should be considered in future research.
The finding that children with ADHD exhibited greater delay discounting on the real-time task than did controls (d = 0.65) is consistent with theoretical models that emphasize altered reinforcement sensitivity (Luman et al., 2010) and with prior research (Rosch & Mostofsky, 2016; Scheres, Tontsch, & Thoeny, 2013; Scheres, Tontsch, Thoeny, & Kaczkurkin, 2010; Schweitzer & Sulzer-Azaroff, 1995). Also consistent with our previous study (Rosch & Mostofsky, 2016), diagnostic group differences were specific to girls with ADHD and we did not observe significant diagnostic group differences on the classic delay discounting task. In addition, the correlation between the cognitive load effect on response control and delay discounting on the real-time discounting task (r = -.502) was twice as strong as for the classic discounting task (r = -.215) among children with ADHD. Altogether, these findings suggest that the real-time and classic discounting tasks may capture different aspects of delay discounting in children with ADHD, as discussed in greater detail in our previous paper (Rosch & Mostofsky, 2016). The classic discounting task was included to allow for comparison with previous studies as this task is commonly used whereas the real-time task is novel. We did not intend to compare the impact of specific aspects of each task, which differ in many ways (i.e., type of reward, length of delays, task duration, experience of waiting in-between choices, etc.), and are therefore unable to determine the precise mechanisms that lead to differential sensitivity of these tasks to differentiating children with ADHD from controls.
Among the full sample, children who displayed poorer response control with increased cognitive load also demonstrated less improvement in response control with motivational contingencies. A detrimental effect of cognitive load on response control may indicate greater executive dysfunction whereas a greater effect of motivational contingencies on response control is thought to reflect increased responsiveness to reward (Fosco, Hawk, Rosch, & Bubnik, 2015). Thus, our findings suggest that individuals with poorer cognitive control tend to show a diminished response to reward, regardless of diagnosis. Multiple-pathway models of ADHD identify neural pathways involved in cool executive functions (EF), which refer to top-down cognitive processes as well those involved in hot EF, which have a motivational, emotional component (Kelly, Scheres, Sonuga-Barke, & Castellanos, 2007), both of which may be deficient in ADHD. One possible interpretation of this finding is that children with greater executive dysfunction are less able to improve their performance with motivational contingencies due to a primary weakness in cool EF.
Cognitive control is crucial in the stages of decision-making that involve comparing and deciding between choices as well as those that tap into reinforcement learning processes (Sonuga-Barke et al., 2015), both of which would be implicated in decisions between smaller-sooner and larger-later rewards. The complex GNG task requires significant cognitive control due to the greater demands on working memory (i.e., counting green spaceships and monitoring whether the count is even or odd) to guide response inhibition (i.e., to select or inhibit response to select a red spaceship). Our findings are consistent with previous studies demonstrating a relationship between neurocognitive deficits and delay discounting in ADHD in that we show that the effect of increased cognitive load on response control (placing demands on working memory and cognitive control) predicted greater delay discounting, but this relationship was specific to children with ADHD and a real-time discounting task. These findings are also in line with a recent study demonstrating that greater working memory load increased delay discounting among adults with externalizing psychopathology (Finn, Gunn, & Gerst, 2015). Furthermore, disruptions in the underlying neurobiology in the choice circuits in lateral prefrontal cortex and parietal cortex (Kable & Glimcher, 2009), particularly the dorsolateral PFC, may interfere with holding information about different choice options in mind and reflecting on the relative value of each, resulting in greater delay discounting (Sonuga-Barke & Fairchild, 2012).
Contrary to our hypothesis, the motivational effect on response control was not significantly correlated with delay discounting in ADHD, and was reliably smaller than the relationship between the cognitive control effect on response control and delay discounting. Furthermore, the relationship between the cognitive control effect on response control and delay discounting remained significant after accounting for the motivational effect on response control. This pattern of findings might suggest that poor cognitive control may primarily contribute to greater delay discounting in ADHD rather than atypical reward sensitivity as assessed by our motivational GNG task. However, it is also possible that our motivational GNG task is not isolating the effect of reward because it also incorporates an element of response cost, particularly with regard to response inhibition (i.e., lost money for commission errors). Future studies should attempt to isolate the effect of reward on response control when investigating associations with delay discounting. Additionally, perhaps a more direct measure of reward processing or valuation may be more strongly related to discounting rather than the effects of reward on a cognitive process.
Interpretation of these collective findings in relation to computational models of delay discounting is important for guiding future research. Many computation models now include at least three components: control, immediate valuation, and future valuation (see review by Peters & Buchel, 2011). Our findings suggest that greater delay discounting in ADHD may be due in part to weaknesses in cognitive control but not atypical reward sensitivity (perhaps as a proxy for immediate reward valuation). It will be important for future studies to consider alternative measures of reward sensitivity and to incorporate time estimation measures to better characterize immediate and future valuation processes and their contribution to delay discounting in ADHD.
It is important to address the limitations of this study with regard to the sample and methods. First, due to our small sample of girls, this study was underpowered to examine whether these relationships differed for girls and boys with ADHD. Instead, this study aimed to investigate if cognitive and motivational effects on response control were associated with greater delay discounting as a result of a broader neurocognitive profile rather than a sex-specific relationship. Future studies should expand female samples in order to examine if these associations are observed to a similar extent in both boys and girls with ADHD. Second, participants were pooled from a larger sample enrolled in a neuroimaging study wherein participants were recruited primarily from local schools, which may influence sample characteristics such as IQ and SES in comparison to clinic samples. In particular, the average IQ of our ADHD sample (mean FSIQ = 110) is relatively high compared to other studies in the literature (e.g., Parke, Thaler, Etcoff, & Allen, 2015 report an average FSIQ of 102). While this reduces the potential confound of IQ differences often present in delay discounting studies of individuals with ADHD, it may also limit the generalizability of our findings. Third, although we screened for learning disabilities, it is possible that participants in our sample could have a learning disability as this was not comprehensively evaluated in the context of this study. Fourth, the only comorbid condition explicitly permitted in our sample of children with ADHD was ODD, despite the frequent association of ADHD with various comorbidities (Rowland, Lesesne, & Abramowitz, 2002). Exploring the role of comorbidities in terms of cognitive and motivational effects on response control and delay discounting in future research may improve our ability to generalize these findings to the broader population of individuals with ADHD. Finally, we did not fully counterbalance the order of the tasks, although there was some randomization inherent in the study design that reduces the potential for order effects.
In summary, this study provides new information regarding the relationship between cognitive and motivational effects on response control and greater delay discounting in ADHD. Although delay discounting is an important phenomenon guiding our actions on a daily basis, it is not often considered within the context of ADHD assessment or treatment. Thus, an important implication for clinicians working with individuals with ADHD is that children presenting with greater executive dysfunction, which is often assessed when considering a diagnosis of ADHD, are more likely to display a stronger preference for immediate reward. Given the effectiveness of behavioral treatments emphasizing contingency management through the use of reinforcement and punishment, this information may be useful in guiding treatment planning. A valuable area for future research is to examine whether delay discounting is related to an individual’s response to contingency management. Also, given the implication of interacting neural pathways underlying both cognition and motivation in contributing to our understanding of ADHD (Sonuga-Barke, 2002, 2003), delay discounting (Peters & Buchel, 2011), and decision-making (Sonuga-Barke et al., 2015), future research including neuroimaging measures may be particularly informative. Collectively, these findings contribute to our understanding of deficient impulse control in children with ADHD both in terms of motor response control and reward-based decision-making, and the relationships between these neurocognitive processes.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Acknowledgments
This work was supported in part by grants from the National Institute of Mental Health (RO1 MH078160; RO1 MH085328, K23 MH101322) and the Johns Hopkins University School of Medicine Institute for Clinical and Translational Research National Institutes of Health/National Center for Research Resources Clinical and Translational Science Award program UL1 TR 000424-06.
Footnotes
After selecting for all other inclusionary criteria, only one participant met for the hyperactive/impulsive subtype of ADHD. This participant was excluded in an effort to create a more uniform sample and avoid potential diagnostic confounds.
Data for the current study was collected as a part of a larger laboratory study, which recently transitioned from using the DICA-IV to the Kiddie Schedule for Affective Disorders and Schizophrenia for School Aged Children Present Lifetime version (KSADS-PL; Kaufman et al., 2013). Among the subset of participants selected for inclusion in the present study, one had been assessed for diagnostic status using the KSADS-PL. All other participants (n = 65) were diagnosed using the DICA-IV.
The authors do not have any conflicts of interest to disclose.
Contributor Information
Mary K. Martinelli, Center for Neurodevelopmental and Imaging Research, Kennedy Krieger Institute, Baltimore, Maryland
Stewart H. Mostofsky, Center for Neurodevelopmental and Imaging Research and Center for Autism and Related Disorders, Kennedy Krieger Institute, Baltimore, Maryland and Departments of Neurology and Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland
Keri S. Rosch, Center for Neurodevelopmental and Imaging Research and Department of Neuropsychology, Kennedy Krieger Institute, Baltimore, Maryland and Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bobova L, Finn PR, Rickert ME, Lucas J. Disinhibitory psychopathology and delay discounting in alcohol dependence: personality and cognitive correlates. Experimental and Clinical Psychopharmacology. 2009;17:51–61. doi: 10.1037/a0014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubnik MG, Hawk LW, Pelham WE, Waxmonsky JG, Rosch KS. Reinforcement enhances vigilance among children with ADHD: Comparisons to typically developing children and to the effects of methylphenidate. Journal of Abnormal Child Psychology. 2015;43:149–161. doi: 10.1007/s10802-014-9891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends in cognitive sciences. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Chapman GB, Elstein AS. Valuing the future: temporal discounting of health and money. Medical Decision Making. 1995;15:373–386. doi: 10.1177/0272989X9501500408. [DOI] [PubMed] [Google Scholar]
- Coghill DR, Seth S, Matthews K. A comprehensive assessment of memory, delay aversion, timing, inhibition, decision making and variability in attention deficit hyperactivity disorder: advancing beyond the three-pathway models. Psychological Medicine. 2013:1–13. doi: 10.1017/S0033291713002547. [DOI] [PubMed] [Google Scholar]
- Cohen D. Statistical power analyses for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Conners CK. Conners’ Rating Scales- Revised. Toronto: Multi-Health Systems, Inc; 2002. [Google Scholar]
- Conners CK. Conners 3. North Tonawanda, NY: Multi-Health Systems, Inc; 2008. [Google Scholar]
- Crunelle CL, Veltman DJ, van Emmerik-van Oortmerssen K, Booij J, van den Brink W. Impulsivity in adult ADHD patients with and without cocaine dependence. Drug and Alcohol Dependence. 2013;129:18–24. doi: 10.1016/j.drugalcdep.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Demurie E, Roeyers H, Baeyens D, Sonuga-Barke EJ. Domain-general and domain-specific aspects of temporal discounting in children with ADHD and autism spectrum disorders (ASD): a proof of concept study. Research in Developmental Disabilities. 2013;34:1870–1880. doi: 10.1016/j.ridd.2013.03.011. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale—IV. New York, NY: Guilford Press; 1998. [Google Scholar]
- Finn PR, Gunn RL, Gerst KR. The effects of a working memory load on delay discounting in those with externalizing psychopathology. Clinical psychological science. 2015;3:202–214. doi: 10.1177/2167702614542279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forzano LB, Michels JL, Sorama M, Etopio AL, English EJ. Self-control and impulsiveness in adult humans: Comparison of qualitatively different consumable reinforcers using a new methodology. The Psychological Record. 2014;64:719–730. doi: 10.1007/s40732-014-0038-7. [DOI] [Google Scholar]
- Fosco WD, Hawk LW, Jr, Rosch KS, Bubnik MG. Evaluating cognitive and motivational accounts of greater reinforcement effects among children with attention-deficit/hyperactivity disorder. Behavioral and Brain Functions. 2015;11:20. doi: 10.1186/s12993-015-0065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Claus ED. Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychological Review. 2006;113:300–326. doi: 10.1037/0033-295x.113.2.300. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Scheres A, Sherman SJ. Understanding decision-making deficits in neurological conditions: insights from models of natural action selection. Philosophical Transactions of the Royal Society of London Series B, Biological sciences. 2007;362:1641–1654. doi: 10.1098/rstb.2007.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel JE, DeHart WB, Madden GJ, Odum AL. Impulsivity and cigarette smoking: discounting of monetary and consumable outcomes in current and non-smokers. Psychopharmacology. 2014;231:4517–4526. doi: 10.1007/s00213-014-3597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getahun D, Jacobsen SJ, Fassett MJ, Chen W, Demissie K, Rhoads GG. Recent trends in childhood attention-deficit/hyperactivity disorder. JAMA pediatrics. 2013;167:282–288. doi: 10.1001/2013.jamapediatrics.401. [DOI] [PubMed] [Google Scholar]
- Gupta R, Kar BR. Development of attentional processes in ADHD and normal children. Progress in Brain Research. 2009;176:259–276. doi: 10.1016/S0079-6123(09)17614-8. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University, Department of Sociology; 1975. [Google Scholar]
- Jensen PS, Hinshaw SP, Kraemer HC, Lenora N, Newcorn JH, Abikoff HB, Vitiello B, et al. ADHD comorbidity findings from the MTA study: Comparing comorbid subgroups. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:147–158. doi: 10.1097/00004583-200102000-00009. [DOI] [PubMed] [Google Scholar]
- Johansen EB, Aase H, Meyer A, Sagvolden T. Attention-deficit/hyperactivity disorder (ADHD) behaviour explained by dysfunctioning reinforcement and extinction processes. Behavioural Brain Research. 2002;130:37–45. doi: 10.1016/s0166-4328(01)00434-x. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron. 2009;63:733–745. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Huang-Pollock CL. Examining relationships between executive functioning and delay aversion in attention deficit hyperactivity disorder. Journal of Clinical Child and Adolescent Psychology. 2011;40:837–847. doi: 10.1080/15374416.2011.614578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Axelson D, Perepletchikova F, Brent D, Ryan N. Kiddie Schedule for Affective Disorders and Schizophrenia for School-Aged Children – Lifetime Version (Kiddie-SADS-PL 2013 Working Draft) Pittsburgh Pennsylvania: Western Psychiatric Institute and Clinic and Yale University; 2013. [Google Scholar]
- Kelly AMC, Scheres A, Sonuga-Barke EJ, Castellanos FX. Functional neuroimaging of reward and motivational pathways in ADHD. In: Fitzgerald M, Bellgrove M, Gill M, editors. The Handbook of Attention Deficit Hyperactivity Disorder. Hoboken, NJ: John Wiley & Sons Ltd; 2007. pp. 209–235. [Google Scholar]
- Killeen PR. The arithmetic of discounting. Journal of the Experimental Analysis of Behavior. 2015;103:249–259. doi: 10.1002/jeab.130. [DOI] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clinical Psychology Review. 2005;25:183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Luman M, Tripp G, Scheres A. Identifying the neurobiology of altered reinforcement sensitivity in ADHD: a review and research agenda. Neuroscience and Biobehavioral Reviews. 2010;34:744–754. doi: 10.1016/j.neubiorev.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Marco R, Miranda A, Schlotz W, Melia A, Mulligan A, Muller U, Sonuga-Barke EJ, et al. Delay and reward choice in ADHD: An experimental test of the role of delay aversion. Neuropsychology. 2009;23:367–380. doi: 10.1037/a0014914. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Schafer JG, Abrams MT, Goldberg MC, Flower AA, Boyce A, Pekar JJ. fMRI evidence that the neural basis of response inhibition is task-dependent. Brain Research Cognitive Brain Research. 2003;17:419–430. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L. Discounting of delayed rewards: Models of individual choice. Journal of the Experimental Analysis of Behavior. 1995;64:263–276. doi: 10.1901/jeab.1995.64-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biological Psychiatry. 2005;57:1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Parke EM, Thaler NS, Etcoff LM, Allen DN. Intellectual profiles in children with ADHD and comorbid learning and motor disorders. Journal of Attention Disorders. 2015 doi: 10.1177/1087054715576343. [DOI] [PubMed] [Google Scholar]
- Patros CH, Alderson RM, Kasper LJ, Tarle SJ, Lea SE, Hudec KL. Choice-impulsivity in children and adolescents with attention-deficit/hyperactivity disorder (ADHD): A meta-analytic review. Clinical Psychology Review. 2015 doi: 10.1016/j.cpr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Patros CH, Alderson RM, Lea SE, Tarle SJ, Kasper LJ, Hudec KL. Visuospatial working memory underlies choice-impulsivity in boys with attention-deficit/hyperactivity disorder. Research in Developmental Disabilities. 2015;38:134–144. doi: 10.1016/j.ridd.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Peters J, Buchel C. The neural mechanisms of inter-temporal decision-making: understanding variability. Trends in cognitive sciences. 2011;15:227–239. doi: 10.1016/j.tics.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Reed DD, Kaplan BA, Brewer AT. A tutorial on the use of Excel 2010 and Excel for Mac 2011 for conducting delay-discounting analyses. Journal of Applied Behavior Analysis. 2012;45:375–386. doi: 10.1901/jaba.2012.45-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W, Welner Z, Herjanic B. The Diagnostic Interview for Children and Adolescents-IV. North Tonawanda: Multi-Health Systems; 1997. [Google Scholar]
- Reynolds B, Penfold RB, Patak M. Dimensions of impulsive behavior in adolescents: laboratory behavioral assessments. Exp Clin Psychopharmacol. 2008;16:124–131. doi: 10.1037/1064-1297.16.2.124. [DOI] [PubMed] [Google Scholar]
- Rosch KS, Dirlikov B, Mostofsky SH. Reduced intrasubject variability with reinforcement in boys, but not girls, with ADHD: Associations with prefrontal anatomy. Biological Psychology. 2015;110:12–23. doi: 10.1016/j.biopsycho.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch KS, Fosco WD, Pelham WE, Jr, Waxmonsky JG, Bubnik MG, Hawk LW., Jr Reinforcement and stimulant medication ameliorate deficient response inhibition in children with attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2016;44:309–321. doi: 10.1007/s10802-015-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch KS, Mostofsky SH. Increased delay discounting on a novel real-time task among girls, but not boys, with ADHD. Journal of the International Neuropsychological Society. 2016;22:12–23. doi: 10.1017/S1355617715001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland AS, Lesesne CA, Abramowitz AJ. The epidemiology of attention-deficit/hyperactivity disorder (ADHD): a public health view. Mental Retardation and Developmental Disabilities Research Reviews. 2002;8:162–170. doi: 10.1002/mrdd.10036. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences. 2005;28:397–419. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Scheres A, Dijkstra M, Ainslie E, Balkan J, Reynolds B, Sonuga-Barke EJ, Castellanos FX. Temporal and probabilistic discounting of rewards in children and adolescents: effects of age and ADHD symptoms. Neuropsychologia. 2006;44:2092–2103. doi: 10.1016/j.neuropsychologia.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Scheres A, Hamaker EL. What we can and cannot conclude about the relationship between steep temporal reward discounting and hyperactivity-impulsivity symptoms in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2010;68:e17–18. doi: 10.1016/j.biopsych.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Scheres A, Lee A, Sumiya M. Temporal reward discounting and ADHD: task and symptom specific effects. Journal of Neural Transmission. 2008;115:221–226. doi: 10.1007/s00702-007-0813-6. [DOI] [PubMed] [Google Scholar]
- Scheres A, Tontsch C, Thoeny AL. Steep temporal reward discounting in ADHD-Combined type: Acting upon feelings. Psychiatry Research. 2013;209:207–213. doi: 10.1016/j.psychres.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Scheres A, Tontsch C, Thoeny AL, Kaczkurkin A. Temporal reward discounting in attention-deficit/hyperactivity disorder: the contribution of symptom domains, reward magnitude, and session length. Biological Psychiatry. 2010;67:641–648. doi: 10.1016/j.biopsych.2009.10.033. [DOI] [PubMed] [Google Scholar]
- Scheres A, Tontsch C, Thoeny AL, Sumiya M. Temporal reward discounting in children, adolescents, and emerging adults during an experiential task. Frontiers in Psychology. 2014;5 doi: 10.3389/fpsyg.2014.00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer JB, Sulzer-Azaroff B. Self-control in boys with attention deficit hyperactivity disorder: effects of added stimulation and time. Journal of Child Psychology and Psychiatry. 1995;36:671–686. doi: 10.1111/j.1469-7610.1995.tb02321.x. [DOI] [PubMed] [Google Scholar]
- Seymour KE, Mostofsky SH, Rosch KS. Cognitive Load Differentially Impacts Response Control in Girls and Boys with ADHD. Journal of Abnormal Child Psychology. 2016;44:141–154. doi: 10.1007/s10802-015-9976-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamosh NA, Gray JR. Delay discounting and intelligence: A meta-analysis. Intelligence. 2008;36:289–305. doi: 10.1016/j.intell.2007.09.004. [DOI] [Google Scholar]
- Shiels K, Hawk LW, Lysczek CL, Tannock R, Pelham WE, Spencer SV, Waschbusch DA. The effects of incentives on visual-spatial working memory in children with attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2008;36:903–913. doi: 10.1007/s10802-008-9221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels K, Hawk LW, Reynolds B, Mazzullo RJ, Rhodes JD, Pelham WE, Gangloff BP, et al. Effects of methylphenidate on discounting of delayed rewards in attention deficit/hyperactivity disorder. Exp Clin Psychopharmacol. 2009;17:291–301. doi: 10.1037/a0017259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels Rosch K, Dirlikov B, Mostofsky SH. Increased intrasubject variability in boys with ADHD across tests of motor and cognitive control. Journal of Abnormal Child Psychology. 2013;41:485–495. doi: 10.1007/s10802-012-9690-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjowall D, Roth L, Lindqvist S, Thorell LB. Multiple deficits in ADHD: executive dysfunction, delay aversion, reaction time variability, and emotional deficits. Journal of child psychology and psychiatry, and allied disciplines. 2013;54:619–627. doi: 10.1111/jcpp.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV, Abikoff H, Sonuga-Barke EJ, Schachar R, Logan GD, Wigal T, Turkel E, et al. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. Journal of Abnormal Child Psychology. 2001;29:215–232. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Gilbert SN, Raj A, Zhu J, Pope-Boyd S, Stepak B, Newcorn JH, et al. Neurocognitive functioning in AD/HD, predominantly inattentive and combined subtypes. Journal of Abnormal Child Psychology. 2007;35:729–744. doi: 10.1007/s10802-007-9123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Psychological heterogeneity in AD/HD--a dual pathway model of behaviour and cognition. Behavioural Brain Research. 2002;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neuroscience and Biobehavioral Reviews. 2003;27:593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biological Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Bitsakou P, Thompson M. Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:345–355. doi: 10.1016/j.jaac.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Cortese S, Fairchild G, Stringaris A. Annual Research Review: Transdiagnostic neuroscience of child and adolescent mental disorders – differentiating decision making in attention-deficit/hyperactivity disorder, conduct disorder, depression, and anxiety. Journal of Child Psychology and Psychiatry. 2015;57:321–349. doi: 10.1111/jcpp.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Fairchild G. Neuroeconomics of attention-deficit/hyperactivity disorder: differential influences of medial, dorsal, and ventral prefrontal brain networks on suboptimal decision making? Biological Psychiatry. 2012;72:126–133. doi: 10.1016/j.biopsych.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Strand MT, Hawk LW, Jr, Bubnik M, Shiels K, Pelham WE, Jr, Waxmonsky JG. Improving working memory in children with attention-deficit/hyperactivity disorder: the separate and combined effects of incentives and stimulant medication. Journal of Abnormal Child Psychology. 2012;40:1193–1207. doi: 10.1007/s10802-012-9627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hulst BM, de Zeeuw P, Durston S. Distinct neuropsychological profiles within ADHD: a latent class analysis of cognitive control, reward sensitivity and timing. Psychological Medicine. 2015;45:735–745. doi: 10.1017/s0033291714001792. [DOI] [PubMed] [Google Scholar]
- Vaurio RG, Simmonds DJ, Mostofsky SH. Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia. 2009;47:2389–2396. doi: 10.1016/j.neuropsychologia.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler DL. Wechsler Individual Achievement Test - Second Edition (WIAT-II) San Antonio, TX: The Psychological Corporation; 2002. [Google Scholar]
- Wechsler DL. Wechsler Intelligence Scale for Children - Fourth Edition (WISC-IV) San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Wilson VB, Mitchell SH, Musser ED, Schmitt CF, Nigg JT. Delay discounting of reward in ADHD: application in young children. Journal of child psychology and psychiatry, and allied disciplines. 2011;52:256–264. doi: 10.1111/j.1469-7610.2010.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodka EL, Mahone EM, Blankner JG, Larson JC, Fotedar S, Denckla MB, Mostofsky SH. Evidence that response inhibition is a primary deficit in ADHD. Journal of Clinical and Experimental Neuropsychology. 2007;29:345–356. doi: 10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]