Abstract
Pump thrombosis is a dreaded complication of left ventricular assist device (LVADs). We completed a systematic review to evaluate the efficacy and complications associated with medical management of LVAD thrombosis. Databases were searched using the terms “vad*” or “ventricular assist device” or “heart assist device” and “thrombus” or “thrombosis” or “thromboembolism”. Of 2383 manuscripts, 49 articles met the inclusion criteria. The risk of partial or no resolution of LVAD thrombosis did not significantly differ between thrombolytic and non-thrombolytic regimens (OR 0.48; 95% CI 0.20–1.16). When response to therapy was evaluated based upon pump type, there were no significant differences in how patients with a HMII or HVAD responded to thrombolytic or non-thrombolytic treatment. Pooled risk of major bleeding in the thrombolytic group was 29% (95% CI 0.17–0.44) and 12% (95% CI 0.01–0.57) in the non-thrombolytic group. Odds of death did not differ between thrombolytic and non-thrombolytic regimens (OR 1.28; 95% CI 0.42–3.89). Although thrombolytic and non-thrombolytic treatment similarly resolved LVAD thrombosis, major hemorrhage may be increased with use of thrombolysis. Randomized clinical trials comparing thrombolytic and non-thrombolytic treatment of LVAD thrombosis are needed to establish the most effective and safe option for patients who are not surgical candidates.
Keywords: Pump thrombosis, ventricular assist device, thrombolysis, direct thrombin inhibition, platelet GP IIb/IIIa receptor inhibition
Introduction
Approximately 5.1 million persons in the United States have heart failure, and the prevalence continues to rise.1 With the increasing number of advanced heart failure patients and a lack of heart transplant donors, mechanical circulatory support devices are increasingly used. Indications for mechanical circulatory support include bridge to transplantation, bridge to recovery as well as destination therapy. Left ventricular assist devices (LVADs), one form of mechanical circulatory support, have dramatically improved patients’ overall survival and quality of life.2 With an improvement in overall survival of patients supported with LVADs and an increasing duration of support on LVADs, LVAD related complications must be minimized. Pump thrombosis is a dreaded complication of both short term and long term use of LVADs. LVAD thrombosis occurs in 2–13% of adult patients with a continuous-flow LVAD (axial-flow 4–13%, centrifugal-flow 8%)3,4 and 18% of pediatric patients with a paracorporeal device.5 Pump thrombosis denotes the development of clot within the flow path of any component of the pump, including the titanium inflow cannula, the rotor and the outflow graft. Thrombus can originate in the pump or travel from the left atrium or left ventricle, and lodge in the pump components.6 Pump thrombosis can lead to thromboembolic stroke, peripheral thromboembolism, LVAD malfunction with reduced systemic flows, LVAD failure with life-threatening hemodynamic impairment, cardiogenic shock, and death.7
Successful management of LVAD thrombosis has included surgical and pharmacological therapies. Invasive management such as device exchange8,9 and catheter-directed thrombectomy10 have been described. There are no studies in literature comparing the efficacy or adverse outcomes related to pharmacological treatment of LVAD thrombosis. Guidelines have outlined diagnosis and management strategies for LVAD thrombosis,6 but are based on expert or consensus opinion. We completed a systematic review of the literature to evaluate the efficacy and complications associated with medical management strategies for adults with LVAD thrombosis.
Materials and Methods
The current analysis conforms to standard guidelines and was written according to the Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) statement.11 Pubmed, SCOPUS, Ovid Medline, Cochrane and the Web of Science were searched through July 15th, 2016. Studies were identified using the following medical subject headings and keywords including “vad*” or “ventricular assist device” or “heart assist device” and “thrombus” or “thrombosis” or “thromboembolism”. Studies were included if they reported patients > 18 years of age with confirmed or suspected LVAD thrombosis of continuous flow devices. Confirmed pump thrombosis was defined as a thrombus on the blood-contacting surfaces of the LVAD, its inflow cannula, or its outflow conduit at pump replacement, urgent transplantation, or autopsy. Suspected pump thrombosis was defined as a clinical diagnosis of pump-related malfunction and hemolysis as reported by the individual publications.12 Exclusion criteria included studies describing pulsatile LVADs, thrombosis resulting due to heparin-induced thrombocytopenia, studies not published in the English language, and abstracts without published full text articles. Articles were also excluded if they did not contain information about the thrombotic events, the type of medical intervention, and if the thrombus resolved. Two reviewers (GD and NE) independently evaluated the titles and abstracts to determine eligibility for inclusion. If either reviewer believed the article was eligible, the full manuscript was reviewed. Eligibility was agreed upon through discussion between the reviewers. A third reviewer (LBK) was used if disagreements about eligibility occurred. If additional information pertaining to the published articles was needed, the authors of the respective article were contacted. The Newcastle-Ottawa scale was used to assess study quality and risk of bias due to the non-randomized studies included in the systematic review.13 Despite the lack of a control group in most studies, the cohort tool was used as recommended by the Cochrane Collaboration.
Data was independently abstracted by both reviewers. Study type, patient demographics, device name, and type and duration of anticoagulation were abstracted. Outcomes including resolution of thrombus, major or minor bleeding, need for escalation of care and mortality were recorded. Complete thrombus resolution was defined as clinical improvement, along with improvement in VAD parameters as well as laboratory parameters for hemolysis. INTERMACS adverse event definitions were used for major and minor bleeding. Data tables were exchanged and discrepancies were discussed.
Statistical Analysis
Percentages and odds ratios (OR) with 95% confidence limits were used to describe the data. For the nine cohort studies, the study was assumed to be random to estimate the overall log of odds ratio for thrombolytic vs. non-thrombolytic treatments. A half was added to all four numbers in the two-by-two table if there were a zero so that this could be calculated. Different assumptions can be made about the variation. Due to the sparse data, in addition to a Normal-Normal (NN) model, we also explored Hypergeometric-Normal (HN) model and Binomial-Normal (BN) model.14 We performed a NN model on the nine cohort studies. HN and BN models could not be fit. A sensitivity analysis was also performed by excluding four cohort studies: Oezpeker 2016,15 Rothenburger 2002,16 Tellor 2014,17 Scandroglio 201618 since these studies only had one group of treatment. A BN model was performed on the cases reports and case series. The pooled risk was compared with 0.5. The OR or pooled risk and 95% confidence interval (CI) were estimated and forest plot was shown. Funnel plots were employed to check publication bias. Study weights were calculated as 1 over the sum of variance and estimated amount of total heterogeneity. P≤0.05 was considered significant. Statistical analysis and graphs were performed using SAS 9.4 (SAS Institute, Cary, North Carolina) and R metafor package.19
Results
Database searches identified 2383 manuscripts, of which 2274 were excluded after title and abstract screening (Figure 1). The full text of 109 manuscripts were reviewed. The most common reasons for article exclusion were no medical intervention (28/60, 47%), device not currently in use (9/60, 15%), lack of information on thrombosis (8/60, 13%), lack of information on medical intervention or patient outcomes (7/60, 12%) and unknown device type (5/60, 8%). Fourteen recurrent events were excluded from the analysis. The 49 included studies (40 case reports and case series and 9 retrospective cohort studies) reported 238 patients with thrombotic events (Table 1, Table 3). Retrospective cohort studies comprised 18% (9/49) of the included studies (Table 3).4,15–18,20–23 None of the these studies were controlled trials and thus were deemed of moderate quality. The most reported devices included HVAD (120/216, 56%), HeartMate II (76/216, 35%) and MicroMed DeBakey (14/216, 6%). A majority of the LVADs in adults were used long term, but a range of implantation time between 1 day and 1713 days was reported. Median time since implantation for thrombolytic and non-thrombolytic regimens was 186 and 146 days respectively. Median follow-up period of all studies combined was 180 days (range 1 – 1107 days).
Figure 1.

Preferred reporting items of systematic reviews And meta-analysis (PRISMA) study selection diagram
Table 1.
Study characteristics of case reports and case series
| Study | Patients (n) |
LVAD Type |
Anti platelet therapy |
INR at event |
Goal INR | Intervention | Thrombosis Resolution |
Escalati on of care |
Minor bleeding |
Major bleedin g |
Death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Increased INR goal alone | |||||||||||
| Sacher24 2013 |
1 | HM II | ASA 81 mg + Dipridam ole 75 mg TID |
2.0 – 2.5 | Increased INR goal to 2.5 – 3.5 + ASA 325 mg daily |
CR | N | N | N | N | |
| Heparin Monotherapy | |||||||||||
|
Kim JB28 2014 |
1 | HM II | None | 1.7 | 1.7 – 2.0 | IV UFH | NoR | Y – Tandem heart |
N | N | Y |
| Nakajima29 2014 |
1 | HM II | ASA 100 mg |
1.5 – 2.0 | IV UFH | NoR | Y – CABG |
N | N | N | |
| Smith31 2013 |
1 | HM II | ASA + Dipridam ole + pentoxifyl line |
2.5 | IV UFH | NoR | Y – Pump exchang e |
N | N | N | |
| Szarszoi30 2012 |
1 | HM II | IV UFH + increased INR goal |
NoR | N | N | N | Y | |||
| Santise27 2012 |
1 | HVAD | ASA | 2.4 | 2 – 3 | IV UFH + ASA 300 mg |
CR | N | N | N | N |
| Morici26 2016 |
1* | HVAD | ASA 100 mg + Dipyridam ole 800 mg |
2– 3 | IV UFH + ASA 300 mg |
NoR | Y – Pump Exchang e |
N | N | N | |
| Bistola25 2016 |
1 | CF VAD |
ASA | 2.65 | 2 – 3 | IV UFH + ASA 325 mg |
PR | N | N | N | N |
| Direct Thrombin inhibitor Monotherapy | |||||||||||
|
Badiye A40 2014 |
4 | HM II | ASA 81 – 325 mg ± Dipyridam ole 75 mg |
2 – 3 | IV Argatroban | 3/4 – CR ¼ - PR |
¼ - HT | N | 2/4 – SAH, pericar dial hemorr hage |
N | |
| Meyer41 2008 |
1 | HM II | ASA 100 mg |
Subthe rapeuti c |
2.5 – 3.0 | IV Hirudin | PR | N | N | N | N |
| Sylvia32 2014 |
10 | HM II | ASA (100%) Plavix (40%) Dipridamole (30%) |
2.96 (mean) |
9/10 - IV Bivalirudin 1/10 – IV UFH + IV Bivalirudin + IV eptifibatide |
2/9 – NoR 7/9– CR 1/1 - CR |
3/9 – pump exchang e 2/9 – HT 4/9 – N 1/1 - N |
N | N | N | |
| Heparin + Glycoprotein IIb/IIIa inhibitor and/or Direct thrombin inhibitor | |||||||||||
|
Al-Quthami AH33 2012 |
2 | HM II | ASA 325 mg |
1.3 1.4 |
IV UFH + IV Eptifibatide |
2/2 - CR | N | N | 2/2 – GI bleed |
N | |
| Bellumkon da35 2014 |
4 | HM II | ASA | therap eutic |
1.8 – 3 | IV UFH + IV Eptifibatide |
¼ - CR ¾ - NoR |
2/4 – pump exchang e |
N | ¼ - stroke |
1/4 |
|
Blais DM34 2008 |
1 | HM II | ASA 81mg + persantin e 75 mg TID |
2.1 | 2 – 3 | IV UFH + IV Eptifibatide + IV Argatroban |
CR | N | N | N | N |
| Jennings38 2012 |
1 | HM II | ASA | 2 – 3 | IV UFH + IV Eptifibatide |
CR | N | N | N | N | |
| Sarsam36 2013 |
1 | HM II |
IV UFH + IV Bivalirudin |
CR | N | N | N | N | |||
| Thomas39 2008 |
1 | HVAD | ASA 150 mg |
3.2 | 2.5 – 3.5 | IV UFH + IV tirofiban + Plavix |
CR | N | N | Y – Menstr ual loss |
N |
|
Freed BH37 2011 |
1 | HVAD | None | Subthe rapeuti c |
IV UFH + IV Eptifibatide |
PR | Y – thrombe ctomy |
N | N | N | |
| Heparin and Thrombolytics | |||||||||||
| Tschirkov62 2007 |
1 | Berlin heart INCOR syste m |
IV UFH + IV reteplase |
CR | N | N | N | N | |||
| Russo60 2002 |
1 | Micro Med DeBak ey |
ASA + Dipyridam ole + Pentoxifyl line |
3.3 | IV UFH + Intra Ventricular rtPA |
NoR | N | Y – Hematuri a |
N | N | |
| Jahanyar58 2007 |
1 | Micro Med DeBak ey |
None | IV UFH + IV rtPA X 4 |
CR | N | N | N | N | ||
|
Delgado R 3rd57 2005 |
2 | Jarvik 2000 |
None | ID UFH + IV rtPA | 2/2 - CR | N | N | N | ½ | ||
| Kapur59 2014 |
1 | HM II | None | IV UFH + intra ventricular Alteplase |
CR | N | N | N | N | ||
| Agarwal56 2015 |
1 | HM II | ASA | 1.6 | 2 – 3 | IV UFH + IV Alteplase |
CR | N | N | Y – Cannulat ion site |
N |
| Tang61 2013 |
1 | HM II | 7.0 | IV UFH +intra ventricular rtPA + IV rtPA |
PR | Y – Pump exchang e |
N | Y - ICH | Y | ||
| Santise27 2012 |
1 | HVAD | ASA | 1.3 | 2 – 3 | IV UFH + intra ventricular rtPA |
CR | N | N | N | N |
| Aissaoui43 2011 |
2 | HVAD | ASA | 2.1 (mean) |
2 – 3 | IV UFH + IV rtPA | 1 – CR 1 - NoR |
Y – pump exchang e |
N | 1 – Drivelin e site bleed |
N |
| Heparin + Thrombolytics + Glycoprotein IIb/IIIa inhibitor and/or Direct thrombin inhibitor | |||||||||||
| sclendorf44 2014 |
8 | HM II | ASA + Plavix + Dipyidam ole |
2.11 (mean) |
>2.0 | 6/8 – IV UFH + Intra ventricular rtPA 1/8 – IV UFH + IV GPIIb/IIIa inhi + Intraventricular rtPA 1/8 – IV UFH + IV GPIIb/IIIa inhi + IV Bivalirudin + Intraventricular rtPA |
2/6 - CR 4/6 - NoR 1/1 -CR 1/1 – CR |
1/6 – HT 1/6 – pump exchang e N N |
N N N |
3/6 – ICH/em bolic stroke N N |
2/6 N N |
| Thenappan 64 2013 |
2 | HM II | 1.5 (mean) |
IV UFH + IV eptifibatide + Intraventricular rtPA |
CR | N | ½ - Y – groin hematom a |
N | N | ||
| Muthiah42 2013 |
5 | HVAD | ASA – 100% Plavix – 80% Dipyridam ole – 20% |
2.72 (mean) |
2 – 3 | 1/5 - IV tirofiban 3/5 - IV rtPA 1/5 - IV UFH + IV tirofiban + IV rtPA |
1/1 – CR 3/3– PR 1/5 - CR |
N N N |
1/1– Epistaxis 1/3 – epistaxis N |
1/5 – Hemothorax N N |
N 2/3 N |
| Webber63 2016 |
1 | HVAD | ASA + Plavix |
2.74 | 2 – 3 | IV UFH + IV Argatroban + IV rtPA |
PR | N | N | N | N |
| Thrombolytics alone | |||||||||||
| Ninios51 2010 |
1 | Jarvik 2000 |
ASA 100 mg |
3.3 | 3.0 – 3.5 | IV rtPA | NoR | N | N | Y – Splenic Hemat oma |
N |
| Hayes47 2008 |
1 | Jarvik 2000 |
ASA | IV Tenecteplase | CR | N | N | Y – ICH, IV site |
Y | ||
| Wilhelm54 2005 |
4 | Micro Med DeBak ey |
ASA 300 mg + Dipridam ole 75 mg (100%) Plavix 75 mg |
2.5 – 4.5 | IV rtPA X 6 | CR | N | 1/6 - epistaxis |
2/6 – Pericar dial hemat oma, device hemat oma |
N | |
|
Dalén M45 2014 |
1 | HVAD | ASA 160 mg |
2.4 | 2 – 3 | IV Alteplase |
CR | N | N | N | N |
|
Dimarakis I 46 2014 |
1 | HVAD | None | 1.6 | IV rtPA | PR | Y – Pump exchang e |
N | Y – SAH | N | |
| Jabbar48 2013 |
1 | HVAD | None | Therap eutic |
2.5 – 3 | IV Alteplase | CR | N | N | N | N |
| Kamouh49 2012 |
1 | HVAD | ASA 162 mg |
1.55 | 2.0 – 2.5 | IV Alteplase | CR | N | Y – arm hematom a |
N | N |
| Kiernan50 2011 |
1 | HVAD | None | 1.4 | Intra ventricular Alteplase |
CR | N | N | N | N | |
| Paluszkiewi cz53 2014 |
1 | HVAD | IV tenecteplase X 3 |
CR | N | N | N | N | |||
| Raffa52 2015 |
4 | HVAD | ASA 100 mg -> 325 mg |
1.86 ± 0.56 |
2 – 3 | Intraventricular rtPA |
4/4 – CR | N | N | ¼ - Y | ¾ - Y |
ASA – Aspirin, CR – complete resolution, HM II – HeartMate II, HT – Heart Transplant,HVAD – HeartWare ventricular assist device, ICH – Intra cranial hemorrhage, ID – Intra device, INR – international normalized ratio, IV – Intravenous, NoR – No resolution, N - No, PR – Partial resolution,, rtPA – recombinant tissue plasminogen activator, SAH – Subarachnoid Hemorrhage, TID – three times daily, UFH – unfractionated heparin, Y – Yes.
1 patient with recurrent thrombotic events requiring treatment
Table 3.
Patient and device characteristics and quality assessment of Retrospective cohort studies:
| Study | Patients (n) |
Patients with events, n |
Device type, n |
BTT/DT (%) |
Age in years, mean (SD) or Median (IQR) |
Time since implantation, median (Range), in days |
Quality assessment (out of 9) |
|---|---|---|---|---|---|---|---|
| Hasin23 2014 |
115 | 8 | HM II | 37% -BTT 63% - DT |
59 ± 13 | 260 (57 – 796) | 6 |
| Najjar4 2014 |
382 | 30 | HVAD | 100% - BTT | 50.3 ± 11.3 | 289 (18 – 1293) | 6 |
| Rothenburger18 2002 |
22 | 8 | MicroMed DeBakey |
43.7 ± 14.3 | 5 | ||
| Tellor17 2014 |
206 | 17* | 16 – HM II 1 - HVAD |
94% - BTT | 52 (32 – 70) | 47.34 (3.9 – 397.7) |
5 |
| Ertugay20 2016 |
163 | 15 | 3 – HM II 12 – HVAD |
50.7 ± 13 | 259 (8 – 585) | 7 | |
| Oezpeker15 2016 |
473 | 52$ | 7 – HMII 22 - HVAD |
86.2% - BTT 14.8% - DT |
55 (44 – 62) | 362 (242 – 625) | 7 |
| Saeed21 2016 |
91 | 13# | HVAD | 70% - BTT 30% - DT |
55 ± 14 | 467 (11 – 937) | 7 |
| Scandroglio16 2016 |
524 | 100@ | HVAD | Pre Pump – 58 ± 13 Intra Pump – 52 ± 13 Post Pump – 48 ± 12 |
293 (41 – 742) | 7 | |
| Upshaw22 2016 |
125 | 25 | 21 - HMII 4 - HVAD |
68% - BTT 20% - DT |
58 (53 – 64) | 170 (41 – 396) | 7 |
BTT – Bridge to transplantation, DT- Destination therapy, HM II- Heartmate II, HVAD – HeartWare, SD – Standard deviation
Recurrent events were excluded from the analysis
There were 17 patients with 22 events
52 patients in the cohort had pump thrombosis, however only 29 patients received medical treatment and would be included in this study.
There were 13 patients with 21 events, 1 developed PT in the setting of HIT and 2 did not receive medical interventions and thus were excluded from our study
Only 18 patients (9 with pre pump thrombosis, 9 with intra pump thrombosis) that received medical therapy were included
Tables 1 and 2 outline the antithrombotic management of adult patients admitted with LVAD related pump thrombosis. Sacher et al reported a single patient who had complete resolution of symptoms without bleeding when the INR goal was increased from 2.0–2.5 to 2.5– 3.5 and aspirin was increased to 325 mg daily.24 Of the other reports, unfractionated heparin (UFH) was the most commonly used agent. It was used either alone or in combination with thrombolytic and/or GPIIb/IIIa inhibitor or direct thrombin inhibitor agents in most adults, with goal activated partial thromboplastin times (aPTTs) between 60 and 90s. Thirty one patients were treated with IV UFH alone with complete thrombus resolution in only 23% (7/31) of patients. Escalation of care to pump exchange or heart transplant occurred in 48% (15/31) of these patients. No minor bleeding events occurred while major bleeding events were reported in 10% (3/31) of patients.4,20–23,25–31 IV UFH was used in combination with a GPIIb/IIIa inhibitor and/or a direct thrombin inhibitor in the management of 51 events resulting in complete resolution in 49% (25/51) of the cases and no or partial resolution in 51% (26/51) cases. Major bleeding events were noted in 35% (18/51) cases.4,17,20–23,26,32–39 GPIIb/IIIa inhibitor or direct thrombin inhibitor therapy alone (goal PTT of 50– 75 sec) was used in 39 patients with pump thrombosis resulting in complete thrombus resolution in 49% (19/39) of patients. Major bleeding events were noted in 10% (4/39) of patients and there was one reported death.17,18,22,32,40–42
Table 2.
Summary Table of outcomes of patients treated with thrombolytic and non-thrombolytic regimens
| Thrombolytic Regimens | Non-Thrombolytic Regimen | ||||
|---|---|---|---|---|---|
| Outcome | Alone or Dual therapy N=98 (%) |
Triple or Quadruple therapy N=18 (%) |
IV UFH alone N = 31 (%) |
DTI or GpIIb/IIIa inhibitor alone N= 39 (%) |
IV UFH + DTI or GpIIb/IIIa inhibitor N=51 (%) |
|
Complete resolution |
65 (66) | 10 (56) | 7 (23) | 19 (49) | 25 (49) |
|
Major Bleed |
19 (19) | 6 (33) | 3 (10) | 4 (10) | 18 (35) |
| GI Bleed | 0 (0) | 2 (11) | 2 (6) | 2 (5) | 6 (12) |
| ICH | 11 (11) | 3 (17) | 1 (3) | 1 (3) | 9 (18) |
| Death | 20 (20) | 2 (11) | 4 (13) | 1 (3) | 10 (20) |
Thrombolytic therapies were used either alone (Intravenous [IV] or intra-ventricular) or as a dual therapy (along with UFH or GPIIb/IIIa inhibitor or direct thrombin inhibitor) in 98 patients resulting in a complete resolution in 66% (65/98) of patients. Major bleeding was reported in 19% (19/98) patients. Likewise, minor bleeding occurred in 19% (19/98) patients. There were 20 reported deaths in this group of patients.4,15,20–23,26,27,42–62 Thrombolytics were used as a triple or a quadruple drug therapy (i.e. in combination with IV UFH and either GPIIb/IIIa inhibitor and/or direct thrombin inhibitor) in 18 patients resulting in a complete resolution in 56% (10/18) of patients. There were 6 major bleeding events and 1 minor bleeding event.4,20,22,42,44,63,64 Overall, 16% (37/238) of reported patients died among all the medical regimens.
Because individual patient information was presented in the case report and case series, outcomes were aggregated to assess thrombus resolution, risk of bleeding, and death. Overall, treatment with a thrombolytic regimen failed to completely resolve the thrombus in 31% of patients [pooled risk for partial or no thrombus resolution was 0.22 (95% CI 0.06–0.55)] (Figure 2A) whereas non-thrombolytic regimens reported 40% of patients with partial or no response to therapy [pooled risk 0.43 (95% CI 0.23–0.65)] (Figure 2B). Funnel plot analysis did not show significant publication bias. Pooled risk of major bleeding in the in the thrombolytic group was 29% (95% CI 0.17–0.44), P<0.01 (Figure 3A) and non-thrombolytic group was 12% (95% CI 0.01–0.57), P<0.1 (Figure 3B). The pooled risk of death was 20% for thrombolytic regimens (95% CI 0.06–0.47, p<0.05) and 6% (95% CI 0.003–0.58, p<0.1) for non-thrombolytic regimens.
Figure 2.

Risk estimates of partial or no resolution of thrombus from case reports and case series using thrombolytic (A) and non-thrombolytic (B) treatment regimens
Figure 3.
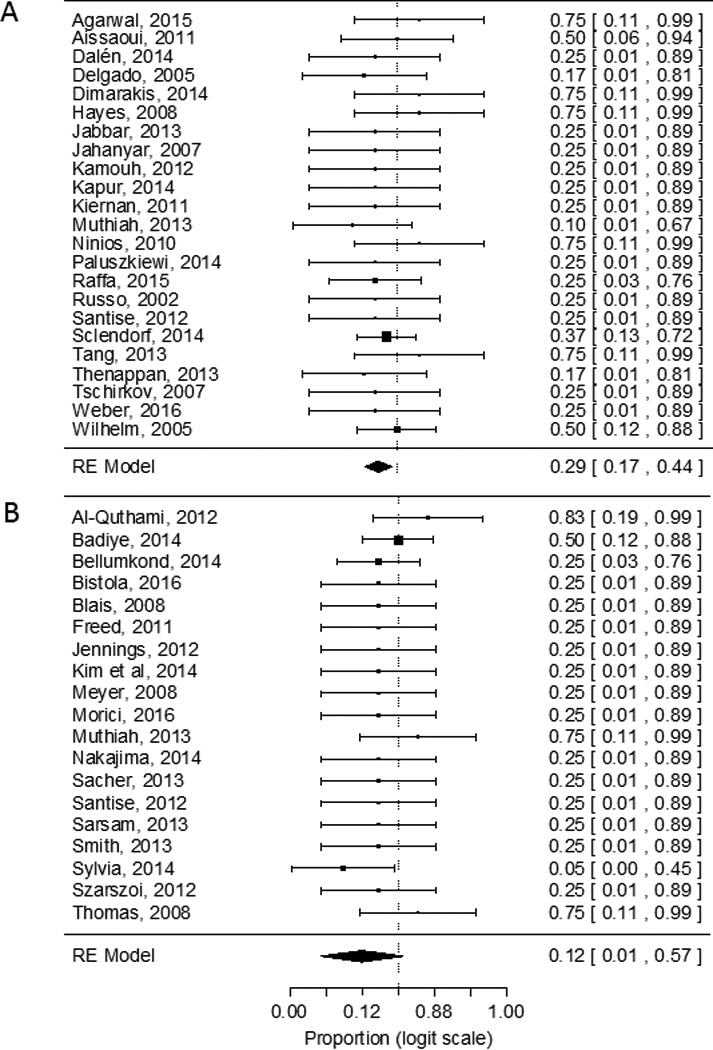
Risk estimates of major bleeding from case reports and series using thrombolytic (A) and non-thrombolytic (B) regimens
When response to therapy was evaluated based upon pump type, there were no significant differences in how patients with a HMII or HVAD responded to thrombolytic or non-thrombolytic treatment in resolution [non-thrombolytic regimen (no or partial resolution) OR 0.94, (95% CI 0.30–2.97); thrombolytic regimen OR 1.18 (95% CI 0.35–3.99)]. Risk of major hemorrhage, minor hemorrhage, intracranial hemorrhage (ICH) or death did not significantly differ likely due to the limited number of events within the groups. The number of patients in each treatment arm was too small to allow for comparison between the different treatments (i.e. heparin monotherapy vs. direct thrombin inhibitors) within the thrombolytic and non-thrombolytic regimen groups.
Because the cohort studies reported patients treated with thrombolytic and non-thrombolytic regimens, the treatment regimens could be compared. There was not a statistically significant difference between thrombolytic and non-thrombolytic regimen for resolving the pump thrombotic events (OR 0.48; 95% CI 0.20–1.16) (Figure 4). No statistically significant difference in major bleeding was found between patients treated with thrombolytic and non-thrombolytic regimens (OR 1.95; 95% CI 0.69–5.53) (Figure 5). Mortality was similar in thrombolytic and non-thrombolytic regimens [14% (12/87) vs 16% (12/74); OR 1.28 (95% CI 0.42–3.89).
Figure 4.

Comparison of thrombus resolution using thrombolytic and non-thrombolytic regimens from cohort studies
Figure 5.

Comparison of major bleeding using thrombolytic and non-thrombolytic regimens in cohort studies
Out of the 9 cohort studies, four had data for only one treatment (thrombolytic or non-thrombolytic) available. Therefore, we performed a sensitivity analysis of the 5 studies with comparative results. HN model showed that thrombolytic regimens were 3.57 times more likely to have major bleed than non-thrombolytic regimes (95% CI 1.07–11.88, P<0.05) although NN model (OR 2.71; 95% CI 0.83–8.86, P<0.1) and BN model (OR 2.40; 95% CI 0.96–6.03, P<0.1) were only marginally significant.
Discussion
Data from the REMATCH trial indicates that LVAD implantation increase survival and quality of life as compared to optimal medical management alone.65 The survival of patients with continuous flow LVADs continues to improve, but these patients still remain at a high risk for fatal complications like pump thrombosis. The incidence of pump thrombosis reported in the initial and extended clinical trials of continuous flow devices ranged from 0.014 to 0.03 events per patient-year, but increased incidence of pump thrombosis have been noted.2,66–68 In the setting of suspected thrombosis, surgical device exchange or urgent heart transplantation represent the most definitive treatment modalities. However, cardiothoracic surgery is not without risks. An additional surgery for pump exchange can result in formation of scar tissue and adhesions, which can increase the duration and risk of bleeding during subsequent surgery for heart transplantation.69 Therefore, it is important to explore medical management strategies to treat pump thrombosis for transplant candidates or patients who cannot withstand surgery. Several studies have compared surgical with medical management for pump thrombosis.4,12,70 However, no previous reported studies have compared medical treatment of pump thrombosis.
Our systematic review and meta-analysis shows that data regarding the efficacy and safety of medical management of LVAD thrombosis is limited to case reports, case series or small single institute cohort studies. Jennings and Weeks recently summarized the case reports and series of medical and surgical treatment of LVAD thrombosis and highlighted that management strategies varied widely between institutions.69 However, comparison between the different treatment regimens was not reported. Complete thrombus resolution occurred in 65% of patients receiving a thrombolytic regimen and in 43% of patients who received a non-thrombolytic regimen. The ineffective nature of heparin monotherapy to resolve LVAD thrombosis likely affected the estimate of the non-thrombolytic regimens. The cohort studies showed that no or partial resolution of LVAD thrombosis did not significantly differ between thrombolytic and non-thrombolytic regimens (OR 0.48; 95% CI 0.20–1.16). Case reports and series showed that the pooled risk of major bleeding in the thrombolytic regimens was 29% and 12% in the non-thrombolytic regimens. A 3.57 times increased odds of major bleeding was found for thrombolytic regimens in the sensitivity analysis using HN model. However, the NN model and BN model were only marginally significant indicating the instability of the model. There were no differences in risk of death in the two groups. Due to the uncontrolled nature of the comparison, it is possible that severity of illness, severity of the thrombosis, or duration of symptoms were worse in patients who received thrombolytics which could have influenced patients’ mortality outside of bleeding and resolution of the pump thrombosis. Randomized or controlled prospective studies would be needed to control for these confounding factors. The limited number of patients at each site and institutional preferences may make completion of a randomized trial difficult. Based on the available evidence, providers should understand that the use of thrombolytic therapy, either intraventricular or systemic, is likely associated with an increased risk of major hemorrhage.
Combining outcomes of patients with LVAD thrombosis allows a summary of the published knowledge base for treatment of these patients and is hypothesis-generating for future studies. This approach has limitations, however. The strength of the conclusions depends upon the quality of the available literature which is limited by the inherent biases associated with case reports and series. No randomized control trials have been conducted to date. Publication bias is likely in case reports and case series as researchers and journals are more likely to publish effective therapies. Almost all of the reports did not have a comparison group and numbers of reported patients were low. The definitions used for the diagnosis as well as the resolution of pump thrombosis varied between different studies. Ramp studies were not often performed. Moreover, the dosages, route of administration and the durations of use of the antithrombotic regimens also varied widely between institutions. Patient characteristics among the studies differed and the studies ranged over 10 years which could have also biased the results. It is also possible that the outcomes and adverse events may have been misclassified between treatment regimens based on the reviewers’ interpretation of the data. However, the data was meticulously reviewed by two different reviewers and compared in order to minimize this possibility. Moreover, in most studies medical management was used in patients that were hemodynamically stable at the time of presentation. Thus it is difficult to extrapolate the results of this analysis to high risk group of patients. Further studies are needed to determine the role of medical management in such patient population in comparison to surgical management. Despite these limitations, this study systematically assessed the efficacy and safety of the various medical regimens for the management of pump thrombosis, provides data to clarify the role of non-surgical management of pump thrombosis, and a basis for future prospective or randomized studies.
In conclusion, our systematic review of 49 studies consisting of 238 continuous-flow LVAD patients discusses existing therapeutic options and provides estimate of resolution of pump thrombosis and major hemorrhage associated with thrombolytic and non-thrombolytic regimens. Additional prospective studies evaluating the dosing and route of administration of thrombolysis and controlled comparison to anticoagulant and/or antiplatelet therapies are needed to define optimal management strategies for this vexing condition.
Table 4.
Management and outcomes in adult LVAD patients with thrombosis reported in retrospective cohort studies
| Study | Antiplatelet | INR at event Mean ± SD, median (range) |
LDH at event, mean ± SD in U/L |
Medical interventions |
Thrombosis Resolution |
Escalation of care |
Minor bleeding |
Major bleeding |
Death |
|---|---|---|---|---|---|---|---|---|---|
| Hasin23 2014 |
ASA 81 – 325 mg (6/8) Plavix 75 mg (1/8) |
1.7 ± 0.4 |
2456 | IV UFH X 3–5 days (2/8) IV UFH X 3–16 days + Plavix 75 mg (4/8) IV UFH X 21 days + IV eptifibatide X 8 days + Plavix 150 mg (1/8) IV UFH X 19 days + IV alteplase 30 mg (1/8) |
PR – 2/2 CR – ¼, PR – ¾ CR PR |
None | None | Yes – GI bleed None Yes – GI bleed Yes - CVA |
None |
| Najjar4 2014 |
ASA 325 mg (52%) |
1.9 (0.9 – 3.4) |
317 ± 190 |
IV tPA (15 – 100 mg) + IV UFH + eptifibatide (8/30 – 27%) IV UFH (5/30 – 17%) IV tPA alone or dual therapy† (11/30 – 37%) IV eptifibatide alone or with IV UFH (6/30 – 20%) |
CR – 37.5% (3/8) NoR – 50% (4/8) Death – 12.5% (1/8) NoR – 100% (5/5) CR – 82% (9/11) CR – 50% (3/6) |
Yes – 14 (12 – pump exchange, 2 – Heart transplant) |
None | Yes – 2 – Hemorrhagic CVA 2 – GI bleed 1 – ICD pocket bleed |
5 – total deaths (4 – following pump exchange 1 – Hemorrhagic stroke) |
| Rothenburger18 2002 |
ASA 300 mg + Dipyridamole 75 mg TID |
IV UFH (PTT 100s) + IV rtPA 100 mg |
CR | None | Yes - epistaxis (50%) |
None | None | ||
| Tellor17 2014 |
ASA 325 mg – 76% ASA 81 mg – 24% |
1.17 – 6.0 |
1564 (361 – 3960) |
+ IV UFH + IV eptifibatide – (13/18) IV eptifibatide + IV bivalirudin (3/18) IV eptifibatide (2/18) |
CR – 4/13 NoR- 9/13 CR – 1/3 NoR – 2/3 NoR – 2/2 |
Yes – pump exchange in 18%, heart transplant in 12% |
1 - epistaxis 1 - hematuria 0 |
3 – ICH, 3 – GI bleed 1 – GI bleed 1 – GI bleed |
Yes – 4/13 Yes - 1/3 Yes – 1/2 |
| Ertugay20 2016 |
ASA (9/15) ASA + Plavix (3/15) Plavix (3/15) |
2.3 (1.0 – 4.4) |
1271 ± 1016 |
IV UFH - (8/21) IV UFH + rtPA (4/21) IV UFH + Tirofiban (6/21) IV UFH + Tirofiban + rtPA (3/21) |
CR – 4/8 NoR – 4/8 CR – 2/4 NoR – 2/4 CR – 2/6 NoR – 4/6 CR – 2/3 NoR – 1/3 |
Yes – Heart Transplant (2/4), Pump Exchange (2/4) Yes – Pump Exchange (1/4) Yes – pump exchange (1/6) Yes – Pump exchange (1/3) |
None | None Yes – 2 Hemorrhagic CVA Yes – 3 Hemorrhagic CVA None |
0/8 3/4 3/6 0/3 |
| Oezpeker15 2016 |
ASA 100 mg | 2.3 (1.7 – 2.6) |
IV UFH + IV rtPA (29) |
CR – 18 /29 NoR – 11/29 |
Yes – Pump Exchange (11/29) |
11 – catheter site bleeding |
2 - ICH | 6/29 | |
| Saeed21 2016 |
ASA 100 mg | IV UFH (1/10) IV UFH + IV tirofiban (3/10) IV rtPA + IV UFH (6/10) |
CR (1/1) CR (3/3) CR (2/6) PR (4/6) |
None None None |
0/1 0/3 0/6 |
0/1 0/3 Yes - 1 |
0/1 0/3 0/6 |
||
| Scandroglio16 2016 |
ASA | Pre pump – 2 ± 0.9 Intra Pump – 2.5 ± 1 Post Pump – 3.2 ± 1 |
Pre Pump – 516 (359 – 910) Intra pump – 1495 (1007 – 2548) Post Pump – 426 (299 – 541) |
Pre Pump – IV Tirofiban (9 /26) Intra Pump – IV Tirofiban (9/70) Post Pump – No Medical treatment |
CR (5/9) NoR (4/9) CR (3/9) NoR (6/9) |
Yes – 2 – Pump Exchange 2 – Washout manouvre |
1 -GI Bleed |
None | None |
| Upshaw22 2016 |
ASA 325 mg | 2.6 (1.9 – 3) |
1152 (1082 – 1768) |
IV UFH (4/25) IV UFH + IV alteplase (2/25) IV UFH + IV Eptifibatide (5/25) IV UFH + IV Bivalirudin (5/25) IV Eptifibatide + IV Bivalirudin (3/25) IV Bivalirudin (4/25) IV Bivalirudin + IV Alteplase (1/25) IV Bivalirudin + IV Eptifibatide + IV alteplase (1/25) |
PR (2/4) NoR (2/4) CR (2/2) CR (4/5) NoR (1/5) PR (4/5) NoR (1/5) CR (0/3) NoR (3/3) PR (2/4) NoR (2/4) PR (1/1) NoR (1/1) |
Yes – pump exchange (2/4) None None None Yes – Pump Exchange (3/3) Yes – Pump Exchange (2/4) None None |
None None None None None None None None |
ICH (1/4) None 3/5 – 1 ICH, 2 major bleeds 1 - ICH 1/3-major bleed None 1 – Major bleed 1 -ICH |
None None 1/5 0/5 0/3 None None Yes |
ASA – aspirin, CR – complete resolution, CVA – cerebrovascular accident, GI – gastro intestinal, ICH – intracranial hemorrhage, INR- international normalized ratio, IV – intra venous, NoR – non resolution, PR – partial resolution, PTT – partial thromboplastin time, rtPA – recombinant tissue plasminogen activator, TID – Three times daily, UFH – unfractionated heparin,
Dual therapy – IV rtPA + IV UFH or IV eptifibatide
Acknowledgments
Funding: Funding provided by the Blood Center Research Foundation
We appreciate the assistance of the reference librarians at the Medical College of Wisconsin with the database search.
ABBREVIATIONS AND ACRONYMS
- aPTTs
Activated partial thromboplastin times
- CI
Confidence interval
- HVAD
HeartWare ventricular assist device
- INR
International normalized ratio
- IV
Intravenous
- LVAD
Left ventricular assist device
- OR
Odds ratio
- rtPA
recombinant tissue plasminogen activator
- SD
Standard deviation
- UFH
Unfractionated heparin
Footnotes
Conflict of interest: None declared
Contributions: Geetanjali Dang was responsible for design, data abstraction, data analysis, manuscript writing, critical appraisal and final approval; Narendranath Epperla was responsible for design, data abstraction, critical appraisal and final approval; Vijayadershan Mupiddi was responsible for data abstraction, critical appraisal and final approval. Natasha Sahr, Amy Pan and Pippa Simpson were responsible for data analysis, critical appraisal and final approval. Lisa Baumann Kreuziger was responsible for design, manuscript writing, critical appraisal and final approval.
Disclosures: None
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: A report from the american heart association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 3.Baumann Kreuziger LM, Kim B, Wieselthaler GM. Antithrombotic therapy for left ventricular assist devices in adults: A systematic review. J Thromb Haemost. 2015;13(6):946–955. doi: 10.1111/jth.12948. [DOI] [PubMed] [Google Scholar]
- 4.Najjar SS, Slaughter MS, Pagani FD, et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. Journal of Heart and Lung Transplantation. 2014;33(1):23–34. doi: 10.1016/j.healun.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Hetzer R, Loebe M, Potapov EV, et al. Circulatory support with pneumatic paracorporeal ventricular assist device in infants and children. Ann Thorac Surg. 1998;66(5):1498–1506. doi: 10.1016/s0003-4975(98)00914-x. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein DJ, John R, Salerno C, et al. Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant. 2013;32(7):667–670. doi: 10.1016/j.healun.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Bartoli CR, Ailawadi G, Kern JA. Diagnosis, nonsurgical management, and prevention of LVAD thrombosis. J Card Surg. 2014;29(1):83–94. doi: 10.1111/jocs.12238. [DOI] [PubMed] [Google Scholar]
- 8.Moazami N, Milano CA, John R, et al. Pump replacement for left ventricular assist device failure can be done safely and is associated with low mortality. Ann Thorac Surg. 2013;95(2):500–505. doi: 10.1016/j.athoracsur.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Stulak JM, Cowger J, Haft JW, Romano MA, Aaronson KD, Pagani FD. Device exchange after primary left ventricular assist device implantation: Indications and outcomes. Ann Thorac Surg. 2013;95(4):1262–1267. doi: 10.1016/j.athoracsur.2012.08.031. discussion 1267–8. [DOI] [PubMed] [Google Scholar]
- 10.Barbieri A, Bertelli L, Sangiorgi GM. Novel application of angiojet rheolytic thrombectomy for massive thrombosis of the native aortic valve and jarvick 2000 ventricular assist device in a patient with end-stage heart failure. Catheter Cardiovasc Interv. 2011;78(6):958–961. doi: 10.1002/ccd.23078. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151(4):264–269. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 12.Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370(1):33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 14.Stijnen T, Hamza TH, Özdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29(29):3046–3067. doi: 10.1002/sim.4040. [DOI] [PubMed] [Google Scholar]
- 15.Oezpeker C, Zittermann A, Ensminger S, et al. Systemic thrombolysis versus device exchange for pump thrombosis management: A single-center experience. ASAIO J. 2016;62(3):246–251. doi: 10.1097/MAT.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 16.Scandroglio AM, Kaufmann F, Pieri M, et al. Diagnosis and treatment algorithm for blood flow obstructions in patients with left ventricular assist device. J Am Coll Cardiol. 2016;67(23):2758–2768. doi: 10.1016/j.jacc.2016.03.573. [DOI] [PubMed] [Google Scholar]
- 17.Tellor BR, Smith JR, Prasad SM, Joseph SM, Silvestry SC. The use of eptifibatide for suspected pump thrombus or thrombosis in patients with left ventricular assist devices. Journal of Heart and Lung Transplantation. 2014;33(1):94–101. doi: 10.1016/j.healun.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Rothenburger M, Wilhelm MJ, Hammel D, et al. Treatment of thrombus formation associated with the MicroMed DeBakey VAD using recombinant tissue plasminogen activator. Circulation. 2002;106(12 Suppl 1):189–192. [PubMed] [Google Scholar]
- 19.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 20.Ertugay S, Engin C, Daylan A, et al. Outcomes of various treatment strategies for patients with continuous-flow ventricular assist device thrombosis: A retrospective analysis. ASAIO Journal. 2016 doi: 10.1097/MAT.0000000000000397. [DOI] [PubMed] [Google Scholar]
- 21.Saeed D, Maxhera B, Albert A, Westenfeld R, Hoffmann T, Lichtenberg A. Conservative approaches for HeartWare ventricular assist device pump thrombosis may improve the outcome compared with immediate surgical approaches. Interact Cardiovasc Thorac Surg. 2016;23(1):90–95. doi: 10.1093/icvts/ivw063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Upshaw JN, Kiernan MS, Morine KJ, Kapur NK, DeNofrio D. Incidence, management, and outcome of suspected continuous-flow left ventricular assist device thrombosis. ASAIO J. 2016;62(1):33–39. doi: 10.1097/MAT.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 23.Hasin T, Deo S, Maleszewski JJ, et al. The role of medical management for acute intravascular hemolysis in patients supported on axial flow LVAD. ASAIO Journal. 2014;60(1):9–14. doi: 10.1097/MAT.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 24.Sacher VY, Andreopoulos F, Pham SM. Nonoperative management of aortic valve thrombus in a patient with left ventricular assist device. Artif Organs. 2013;37(8):742–743. doi: 10.1111/aor.12063. [DOI] [PubMed] [Google Scholar]
- 25.Bistola V, Parissis JT, Lekakis J, Filippatos G. Non-obstructive left ventricular assist device outflow thrombus: What is the appropriate management? Int J Cardiol. 2016;214:33–34. doi: 10.1016/j.ijcard.2016.03.164. [DOI] [PubMed] [Google Scholar]
- 26.Morici N, Perna E, Cipriani M, et al. Ticagrelor for left ventricular assist device thrombosis: A new therapeutic option to be evaluated with caution. Int J Cardiol. 2016;221:58–59. doi: 10.1016/j.ijcard.2016.06.304. [DOI] [PubMed] [Google Scholar]
- 27.Santise G, Sciacca S, Baglini R, Clemenza F, Pilato M. Can learning to interpret pump messages help lead to an early diagnosis of HeartWare ventricular assist device thrombosis? ASAIO J. 2012;58(6):629–632. doi: 10.1097/MAT.0b013e31826a87bc. [DOI] [PubMed] [Google Scholar]
- 28.Kim JB, Rhee J-, Brenner DA, et al. Continuous flow left ventricular assist device placement complicated by aortic valve thrombus and myocardial infarction. Int J Cardiol. 2014 doi: 10.1016/j.ijcard.2014.07.248. [DOI] [PubMed] [Google Scholar]
- 29.Nakajima S, Seguchi O, Murata Y, et al. Left coronary artery occlusion caused by a large thrombus on the left coronary cusp in a patient with a continuous-flow ventricular assist device. Journal of Artificial Organs. 2014;17(2):197–201. doi: 10.1007/s10047-014-0758-0. [DOI] [PubMed] [Google Scholar]
- 30.Szarszoi O, Maly J, Turek D, et al. Implantation of left ventricular assist device complicated by undiagnosed thrombophilia. Texas Heart Institute Journal. 2012;39(5):615–617. [PMC free article] [PubMed] [Google Scholar]
- 31.Smith MC, Nielsen VG. Detection of carboxyhemefibrinogen and methemefibrinogen in a patient with thrombosis of a HeartMate II ventricular assist device. ASAIO Journal. 2013;59(1):93–95. doi: 10.1097/MAT.0b013e31827986e6. [DOI] [PubMed] [Google Scholar]
- 32.Sylvia L, Ordway L, Pham DT, DeNofrio D, Kiernan M. Bivalirudin for treatment of lvad thrombosis: A case series. ASAIO Journal. 2014 doi: 10.1097/MAT.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 33.Al-Quthami AH, Jumean M, Kociol R, et al. Eptifibatide for the treatment of HeartMate II left ventricular assist device thrombosis. Circulation: Heart Failure. 2012;5(4):e68–e70. doi: 10.1161/CIRCHEARTFAILURE.112.966804. [DOI] [PubMed] [Google Scholar]
- 34.Blais DM, Sun B, Vesco P, Louis LB, Sai-Sudhakar C, Firstenberg MS. Profound thrombocytopenia with glycoprotein IIb/IIIa inhibitors plus heparin for pump thrombus. Journal of Heart & Lung Transplantation. 2008;27(12):1361–1362. doi: 10.1016/j.healun.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Bellumkonda L, Subrahmanyan L, Jacoby D, Bonde P. Left ventricular assist device pump thrombosis: Is there a role for glycoprotein IIb/IIIa inhibitors? ASAIO Journal. 2014;60(1):134–136. doi: 10.1097/MAT.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 36.Sarsam SH, Civitello AB, Agunanne EE, Delgado RM. Bivalirudin for treatment of aortic valve thrombosis after left ventricular assist device implantation. ASAIO Journal. 2013;59(4):448–449. doi: 10.1097/MAT.0b013e3182937a65. [DOI] [PubMed] [Google Scholar]
- 37.Freed BH, Jeevanandam V, Jolly N. Aortic root and valve thrombosis after implantation of a left ventricular assist device. J Invasive Cardiol. 2011;23(4):E63–E65. [PubMed] [Google Scholar]
- 38.Jennings DL, Cabrera R, Wang DD, Tita C. Successful treatment of a continuous flow left ventricular assist device thrombosis with eptifibatide. ASAIO Journal. 2012;58(6):633–635. doi: 10.1097/MAT.0b013e318271bde2. [DOI] [PubMed] [Google Scholar]
- 39.Thomas MD, Wood C, Lovett M, Dembo L, O'Driscoll G. Successful treatment of rotary pump thrombus with the glycoprotein IIb/IIIa inhibitor tirofiban. Journal of Heart & Lung Transplantation. 2008;27(8):925–927. doi: 10.1016/j.healun.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Badiye A, Hernandez GA, Chaparro S. Argatroban as novel therapy for suspected thrombosis in patients with continuous-flow left ventricle assist device and hemolysis. ASAIO Journal. 2014;60(3):361–365. doi: 10.1097/MAT.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 41.Meyer AL, Kuehn C, Weidemann J, et al. Thrombus formation in a HeartMate II left ventricular assist device. Journal of Thoracic & Cardiovascular Surgery. 2008;135(1):203–204. doi: 10.1016/j.jtcvs.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 42.Muthiah K, Robson D, Macdonald PS, et al. Thrombolysis for suspected intrapump thrombosis in patients with continuous flow centrifugal left ventricular assist device. Artif Organs. 2013;37(3):313–318. doi: 10.1111/j.1525-1594.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 43.Aissaoui N, Börgermann J, Gummert J, Morshuis M. HeartWare continuous-flow ventricular assist device thrombosis: The bad oeynhausen experience. J Thorac Cardiovasc Surg. 2012;143(4):e37–e39. doi: 10.1016/j.jtcvs.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 44.Schlendorf K, Patel CB, Gehrig T, et al. Thrombolytic therapy for thrombosis of continuous flow ventricular assist devices. J Card Fail. 2014;20(2):91–97. doi: 10.1016/j.cardfail.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Dalén M, Sartipy U, Corbascio M, Lund LH, Grinnemo K- HeartWare left ventricular assist device thrombosis in aspirin non-responder. Asian Cardiovascular and Thoracic Annals. 2014;22(2):203–204. doi: 10.1177/0218492312467993. [DOI] [PubMed] [Google Scholar]
- 46.Dimarakis I, Shaw S, Venkateswaran R. HeartWare left ventricular assist device thrombosis including outflow graft. J Thorac Cardiovasc Surg. 2014 doi: 10.1016/j.jtcvs.2014.01.049. [DOI] [PubMed] [Google Scholar]
- 47.Hayes H, Dembo L, Larbalestier R, O'Driscoll G. Successful treatment of ventricular assist device associated ventricular thrombus with systemic tenecteplase. Heart, Lung & Circulation. 2008;17(3):253–255. doi: 10.1016/j.hlc.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 48.Jabbar AA, Yau R, Frazier OH, Delgado R., 3rd Direct thrombolytic therapy for thrombosis of a centrifugal flow left ventricular assist device. ASAIO Journal. 2013;59(5):530–532. doi: 10.1097/MAT.0b013e3182a39628. [DOI] [PubMed] [Google Scholar]
- 49.Kamouh A, John R, Eckman P. Successful treatment of early thrombosis of HeartWare left ventricular assist device with intraventricular thrombolytics. Ann Thorac Surg. 2012;94(1):281–283. doi: 10.1016/j.athoracsur.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 50.Kiernan MS, Pham DT, DeNofrio D, Kapur NK. Management of HeartWare left ventricular assist device thrombosis using intracavitary thrombolytics. Journal of Thoracic & Cardiovascular Surgery. 2011;142(3):712–714. doi: 10.1016/j.jtcvs.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 51.Ninios V, Visouli A, Pitsis A. Repeated successful thrombolysis of a jarvik 2000 left ventricular assist device in a patient with noncompaction cardiomyopathy. Circulation. 2010;121(3):e13–e14. doi: 10.1161/CIR.0b013e3181cdb410. [DOI] [PubMed] [Google Scholar]
- 52.Raffa GM, D'Ancona G, Romano G, et al. Should device replacement be the first choice strategy in continuous-flow left ventricle assist device thrombosis? analysis of 9 events and results after endoventricular thrombolysis. Int J Cardiol. 2015;(178):159–161. doi: 10.1016/j.ijcard.2014.10.169. [DOI] [PubMed] [Google Scholar]
- 53.Paluszkiewicz L, Morshuis M, Hakim-Meibodi K, Ensminger S, Gummert J. CASE REPORT successful repeated thrombolysis in a patient with HeartWare thrombosis–the importance of doppler flow pattern. Kardiochirurgia i Torakochirurgia Polska. 2014;11(4):428–431. doi: 10.5114/kitp.2014.47346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilhelm MJ, Hammel D, Schmid C, et al. Long-term support of 9 patients with the DeBakey VAD for more than 200 days. Journal of Thoracic & Cardiovascular Surgery. 2005;130(4):1122–1129. doi: 10.1016/j.jtcvs.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 55.Rothenburger M, Wilhelm M, Schmid C, et al. Thrombolytic therapy with recombinant tissue plasminogen activator in patients with thromboembolism inside the deBakey-NASA left ventricular assist device. Circulation. 2001;104(17):713–714. [Google Scholar]
- 56.Agarwal R, Raina A, Lasorda DM, et al. Successful treatment of acute left ventricular assist device thrombosis and cardiogenic shock with intraventricular thrombolysis and a tandem heart. ASAIO J. 2015;61(1):98–101. doi: 10.1097/MAT.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 57.Delgado R, 3rd, Frazier OH, Myers TJ, et al. Direct thrombolytic therapy for intraventricular thrombosis in patients with the jarvik 2000 left ventricular assist device. Journal of Heart & Lung Transplantation. 2005;24(2):231–233. doi: 10.1016/j.healun.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 58.Jahanyar J, Noon GP, Koerner MM, et al. Recurrent device thrombi during mechanical circulatory support with an axial-flow pump is a treatable condition and does not preclude successful long-term support. Journal of Heart & Lung Transplantation. 2007;26(2):200–203. doi: 10.1016/j.healun.2006.11.602. [DOI] [PubMed] [Google Scholar]
- 59.Kapur NK, Upshaw J, Kiernan MS, Pham DT. Left ventricular assist device thrombosis presenting as an acute coronary syndrome. Journal of Thoracic & Cardiovascular Surgery. 2014;147(6):e72–e73. doi: 10.1016/j.jtcvs.2014.02.037. [DOI] [PubMed] [Google Scholar]
- 60.Russo C, De Biase AM, Bruschi G, Agati S, Vitali E. Successful intraventricular thrombolysis during ventricular assist device support. Ann Thorac Surg. 2002;73(5):1628–1629. doi: 10.1016/s0003-4975(01)03437-3. [DOI] [PubMed] [Google Scholar]
- 61.Tang GH, Kim MC, Pinney SP, Anyanwu AC. Failed repeated thrombolysis requiring left ventricular assist device pump exchange. Catheterization & Cardiovascular Interventions. 2013;81(6):1072–1074. doi: 10.1002/ccd.24523. [DOI] [PubMed] [Google Scholar]
- 62.Tschirkov A, Nikolov D, Tasheva I, Papantchev V. Successful fibrinolysis after acute left ventricular assist device thrombosis. The Journal of heart and lung transplantation. 2007;26(5):553–555. doi: 10.1016/j.healun.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 63.Webber BT, Panos AL, Rodriguez-Blanco YF. Intravenous thrombolytic therapy for patients with ventricular assist device thrombosis: An attempt to avoid reoperation. Annals of Cardiac Anaesthesia. 2016;19(1):192–196. doi: 10.4103/0971-9784.173047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thenappan T, Anderson AS, Jeevanadham V, Rich JD, Shah AP. Treatment of left ventricular assist device thrombosis with extended catheter-directed intraventricular thrombolytic therapy. Circulation: Heart Failure. 2013;6(3):e27–e29. doi: 10.1161/CIRCHEARTFAILURE.113.000013. [DOI] [PubMed] [Google Scholar]
- 65.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 66.Aaronson KD, Slaughter MS, Miller LW, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125(25):3191–3200. doi: 10.1161/CIRCULATIONAHA.111.058412. [DOI] [PubMed] [Google Scholar]
- 67.Pagani FD, Miller LW, Russell SD, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54(4):312–321. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 68.Boyle AJ, Russell SD, Teuteberg JJ, et al. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: Analysis of outpatient anti-coagulation. The Journal of heart and lung transplantation. 2009;28(9):881–887. doi: 10.1016/j.healun.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 69.Jennings DL, Weeks PA. Thrombosis in Continuous-Flow left ventricular assist devices: Pathophysiology, prevention, and pharmacologic management. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2015;35(1):79–98. doi: 10.1002/phar.1501. [DOI] [PubMed] [Google Scholar]
- 70.Ballew C, Benton E, Groves D, et al. Comparing survival of HMII patients with elevated LDH: Implications for medical and surgical management. The Journal of Heart and Lung Transplantation. 2013;32(4):S38. [Google Scholar]


