Abstract
Background
Complement plays a major role in inflammatory diseases but its involvement and mechanisms of activation in chronic rhinosinusitis (CRS) are not known.
Objectives
Following earlier studies discovering autoantibodies in CRS, we sought to investigate the nature, extent, and location of complement activation in nasal tissue of patients with CRS. Specifically, we were interested in whether antibody-mediated activation via the classical pathway was a major mechanism for complement activation in CRS.
Methods
Nasal tissue was obtained from patients with CRS and control patients. Tissue homogenates were analyzed for complement activation products (ELISA-C5b-9, C4d, activated C1 and C5a) and major complement fixing antibodies (Luminex). Tissue sections were stained for C5b-9, C4d, and laminin. Antibodies were purified using protein A/G columns from nasal polyps (NP), matching patient serum and control serum, and assayed for basement membrane binding via ELISA.
Results
C5b-9 was significantly increased in NP tissue compared to UT (uncinate tissue) of CRS with NP (CRSwNP) and CRS without NP (CRSsNP) (p<0.01). Similarly, C4d was increased in NP compared to UT of CRSwNP, CRSsNP and control (p<0.05). Activated C1 was also increased in NP tissue compared to UT of CRSsNP and control (p<0.05) and was correlated with C5a (p<0.01), local immunoglobulins, especially IgM (p<0.0001) and anti-dsDNA IgG (p<0.05). Immunofluorescence showed that C5b-9 and C4d deposition occurred linearly along the epithelial basement membrane. NP tissue extracts had significantly more anti-basement membrane antibodies than sera from CRSwNP and control patients (p<0.0001).
Conclusion
C5b-9, C4d and activated C1 were significantly increased locally in NP tissue. C5b-9 and C4d were almost universally deposited linearly along the basement membrane of NP tissue. Furthermore, activated C1 was best correlated with local immunoglobulin levels and C5a levels. Together, these data suggest that the classical pathway plays a major role in complement activation in CRS.
Keywords: Chronic rhinosinusitis, complement, basement membrane, antibodies, classical complement pathway
Capsule summary
Complement activation was elevated in CRSwNP tissue, and complement was deposited at the epithelial basement membrane. We conclude that the classical antibody-mediated complement pathway had a significant contribution to complement activation in nasal polyps.
Introduction
Chronic rhinosinusitis (CRS) is characterized by chronic inflammation of the sinonasal mucosa that persists for at least 12 weeks.(1–3) Despite the high prevalence and the impact on quality of life, the etiology and pathogenesis that underpin the inflammation in CRS are not well understood.(4, 5) Chronic rhinosinusitis with nasal polyps (CRSwNP) is characterized by a prominent type 2 infiltrate with elevated numbers of eosinophils but more recent evidence suggests that there is also B-cell dysregulation as evidenced by plasma cell proliferation, increased antibodies, increased local class switching, and the presence of a multitude of autoantibodies.(6–11) However, it remains unknown whether this prominent antibody response plays a role in the barrier dysfunction and pathogenic inflammation associated with CRS.(12)
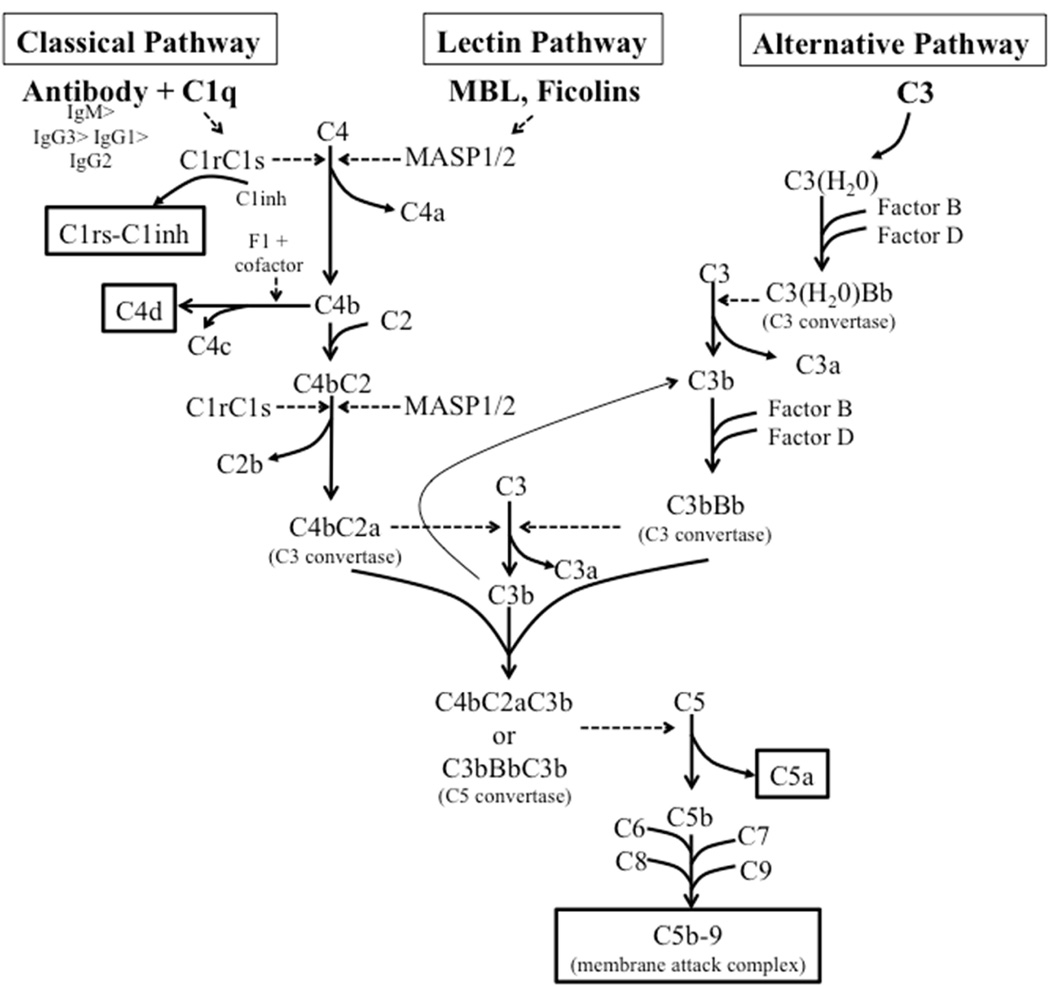
As excessive antibody-mediated complement activation is frequently a hallmark for a pathologic autoantibody response, we sought to investigate whether evidence for classical-pathway activation could be found in CRSwNP. The complement system represents a major effector mechanism linking the adaptive and innate immune responses. However, the complement system has three described routes of activation: the classical, the alternative, and the lectin pathways.(13) All three pathways of activation eventually lead to the formation of C3 convertases that cleave C3 to C3a and C3b, and initiate a cascade ending with the formation of C5b-9 (terminal complement complex or membrane attack complex (MAC) that induces cell lysis in non-nucleated cells and can be detected by ELISA and immunohistochemistry (Fig 1).(13) The lectin pathway is initiated with the activation of lectin proteins, including mannose-binding lectin (MBL) and ficolins upon their binding to pathogen-associated molecular patterns (PAMPs). Both the classical and lectin pathways result in the activation of C4 and generate the complement split product C4d.(14) C4d rapidly forms a covalent bond with any nearby protein or carbohydrate within microseconds, and thus it binds in proximity with the site of activation.(14) Like C5b-9, C4d can also be detected by ELISA and immunohistochemistry. The classical pathway is initiated by binding of C1q to antigen-antibody complexes, which leads to conformational changes in the C1q molecule that releases C1r and C1s that covalently bind to the C1inhibitor (C1inh). The soluble C1rs-C1inh complex can be detected by ELISA and is highly specific for activation of the classical pathway.(15) In contrast, the alternative pathway is initiated by the spontaneous hydrolysis of C3, C3(H2O) and does not involve the C1 or C4 molecules.
Figure 1.
The complement activation pathway. The three major pathways of complement activation; classical, lectin and alternative, all lead to the formation of C3 convertases which leads to the generation of the anaphylatoxins C3a and C5a and the formation of the lytic membrane attack complex (C5b-9). We evaluated the complement activation neoepitopes highlighted by the boxes. The anaphylatoxin C5a was also evaluated. Dotted arrows indicate enzymatic processes. MBL, mannose-binding lectin; MASP, MBL-associated serine protease.(68)
Although complement is best known for its role in clearing invading microorganisms through targeted lysis, there is growing evidence for a pathogenic role in transplant rejection, cancer, allergic inflammatory diseases, asthma and autoimmune disorders.(16, 17) In these conditions there is either an excessive activation or an inadequate dampening of complement activation that exacerbates inflammatory cell infiltration and tissue damage.(18) Complement deposition is well described in autoimmune diseases where it deposits in the glomerular basement membrane of kidneys, as seen in, systemic lupus erythematosus (SLE), and cryoglobulinemia.(16, 19) Separately, complement deposition is also described in the thyroid follicular basement membranes of Hashimoto’s thyroiditis and Graves disease(20) and skin of autoimmune blistering diseases such as bullous pemphigoid, epidermolysis bullosa acquisita and mucous membrane pemphigoid.(21–23) In these skin diseases, the autoantigens are involved in cell–cell or cell–matrix adhesion in the basement membrane of the skin.
In the present study, we evaluated the complement activation products C1rs-C1inh, C4d, C5b-9, and C5a to elucidate the mechanisms of complement activation in CRS. We further explore the relationship of these complement activation products and the previously described phenomena of eosinophilia and anti-dsDNA autoantibody responses observed in CRSwNP tissue.
Methods
Patients and tissue sample collection
Patients with CRS and non-CRS control subjects were recruited from the Otolaryngology and Allergy-Immunology clinics at Northwestern Medicine. In patients with CRS, uncinate tissue (UT) and nasal polyp (NP) were obtained during routine functional endoscopic sinus surgery. CRS patients met the criteria for CRS, as defined by the American Academy of Otolaryngology–Head and Neck Surgery Chronic Rhinosinusitis Task Force.(24) Tissue samples from non-CRS control subjects were obtained during endoscopic skull-based tumor excisions, intranasal procedures for obstructive sleep apnea, or approaches to the orbit. Details of subject characteristics and specimens analyzed are shown in Table I. All subjects provided informed consent, and the study was approved by the Institutional Review Board of Northwestern University Feinberg School of Medicine.
Table I.
Characteristics of subjects providing sinus tissue.
| Control Subjects | Patients with CRSsNP | Patients with CRSwNP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject total | n = 35 (16 M/19 F) | n = 30 (16 M/14 F) | n =111 (66 M/45 F) | ||||||
| Age (y), median (range) | 50 (25–78) | 41 (19–63) | 47 (20–74) | ||||||
| Y | N | U | Y | N | U | Y | N | U | |
| Atopy | 6 | 25 | 4 | 15 | 12 | 3 | 68 | 33 | 10 |
| Asthma | 1 | 34 | 0 | 7 | 22 | 1 | 59 | 49 | 3 |
| Nasal Steroids | 4 | 30 | 1 | 6 | 22 | 1 | 28 | 77 | 7 |
| Inhaled Steroids | 0 | 34 | 1 | 3 | 26 | 1 | 20 | 84 | 7 |
| Systemic Steroids | 1 | 33 | 1 | 3 | 26 | 1 | 37 | 67 | 7 |
| Methodology used | Control UT | CRSsNP UT | CRSwNP UT* | CRSwNP NP* |
|---|---|---|---|---|
| C1rs-C1inh, C5b-9, C4d ELISA |
n = 12 (4 M/8 F) | n = 24 (13 M/10 F) | n = 27 (18 M/9 F) | n = 55 (33 M/22 F) |
| Age (y), median (range) | 54 (27–78) | 39 (19–61) | 47 (20–72) | 49 (20–74) |
| Immunofluorescence | n = 7 (4 M/3 F) | n = 6 (3 M/3 F) | n = 13 (7 M/6 F) | |
| Age (y), median (range) | 42 (25–68) | 44 (35–63) | 38 (27–72) | |
| C5a ELISA | n = 12 (6 M/6 F) | n = 11 (6 M/5 F) | n = 11 (7M/4F) | n = 51 (31 M/20 F) |
| Age (y), median (range) | 55 (27–78) | 27 (19–61) | 51 (20–72) | 48 (20–72) |
| Isotyping Multiplex | n = 71 (41 M/30 F) | |||
| Age (y), median (range) | 47 (20–74) | |||
| Other markers assessed | ||||
| ECP (ng/mg of protein), mean (range) |
61.6 (0.4–224.5) | 139.9 (1.5–1394.3) | 1042.6 (10–7077.7) | 1866.0 (3.3–21968.7) |
| dsDNA IgG (IU/mg of protein), mean (range) |
107.9 (1.3–438.9) | 309.8 (16.1–2234.9) | 67.9 (5.9–259.5) | 529.0 (1.3–6332.3) |
| Control Serum antibodies |
CRSwNP serum and NP antibodies |
|
|---|---|---|
| Anti-Matrigel ELISA | n = 11 (5 M/6 F) | n = 16 (10 M/6 F) |
| Age (y), median (range) | 41 (25–63) | 44 (31–74) |
F, Female; M, male; N, no; U, unknown; Y, yes.
Some patients had both UT and polyp tissues used in these studies and are represented in both groups.
Measurement of complement components C5b-9, C4d, C1 and C5a
Homogenates from freshly obtained sinus tissue specimens were prepared as previously described.(25) Homogenates were analyzed for C5b-9, C4d and C5a using ELISA kits (Quidel Corporation, San Diego, CA, and Abcam, Cambridge, MA respectively) according to manufacturer’s instructions. The activated C1 neoepitope C1rs-inh was measured with a sandwich ELISA as previously described.(15) Specifically, microtiter plates were coated overnight with mouse anti human activated C1inh, monoclonal antibody (Kok-12, Sanquin Blood Supply Foundation, Amsterdam, The Netherlands), samples and control tissue extracts were incubated on the plates, washed and were further incubated with a cocktail of goat anti-human C1s and anti-C1r secondary antibodies (Quidel and Nordic immunology, Susteren, The Netherlands, respectively). Finally complexes were detected with mouse anti-goat IgG-HRP (Jackson ImmunoResearch, West Grove, PA). Human serum activated with heat-aggregated human IgG (Sigma) was used as a standard and was defined to contain 1,000 arbitrary units (AU)/ml.(15) All ELISA results were further normalized to the total protein measured in the tissue homogenate as previously described.(6) The sensitivity limit for C5b-9 was 3.7 ng/ml, C4d was 1 ng/ml, and activated C1 was 0.12AU/µl and C5a was 31 pg/µl respectively. For concentrations below the limit of detection we assigned a value of zero for analysis. In instances where data are presented in a log scale, a value corresponding to 1/10 of the limit of detection was utilized.
Anti-dsDNA IgG and ECP Elisa
Homogenates were analyzed for Anti-dsDNA IgG and ECP using ELISA kits (ALPCO, Salem, NH and MBL, Nagoya, Japan) according to manufacturer’s instructions as previously described.(6)
Tissue staining and microscopy
Cryosections of tissue (5–10 micron thickness) were fixed in 1% formaldehyde in PIPES buffer (Sigma-Aldrich, St. Louis, MO). Sections were blocked with donkey serum and subsequently stained with mouse anti-human C5b-9 (aE11, Santa Cruz Biotechnology) followed by AlexaFluor647 conjugated donkey anti-mouse Ig (Jackson ImmunoResearch) and by rabbit polyclonal anti-human C4d (Abcam) followed by Rhodamine (TRITC)-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch), or rabbit anti-human laminin (Rabbit polyclonal, Abcam) followed by AlexaFluor488 conjugated donkey anti-rabbit IgG (Invitrogen). Spectral imaging of C5b-9, C4d and/or Laminin expression was performed on a Nikon A1R laser scanning confocal microscope equipped with a 20x, 40X or a 100× objective and Nikon Elements Software. For the 100X objective, 4 color (DAPI, AlexaFluor488, TRITC, AlexaFluor647) image stacks containing 20–40 sections in the Z plane in 0.5 µm steps were acquired and deconvolved using softWoRx software (Applied Precision Inc., Mississauga, ON, Canada) on a DeltaVision inverted microscope. Whole tissue section images were made by stitching 20x images taken from an entire tissue section. Specific signal was defined by imaging control slides without primary antibody to define background autofluorescence. Semiquantitative analysis of the percentage of epithelium lined with complement was determined using the freehand line measuring tool in Image J (NIH, Bethesda, MD). (26)
Measurement of antibody isotypes
Antibody levels were measured in NP tissue lysates using the human immunoglobulin isotyping multiplex kit according to the manufacturer’s instructions (EMD Millipore, Billerica, MA). Total immunoglobulin was calculated by adding the amount of all isotypes quantified on the Luminex 200 instrument (ThermoFisher Scientific, Waltham, MA). All results were normalized to total protein measured in the tissue homogenate as previously described.(6)
Antibody purification from serum and tissue extracts
Antibodies were extracted using Pierce® Protein A/G agarose beads (ThermoFisher Scientific) according to the manufacturer’s instructions. Protein A/G specifically bind to human antibodies of most isotypes, especially IgG but not IgD(27). In brief, 300µl serum or polyp extract was added to 200 µl agarose beads and incubated overnight at 4 °C on rotator. Be ads were washed with Triton and eluted with elution buffer. Purified antibodies were buffer exchanged into PBS using Zeba Spin Desalting Columns (ThermoFisher Scientific). Purified antibodies were characterized by SDS-PAGE electrophoresis followed by Coomassie blue staining. Antibody concentration was measured using a NanoDrop1000 (ThermoFisher Scientific) and diluted in PBS accordingly to a concentration of 5mg/µl with PBS.
Anti-Matrigel ELISA
Matrigel ELISA was adapted from Orjuela, A. et al.(28) Microtiter 96 well plates were coated with 50µl of 500µg/µl of Matrigel® matrix (BD Biosciences) in cold PBS and incubated overnight at 4°C. The plates were washed and blocked with 200µl of PBS containing 3% bovine serum albumin for one hour at room temperature. Following blocking, the plates were washed and 50µl of 5 µg/µl of purified antibodies were added to the wells and incubated at 37°C for one hour. The plates were then washed, and bound antibodies were detected using a cocktail of HRP-conjugated goat anti-human IgM and HRP-conjugated goat anti-human IgG (Bethyl Laboratories, Inc.). To ensure comparable OD450 of measured samples, all samples were run on the same day and on the same plate.
Statistical analysis
All data are reported as mean ± SEM, and analyses was done with GraphPad Prism version 6.0d software (GraphPad Software, San Diego, CA). Differences among groups were analyzed by using the 1-way ANOVA Kruskal-Wallis test, with a Dunn correction for multiple comparisons. Correlations were assessed using a nonparametric Spearman rank correlation coefficient. A P value of less than 0.05 was considered significant.
For comparison of clinical and demographic factors associated with high and low complement activation the population was divided into tertiles. The top tertile was compared to the bottom tertile using the chi-square contingency tables for categorical variables and t-test for continuous variables.
Results
Complement was activated in nasal polyp tissue
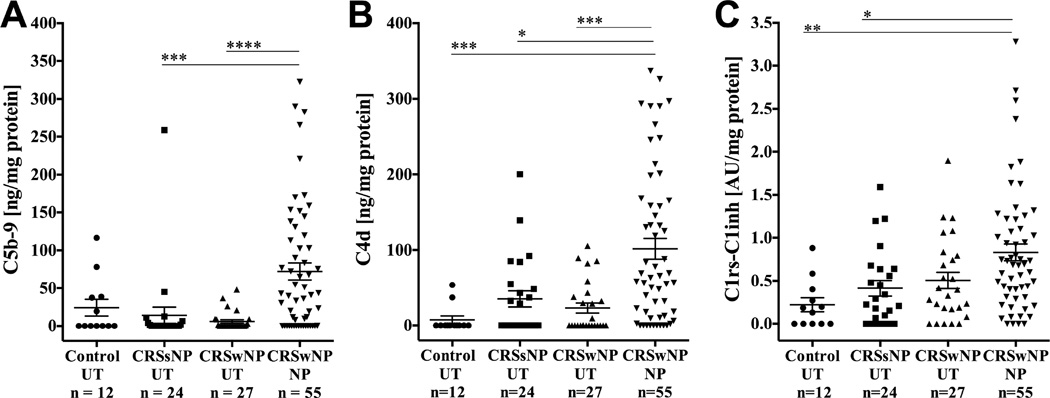
UT tissue were collected from 12 control subjects, 24 subjects with CRSsNP and 27 subjects with CRSwNP and NP tissues were collected from 55 subjects with CRSwNP. We first assessed formation of the terminal complement complex by measuring levels of C5b-9. We found that complement C5b-9 was elevated in NP tissue compared to UT tissue from CRSwNP and CRSsNP (p<0.0001 and p<0.001, respectively) (Fig 2, A).
Figure 2.
Complement activation products C5b-9, C4d and C1rs-C1inh were elevated in NP compared to UT. Tissue homogenates were tested by ELISA and levels were normalized to total protein for A) C5b-9, B) C4d and C) C1rs-C1inh. Data represents means ± SEMs. *P<0.05, **P<0.01, *** P<0.001, and ****P<0.0001, Kruskal-Wallis test.
We next quantified C4d in the same sinonasal tissues extracts. C4d was also elevated in NP tissue compared to UT tissue from CRSwNP, CRSsNP and control (p=0.0003, P=0.0106 and P=0.0001, respectively) (Fig 2,B).
Despite common use of C4d as a surrogate marker for antibody mediated tissue rejection, C4d actually indicates that complement activation was triggered by either the classical or lectin pathways. Therefore, to further resolve whether the complement activation occurred through the classical pathway, we analyzed the activation of C1 by measuring the complex containing C1r–C1s and C1-inh (C1rs-C1inh). Significantly elevated levels of C1rs-C1inh complexes were found in NP tissue compared to UT tissue from CRSsNP and control patients (p=0.0199 and p=0.0027, respectively) (Fig 2,C). In addition, the levels of complement components C5b-9, C4d and C1rs-C1inh complexes in sinonasal tissue were significantly correlated (Figure E1) in these homogenates. Together, these findings suggest that total and classical pathway mediated complement activation occurred at significantly higher levels in NP tissue. In order to further evaluate the association of these complement products with demographic and clinical factors prospectively collected on patients with CRSwNP, the patients in the top tertile of C1rs-C1inh, C4d, and C5b-9 expressing polyps were compared to those in the bottom tertile in polyp tissue. We found asthma was weakly associated with low C1rs-C1inh and C4d but otherwise, no significant associations were found with age, sex, radiographic severity, medication use, AERD or history of sinus surgery (Table E1).
Complement was linearly deposited along the epithelial basement membrane of polyp tissue
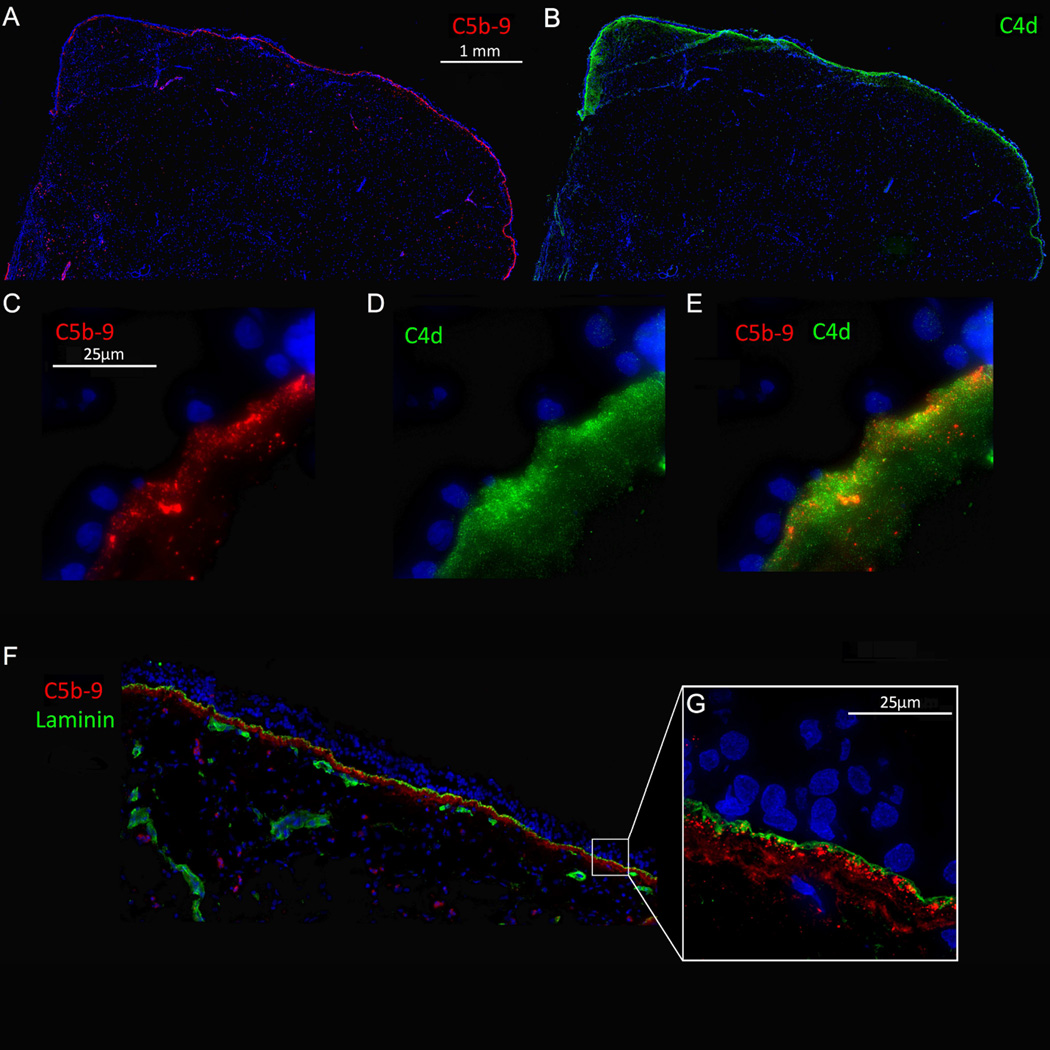
Since both C5b-9 and C4d were elevated in polyp tissue (Fig 2), we next investigated where complement was being activated. In contrast to C5b-9, which exists as soluble and membrane-associated complexes,(29) C4d is surface bound and is found in close proximity to the site of activation.(14) Laminin, a component of the basement membrane, was also used to visualize the basement membrane. Immunofluorescent imaging revealed that both C5b-9 and C4d were linearly deposited along the epithelial basement membrane (Fig 3) and along most glandular epithelium (data not shown). Microscopically, C5b-9 and C4d were located on the basal side of laminin with C4d being slightly more diffusely deposited (Fig 3). To evaluate the extent of epithelial basement membrane complement deposition, the percent of epithelium lined with C5b-9 was analyzed using stitched images from sectioned tissue. In NP tissue, 87 % of the epithelium was lined with C5b-9 whereas UT tissue of CRSsNP and control UT had significantly lower subbasement membrane complement deposition (49%, p<0.05 and 16%, p<0.001, respectively) (Fig 4). Together, these findings suggest that the majority of complement was being activated and deposited in the vicinity of the epithelial basement membrane.
Figure 3.
Complement components C5b-9 and C4d are deposited linearly along the basement membrane of the nasal epithelium. OCT embedded tissue sections were stained for C5b-9 in red (A, C, E and F), C4d in green (B, D and E) and Laminin in green (F and G). Representative image of NP tissue.
Figure 4.
Percentage of nasal epithelium lined with complement. Stitched whole tissue section images were analyzed for positive subepithelial C5b-9 staining using Image J and represented as a percentage of the length of epithelium visualized. Data represents means ± SEMs. *P<0.05, and *** P<0.001, Kruskal-Wallis test.
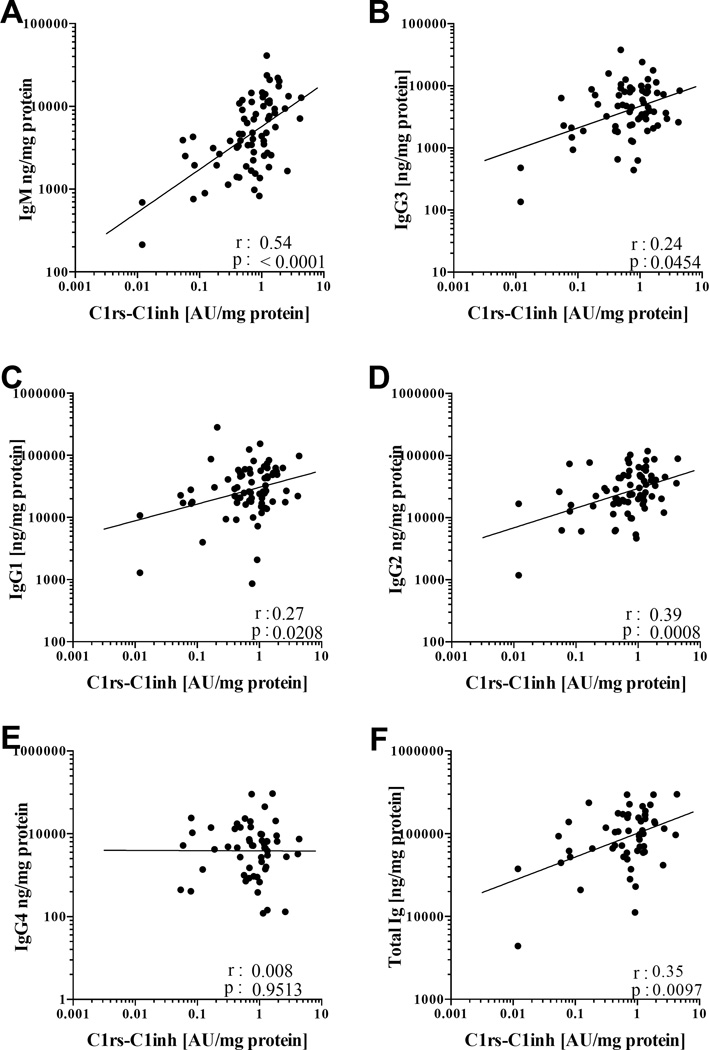
IgM was most correlated with complement activation
Because the above results indicated that complement activation in NP appeared to be occurring mainly through the classical pathway, we further tested the hypothesis that our previously reported local elevations of immunoglobulins in NP were associated with complement activation. NP tissue homogenates were analyzed using the human immunoglobulin isotyping multiplex kit to quantitate IgM, IgG1, IgG2, IgG3, IgG4 and IgA. Only the classical pathway-specific activation product C1rs-C1inh levels were significantly correlated with total immunoglobulin (p=0.0097, Spearman r= 0.35), total IgM (p<0.0001, Spearman r= 0.54), total IgG3, IgG1 and IgG2 (p=0.045, Spearman r= 0.24; p=0.021, Spearman r= 0.27 and p=0.0008, Spearman r= 0.39 respectively; Fig 5) and total IgA (p<0.0076, Spearman r=0.31; data not shown). No significant correlations were detected between C4d and C5b-9 levels and the individual immunoglobulin levels. These data may suggest that the locally elevated levels of complement fixing antibodies may play a significant role in activating the classical pathway in CRSwNP.
Figure 5.
Correlations between complement fixing antibody isotypes and C1rs-C1inh- activation product of the classical pathway in NP tissue. A, Correlation between IgM and C1rs-C1inh. B, Correlation between IgG3 and C1rs-C1inh. C, Correlation between IgG1 and C1rs-C1inh. D, Correlation between IgG2 and C1rs-C1inh. E, Correlation between IgG4 and C1rs-C1inh. F, Correlation between total immunoglobulin and C1rs-C1inh. (Spearman’s coefficient)
Relationship with Anaphylatoxin C5a, ECP and anti-dsDNA
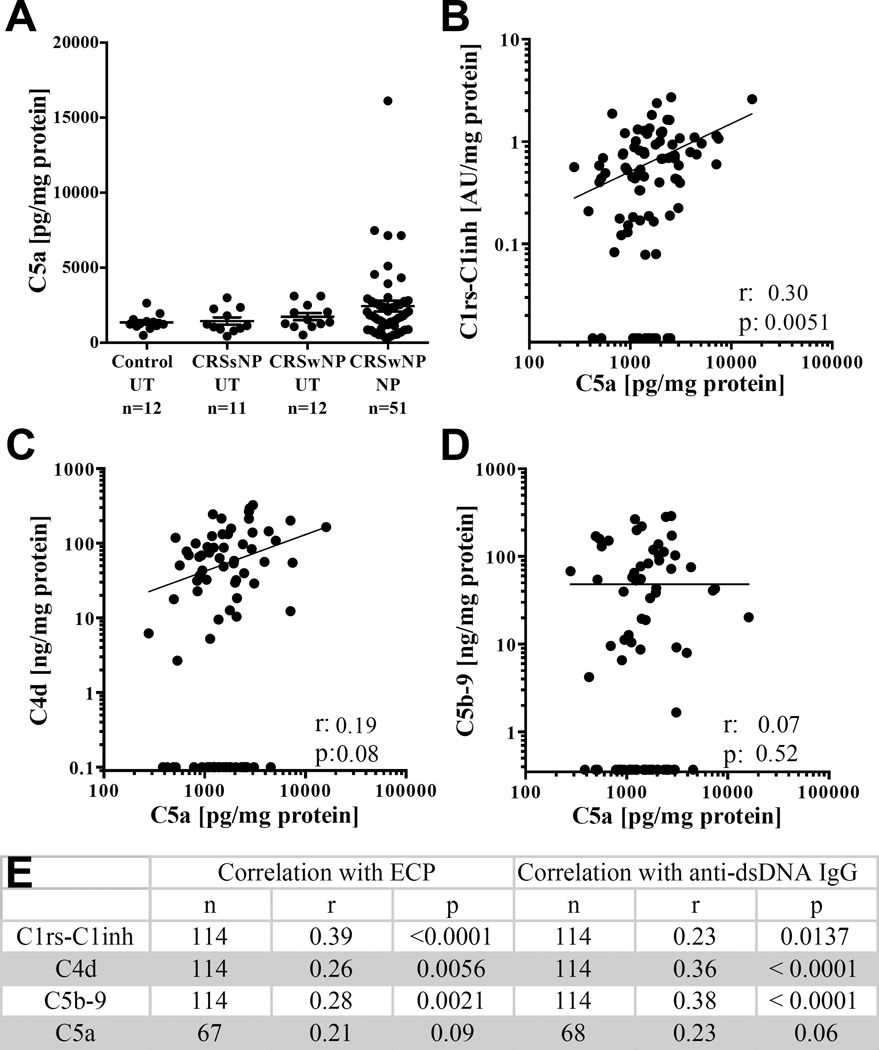
Anaphylatoxins play a critical role in supporting inflammation and activation of cells that express anaphylatoxin receptors. Unlike C5b-9 and C4d previously analyzed, the anaphylatoxins are soluble proteins and are rapidly cleared from the circulation in vivo.(30, 31) Since C5a is highly unstable and is degraded quickly into C5a des-Arg, the ELISA used measures both C5a and C5a des-Arg. While the des-Arg form of C5a is more stable, its serum half life is still only about 2 min.(31) We thus interpret anaphylatoxins as evidence for ongoing complement activation at the time of sample acquisition during surgery. Although elevated levels of C5a were detected in NP tissue compared to UT tissue, the differences were not statistically significant (Fig 6, A). However, when correlations between C5a and the other complement activation products were investigated, we found only C1rs-C1inh was significantly correlated with C5a (Fig 6, B–D), suggesting that ongoing complement activation was best correlated with initiation of the classical pathway.
Figure 6.
C5a levels in sinonasal tissue and correlations with complement activation products. A. Levels of C5a B. Correlation between C1rs-C1inh and C5a. C, Correlation between C4d and C5a. D, Correlation between C5b-9 and C5a. E, Correlations between activated C1, C4d, c5b-9 and C5a with ECP and anti-dsDNA IgG (Spearman’s coefficient).
Further analysis was performed to evaluate the relationship of our complement findings with other well-characterized immunologic features of CRSwNP namely the pronounced type-2 inflammation with eosinophilia(32) and the elevated anti-dsDNA IgG antibodies.(6, 33) We therefore correlated tissue ECP and Anti-dsDNA IgG with C1rs-C1inh, C4d, C5b-9 and C5a. The complement activation products all had weak but highly significantly correlations with ECP and Anti-dsDNA IgG except for C5a which was not significantly correlated with ECP or Anti-dsDNA IgG (Fig 6E). However, due to limitations in the availability of tissue homogenate, we were unable to analyze all the specimens for C5a which may have affected the power to detect a significant association.
Elevated levels of anti-basement membrane antibodies in polyp tissue
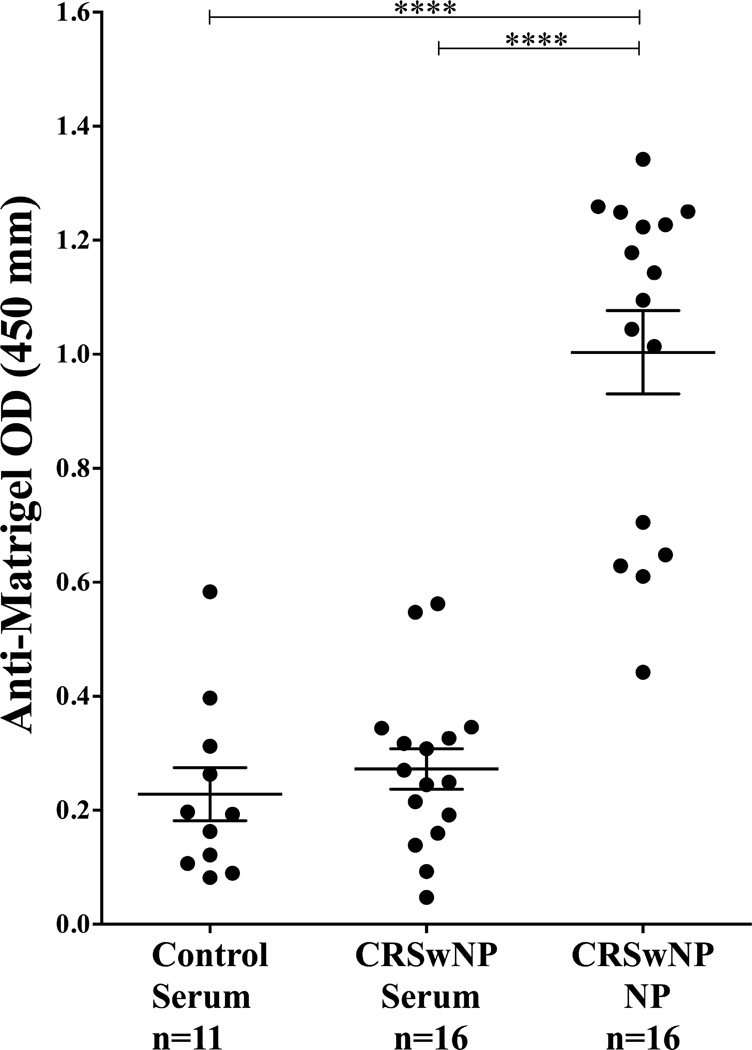
To further understand why complement was activated and deposited at the basement membrane, we tested the hypothesis that there was local autoreactivity against basement membrane components as previously found in a subset of SLE.(34) Basement membrane reactivity was assessed using an ELISA to detect anti-Matrigel matrix antibodies.(28) Matrigel matrix is a reconstituted basement membrane preparation that is extracted from the Engelbreth-Holm-Swarm mouse sarcoma, a tumor rich in extracellular matrix protein. Antibodies were purified from NP tissue extracts and compared with serum from the same CRSwNP patients, along with a random set of serum from control patients. Total tissue immunoglobulin from control patients was low and insufficient immunoglobulin was isolated to perform this analysis. Significant and strikingly higher concentrations of anti-Matrigel matrix IgG and IgM antibodies were detected in NP extracts compared to serum from normal and CRSwNP patients (p<0.0001) (Fig 7). From these data, we infer that the immunoglobulins within a nasal polyp are significantly more autoreactive to basement membrane components than those in the sera of both control and CRSwNP patients.
Figure 7.
Elevated levels of anti-basement membrane antibodies in NP tissue. Antibodies were purified from NP tissue homogenates and compared with antibodies purified from serum from the same CRSwNP and control patients. 5mg/µl of total extracted antibody were tested on an anti-Matrigel ELISA. Data represents means ± SEMs. ****P<0.0001, Kruskal-Wallis test.
Discussion
In this study, we investigated the activation and location of complement activation in CRS tissue. We demonstrated that NP tissue had elevated levels of the complement activation products C4d and C1rs-C1inh compared to normal and CRS UT tissues (Figure 2). Although C5b-9 was similarly elevated, differences were not significant comparing NP tissue to control UT. In addition, these markers also correlated significantly with each other (Figure E1). Similar to what was previously shown by Van Zele et al., (35) we found that C5b-9 is deposited at the basement membrane of the epithelium almost universally in NP tissue, with 87 % of NP epithelium lined with C5b-9. Since C5b-9 is a stable neoepitope that exists as a soluble and surface-bound form, local deposition may result from remote activation. We thus further evaluated C4d, a product that is formed from the degradation of surface bound C4b. C4b is highly reactive and binds covalently to nearby surfaces within microseconds of activation; therefore C4d is found in close proximity of the site of complement activation and its covalent bond does not break spontaneously.(14) We demonstrated that C4d was similarly deposited along the basement membrane suggesting that complement activation via the classical or lectin pathway was occurring in the vicinity of the basement membrane (Fig 3). Together, these findings are the first studies demonstrating that complement activation via the classical antibody-mediated pathway is increased in NP tissue. Our C4d findings also further suggest that complement activation is occurring along the epithelial basement membrane.
Given these findings, we evaluated the correlation between activation products and antibodies in NP tissue. In prior studies by Hulse et al, we had found locally elevated levels of IgG, IgA, and IgM along with elevated levels of B-cells and plasmablasts in NP tissue.(7) We found that C1rs-C1inh was significantly correlated with the complement fixing antibody isotypes IgM and IgG1, IgG2 and IgG3 but to a lesser extent (Fig 5). These data may suggest that the locally elevated levels of complement fixing antibodies may play a significant role in activating the classical pathway in CRSwNP. Interestingly, IgM, the isotype that activates complement most efficiently,(36) was also best correlated with C1rs-C1inh levels (Fig 5A). Unlike C1rs-C1inh, C4d and C5b-9 were not correlated with antibody isotype levels (data not shown). We postulate that given the dynamic nature of complement activation, the long tissue half lives of these covalently bound complexes, and the possibility they may be activated by the other two complement pathways, make them less readily correlated with antibody levels.
We also considered the possibility that autoreactive antibodies could be involved in complement activation since we had previously found a multitude of autoreactive antibodies including anti-double stranded DNA (dsDNA) antibodies and anti-nuclear antibodies present at elevated levels in NP tissue.(37) We found weak but significant correlation between anti-dsDNA IgG and C1rs-C1inh, C4d, C5b-9 but not with C5a (Fig 6). We hypothesize that levels of autoantibody and complement activation products may be affected by disease flares and fluctuate in time as previously found in classic autoimmune diseases like SLE(38), thus weakening intercorrelations. However, since C4d binds to surfaces close to the site of activation, we investigated the presence of anti-basement membrane autoreactive antibodies in NP tissue and compared them to serum from the same patients and control patients. Anti-Matrigel antibodies have been studied in SLE since Matrigel® contains many of the constituent matrix proteins that make up basement membranes.(28) We found markedly high levels of anti-Matrigel autoreactive antibodies present in NP tissue that were not detected at higher levels systemically (Fig. 7). Another possible mechanism for basement membrane complement activation was shown by van Bruggen et al. found that anti-double stranded DNA or anti-nucleosome autoantibody immune complexes could bind to the glomerular basement membrane, whereas purified antibodies did not bind.(39) They attributed this to binding between the positively charged histone moieties within the nucleosome with anionic determinants in the glomerular basement membrane.(39) These findings are reminiscent of recent studies examining serum from SLE patients using autoantigen microarrays. In studies by Zhen et al., elevated levels of antibodies binding basement membrane components (vimentin, myosin, Matrigel, laminin, and heparan sulphate) were separately clustered from antibodies against dsDNA, ssDNA and chromatin, but both were independently associated with more severe disease,(40) suggesting that autoreactivity to basement membrane and nuclear antigens played an important role in lupus severity. We postulate that since both anti-basement membrane and anti-nuclear autoantibodies are similarly found in CRSwNP at elevated levels, complement activation at the basement membrane via the classical pathway may occur through similar mechanisms as have been described in SLE.
Previous studies by Schlosser(41) and Lane(42) have evaluated gene expression of complement factors in CRS tissue. They found that tissue from CRSsNP patients had significantly higher gene expression of factor B, C3 and C5 compared to control patients(41). Indeed, normal nasal epithelial cells have also been shown to produce C3 locally(42). In addition, several airway epithelial cells and cell lines upregulate the expression of C3 in response to cytokines.(43),(44),(45, 46) The study by Schlosser et al. also found C5b-9 deposition in the basement membrane by immunohistochemistry.(41) However, in contrast to our findings, they found that C4d and C3d were deposited on the apical surface of the epithelial cells as well as on endothelial and inflammatory cells within the submucosa.(41) These discrepancies may be due to the use of different antibodies and/or a different fixation protocol. In our prior experience, non-specific binding to cilia or mucus proteins in immunofluorescent immunohistochemistry studies of nasal mucosa occurs frequently and requires caution when concluding ciliary protein expression. A separate study by Van Zele et al. found that the complement anaphylatoxins C3a and C5a were significantly elevated in nasal secretions from CRSwNP patients compared to control patients, and C5b-9 deposition was reported at the basement membrane of mucosal epithelium and blood vessels of NP.(35) Serum C3a and C5a levels were not elevated in NP compared to control patients suggesting the complement activation is a local process.(35) Our study of sinonasal tissue found non-significant but elevated levels of tissue C5a that were positively correlated with ECP. (Fig 6) However our study may not be adequately powered to detect differences in the tissue anaphylatoxins and nasal tissue may contain factors that rapidly degrade C5a given the labile nature of this protein. Together, while prior studies have provided evidence that nasal tissue may produce some complement proteins and that there was evidence for ongoing complement activation in CRSwNP, the mechanism of complement activation was previously unknown.
While the effects of complement activation products on nasal epithelial cells are largely unknown, they have been studied in other epithelial cells. Insertion of C5b-9 into cell membranes will induce cell lysis of non-nucleated cells (such as bacteria and red blood cells).(19) In nucleated cells, C5b-9 is usually sublytic and instead induces cellular activation and/or promotion of tissue injury.(47, 48) For example, C5b-9 has been shown to induce endothelial cell proliferation(49) and collagen synthesis of glomerular epithelial cells.(50) Complement activation products have also been shown to promote epithelial-to-mesenchymal transition (EMT)(51). EMT is a biological process by which epithelial cells lose their cellular polarity and cell-cell adhesion, to assume a mesenchymal cell phenotype. Indeed, prior work has demonstrated evidence for EMT in NP tissue.(52) Tong et al. has shown that C3a induces EMT through activation of the C3a receptor on tubular epithelial cells.(51) Similar to tubular epithelial cells, nasal epithelial cells also express this receptor(53) and C3a may thus similarly promote EMT in CRSwNP. In addition, C5a has been shown to induce production of oncostatin M (OSM) by macrophages.(54) We have recently shown that OSM is a potent cytokine that disrupts epithelial barrier integrity in CRSwNP and other type 2 inflammatory conditions.(55) The activation of complement immediately below the epithelial basement membrane may thus promote the barrier dysfunction observed in CRSwNP through multiple mechanisms.
In addition to effects on epithelial cells, complement activation products have potent pro-inflammatory effects on eosinophils(56), mast cells(57) and macrophages(58) which have been described at elevated levels in CRSwNP. The anaphylatoxins, in particular, are powerful leukocyte chemotactic factors; can induce oxidative burst;(59–61) and can cause degranulation of mast cells.(62) Anaphylatoxin induced histamine release by basophils and mast cells results in vasodilation.(63, 64) In the eosinophilic inflammation that typifies western CRSwNP, C5a plays important roles in upregulating cell adhesion molecules on eosinophils(65) and enhances degranulation.(66) We found that levels of C5a were significantly correlated with the elevated levels of the classical pathway activation product C1rs-C1inh and complement activation was positively correlated with ECP levels (Fig 6). Since the anaphylatoxins like C5a are unstable products and are degraded quickly,(30, 31) these results suggest ongoing complement activation was occurring via the classical pathway. Finally, complement components C3d and iC3b, a degradation product of C5b, play important roles in antibody generation by B cells through interaction with the complement receptor CR2 which forms a co-receptor complex with CD19 and CD81. These complement split products increase the strength of B cell receptor signaling and lower the threshold for B cell activation.(67) Overall, we find it intriguing that autoreactive antibodies may play a role in activating complement adjacent to the basement membrane while complement activation may in turn lead to the dysregulation of B-cells observed in CRSwNP.
In summary, we have shown that the complement activation products were elevated specifically in NP tissue compared to UT tissue of both CRSwNP, CRSsNP and control patients. We found that the activated complement was deposited along the basement membrane of the nasal epithelium and propose that complement may, directly or indirectly, alter epithelial barrier viability and function. Furthermore, our data strongly suggest that the classical complement pathway is a driver of complement activation in CRSwNP. Local elevations of IgM and the presence of anti-basement membrane autoreactive antibodies may initiate this process.
Supplementary Material
Key messages.
Elevated levels of complement C5b-9, C4d and activated C1 were detected in NP tissue compared to UT.
Complement was deposited linearly along the epithelial basement membrane of CRS nasal tissue.
Local, autoreactive, anti-basement membrane antibodies were found at significantly higher levels in NP tissue and may be responsible for complement activation.
Acknowledgments
We thank Professor Thomas Hope for the use of his microscopes, Daniel Stieh for advice on imaging and Jeff Schneider for the advice on the antibody purification.
Funding: This work was supported by NIH grant K23DC012067 (B.K.T), Chronic Rhinosinusitis Integrative Studies Program (CRISP) U19 AI106683 (B.K.T, R.C.K, K.E.H, A.K., R.P.S), R37 HL078860, R01 HL068546, R01 AI072570 and R01 AI104733, the Triological Society/American College of Surgeons (B.K.T) and the Ernest S. Bazley Foundation.
List of Abbreviations
- AU
arbitrary units
- C1inh
C1-inhibitor
- CRS
chronic rhinosinusitis
- CRSsNP
Chronic rhinosinusitis without nasal polyps
- CRSwNP
Chronic rhinosinusitis with nasal polyps
- CR2
complement receptor 2
- EMT
epithelial-to-mesenchymal transition
- FDCs
follicular dendritic cells
- MAC
membrane attack complex
- MASP
MBL-associated serine protease
- MBL
mannose-binding lectin
- NP
nasal polyp
- OSM
oncostatin M
- PAMP
pathogen-associated molecular pattern
- SLE
systemic lupus erythematosus
- UT
Uncinate tissue
- ESS
Endoscopic Sinus Surgery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
References
- 1.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: Establishing definitions for clinical research and patient care. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2004;131(6 Suppl):S1–S62. doi: 10.1016/j.otohns.2004.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi-Peters A, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. American journal of rhinology. 2008;22(6):549–559. doi: 10.2500/ajr.2008.22.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. The Journal of allergy and clinical immunology. 2010;125(2 Suppl 2):S103–S115. doi: 10.1016/j.jaci.2009.12.989. [DOI] [PubMed] [Google Scholar]
- 4.Ference EH, Stubbs V, Lidder AK, Chandra RK, Conley D, Avila PC, et al. Measurement and comparison of health utility assessments in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015;5(10):929–936. doi: 10.1002/alr.21556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orlandi RR, Kingdom TT, Hwang PH, Smith TL, Alt JA, Baroody FM, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22–S209. doi: 10.1002/alr.21695. [DOI] [PubMed] [Google Scholar]
- 6.Tan BK, Li Q-Z, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. Journal of Allergy and Clinical Immunology. 2011;128(6):1198–1206. e1. doi: 10.1016/j.jaci.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hulse KE, Norton JE, Suh L, Zhong Q, Mahdavinia M, Simon P, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol. 2013;131(4):1075–1083. 83 e1–83 e7. doi: 10.1016/j.jaci.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeffe JS, Seshadri S, Hamill KJ, Huang JH, Carter R, Suh L, et al. A role for anti-BP180 autoantibodies in chronic rhinosinusitis. Laryngoscope. 2013;123(9):2104–2111. doi: 10.1002/lary.24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Schryver E, Calus L, Bonte H, Natalie DR, Gould H, Donovan E, et al. The quest for autoreactive antibodies in nasal polyps. Journal of Allergy and Clinical Immunology. doi: 10.1016/j.jaci.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gevaert P, Nouri-Aria KT, Wu H, Harper CE, Takhar P, Fear DJ, et al. Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy. 2013;68(1):55–63. doi: 10.1111/all.12054. [DOI] [PubMed] [Google Scholar]
- 11.Van Zele T, Gevaert P, Holtappels G, van Cauwenberge P, Bachert C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2007;37(12):1840–1847. doi: 10.1111/j.1365-2222.2007.02838.x. [DOI] [PubMed] [Google Scholar]
- 12.Tan BK, Schleimer RP, Kern RC. Perspectives on the etiology of chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2010;18(1):21–26. doi: 10.1097/MOO.0b013e3283350053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janeway CATP, Jr, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5. New York: Garland Science; 2001. [Google Scholar]
- 14.Murata K, Baldwin WM., 3rd Mechanisms of complement activation, C4d deposition, and their contribution to the pathogenesis of antibody-mediated rejection. Transplant Rev (Orlando) 2009;23(3):139–150. doi: 10.1016/j.trre.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fure H, Nielsen EW, Hack CE, Mollnes TE. A Neoepitope-Based Enzyme Immunoassay for Quantification of C1-Inhibitor in Complex with C1r and C1s. Scandinavian Journal of Immunology. 1997;46(6):553–557. doi: 10.1046/j.1365-3083.1997.d01-168.x. [DOI] [PubMed] [Google Scholar]
- 16.Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, Roumenina LT. Complement System Part II: Role in Immunity. Frontiers in Immunology. 2015;6:257. doi: 10.3389/fimmu.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Kohl J. A complex role for complement in allergic asthma. Expert Rev Clin Immunol. 2010;6(2):269–277. doi: 10.1586/eci.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. Journal of immunology. 2013;190(8):3831–3838. doi: 10.4049/jimmunol.1203487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathern DR, Heeger PS. Molecules Great and Small: The Complement System. Clinical Journal of the American Society of Nephrology. 2015 doi: 10.2215/CJN.06230614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weetman AP, Cohen SB, Oleesky DA, Morgan BP. Terminal complement complexes and C1/C1 inhibitor complexes in autoimmune thyroid disease. Clin Exp Immunol. 1989;77(1):25–30. [PMC free article] [PubMed] [Google Scholar]
- 21.Lessey E, Li N, Diaz L, Liu Z. Complement and cutaneous autoimmune blistering diseases. Immunologic Research. 2008;41(3):223–232. doi: 10.1007/s12026-008-8028-y. [DOI] [PubMed] [Google Scholar]
- 22.Jordon RE. Complement Activation In Bullous Skin Diseases. Journal of Investigative Dermatology. 1975;65(1):162–169. doi: 10.1111/1523-1747.ep12598113. [DOI] [PubMed] [Google Scholar]
- 23.Mooney E, Falk RJ, Gammon W. STudies on complement deposits in epidermolysis bullosa acquisita and bullous pemphigoid. Archives of Dermatology. 1992;128(1):58–60. [PubMed] [Google Scholar]
- 24.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. The Journal of allergy and clinical immunology. 2004;114(6 Suppl):155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. The Journal of allergy and clinical immunology. 2008;121(6):1385–1892. 92 e1–92 e2. doi: 10.1016/j.jaci.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Meth. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.TECHNICAL RESOURCE : Binding characteristics of Protein A, Protein G, Protein A/G and Protein L: Thermo Scientific™ Pierce™ Protein Biology. 2004 [Available from: https://fscimage.fishersci.com/cmsassets/downloads/segment/Scientific/pdf/pierce_binding.pdf.
- 28.Orjuela A, Suwanichkul A, Canter D, Minard CG, Devaraj S, Hicks MJ, et al. High Titer Anti-Basement Membrane Antibodies in a Subset of Patients with Pediatric Systemic Lupus Erythematosus. American Journal of Nephrology. 2015;41(3):241–247. doi: 10.1159/000381965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mollnes TE, Lea T, Harboe M, Tschopp J. Monoclonal Antibodies Recognizing a Neoantigen of Poly(C9) Detect the Human Terminal Complement Complex in Tissue and Plasma. Scandinavian Journal of Immunology. 1985;22(2):183–195. doi: 10.1111/j.1365-3083.1985.tb01870.x. [DOI] [PubMed] [Google Scholar]
- 30.Weisdorf DJ, Hammerschmidt DE, Jacob HS, Craddock PR. Rapid in vivo clearance of C5ades arg: a possible protective mechanism against complement-mediated tissue injury. The Journal of laboratory and clinical medicine. 1981;98(6):823–830. [PubMed] [Google Scholar]
- 31.Webster RO, Larsen GL, Henson PM. In vivo clearance and tissue distribution of C5a and C5a des arginine complement fragments in rabbits. J Clin Invest. 1982;70(6):1177–1183. doi: 10.1172/JCI110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology Supplement. 2012;(23):1–298. [PubMed] [Google Scholar]
- 33.De Schryver E, Calus L, Bonte H, Natalie R, Gould H, Donovan E, et al. The quest for autoreactive antibodies in nasal polyps. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan LS, Lapiere J, Chen M, et al. BUllous systemic lupus erythematosus with autoantibodies recognizing multiple skin basement membrane components, bullous pemphigoid antigen 1, laminin-5, laminin-6, and type vii collagen. Archives of Dermatology. 1999;135(5):569–573. doi: 10.1001/archderm.135.5.569. [DOI] [PubMed] [Google Scholar]
- 35.Van Zele T, Coppieters F, Gevaert P, Holtappels G, Van Cauwenberge P, Bachert C. Local complement activation in nasal polyposis. Laryngoscope. 2009;119(9):1753–1758. doi: 10.1002/lary.20484. [DOI] [PubMed] [Google Scholar]
- 36.Schroeder HW, Jr, Cavacini L. Structure and function of immunoglobulins. Journal of Allergy and Clinical Immunology. 2010;125(2, Supplement 2):S41–S52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan B, Brown D, Xu S, Valle D. PHR1 is a vesicle-bound protein abundantly expressed in mature olfactory neurons. Laryngoscope. 2010;120(5):1002–1010. doi: 10.1002/lary.20779. [DOI] [PubMed] [Google Scholar]
- 38.Giles BM, Boackle SA. Linking complement and anti-dsDNA antibodies in the pathogenesis of systemic lupus erythematosus. Immunol Res. 2013;55(1–3):10–21. doi: 10.1007/s12026-012-8345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Bruggen MC, Walgreen B, Rijke TP, Tamboer W, Kramers K, Smeenk RJ, et al. Antigen specificity of anti-nuclear antibodies complexed to nucleosomes determines glomerular basement membrane binding in vivo. European journal of immunology. 1997;27(6):1564–1569. doi: 10.1002/eji.1830270636. [DOI] [PubMed] [Google Scholar]
- 40.Zhen QL, Xie C, Wu T, Mackay M, Aranow C, Putterman C, et al. Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. Journal of Clinical Investigation. 2005;115(12):3428–3439. doi: 10.1172/JCI23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlosser RJ, Mulligan RM, Casey SE, Varela JC, Harvey RJ, Atkinson C. Alterations in gene expression of complement components in chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24(1):21–25. doi: 10.2500/ajra.2010.24.3399. [DOI] [PubMed] [Google Scholar]
- 42.Lane AP, Truong-Tran QA, Myers A, Bickel C, Schleimer RP. Serum amyloid A, properdin, complement 3, and toll-like receptors are expressed locally in human sinonasal tissue. American journal of rhinology. 2006;20(1):117–123. [PMC free article] [PubMed] [Google Scholar]
- 43.Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, et al. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11(10):928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varsano S, Kaminsky M, Kaiser M, Rashkovsky L. Generation of complement C3 and expression of cell membrane complement inhibitory proteins by human bronchial epithelium cell line. Thorax. 2000;55(5):364–369. doi: 10.1136/thorax.55.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christian-Ritter KK, Hill LD, Hoie EB, Zach TL. Effect of interleukin-4 on the synthesis of the third component of complement by pulmonary epithelial cells. Am J Pathol. 1994;144(1):171–176. [PMC free article] [PubMed] [Google Scholar]
- 46.Rothman BL, Despins AW, Kreutzer DL. Cytokine regulation of C3 and C5 production by the human type II pneumocyte cell line, A549. Journal of immunology. 1990;145(2):592–598. [PubMed] [Google Scholar]
- 47.Jane-wit D, Manes TD, Yi T, Qin L, Clark P, Kirkiles-Smith NC, et al. Alloantibody and Complement Promote T Cell-Mediated Cardiac Allograft Vasculopathy Through Noncanonical Nuclear Factor-κB Signaling in Endothelial Cells. Circulation. 2013;128(23):2504–2516. doi: 10.1161/CIRCULATIONAHA.113.002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adler S, Baker PJ, Johnson RJ, Ochi RF, Pritzl P, Couser WG. Complement membrane attack complex stimulates production of reactive oxygen metabolites by cultured rat mesangial cells. The Journal of Clinical Investigation. 77(3):762–767. doi: 10.1172/JCI112372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fosbrink M, Niculescu F, Rus V, Shin ML, Rus H. C5b-9-induced Endothelial Cell Proliferation and Migration Are Dependent on Akt Inactivation of Forkhead Transcription Factor FOXO1. Journal of Biological Chemistry. 2006;281(28):19009–19018. doi: 10.1074/jbc.M602055200. [DOI] [PubMed] [Google Scholar]
- 50.Torbohm I, Schonermark M, Wingen AM, Berger B, Rother K, Hansch GM. C5b-8 and C5b-9 modulate the collagen release of human glomerular epithelial cells. Kidney international. 1990;37(4):1098–1104. doi: 10.1038/ki.1990.91. [DOI] [PubMed] [Google Scholar]
- 51.Tang Z, Lu B, Hatch E, Sacks SH, Sheerin NS. C3a Mediates Epithelial-to-Mesenchymal Transition in Proteinuric Nephropathy. Journal of the American Society of Nephrology : JASN. 2009;20(3):593–603. doi: 10.1681/ASN.2008040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shin H-W, Cho K, Kim DW, Han DH, Khalmuratova R, Kim S-W, et al. Hypoxia-inducible Factor 1 Mediates Nasal Polypogenesis by Inducing Epithelial-to-Mesenchymal Transition. American Journal of Respiratory and Critical Care Medicine. 2012;185(9):944–954. doi: 10.1164/rccm.201109-1706OC. [DOI] [PubMed] [Google Scholar]
- 53.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Tissue-based map of the human proteome. Science. 2015;347(6220) doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 54.Kastl SP, Speidl WS, Kaun C, Katsaros KM, Rega G, Afonyushkin T, et al. In Human Macrophages the Complement Component C5a Induces the Expression of Oncostatin M via AP-1 Activation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(3):498–503. doi: 10.1161/ATVBAHA.107.160580. [DOI] [PubMed] [Google Scholar]
- 55.Pothoven KL, Norton JE, Hulse KE, Suh LA, Carter RG, Rocci E, et al. Oncostatin M promotes mucosal epithelial barrier dysfunction, and its expression is increased in patients with eosinophilic mucosal disease. Journal of Allergy and Clinical Immunology. 2015;136(3):737–746. e4. doi: 10.1016/j.jaci.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kato A. Immunopathology of chronic rhinosinusitis. Allergology international : official journal of the Japanese Society of Allergology. 2015;64(2):121–130. doi: 10.1016/j.alit.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takabayashi T, Kato A, Peters AT, Suh LA, Carter R, Norton J, et al. Glandular mast cells with distinct phenotype are highly elevated in chronic rhinosinusitis with nasal polyps. The Journal of allergy and clinical immunology. 2012;130(2):410–420. e5. doi: 10.1016/j.jaci.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banks CA, Schlosser RJ, Wang EW, Casey SE, Mulligan RM, Mulligan JK. Macrophage Infiltrate Is Elevated in CRSwNP Sinonasal Tissue Regardless of Atopic Status. Otolaryngology -- Head and Neck Surgery. 2014;151(2):215–220. doi: 10.1177/0194599814528672. [DOI] [PubMed] [Google Scholar]
- 59.Murakami Y, Imamichi T, Nagasawa S. Characterization of C3a anaphylatoxin receptor on guinea-pig macrophages. Immunology. 1993;79(4):633–638. [PMC free article] [PubMed] [Google Scholar]
- 60.Elsner J, Oppermann M, Czech W, Dobos G, Schöpf E, Norgauer J, et al. C3a activates reactive oxygen radical species production and intracellular calcium transients in human eosinophils. European journal of immunology. 1994;24(3):518–522. doi: 10.1002/eji.1830240304. [DOI] [PubMed] [Google Scholar]
- 61.Elsner J, Oppermann M, Czech W, Kapp A. C3a activates the respiratory burst in human polymorphonuclear neutrophilic leukocytes via pertussis toxin-sensitive G-proteins. Blood. 1994;83(11):3324–3331. [PubMed] [Google Scholar]
- 62.Woolhiser MR, Brockow K, Metcalfe DD. Activation of human mast cells by aggregated IgG through FcγRI: additive effects of C3a. Clinical Immunology. 2004;110(2):172–180. doi: 10.1016/j.clim.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 63.Kretzschmar T, Jeromin A, Gietz C, Bautsch W, Klos A, Köhl J, et al. Chronic myelogenous leukemia-derived basophilic granulocytes express a functional active receptor for the anaphylatoxin C3a. European journal of immunology. 1993;23(2):558–561. doi: 10.1002/eji.1830230239. [DOI] [PubMed] [Google Scholar]
- 64.El-Lati SG, Church MK, Dahinden CA. Complement Peptides C3a- and C5a–Induced Mediator Release from Dissociated Human Skin Mast Cells. Journal of Investigative Dermatology. 1994;102(5):803–806. doi: 10.1111/1523-1747.ep12378589. [DOI] [PubMed] [Google Scholar]
- 65.DiScipio RG, Schraufstatter IU. The role of the complement anaphylatoxins in the recruitment of eosinophils. International Immunopharmacology. 2007;7(14):1909–1923. doi: 10.1016/j.intimp.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 66.Takafuji S, Tadokoro K, Ito K. Effects of interleukin (IL)-3 and IL-5 on human eosinophil degranulation induced by complement components C3a and C5a. Allergy. 1996;51(8):563–568. doi: 10.1111/j.1398-9995.1996.tb04669.x. [DOI] [PubMed] [Google Scholar]
- 67.Cherukuri A, Cheng PC, Pierce SK. The Role of the CD19/CD21 Complex in B Cell Processing and Presentation of Complement-Tagged Antigens. The Journal of Immunology. 2001;167(1):163–172. doi: 10.4049/jimmunol.167.1.163. [DOI] [PubMed] [Google Scholar]
- 68.Jozsi Ml. Anti-Complement Autoantibodies in Membranoproliferative Glomerulonephritis and Dense Deposit Disease. In: Prabhakar S, editor. An Update on Glomerulopathies - Etiology and Pathogenesis. 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.