Abstract
SAMP8 mice exhibit changes that commonly occur with normal aging late in life, but do so at a much earlier age. These changes include impairments in learning and memory as early as 8 months of age and so the SAMP8 is a useful model to investigate those age-related brain changes that may affect cognition. As brain insulin signaling and memory decline with aging, the SAMP8 model is useful for investigating these changes and interventions that might prevent the decline. This review will summarize the SAMP8 mouse model, highlight changes in brain insulin signaling and its role in memory, and discuss intranasal insulin delivery in investigating effects on insulin metabolism and memory in the SAMP8 mice.
Keywords: SAMP8, insulin, memory
1. Introduction
Senescence accelerated mice (SAM) were first developed and described by the lab of Toshio Takeda in 1981 (Takeda et al., 1981). Selective breeding led to the SAMP8 line after observing memory and cognitive deficits. SAMP8 mice have normal development but have strain-specific characteristics of rapid aging such as hair loss, circadian rhythm disruptions, and a shortened median lifespan reported to be anywhere from 10 to 17.2 months (Flood and Morley 1998). The spontaneous loss of memory can occur as early as 8 months of age (Miyamoto et al., 1986), with severe cognitive deficits by 12 months. SAMP8 mice are a model of sporadic Alzheimer's disease (AD), displaying features that occur early in the pathogenesis of AD, including cortical atrophy, neuronal cell loss, gliosis, oxidative stress, increased brain levels of amyloid-β peptide (Aβ), tau phosphorylation changes, and deficits in learning and memory (Morley et al., 2012b).
Age-associated memory impairment has been theorized to be caused by defects in hippocampal synaptic plasticity, peripheral inflammation, and oxidative stress. Insulin is involved in each of these processes. Furthermore, insulin has been shown to improve memory in young adults (Benedict et al., 2004), Alzheimer's patients (Craft et al., 2012), and SAMP8 mice (Salameh et al., 2015).
2. Role for Insulin Signaling in Aging and Memory
Insulin readily crosses the blood-brain barrier (BBB) and activates insulin receptors in the brain, which are widely expressed on many cell types. Insulin signaling in the brain elicits changes in feeding behavior and in learning and memory. Insulin receptor levels are highest in the hypothalamus and cerebral cortex in humans (Hopkins and Williams 1997), while in rodents the highest levels are in the olfactory bulb and hippocampus, the latter a fundamental site for the acquisition, consolidation, and recollection of information (Marks et al., 1990). Insulin binding to its receptor induces phosphorylation of the receptor, leading to activation of the insulin signaling pathways through phosphorylation of AKT or mitogen activated protein kinase (MAPK). Activation of insulin signaling has neuromodulatory effects, and contributes to a variety of neurobiological processes such as synaptogenesis, nerve growth, and Aβ clearance from the brain (Banks et al., 2012). Alterations in brain insulin signaling are thought to contribute to neurodegenerative diseases, such as AD (Meredith et al., 2015). With age and in patients with AD, there is decreased brain insulin receptor sensitivity and expression as well as decreased phosphorylation of the insulin receptor and downstream substrates (Frolich et al., 1998; Talbot et al., 2012). These alterations result in an insulin-resistant state in the brain.
Even though activation of the insulin signaling cascade has beneficial effects, inhibition of this pathway throughout the body is considered an evolutionarily conserved mechanism for extending lifespan (Kenyon 2005). Klotho is an aging suppressor protein that can extend lifespan when overexpressed (Kuro-o 2011). It prevents or reverses tyrosine phosphorylation of the insulin receptor and is therefore considered an inhibitor of the insulin signaling pathway (Kurosu et al., 2005). Klotho mRNA has been detected in high levels in mouse hippocampus (Nagai et al., 2003). Widespread loss of klotho results in memory impairments and increased oxidative stress in the brain (Nagai et al., 2003). As oxidative stress increases with age, as exhibited by the SAMP8 mice (Morley et al., 2012a), and has been linked to AD (Christen 2000), investigating mediators of oxidative stress in the brain might help delay age-related memory impairments.
Maintenance of insulin sensitivity in peripheral tissues is a predictor of longevity in humans (Barbieri et al., 2008). Understanding the changes that take place in brain insulin signaling during aging and in diseases such as AD will help in alleviating some of the cognitive deficits. Recent work indicates that the SAMP8 mouse has impaired insulin signaling in the central nervous system (CNS), and therefore may be an important model for studying the role of insulin in AD. The remainder of this review will discuss current knowledge of pathological changes in insulin signaling and pharmacological interventions that target the insulin signaling pathway to improve cognition in the SAMP8 mouse.
3. Central Insulin Signaling and Memory in the SAMP8 Mice
To date, a total of 8 articles have been published on the role of insulin in the CNS of SAMP8 mice, with 7 being published within the last 3 years (Table 1). The first of these studies was published in 2000 by Banks et al., investigating whether SAMP8 mice had BBB disruptions and/or alterations in insulin transport across the BBB (Banks et al., 2000). Previous reports had suggested the BBB may be impaired in AD. Banks et al. found a decrease in the weight of the frontal cortex in the aged SAMP8 mice despite an 8% increase in total brain weight of aged male SAMP8 mice compared to young SAMP8 mice. This loss of cortical volume could be related to the deficits in learning and memory observed in this mouse model. There was no disruption of the BBB in aged SAMP8 mice as measured with the serum marker albumin. In addition, there was no change in the kinetics of insulin transport across the BBB in the whole brain of aged SAMP8 mice. Therefore, aging of SAMP8 mice does not affect the permeability of the BBB as measured by albumin or the ability of peripheral insulin to access the brain.
Table 1.
Review of current literature in chronological order on the changes in memory and insulin signaling with various treatments in SAMP8 mice. Other than memory improvements with treatment, molecular key findings related to insulin are listed.
| Authors | Year | Treatment | Key Finding |
|---|---|---|---|
| 1 Banks et al | 2000 | Insulin transport | No change in insulin BBB transport |
| 2 Lin et al | 2013 | Asiaticoside | Increased levels of IDE mRNA |
| 3 Kuang et al | 2014 | Ligustilide | Klotho upregulation and inhibition of insulin signaling |
| 4 Adler et al | 2014 | Pramlintide | Decreased oxidative stress |
| 5 Armbrecht et al | 2015 | Ap protein precursor antisense | Changes in insulin signaling pathways |
| 6 Zhou et al | 2015 | Tetrahydroxystilbene glucoside (TSG) | Decreased levels of insulin and receptors |
| 7 Tong/Yan et al | 2015 | Acarbose | Prevention of decline in insulin and receptors |
| 8 Chen et al | 2015 | 1-deoxynojirimycin (DNJ) | Prevention of decline in insulin and receptors |
A series of studies investigating the effects of compounds found in healing Chinese herbal medicines on central insulin metabolism and cognition then followed. Six-month old male SAMP8 mice were used to study the effects of a daily 3-month oral treatment of asiaticoside (40 or 80 mg/kg/day) on learning and spatial memory (Lin et al., 2013). Asiaticoside treatment significantly improved memory as measured by escape latency and passive avoidance compared to untreated SAMP8 mice. Oxidative stress and Aβ1-42 protein levels were decreased with treatment. Asiaticoside treated SAMP8 animals had increased expression of proteins important in synaptic plasticity as well as increased levels of insulin degrading enzyme (IDE) mRNA levels. Aβ1-42 is also a substrate for IDE. The preservation of IDE levels could be responsible for the decrease in Aβ1-42 levels. However, whether or not CNS insulin levels were altered with asiaticoside treatment was not investigated. The increase in acetylcholine esterase activity observed with age did not occur with asiaticoside treatment, suggesting a preservation of the cholinergics which are important in memory formation. In all, this compound was able to ameliorate some of the age-related deficits that occur in the SAMP8 mouse by 9 months of age.
Ligustilide is the main lipophilic constituent of Umbelliferae medicinal plants. Ten-month old male SAMP8 mice were treated by oral gavage with 10 or 40 mg/kg/day for 8 weeks (Kuang et al., 2014). Ligustilide improved memory deficits in aged SAMP8 mice as measured by passive avoidance and Y-maze alteration. The number of senescent cells was increased with age in the hippocampus and cerebral cortex of SAMP8 mice, but ligustilide prevented this increase. Ligustilide reduced accumulation of changes in AD-associated proteins including Aβ1-42 and tau phosphorylation levels, while also preventing neuronal loss in aged SAMP8 mice. Ligustilide decreased oxidative stress as well as increasing the resistance towards oxidative stress. Concomitantly, ligustilide prevented the decreased expression of klotho found in the aged SAMP8 mice. It is unclear how ligustilide might regulate klotho expression levels. Ligustilide did increase forkhead box O1 (FoxO1) activation which could be a reason why oxidative stress was decreased with treatment. FOXO1 activation allows this transcription factor to remain in the nucleus, altering the regulation many genes involved in cell survival and differentiation and mediate oxidative stress (Akintola and van Heemst 2015). While FOXO1 protein levels are decreased in the liver and pancreas of aged SAMP8 mice (Tomobe et al., 2013; Tresguerres et al., 2013), FOXO1 reactivity or expression levels in brain have not been measured in the aged SAMP8 mouse. FOXO proteins are considered some of the most important transcriptional effectors of the insulin signaling pathway (Martins et al., 2016). Insulin pathway activation causes phosphorylation of FOXO1, excluding it from the nucleus (Martins et al., 2016).
Tetrahydroxystilbene glucoside (TSG) is a main component of the Polygonaceae plant, Polygonatum multiflorum, and may have lifespan extending properties (Zhou et al., 2015). In order to determine how TSG might extend lifespan, SAMP8 mice were treated with 2, 20, and 50 mM TSG each day. SAMP8 survival was improved in the TSG-treated group by about 17% in the 20mM group. TSG improved SAMP8 memory as measured by the Morris water maze. Klotho protein levels and phosphorylation of AKT was increased in the cortex of TSG-treated mice. Indeed, TSG reduced the amount of insulin, insulin receptor, insulin-like growth factor 1 (IGF-1), and IGF-1 receptor in the cortex. Peripheral perturbations in these individual mediators of the insulin signaling pathway are associated with increased longevity (Kenyon 2005).
Amylin is a regulatory satiety peptide that is co-secreted with insulin by the pancreas. It crosses the BBB three times faster and the percent of injected dose/g of brain is more than two-fold greater than insulin (Banks and Kastin 1998). In the CNS, amylin can activate proteins in the insulin signaling cascade, including Akt and MAPK (Moon et al., 2011). Circulating amylin levels are reduced in plasma of human subjects with mild cognitive impairment and AD (Adler et al., 2014). Pramlintide, an amylin analog, was constantly infused via an osmotic mini-pump with 0.24 mg/kg/day for 5 weeks in 6-month old SAMP8 mice (Adler et al., 2014). Pramlintide treated SAMP8 mice had improved memory as measured by the object recognition test. The hippocampus had decreased levels of oxidative stress markers and increased expression levels of proteins involved in synaptic function when treated with pramlintide. The improvement in synaptic function could be related to the reversal of age-related decline in memory observed in the SAMP8 mouse. Although direct changes in the insulin signaling pathway were not investigated in this study, previous studies have shown CNS-specific increases in AKT phosphorylation with amylin treatment (Moon et al., 2012).
Aβ protein precursor (AβPP) levels are increased in aged SAMP8 mice (Morley et al., 2000). 12-month old SAMP8 mice were treated with 3 doses of 6 μg oligonucleotide (random or AβPP antisense) via tail vein injection spaced 2 weeks apart (Armbrecht et al., 2015). The antisense crosses the BBB (Banks et al., 2001) and this treatment paradigm has been shown to decrease AβPP and Aβ levels (Erickson et al., 2012). Memory, as measured in the T maze, was improved with antisense treatment. Antisense treatment changed expression of 944 genes in key pathways including phosphatidylinositol signaling and MAPK signaling. Changes in these pathways contributed to changes in neurotropin signaling, insulin signaling and ErbB signaling. There were 20 genes specifically changed in the insulin signaling pathway with antisense treatment. Therefore, it is possible that alterations in genes involved in insulin signaling contribute to the memory improvements observed in the aged SAMP8 mouse.
Caloric restriction mimetics and inhibitors of α-glucosidase have been investigated as ways to delay aging. Acarbose is one such mimetic and helps control blood glucose levels by slowing the metabolism of sugars. Long-term administration of acarbose delays age-associated diseases and can reverse brain aging. Three-month old SAMP8 mice received 20 mg/kg/day dissolved in drinking water until 9 months of age (Tong et al., 2015; Yan et al., 2015). Serum insulin was decreased in aged SAMP8 mice while acarbose prevented this decrease and improved memory, sensorimotor skills, and anxiety as assessed by multiple behavior tests. Acarbose increased levels of insulin receptors in the hippocampus as well as histone H4 lysine 8 (H4K8) acetylation levels. Acetylation of H4K8 can be modulated by IGF-1 (Sun and D'Ercole 2006), regulates brain-derived neurotrophic factor (BDNF) expression, and enhances learning and memory (Intlekofer et al., 2013). Aged SAMP8 mice had decreased BDNF levels in the hippocampus, which correlated with poor performance on the Morris water maze. Acarbose reversed the age-related decline in BDNF. These results show acarbose can alleviate the declines in insulin signaling, preserving memory by maintaining BDNF levels and acetylation of H4K8.
The effects of another inhibitor of α-glucosidase, 1-deoxynojirimycin (DNJ), was investigated by the same group in aged SAMP8 mice (10 or 20 mg/kg/day) from 3-9 months of age (Chen et al., 2015). Similar results were observed in that chronic DNJ treatment alleviated age-related changes in the SAMP8 mice including the decreased level in serum insulin and hippocampal levels of the insulin receptor, BDNF, and H4K8 acetylation and the increased level of astrocyte activation. Taken together, the acarbose and DNJ studies suggests an important role for α-glucosidase in aging and dysregulation of insulin signaling.
These studies characterize important changes taking place in the brain of aged SAMP8 mice (Table 2) and support the idea that maintaining CNS insulin signaling can lead to improved memory and in one instance, increase lifespan. Importantly, none of these studies investigated direct administration of the agents to the CNS, but rather relied on transport to the brain or peripheral administration of the compounds. Whether the beneficial effect of these treatments is due to alterations in insulin signaling or an independent mechanism of insulin remains to be determined.
Table 2.
Summary of changes observed in aged SAMP8 mice relating to insulin signaling and memory in alphabetical order. Most changes in aged SAMP8 mice were ameliorated with respective treatments listed in Table 1.
| Marker | Direction | Citation |
|---|---|---|
| Acetylcholine esterase activity | ⇧ | Lin 2013 |
| Astrocyte Activation | ⇧ | Tong 2015, Chen 2015 |
| Aβl-42 | ⇧ | Lin 2013, Kuang 2014 |
| AβPP | ⇧ | Armbrecht 2015 |
| BBB | ⇔ | Banks 2000 |
| BDNF | ⇩ | Tong 2015, Chen 2015, Lin 2013 |
| H4K8 acetylation | ⇩ | Tong 2015, Chen 2015, Yan 2015 |
| Hippocampal Insulin Receptor | ⇩ | Tong 2015, Chen 2015, Yan 2015 |
| IDE | ⇩ | Lin 2013 |
| Insulin Transport across BBB | ⇔ | Banks 2000 |
| Klotho | ⇩ | Kuang 2014, Zhou 2015 |
| Neuronal Loss | ⇧ | Kuang 2014 |
| Oxidative Stress | ⇧ | Lin 2013, Kuang 2014, Adler 2014 |
| Senescent Cells | ⇧ | Kuang2014 |
| Serum Insulin | ⇩ | Tong 2015, Chen 2015, Yan 2015 |
| Tau Phosphorylation | ⇧ | Kuang 2014 |
4. Intranasal Delivery
Intranasal delivery was popularized by William H. Frey in the early 1990's as a noninvasive route to bypass the BBB and deliver substrates directly to the brain (Schioth et al., 2012). Because blood levels of the therapeutic are low, side effects mediated by peripheral tissues are reduced. The central versus peripheral effects of insulin on the effect of memory and insulin signaling in the CNS have been parsed out using the intracerebroventricular (ICV) delivery method (Schioth et al., 2012). However, ICV delivery is not a practical, translatable route of delivery of insulin to the brain.
Intranasal delivery of peptides and proteins is proving to be a viable alternative to increase brain levels of therapeutic compounds. After intranasal administration to the level of the cribriform plate, substrates can enter the CNS via olfactory and trigeminal neural pathways (Hanson and Frey 2008). Concentrations in brain of delivered substrates after intranasal administration are often similar to those delivered intravenously. For example, approximately 0.05% Inj/g-brain of the insulin dose delivered intravenously reaches the brain (Banks et al., 1997), whereas after intranasal delivery, approximately 0.1% Inj/g-brain of the insulin dose is delivered to the brain (Salameh et al., 2015). However, the amount of the compound in blood after intranasal administration is often only a fraction of that seen after intravenous administration, thus reducing peripheral tissue exposure. Degradation that can occur in the circulation or in the brain after intranasal delivery can vary for each peptide, protein, or substrate of interest.
Intranasal delivery of various proteins and peptides including glucagon-like peptide 1, leptin, pituitary adenylate cyclase-activating peptide, and insulin have been shown to have beneficial effects on memory (Meredith et al., 2015). Some of these treatments, including intranasal insulin, are currently being investigated in ongoing clinical studies investigating enhancement of memory.
5. Intranasal Insulin Delivery
Intranasal insulin administration is an effective therapeutic treatment for improving memory. Not only does it improve memory in populations that have decreased insulin signaling to begin with, such as patients with AD or mild cognitive impairment (Craft et al., 2012; Reger et al., 2008), but memory is also enhanced in healthy, young populations (Benedict et al., 2004). Improvements in people with AD are observed as early as 15 minutes after the first treatment (Reger et al., 2008) and continue after chronic, daily treatment for up to 4 months (Craft et al., 2012). The Study of Nasal Insulin to Fight Forgetfulness (SNIFF) is a clinical trial currently being conducted in the United States to more fully investigate the long-term benefit of intranasal insulin.
In order to determine how intranasal insulin might enhance memory, the SAMP8 model of age-related memory impairment has been used to investigate memory and biochemical changes taking place after administration. Indeed, memory was improved 24 hours after a single or repetitive 1μg treatment in aged SAMP8 mice (Salameh et al., 2015). In a young, healthy CD-1 mouse cohort, intranasal insulin had no effect on peripheral metabolism or blood glucose levels. Within 5 minutes after administration, insulin was detected throughout the brain with levels being greatest in the hypothalamus > olfactory bulb > hippocampus > cerebellum > cortex (Figure 1). These studies showing similar memory enhancing effects in the SAMP8 mice as observed in the clinical studies argue for continuation in investigation of pathways altered in the brains of SAMP8 mice after intranasal insulin administration.
Figure 1.
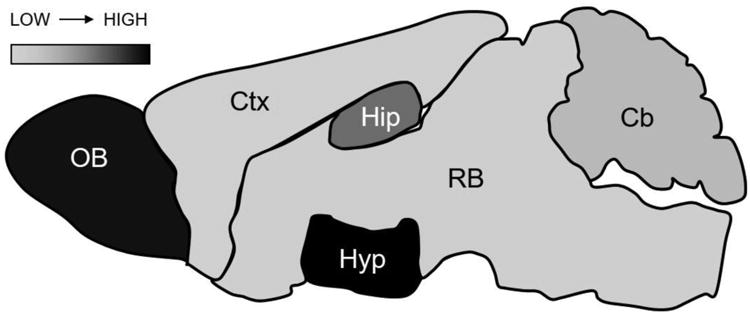
Distribution pattern of insulin after intranasal delivery. Based on previous findings (Salameh et al., 2015), the distribution of insulin after intranasal delivery in young male CD-1 mice differs quite dramatically throughout the brain. Coloring is weighted based on the amount of %Inj/g insulin present in each region. Hyp- hypothalamus > OB- olfactory bulb > Hip- hippocampus > Cb- cerebellum > RB-remaining brain > Ctx- cortex
6. Conclusions
The studies highlighted here using the SAMP8 mouse support the involvement of the insulin signaling pathway in the improvement of memory. Dysregulation of brain insulin signaling is detrimental to cognition and can increase the risk of age-related disorders (Gerozissis 2008; Ghasemi et al., 2013). The klotho studies show that inhibiting the insulin pathway in the CNS leads to an increased lifespan and improved cognition, while the majority of the other treatments presented here support increasing brain insulin levels to enhance memory. Therefore, it appears there is a hormetic effect of insulin in the brain where too much insulin signaling can lead to overactivation and advanced aging, but too little insulin signaling impairs cognition. Maintaining a proper balance of CNS insulin signaling with aging seems crucial in preserving memory.
Indeed, using the intranasal delivery route in the SAMP8 mice for the compounds and peptides summarized in this review could better target the central effects of insulin signaling. In addition, the SAMP8 model could prove useful for studying the anti-aging effects of many compounds currently being investigated in the periphery that have also been shown to enhance memory including rapamycin (Halloran et al., 2012), resveratrol (Porquet et al., 2013), and alpha-lipoic acid (Farr et al., 2003).
Highlights.
SAMP8 mice are a useful model for investigating memory changes due to aging.
SAMP8 mice are a useful model for investigating changes in brain insulin signaling.
SAMP8 mice could be useful for investigating effects of intranasal insulin.
Acknowledgments
This work was supported by the NIH National Institute on Aging T32- AG000057 (EMR) and RO1-AG046619 (WAB). This article results from work supported by resources from the Veterans Affairs Puget Sound Health Care System, Seattle, Washington.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler BL, Yarchoan M, Hwang HM, Louneva N, Blair JA, Palm R, Smith MA, Lee HG, Arnold SE, Casadesus G. Neuroprotective effects of the amylin analogue pramlintide on Alzheimer's disease pathogenesis and cognition. Neurobiology of aging. 2014;35:793–801. doi: 10.1016/j.neurobiolaging.2013.10.076. [DOI] [PubMed] [Google Scholar]
- Akintola AA, van Heemst D. Insulin, aging, and the brain: mechanisms and implications. Front Endocrinol (Lausanne) 2015;6:13. doi: 10.3389/fendo.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbrecht HJ, Siddiqui AM, Green M, Farr SA, Kumar VB, Banks WA, Patrick P, Shah GN, Morley JE. Antisense against Amyloid-beta Protein Precursor Reverses Memory Deficits and Alters Gene Expression in Neurotropic and Insulin-Signaling Pathways in SAMP8 Mice. J Alzheimers Dis. 2015;46:535–548. doi: 10.3233/JAD-142760. [DOI] [PubMed] [Google Scholar]
- Banks WA, Farr SA, Butt W, Kumar VB, Franko MW, Morley JE. Delivery across the blood-brain barrier of antisense directed against amyloid beta: reversal of learning and memory deficits in mice overexpressing amyloid precursor protein. J Pharmacol Exp Ther. 2001;297:1113–1121. [PubMed] [Google Scholar]
- Banks WA, Farr SA, Morley JE. Permeability of the blood-brain barrier to albumin and insulin in the young and aged SAMP8 mouse. J Gerontol A Biol Sci Med Sci. 2000;55:B601–606. doi: 10.1093/gerona/55.12.b601. [DOI] [PubMed] [Google Scholar]
- Banks WA, Jaspan JB, Huang W, Kastin AJ. Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides. 1997;18:1423–1429. doi: 10.1016/s0196-9781(97)00231-3. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides. 1998;19:883–889. doi: 10.1016/s0196-9781(98)00018-7. [DOI] [PubMed] [Google Scholar]
- Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacology & therapeutics. 2012;136:82–93. doi: 10.1016/j.pharmthera.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri M, Gambardella A, Paolisso G, Varricchio M. Metabolic aspects of the extreme longevity. Exp Gerontol. 2008;43:74–78. doi: 10.1016/j.exger.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Chen GH, Tong JJ, Wang F, Hu XQ, Li XW, Tao F, Wei ZJ. Chronic adjunction of 1-deoxynojirimycin protects from age-related behavioral and biochemical changes in the SAMP8 mice. Age (Dordr) 2015;37:102. doi: 10.1007/s11357-015-9839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71:621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Archives of neurology. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Niehoff ML, Farr SA, Morley JE, Dillman LA, Lynch KM, Banks WA. Peripheral administration of antisense oligonucleotides targeting the amyloid-beta protein precursor reverses AbetaPP and LRP-1 overexpression in the aged SAMP8 mouse brain. J Alzheimers Dis. 2012;28:951–960. doi: 10.3233/JAD-2011-111517. [DOI] [PubMed] [Google Scholar]
- Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, Butterfield DA, Morley JE. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. Journal of neurochemistry. 2003;84:1173–1183. doi: 10.1046/j.1471-4159.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE. Learning and memory in the SAMP8 mouse. Neuroscience and biobehavioral reviews. 1998;22:1–20. doi: 10.1016/s0149-7634(96)00063-2. [DOI] [PubMed] [Google Scholar]
- Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Turk A, Hoyer S, Zochling R, Boissl KW, Jellinger K, Riederer P. Brain insulin and insulin receptors in aging and sporadic Alzheimer's disease. J Neural Transm (Vienna) 1998;105:423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- Gerozissis K. Brain insulin, energy and glucose homeostasis; genes, environment and metabolic pathologies. Eur J Pharmacol. 2008;585:38–49. doi: 10.1016/j.ejphar.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Ghasemi R, Dargahi L, Haeri A, Moosavi M, Mohamed Z, Ahmadiani A. Brain insulin dysregulation: implication for neurological and neuropsychiatric disorders. Molecular neurobiology. 2013;47:1045–1065. doi: 10.1007/s12035-013-8404-z. [DOI] [PubMed] [Google Scholar]
- Halloran J, Hussong SA, Burbank R, Podlutskaya N, Fischer KE, Sloane LB, Austad SN, Strong R, Richardson A, Hart MJ, Galvan V. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience. 2012;223:102–113. doi: 10.1016/j.neuroscience.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson LR, Frey WH. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;9(3):S5. doi: 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins DF, Williams G. Insulin receptors are widely distributed in human brain and bind human and porcine insulin with equal affinity. Diabet Med. 1997;14:1044–1050. doi: 10.1002/(SICI)1096-9136(199712)14:12<1044::AID-DIA508>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Intlekofer KA, Berchtold NC, Malvaez M, Carlos AJ, McQuown SC, Cunningham MJ, Wood MA, Cotman CW. Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology. 2013;38:2027–2034. doi: 10.1038/npp.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kuang X, Chen YS, Wang LF, Li YJ, Liu K, Zhang MX, Li LJ, Chen C, He Q, Wang Y, Du JR. Klotho upregulation contributes to the neuroprotection of ligustilide in an Alzheimer's disease mouse model. Neurobiology of aging. 2014;35:169–178. doi: 10.1016/j.neurobiolaging.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Kuro-o M. Klotho and the aging process. Korean J Intern Med. 2011;26:113–122. doi: 10.3904/kjim.2011.26.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Science. Vol. 309. New York, NY: 2005. Suppression of aging in mice by the hormone Klotho; pp. 1829–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Huang R, Zhang S, Wei L, Zhuo L, Wu X, Tang A, Huang Q. Beneficial effects of asiaticoside on cognitive deficits in senescence-accelerated mice. Fitoterapia. 2013;87:69–77. doi: 10.1016/j.fitote.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Marks JL, Porte D, Jr, Stahl WL, Baskin DG. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology. 1990;127:3234–3236. doi: 10.1210/endo-127-6-3234. [DOI] [PubMed] [Google Scholar]
- Martins R, Lithgow GJ, Link W. Long live FOXO: unraveling the role of FOXO proteins in aging and longevity. Aging Cell. 2016;15:196–207. doi: 10.1111/acel.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith ME, Salameh TS, Banks WA. Intranasal Delivery of Proteins and Peptides in the Treatment of Neurodegenerative Diseases. The AAPS journal. 2015;17:780–787. doi: 10.1208/s12248-015-9719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M, Kiyota Y, Yamazaki N, Nagaoka A, Matsuo T, Nagawa Y, Takeda T. Age-related changes in learning and memory in the senescence-accelerated mouse (SAM) Physiology & behavior. 1986;38:399–406. doi: 10.1016/0031-9384(86)90112-5. [DOI] [PubMed] [Google Scholar]
- Moon HS, Chamberland JP, Diakopoulos KN, Fiorenza CG, Ziemke F, Schneider B, Mantzoros CS. Leptin and amylin act in an additive manner to activate overlapping signaling pathways in peripheral tissues: in vitro and ex vivo studies in humans. Diabetes Care. 2011;34:132–138. doi: 10.2337/dc10-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HS, Chamberland JP, Mantzoros CS. Amylin and leptin activate overlapping signalling pathways in an additive manner in mouse GT1-7 hypothalamic, C(2)C(1)(2) muscle and AML12 liver cell lines. Diabetologia. 2012;55:215–225. doi: 10.1007/s00125-011-2332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Armbrecht HJ, Farr SA, Kumar VB. The senescence accelerated mouse (SAMP8) as a model for oxidative stress and Alzheimer's disease. Biochimica et biophysica acta. 2012a;1822:650–656. doi: 10.1016/j.bbadis.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Morley JE, Farr SA, Kumar VB, Armbrecht HJ. The SAMP8 mouse: a model to develop therapeutic interventions for Alzheimer's disease. Curr Pharm Des. 2012b;18:1123–1130. doi: 10.2174/138161212799315795. [DOI] [PubMed] [Google Scholar]
- Morley JE, Kumar VB, Bernardo AE, Farr SA, Uezu K, Tumosa N, Flood JF. Beta-amyloid precursor polypeptide in SAMP8 mice affects learning and memory. Peptides. 2000;21:1761–1767. doi: 10.1016/s0196-9781(00)00342-9. [DOI] [PubMed] [Google Scholar]
- Nagai T, Yamada K, Kim HC, Kim YS, Noda Y, Imura A, Nabeshima Y, Nabeshima T. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J. 2003;17:50–52. doi: 10.1096/fj.02-0448fje. [DOI] [PubMed] [Google Scholar]
- Porquet D, Casadesus G, Bayod S, Vicente A, Canudas AM, Vilaplana J, Pelegri C, Sanfeliu C, Camins A, Pallas M, del Valle J. Dietary resveratrol prevents Alzheimer's markers and increases life span in SAMP8. Age (Dordr) 2013;35:1851–1865. doi: 10.1007/s11357-012-9489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, Fishel MA, Plymate SR, Cherrier MM, Schellenberg GD, Frey WH, 2nd, Craft S. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis. 2008;13:323–331. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salameh TS, Bullock KM, Hujoel IA, Niehoff ML, Wolden-Hanson T, Kim J, Morley JE, Farr SA, Banks WA. Central Nervous System Delivery of Intranasal Insulin: Mechanisms of Uptake and Effects on Cognition. J Alzheimers Dis. 2015;47:715–728. doi: 10.3233/JAD-150307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schioth HB, Craft S, Brooks SJ, Frey WH, 2nd, Benedict C. Brain insulin signaling and Alzheimer's disease: current evidence and future directions. Molecular neurobiology. 2012;46:4–10. doi: 10.1007/s12035-011-8229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LY, D'Ercole AJ. Insulin-like growth factor-I stimulates histone H3 and H4 acetylation in the brain in vivo. Endocrinology. 2006;147:5480–5490. doi: 10.1210/en.2006-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T, Hosokawa M, Takeshita S, Irino M, Higuchi K, Matsushita T, Tomita Y, Yasuhira K, Hamamoto H, Shimizu K, Ishii M, Yamamuro T. A new murine model of accelerated senescence. Mech Ageing Dev. 1981;17:183–194. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. The Journal of clinical investigation. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomobe K, Shinozuka T, Kawashima T, Kawashima-Ohya Y, Nomura Y. Age-related changes of forkhead transcription factor FOXO1 in the liver of senescence-accelerated mouse SAMP8. Arch Gerontol Geriatr. 2013;57:417–422. doi: 10.1016/j.archger.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Tong JJ, Chen GH, Wang F, Li XW, Cao L, Sui X, Tao F, Yan WW, Wei ZJ. Chronic acarbose treatment alleviates age-related behavioral and biochemical changes in SAMP8 mice. Behavioural brain research. 2015;284:138–152. doi: 10.1016/j.bbr.2015.01.052. [DOI] [PubMed] [Google Scholar]
- Tresguerres JA, Cuesta S, Kireev RA, Garcia C, Acuna-Castroviejo D, Vara E. Beneficial effect of melatonin treatment on age-related insulin resistance and on the development of type 2 diabetes. Horm Mol Biol Clin Investig. 2013;16:47–54. doi: 10.1515/hmbci-2013-0041. [DOI] [PubMed] [Google Scholar]
- Yan WW, Chen GH, Wang F, Tong JJ, Tao F. Long-term acarbose administration alleviating the impairment of spatial learning and memory in the SAMP8 mice was associated with alleviated reduction of insulin system and acetylated H4K8. Brain Res. 2015;1603:22–31. doi: 10.1016/j.brainres.2015.01.042. [DOI] [PubMed] [Google Scholar]
- Zhou X, Yang Q, Xie Y, Sun J, Hu J, Qiu P, Cao W, Wang S. Tetrahydroxystilbene glucoside extends mouse life span via upregulating neural klotho and downregulating neural insulin or insulin-like growth factor 1. Neurobiology of aging. 2015;36:1462–1470. doi: 10.1016/j.neurobiolaging.2014.11.002. [DOI] [PubMed] [Google Scholar]


