Sirs
Defective lacrimation is a cardinal feature of familial dysautonomia (FD), an autosomal recessive disease caused by a founder mutation in the IKBKAP gene, first described in 1949 by Riley and Day.1 Patients have reduced secretion of basal, reflex, and emotional tears. Defective lacrimation and decreased corneal sensitivity results in neurotrophic keratopathy with opacities causing visual loss in 27 % of patients. There are no effective treatments. 2
The reason for alacrima in FD is unclear. Normally, lacrimation is mediated by activation of efferent parasympathetic fibers originating in the superior salivary nucleus of the pons travelling with the facial nerve and the greater superficial petrosal nerve to the sphenopalatine ganglion to enter the lacrimal gland via the superior branch of the zygomatic nerve. Enhancing parasympathetic activity with either acetylcholinesterase inhibition or muscarinic agonists in patients with FD results in tear secretion3. This suggests that lacrimal glands are functional with preserved efferent parasympathetic innervation. Thus, we postulated that the lacrimal abnormalities of patients with FD might be due to a selective impairment of the afferent neurons of the tear reflex.
To test this hypothesis, we examined the relationship between corneal sensory thresholds and basal tear production. We hypothesized that, as the defect was mostly in the afferent/sensory pathway, the lacrimal glands could be stimulated directly. We then conducted a single-blind placebo-controlled clinical trial to test whether topical administration of the M3 muscarinic agonist pilocarpine (4%) could stimulate tear production. The Institutional Review Board of the New York University Langone Medical Center approved this study. All patients signed consent form.
We quantified corneal sensitivity using a Cochet-Bonnet esthesiometer. Variable pressure was applied in sequential steps by adjusting the nylon monofilament length from 60-mm to 5-mm to apply pressures from 11 mm/gm to 200 mm/gm to the corneal surface. Results were measured as the minimal length needed until the patient can feel its contact. Measurements were obtained at the four corneal quadrant of each eye and averaged. Sensitivity was graded from from 0 (no sensitivity) to 6 (preserved sensitivity) based on the length of the monofilament.
Proparacaine (0.5%, 2 drops) was administered to anesthetize the corneal surface and prevent reflex tearing. Basal lacrimation was measured with the Schirmer paper-strip test (placed at the outer third lower eyelid conjunctiva for 5 minutes) at baseline, 30 minutes after topical administration of placebo (saline 0.9%, 2 drops), 30 minutes after pilocarpine (saline 4%, 2 drops), and 3 hours after pilocarpine. Values < 15 mm were considered abnormal.
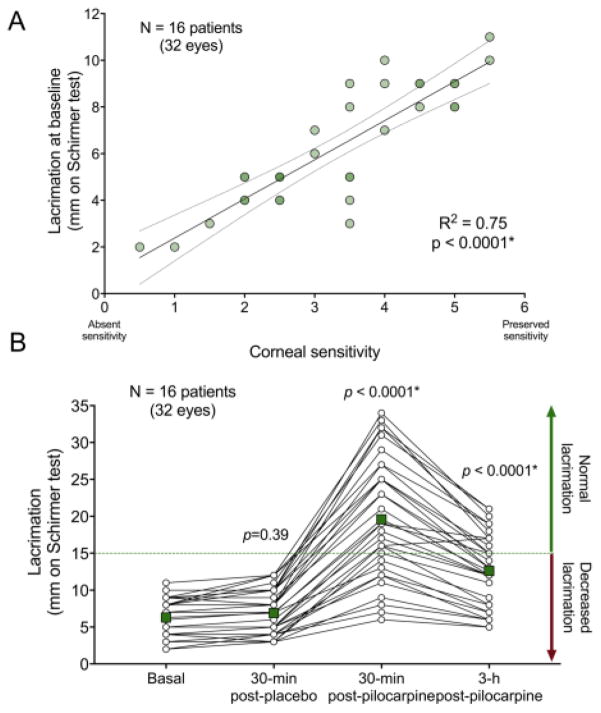
Sixteen patients completed the protocol [11 women, 5 men, 32 eyes; mean age (± standard deviation): 27±8 years]. Mean corneal sensitivity was 3.3±1.3, ranging from 0.5 (almost complete lack of corneal sensitivity) to 5.5 (very mild reduction). Basal tear secretion was 6.3±2.6 mm. Corneal sensitivity was directly related to basal tear secretion (R2=0.75; p<0.0001) (Figure).
Figure.
A. Linear regression showing increasing baseline tear secretion with more preserved corneal sensitivity (R2=0.75; p<0.0001) suggesting that the lack of basal lacrimation in familial dysautonomia is related to the degree of impairment of sensory (afferent) corneal inputs. B. Tear secretion before and after topical eye administration of pilocarpine in familial dysautonomia. Lacrimation was measured with the Schirmer test on four occasions: (1) at baseline, (2) 30-minutes after instillation of placebo, (3) 30-minutes, and (4) 3-hours after pilocarpine instillation. Tear secretion more than tripled after 30-minutes after instillation of piloparpine 4% to the eye (ANOVA with Tukey post-hoc comparisons p<0.0001 versus baseline lacrimation), which persisted after 3 hours (ANOVA with Tukey post-hoc comparisons p<0.0001 vs. baseline lacrimation). Square points represent the average of the 32 eyes at each measurement.
Tear secretion remained stable 30-min after placebo (6.9±3.0 mm; ANOVA and Tukey post-hoc comparison p=0.395 vs. baseline tear secretion). Thirty minutes after topical instillation of pilocarpine, tear secretion increased in 311% to 19.6±8.3 mm (ANOVA and Tukey post-hoc comparison p<0.0001 vs. baseline tear secretion). Three hours post-dose, tear production remained elevated above baseline (12.6±5.1 mm, ANOVA and Tukey post-hoc comparison p<0.0001 vs. baseline tear secretion). Side effects included transient blurred vision for distance (75%) and mild frontal headache (19%).
These results show that patients with FD have functional lacrimal glands, which can be stimulated with topical cholinergic agonists, and that afferent pathways from the cornea regulate, at least in part, basal tear production. Patients with the highest tear production after pilocarpine instillation were the ones with less impaired baseline tear secretion. Therefore, tear secretion, both at baseline and after pilocarpine instillation, appear to be related to corneal sensation. Lack of corneal afferent information explains why these patients develop neurotrophic keratopathy and lack the protective reflex tearing, resulting in frequent injuries and poor healing.
Our findings do not explain the lack of emotional tears, which are the most surprising as responses to emotional stimuli are markedly exaggerated in FD patients.4 Given that the lacrimal glands and the efferent parasympathetic innervation appear preserved, supranuclear pathways may be abnormal. In line with this, von Economo neurons of the insular cortex, important in emotional regulation, are markedly reduced in patients with FD.5
The ability to effectively stimulate tear secretion from the lacrimal gland for several hours with a topical direct-acting muscarinic cholinergic agonist is an important therapeutic development for these patients.
Acknowledgments
Funding: Dysautonomia Foundation and National Institutes of Health (U54NS065736).
Footnotes
Conflict of interest: All authors declare no conflict of interests.
References
- 1.Riley CM, Day RL, Greeley DM, Langford WS. Central autonomic dysfunction with defective lacrimation; report of five cases. Pediatrics. 1949;3(4):468–478. [PubMed] [Google Scholar]
- 2.Mendoza-Santiesteban CE, Hedges TR, 3rd, Norcliffe-Kaufmann L, et al. Clinical neuro-ophthalmic findings in familial dysautonomia. Journal of neuro-ophthalmology: the official journal of the North American Neuro-Ophthalmology Society. 2012;32(1):23–26. doi: 10.1097/WNO.0b013e318230feab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keith CG. Riley-Day Syndrome (congenital familial dysautonomia) The British journal of ophthalmology. 1965;49(12):667–672. doi: 10.1136/bjo.49.12.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norcliffe-Kaufmann L, Axelrod F, Kaufmann H. Afferent baroreflex failure in familial dysautonomia. Neurology. 2010;75(21):1904–1911. doi: 10.1212/WNL.0b013e3181feb283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos M, Uppal N, Butti C, et al. Frontoinsular cortex in familial dysautonomia: a clinicopathologic exploration of the role of von Economo neurons in interoception. 2011 International Familial Dysautonomia Research Conference; 2011; New York, NY. [Google Scholar]