Significance
Angiogenesis, the formation of new blood vessels from existing ones, is essential for embryonic development and necessary for tumor growth and invasion. The Notch signaling pathway regulates angiogenesis where the two Notch ligands, Dll4 and Jagged 1, exert opposite functions on sprouting. We found that the intermediate filament vimentin balances angiogenesis by binding specifically to the proangiogenic Jagged ligands. This binding provides a force-generating mechanism on the Jagged–Notch complex to ensure efficient transendocytosis and Notch activation. The interaction between vimentin and Jagged constitutes a mechanism behind selective regulation of Notch ligands during angiogenesis. The interaction may be amendable by therapeutic intervention and can facilitate strategies targeting a variety of diseases related to Jagged deregulation.
Keywords: vimentin, Notch, angiogenesis, Jagged
Abstract
Notch signaling is a key regulator of angiogenesis, in which sprouting is regulated by an equilibrium between inhibitory Dll4-Notch signaling and promoting Jagged-Notch signaling. Whereas Fringe proteins modify Notch receptors and strengthen their activation by Dll4 ligands, other mechanisms balancing Jagged and Dll4 signaling are yet to be described. The intermediate filament protein vimentin, which has been previously shown to affect vascular integrity and regenerative signaling, is here shown to regulate ligand-specific Notch signaling. Vimentin interacts with Jagged, impedes basal recycling endocytosis of ligands, but is required for efficient receptor ligand transendocytosis and Notch activation upon receptor binding. Analyses of Notch signal activation by using chimeric ligands with swapped intracellular domains (ICDs), demonstrated that the Jagged ICD binds to vimentin and contributes to signaling strength. Vimentin also suppresses expression of Fringe proteins, whereas depletion of vimentin enhances Fringe levels to promote Dll4 signaling. In line with these data, the vasculature in vimentin knockout (VimKO) embryos and placental tissue is underdeveloped with reduced branching. Disrupted angiogenesis in aortic rings from VimKO mice and in endothelial 3D sprouting assays can be rescued by reactivating Notch signaling by recombinant Jagged ligands. Taken together, we reveal a function of vimentin and demonstrate that vimentin regulates Notch ligand signaling activities during angiogenesis.
The intermediate filament (IF) proteins are a large protein family with tissue- and developmental stage-specific expression. Whereas IFs provide cells with mechanical stability, there is mounting evidence indicating that IFs are involved in a range of metabolic, signaling, and regulatory processes that are unrelated to mechanical functions (1–14). The vimentin IF has been shown to act as a scaffold for signaling proteins that regulate epithelial mesenchymal transition (EMT), cancer cell invasion, wound healing and tissue repair, tissue aging, as well as inflammatory signaling (3, 4, 6–8, 10). Vimentin anchors and organizes adhesion molecules as well as actomyosin complexes (15) to regulate cell adhesion and migration (4, 5). Vimentin also influences protein function through regulation of protein trafficking and is involved in the regulation of gene expression (7, 8, 10, 16–18). Vimentin, thus, has regulatory functions especially in dynamic cellular processes. Recently, vimentin has been linked to angiogenesis and vascular homeostasis, and lack of vimentin is associated with defects in vascular tuning, endothelial migration, adhesion, and sprouting, as well as flow-induced arterial remodeling (1, 2). Whereas the above-listed studies determine that vimentin-deficiency could compromise vascular integrity, it is unclear whether there is direct causal relationship between vimentin and vascular development, and molecular links to signaling pathways that regulate angiogenesis have not been identified.
The Notch signaling pathway is a key regulator of angiogenesis. Genetic removal of Notch components results in disorganized and nonfunctional tissue and embryonic lethality (19–25). Deregulation of ligands or alterations in Notch activity is associated with pathological angiogenesis and disturbances in vascular remodelling (19–21, 26–30). The Notch ligands Dll4 and Jagged 1 have opposing roles during angiogenesis (19, 25). Dll4 and Jagged 1 signaling regulates tip cell versus stalk cell selection in the branching endothelium, and the creation of new branching points (22, 31). VEGF-VEGFR2 signaling is a key driver of angiogenesis and induces Dll4 expression in the tip cell (32). Dll4 activation of Notch in neighboring cells reduces expression of VEGFR2 and inhibits tip cell selection. In contrast to Dll4, Jagged-Notch signaling promotes tip cell selection and sprouting by antagonizing Dll4-Notch signaling (31, 33–36). How the competition between the ligands is balanced and how ligand-receptor signaling specificity is achieved is under intense investigation (31, 33, 34, 37, 38). Modification of the Notch receptor by the Fringe family of glycosaminyltransferases has been shown to enhance Dll4-Notch signaling and to suppress sprouting (31). However, other mechanisms that balance the competition between Jagged and Dll4 signaling are still to be elucidated.
Notch signaling is elicited by ligand binding to the Notch receptor on neighboring cells, which leads to proteolytic processing of the receptor and release of the Notch intracellular domain (NICD), which translocates to the nucleus and regulates transcription of target genes (37, 39, 40). Notch signaling is critically regulated by protein trafficking (41–43). In addition to controlling number of ligands and receptors on the cell surface, ligand endocytosis is required for receptor activation (41). Two separate models for the requirement of endocytosis have been suggested: the first model suggests that recycling is important for maturation of the ligands to become active, whereas according to the second model, transendocytosis of the extracellular domain of the Notch receptor (NECD) is needed to produce a strain on the receptor. This strain is termed the pulling force (41, 42, 44, 45) and is thought to reveal the cleavage site and initiate proteolytic processing of the receptor with subsequent release of the NICD.
Here we demonstrate a previously unexplored link between vimentin and Notch signaling in the vasculature. We show that vimentin not only interacts with Jagged, but also regulates Jagged-mediated receptor transendocytosis, expression of Fringe genes, and ligand-specific Notch activation. Our data suggest that vimentin is important for balancing Jagged and Dll4 signaling and in the absence of vimentin, Dll4-Notch signaling dominates to suppress angiogenesis.
Methods
For cell culture, quantitative PCR (qPCR), Western blotting, immunofluorescence, proximity ligation assay (PLA), Notch activity, and Jagged trafficking assays, please see SI Methods. Animal experiments were approved by and carried out in accordance with the National Animal Experiment Board of Finland and conformed to the regulations set by The Finnish Act on Animal Experimentation.
Aortic Ring Assay.
Eight-week-old vimentin wild-type, heterozygous, or null Sv129/Pas mice were killed and aortae harvested. Rings were embedded into 1.5 mg/mL collagen type I and once the collagen had polymerized, fed with Opti-MEM containing 2.5% FBS and 40 ng/mL VEGF. Aortic ring assays were incubated at 37 °C with 5% CO2, and after 4 d, media were replaced. After 6 total days of incubation, aortic ring assays were fixed with 4% paraformaldehyde in PBS for 20 min and rinsed two times with Tris-glycine.
Vimentin Null Aortic Rings with Notch Ligands.
A total of 1 μg of Recombinant Delta-like ligand 1 (Dll1) (R&D Systems, 5026-DL-050), Delta-like ligand 4 (Dll4) (R&D Systems, 1506-D4-050), Jagged (R&D Systems, 599-JG-100), or IgG Fc control (Jackson Immunoresearch, 009–000-008) were coupled to 10 μL Protein A agarose beads (Roche, 11719408001), diluted in PBS to give a final volume of 100 μL. The bead–ligand mixtures were gently rotated for 30 min at room temperature (RT), and the entire 100 μL mixture was added to 900 μL collagen type I, on ice, for a final collagen concentration of 2 mg/mL. Aortae were harvested from vimentin null mice, as described above. A total of 70 μL of the collagen–bead mixture was added per well of a full area 96-well plate, three wells at a time, and 0.5-mm aortic rings were oriented so the lumen was visible. After the collagen polymerized, aortic rings were fed with 150 μL Opti-MEM containing 2.5% FBS and 40 ng/mL VEGF and incubated at 37 °C with 5% CO2. After 4 d of incubation, the media were replaced. Rings were fixed after 7 d with 4% paraformaldehyde in PBS and rinsed twice with Tris-glycine.
Quantification of Aortic Ring Sprouting Responses.
To quantify endothelial sprouting responses, four images were taken of sprouting structures originating from the upper edge of the ring using an Olympus CKX41 inverted microscope equipped with a Q color 3 Olympus camera. Average sprout length was quantified using ImagePro Analyzer software by measuring the distance from the edge of the ring to the tip of invading multicellular structures. The average number of endothelial sprouts per ring was quantified from the four images. Error bars represent SEM.
Immunostaining of Aortic Rings.
Aortic rings stored in Tris-glycine were permeabilized for 30 min with 0.5% Triton X-100 in PBS, then blocked overnight at 4 °C with blocking buffer (0.1% Triton X-100, 1% BSA, and 1% goat serum in TBS). Rings were immunostained using primary antibodies directed against PECAM-1 (Santa Cruz, sc-1505R), VE-cadherin (Enzo Life Sciences, ALX-210-232-C100), or rabbit IgG as a control (Invitrogen, 02-6102), followed by incubation with Alexa-594 or Alexa-488 conjugated secondary antibodies (Invitrogen, A11012 and A11008) and FITC-conjugated phalloidin (Invitrogen, A12379) or cy3-conjugated alpha smooth muscle actin (Sigma, C6198), respectively. Cell nuclei were stained with 1.09 μM DAPI (Invitrogen, D1306). Z stacks of 1 µm step-size compressed images of aortic rings were taken using a Nikon TI A1R inverted confocal microscope at 40× magnification. (Scale bar, 100 μm.)
Receptor Binding and Ligand Transendocytosis.
Cells were plated on 12-well plates, or coverslips for immunofluorescence, a few days in advance. rN1ECD (R&D Systems, cat. no. 1057-TK) (1 µg/mL) and Alexa-Fluor 488 goat anti-human IgG (Invitrogen, cat. no. A11013) (1:200) were diluted in sterile PBS and incubated on rotation in +4 °C for 1 h. Simultaneously, the cells were blocked with DMEM containing 10% goat serum and 1% BSA for 45 min in +37 °C. The rN1ECD–Alexa-488 solution was further diluted in blocking solution at a ratio of 1:5 and this solution was added to the cells, followed by incubation in 37 °C for 2 h. The cells were then detached, centrifuged (450 × g, 5 min) and quenched with 200 µg/mL Trypan blue in PBS for 5 min at RT. Then the cells were centrifuged again and excess Trypan blue was removed. The cells were resuspended in PBS and analyzed by FACS. For immunofluorescence, the incubation was followed by fixation and immunocytochemistry was conducted as stated above. To produce resistance to Jagged-mediated endocytosis and mimic force-dependent ligand-mediated receptor endocytosis and Notch activation, the N1ECD was coupled to fluorescent protein A agarose beads (N1ECDPrtA) (42).
Chorion Allantois Membrane Angiogenic Assay.
Matrigel plugs (V = 150 µL) were mixed with H2O, 50 ng/mL FGF, 0.2 mg/mL DAPT, 10 µM WFA, as well as with both DAPT and WFA, and allowed to solidify on top of precut 1.5 cm2 pieces of 500-µm nylon mesh. Nylon mesh with Matrigel was placed on chorion allantois membrane (CAM) on top of major blood vessel and incubated for 5 d. After 5 d, the embryos were visualized live by a Canon 6D SLR, with a 24-105Lf4 lens at maximum focal length using a 25-mm extension tube. Angiogenic branches in Matrigel were quantified via optical visualization.
SI Methods
Cell Culture.
Mouse embryonic fibroblasts (MEFs), human adrenal carcinoma SW13 cells, human embryonic kidney 293 cells stably expressing full-length Notch 1 receptor (293FLN1) and in 3T3 mouse fibroblast stably overexpressing Jagged 1 (3T3J) were grown in DMEM, supplemented with 10% FBS, 2 mM glutamine, penicillin (100 units/mL), and streptomycin (100 μg/mL). The 293 FLN1 and 3T3J cells were also supplemented with 1 µg/mL puromycin. Human umbilical vein endothelial cells (HUVECs) were grown in endothelial cell growth media (Promocell) supplemented with 10% FCS.
Ligand Swaps.
Ligand swaps were generated by PCR amplification using primers with overhangs for the insert (respective ligand ICD) and vector. PCR was performed using KOD polymerase (Millipore) using standard techniques and a thermocycler (Eppendorf). PCR products were gel purified and recombined (Gibson Assembly kit, NEB) and then transformed into electrocompetent bacteria. Electroporation was performed in 0.1-mm cuvettes at 1.35 kV, 10 µF, 600 Ω using an Eppendorf electroporator (2510). Bacterial colonies were screened by PCR and positive clones were sequence verified at the sequencing facility of the Turku Centre for Biotechnology.
Luciferase Reporter Assay.
The 293 FLN1 cells were transfected with 12xCSL-luc and CMV–β-galactosidase using electroporation. Depending on the experiment, the Notch signal-sending cells were also cotransfections with VimS4,6,7,8,9A, VimS4,6,7,8,9D, VimWT, or empty vector. After 2–24 h, the cells were lysed in Cell Culture Lysis Reagent (Promega) and analyzed for luciferase activity using a Luciferase assay system (Promega) with a Luminoskan ascent microplate luminometer (Thermo Scientific). β-Galactosidase activity was measured using a Multiskan Ascent (Thermo Scientific).
Transfection.
For transfection, MEF, SW13, 3T3J, and 293FLN1 cells were pelleted and resuspended in Opti-MEM (Invitrogen) together with the desired plasmid and electroporated at 220 V and 975 µF. The transfected cells were plated and harvested after 24 h for further analysis.
HUVECs were transfected in 12-well plates using Lipofectamine LTX with Plus reagent (Life Technologies). On the day of transfection, 1 μg of DNA and 1 µL Plus reagent were diluted in 250 μL Opti-MEM medium without serum and incubated for 5 min at room temperature (RT). After a 5-min incubation, the solution was combined with 2.5 μL Lipofectamine LTX and incubated for 25 min at RT. After a 25-min incubation, the DNA–Plus–LTX complexes were added dropwise to the cells. After 4 h, the medium was changed.
Western Blotting.
Proteins were separated by SDS/PAGE and transferred to a Protran nitrocellulose membrane (Perkin-Elmer) using a wet transfer apparatus (Amersham Biosciences). Nonspecific binding was blocked by incubating the membranes in 5% nonfat dry milk at RT for 1 h. Membranes were incubated with primary antibody overnight at 4 °C, followed by incubation with a secondary antibody, coupled to horseradish peroxidase, for 1 h at RT. Proteins were detected using Western Lightning Plus-ECL Enhanced chemiluminescence substrate (Perkin-Elmer) and following the manufacturer’s instructions.
Antibodies.
The following antibodies were used: anti-Notch 1 (cleaved-Val-1754 rabbit) (Sigma-Aldrich), β-actin (rabbit) (Cell Signaling Technology), cleaved Notch 1 (rabbit Val1744) (Cell Signaling Technology), CD31 (PECAM-1) (BD Biosciences), Notch 1 (C20 rabbit for WB and goat for IP) (Santa Cruz Biotechnology), Jagged 1 (rabbit 28H8) (Cell Signaling Technology), Jagged 1 (C20) (Santa Cruz Biotechnology), Delta (C20 rabbit) (Santa Cruz Biotechnology), anti-Dll4 (rabbit) (Sigma-Aldrich), and vimentin (D21H3) (Cell Signaling Technology).
Surface Protein Detection by Biotinylation.
The cells were placed on ice and washed three times with cold PBS, followed by surface labeling of membrane proteins with 0.5 mg/mL EZ-link sulfo-NHS-SS-biotin in PBS for 30 min at 4 °C. Thereafter, the cells were washed three times with cold 0.1 M glycine in PBS followed by three washes with PBS. The samples were lysed in immunoprecipitation (IP) buffer [50 mM Tris pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholic acid, 0.05% SDS, 5 mM EDTA, 5 mM EGTA and protease inhibitor (Complete protease inhibitor mixture, Roche)] for 30 min on ice, followed by centrifugation at 15,000 × g for 10 min at 4 °C. The pellet was discarded. Immunoprecipitation of biotinylated proteins was carried out using streptavidin agarose beads and incubating for 4 h at 4 °C. Finally, the pellet was washed three times with IP buffer and resuspended in Laemmli sample buffer for analysis.
Recycling Assay.
Cells were serum starved for 1 h before surface labeling of membrane proteins with 0.5 mg/mL EZ-link sulfo-NHS-SS-biotin in PBS for 30 min at 4 °C. The biotin solution was removed and cells were washed with PBS, followed by addition of DMEM without serum and incubation at 37 °C for 30 min to allow internalization of biotinylated surface proteins. After internalization, the cells were placed on ice and the media were removed, cold MESNA (60 mM MESNA, 50 mM Tris⋅HCl pH: 8.6–8.8, 100 mM NaCl) was added and the cells were incubated on ice for 30 min to remove biotin from the cell surface. After removal of MESNA, 100 mM iodoacetamide in PBS (IAA) was added for 15 min to quench the remaining MESNA. Warm DMEM containing serum was added to the cells and the cells were incubated at 37 °C to allow recycling during the indicated time points. After recycling, the media were removed, followed by another cycle of MESNA and IAA incubations. The cells were lysed in immunoprecipitation (IP) buffer [50 mM Tris pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholic acid, 0.05% SDS, 5 mM EDTA, 5 mM EGTA and protease inhibitor (Complete protease inhibitor mixture, Roche)] for 30 min on ice, followed by centrifugation at 15,000 × g for 10 min at 4 °C. Immunoprecipitation of biotinylated proteins was carried out using streptavidin agarose beads and incubating for 4 h at 4 °C. Finally, the pellet was washed three times with IP buffer and resuspended in Laemmli sample buffer for analysis.
Immunofluorescence.
Cells grown on coverslips were fixed in 3% paraformaldehyde and permeabilized with 0.1% Triton X-100 in PBS for 5 min at RT. Nonspecific binding sites were blocked by incubation in 3% BSA in PBS with 0.05% Triton X-100 for 30–60 min at RT. Cells were stained with primary antibodies overnight at 4 °C or 2 h at RT, after which coverslips were rinsed three times with PBS and stained for 40–60 min with fluorescence tag-labeled secondary antibodies. Cells were counterstained with DAPI and washed three times in PBS before mounting with Prolong Gold (Invitrogen). Images were collected using a Zeiss LSM510 META laser scanning microscope.
Immunohistochemistry.
For whole-mount immunohistochemistry, embryos were dissected in PBS and fixed in 4% paraformaldehyde overnight. The following day, embryos were rinsed three times in PBS, dehydrated in a series of increasing ethanol concentration, and kept at −20 °C before analysis. Embryos were then bleached by incubation in 5% H2O2 in methanol for 5 h at room temperature, where the solution was changed every hour. Bleaching was stopped by three 15-min washes in 100% methanol, and the embryos were rehydrated in a series of PBS. Following rehydration, embryos were incubated in PBSMT (PBS with 3% nonfat dry milk and 0.1% Triton X-100) twice for 1 h at room temperature before incubation with primary antibody in PBSMT at 4 °C overnight. The following day, embryos were subjected to five 1-h washes in PBSMT and incubated with biotinylated secondary antibody in PBSMT at 4 °C overnight. The following day, embryos were washed five times in PBSMT before incubation with ABC reagent (Vector Laboratories) in PBSMT at 4 °C overnight. Embryos were then washed five times in PBSMT, once in PBT (PBS with 0.2% BSA and 0.1% Triton X-100), and staining was achieved by incubation in DAB staining solution (Vector Laboratories). Following washes in PBT and PBS, the embryos were postfixed in 0.1% glutaraldehyde, 2% paraformaldehyde in PBS, and transferred to 70% glycerol.
Analyzes of Angiogenic Patterning in VimWT and VimKO Embryos.
Four litters of WT pups (18 embryos in total) and four litters of VimKO pups (12 embryos in total) were stained and used for analyses. In addition, 30 pups were used for the initial observation and setup of the staining. Fifty percent of the VimKO embryos showed a clear phenotype.
Colocalization Analysis.
For colocalization analysis, MEF WT and VimKO cells were seeded on glass coverslips in 24-well plates and transfected with Rab4-GFP or Rab11 constructs using Lipofectamine LTX according to the manufacturer’s instructions. Two days after transfection, the cells were fixed and labeled according to the immunofluorescence protocol. The samples were imaged with a Leica SP5 laser scanning confocal microscope using a 60×/1.4 Plan Apochromat oil immersion objective. Colocalization of Jagged 1 and Rab4 or Rab11 was analyzed using Fiji ImageJ software and associated plugins. The 3,072 × 3,072 resolution images with 80-nm pixel sizes were used for the analysis. The subtract background function was used with 200-pixel rolling ball radius for each image. Images showing colocalization and Manders’ colocalization coefficients were calculated using the Colocalization Threshold. Pseudocolor images showing colocalization amounts were created from Colocalization Threshold colocalization images by removing green and red, turning the image into grayscale, and changing the lookup table to rainbow. Zero–zero pixels were not included in the threshold calculation. Pearson’s colocalization coefficients were calculated with the Colocalization Test.
Three-Dimensional Matrigel Angiogenesis Assay.
The 96-well plates with round bottoms were coated with 0.5% agarose in H2O by pipetting 80 μL of warm agarose solution to the wells and quickly turning the plates over to remove excess agarose liquid, leaving a coating of agarose in the wells. The 96-well plates were incubated for 24 h in 37 °C. HUVECs, transfected 48 h previously, were then plated to the 96-well plate, adding 4,000 cells per well in 80 μL of medium. Due to the agarose coating, the cells form spheroids instead of attaching to the bottom of the plate. The next day, spheroids were collected. A total of 8–10 spheroids were mixed with a fibrinogen-HBSS mixture (HBSS, 200 units/mL thrombin, 10 mg/mL aprotinin) and 80 μL was plated carefully as a drop in the middle of a 12-well plate. The fibrinogen gel drops were allowed to solidify for 1 h followed by addition of medium. The spheroids were then visualized using a light microscope during the following 4 d.
Vesicle Tracking.
Immunofluorescence tracking of vesicles was performed on WT and Vim−/− MEFs. Jagged 1 was overexpressed in both cell types. A total of 50,000 cells were plated onto 35-mm glass-bottomed plates and transfected with Jagged 1 after 24 h using Lipofectamine LTX according to the manufacturer’s instructions. Before labeling, human Alexa-488 secondary antibody was incubated with rN1ECD as described above and the cells were simultaneously blocked with DMEM containing 10% goat serum and 1% BSA for 45 min in 37 °C. Next, the cells were incubated for 2 h in 37 °C with medium containing the rN1ECD–Alexa-488 complex to allow internalization. New complete DMEM was changed to the cells. Alexa-488 signal from the brighter Jagged 1 overexpressing cells were imaged with Leica SP5 confocal microscope with 63× oil objective. Images were taken in 512 × 512 resolution and with 600-ms time intervals. Images were analyzed with CellProfiler software using the LAP tracking algorithm. Statistics were further analyzed and movement track images produced with R statistics software.
Fingerprint Assay.
The 96-well glass-bottom microplates (sensoplate no. 655892, Greiner Bio-One) were coated overnight at RT with 50 µg/mL protein G (Zymed) in PBS. The plates were washed three times with PBS and blocked with 10 mg/mL BSA in PBS for 2 h at RT. The blocked plates were washed three times with PBS and incubated with rN1ECD (R&D Systems, cat. no. 1057-TK) at concentrations of 1 µg/mL in 0.1% BSA in PBS for 2 h at RT. After a final washing with PBS and cell culture medium 10,000 MEF WT or Vim−/− cells were plated per well. The cells were allowed to attach overnight followed by three washes with HBSS containing Mg2+ and Ca2+. The cells were incubated with 3,3-dithio-bis-succinimidylproprionate at 37 °C for 30 min in HBSS and the cross-linking reaction was quenched with 50 mM Tris⋅HCl (pH 7.5) for 10 min. Cells were extracted with 0.1% (wt/vol) SDS in PBS for 5 min at RT, followed by washing with PBS and briefly rinsing with acetone before fixation with acetone for 5 min at RT. Finally, the wells were washed three times with PBS at RT followed by immunofluorescence staining of Jagged 1 and NotchECD.
Proximity Ligation Assay.
MEF cell samples were fixed for 10 min with methanol and 1 min with acetone and incubated with vimentin together with Dll4 or Jagged 1 primary antibodies for 75 min at RT (3 μL antibody per 100 μL blocking solution). Thereafter, the assays were continued using Duolink DUO92102 reagents (Sigma-Aldrich) according to manufacturer’s instructions. Finally, samples were mounted with DAPI-containing medium (Duolink DUO82040, Sigma-Aldrich) and imaged using the Zeiss LSM780 confocal microscope. The number of PLA signals per cell were quantified for three separate experiments with a minimium of 25 cells per experiment. The statistical significance was determined using GraphPad Prism and shown as SEM in the figure. P values were determined using an unpaired parametric t test.
qPCR.
The RNeasy kit (Qiagen) was used to isolate RNA from MEFs. DNase treatment of 1 μg of each RNA sample was carried out by using RQ1 DNase (Promega M610A). The DNase-treated samples were reverse transcribed to cDNA by using the M-MLV Reverse transcriptase protocol (Promega). Kapa Probe Fast qPCR Master Mix (Kapa Biosystems) was used and quantitative RT-PCR was performed with the 7900HT Fast Real-Time PCR System (Applied Biosystems). Primers for mouse Hes1, Hey1, Jagged1, and Lunatic Fringe were designed using Universal Probe Library Assay Design Center (Roche Applied Biosciences). The primers for Hes1, Hey1, Jagged1, and Lunatic Fringe were purchased from Oligomer and were as follows: Hes1 forward 5′-ACACCGGACAAACCAAAGAC-3′, Hes1 reverse 5′-CGCCTCTTCTCCATGATAGG-3′; Hey1 forward 5′-ACCATCGAGGTGGAAAAGG-3′, Hey1 reverse 5′-CTTCTCGATGATGCCTCTCC-3′; Jagged1 forward 5′-TGGCCGAGGTCCTACACTT-3′, Jagged1 reverse 5′-GCCTTTTCAATTATGCTATCAGG-3′; and Lunatic Fringe forward 5′-CCACTCCCACCTAGAGAACTT-3′, Lunatic Fringe reverse 5′-CGTTCCGCTTGTTCTCAAAC-3′. Taqman probes from Roche were used: no. 99 for Hes1, no. 72 for Hey1, no. 22 for Jagged1, and no. 77 for Lunatic Fringe. Relative quantities of Hes1, Hey1, Jagged1, and Lunatic Fringe transcripts were normalized against their own GADPH. Experiments were repeated at least three times. The results were analyzed with SDS 2.3 and RQ manager software (Applied Biosystems) and statistics were analyzed using GraphPad Prism 5.
Results
Vimentin Interacts with Jagged.
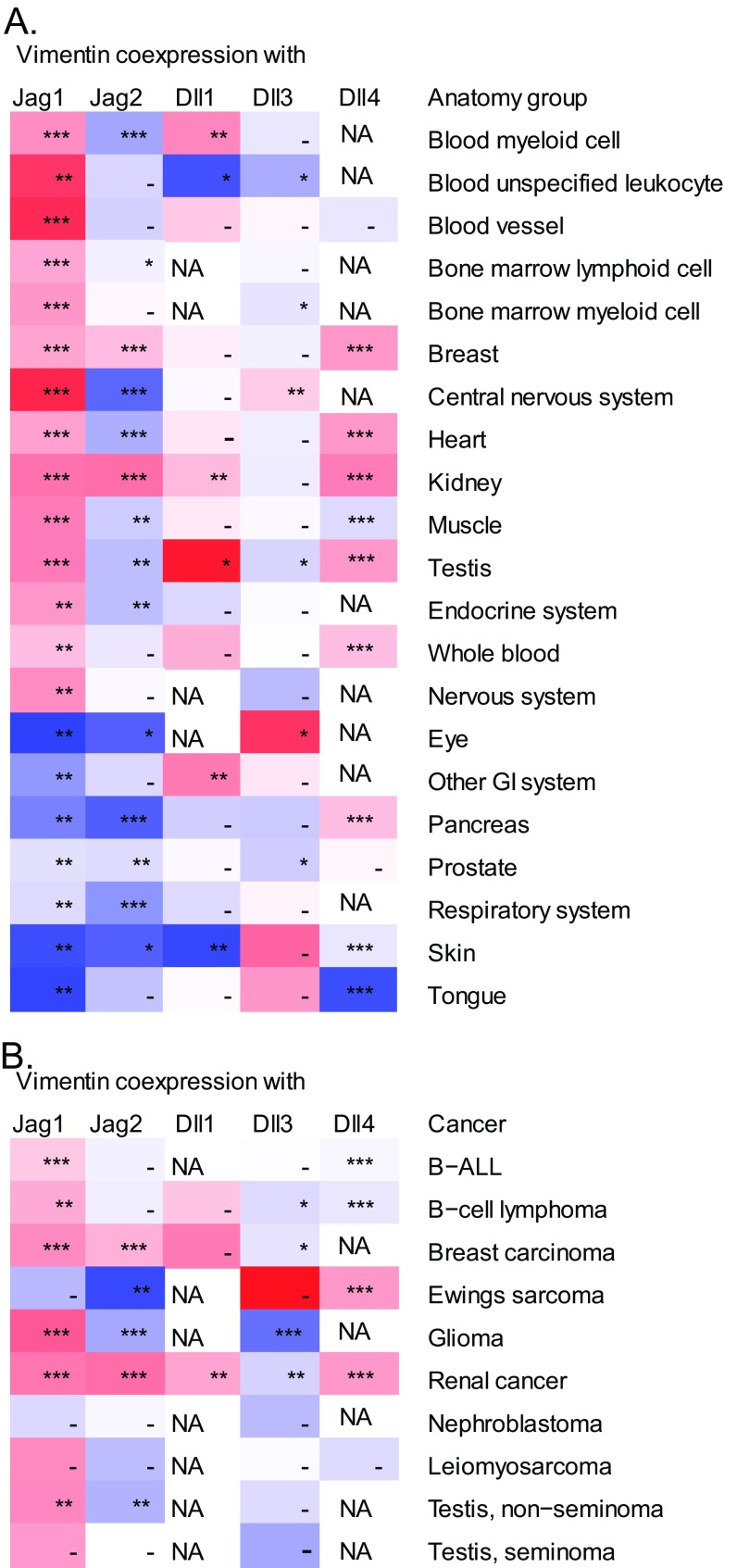
We have previously shown that loss of the astrocytic intermediate filament cytoskeleton, composed of GFAP, vimentin, and nestin, affects Jagged-mediated Notch signaling during neurogenesis (13). As genes that are coexpressed in certain tissues have a higher probability to share a functional relationship (46), we screened tissue expression data in the GeneSapiens database (47) and found a strong positive correlation between the expression of vimentin and Jagged ligands in several tissues, e.g., in blood, breast, leukocytes, central nervous system, heart, kidney, muscle, and testis (Fig. S1A). A significant correlation was also evident in several types of cancer (Fig. S1B). The data suggest the notion of a functional relationship between Jagged and vimentin.
Fig. S1.
Vimentin and Jagged 1 correlate positively in several tissues (A) and cancers (B). mRNA correlations between vimentin and Jagged 1 (Jag1), Jagged 2 (Jag2), Delta-like 1 (Dll1), Delta-like 3 (Dll3), and Delta-like 4 (Dll4) were extracted from the GeneSapiens database. Red colors indicate a more positive correlation and blue, a more negative correlation. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
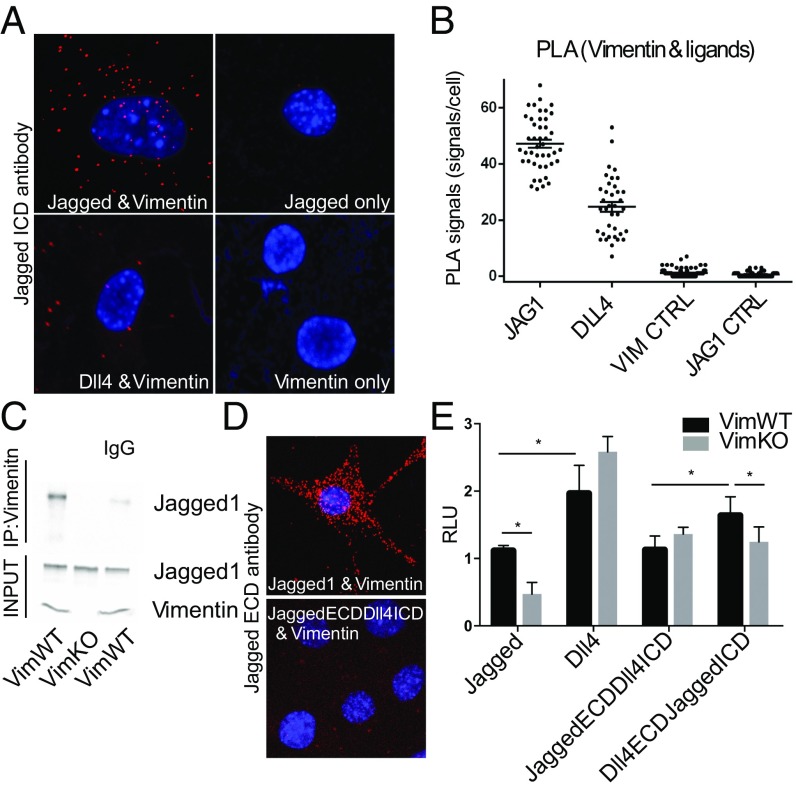
To elucidate the mechanisms and nature of the interrelationship between Jagged and vimentin, we first analyzed whether the proteins physically interacted. Proximity ligation assays demonstrated that vimentin interacts with Jagged 1. Interactions between vimentin and Dll4 were significantly less prominent (Fig. 1 A and B). Coimmunoprecipitation assays further confirmed the interaction between Jagged and vimentin (Fig. 1C, and Fig. S2A). Vimentin interacts with membrane-tethered proteins through PDZ motifs (48) and Jagged and Dll carry distinct PDZ sequences in their ICDs (49–51). Whereas the ECDs are important for receptor binding, the ICDs play important roles in signal activation through posttranslational modifications (37, 52) and interactions with endocytic regulators (41–43). To elucidate whether the ICDs of Dll4 and Jagged 1 influence vimentin binding and Notch signaling, we swapped the ICDs of Dll4 and Jagged 1 (53) to generate the chimeric variants: JaggedECDDll4ICD and Dll4ECDJaggedICD. Swapping the ICD of Jagged with that of Dll4 led to loss of vimentin interaction as demonstrated by loss of the PLA signal (Fig. 1D). We next expressed Dll4 and Jagged, and the chimeric ligands in mouse embryonic fibroblasts (MEFs) derived from WT (VimWT) and vimentin knockout (VimKO) mice and cocultured them with 293HEK reporter cells stably expressing full-length Notch 1 (FLN reporter cells) and then measured signal activation in FLN reporter cells stably expressing full-length Notch 1 using the CSL (CBF-1, suppressor of hairless, Lag-2)-based luciferase reporter system (FLN reporter cells) (13). Transfection efficiency and expression levels of the different proteins were confirmed by immunofluorescence and Western blot (WB). VimWT MEFs expressing Dll4 were significantly stronger signal activators than MEFs expressing Jagged (Fig. 1E). However, whereas Dll4 signaling was unaffected by vimentin depletion, Jagged signaling was significantly reduced in VimKO MEFS (Fig. 1E). Further, signaling from the Dll4ECJaggedICD-expressing MEFs was significantly higher than from cells expressing JaggedECDll4ICD. Signaling from JaggedECDll4ICD was not significantly different in VimWT and VimKO cells. However, signaling from Dll4ECJaggedICD was significantly reduced in VimKO cells (Fig. 1E). The data suggest that Jagged ICD contributes to signaling strength and vimentin is required for efficient Jagged signaling.
Fig. 1.
Jagged interacts with vimentin and the intracellular domain of Jagged potentiates signal activation. (A) In situ PLA was used to demonstrate physical interaction between vimentin and Jagged versus Dll4. Vimentin or Jagged were used as negative controls. (B) Number of PLA signals per cell. Quantification of signals is shown from three separate experiments. P values are <0.0001 for all groups compared with Jagged. (C) Jagged coimmunoprecipitates with vimentin from VimWT MEFs. (D) PLA using an antibody against the Jagged 1 extracellular domain shows strong PLA interaction between Jagged and vimentin, but the chimeric ligand JaggedECDDll4ICD where the intracellular domain of Jagged is switched to Dll4 shows no visible interaction. (E) The signal sending potential of Dll4, Jagged, and chimeric ligands, JaggedECDDll4ICD and Dll4ECDJaggedICD, was measured by coculturing ligand expressing VimKO and VimWT MEF cells with 293HEK cells expressing the Notch 1 receptor (293HEK-FLN1) using a luciferase-based reporter system. Values represent means of three separate experiments including three experimental repetitions ± SEM. Statistical significance was determined using one-way ANOVA and Bonferroni post hoc test, P < 0.05. RLU, relative light unit.
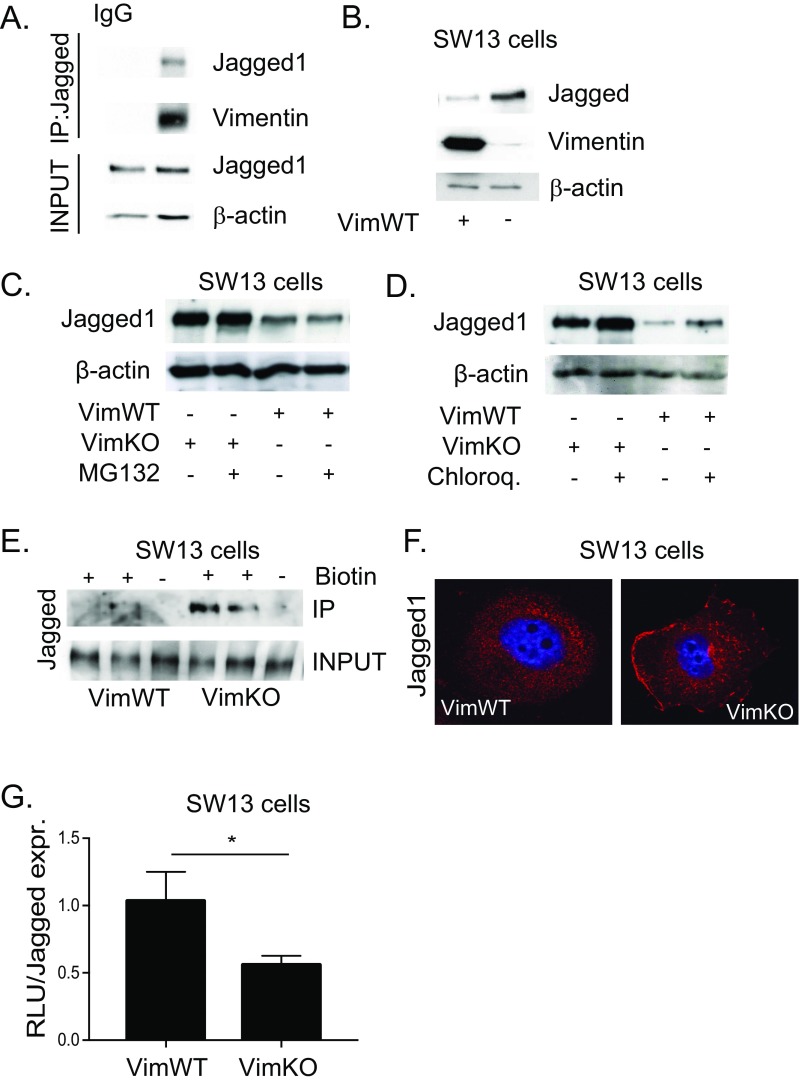
Fig. S2.
Vimentin does not modulate Jagged turnover. (A) Vimentin coimmunoprecipitates with Jagged in 293 HEK cells transfected with vimentin. (B) Representative immunoblot shows Jagged 1 protein levels in vimentin-negative and -positive SW13 cells. (C) Representative images showing Jagged 1 protein expression in vimentin-negative and -positive SW13 cells in the presence of the proteosome inhibitor MG132 (20 µM, 6 h). (D) Representative immunoblot showing Jagged 1 protein expression in vimentin-negative and -positive SW13 cells in the presence of the lysosomal inhibitor chloroquine (20 µM, 16 h). (E) Cell surface proteins in SW13 cells were labeled with biotin and immunoprecipitated using streptavidin agarose beads. Immunoblotting was performed with an antibody detecting Jagged 1. (F) Representative confocal microscopy images showing Jagged 1 immunofluorescence in vimentin-negative and -positive SW13 cells. (G) Jagged signal sending potential measured by coculturing vimentin-negative and -positive SW13 cells with 293HEK cells expressing the Notch 1 receptor (293HEK-FLN1) using a luciferase-based reporter system. Jagged signal sending potential as related to surface levels of Jagged. Values represent means ± SEM. Statistical significance was determined using Student’s t test, P < 0.05. RLU, relative light unit.
Vimentin Regulates Jagged Recycling and Surface Levels.
To gain further insight into a potential functional link between vimentin and Jagged, we analyzed Jagged protein levels in VimWT and VimKO MEFs. The protein levels of Jagged in MEFs were unaffected by the absence of vimentin (Fig. 2A), but were surprisingly enhanced in vimentin-negative SW13 cells (Fig. S2B), demonstrating a cell-type dependent effect. However, we observed no difference in proteasomal (Fig. S2C) nor lysosomal degradation of Jagged 1 in the absence of vimentin in SW13 cells (Fig. S2D), demonstrating that vimentin does not directly affect Jagged turnover.
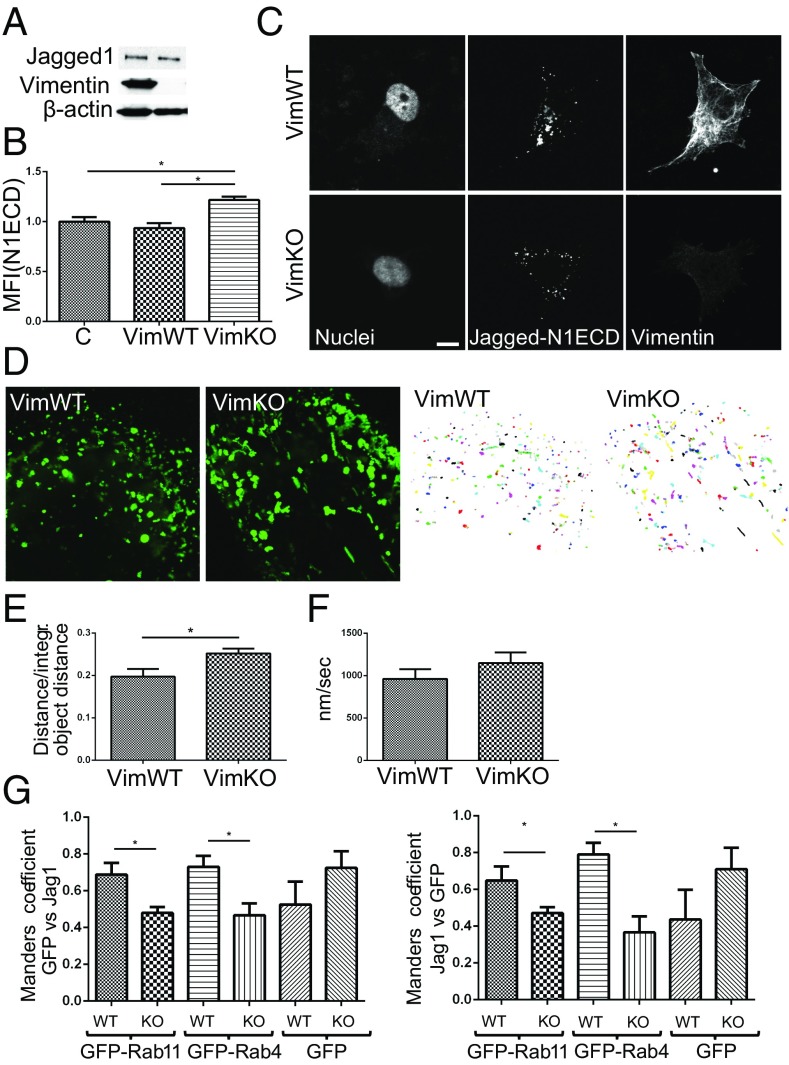
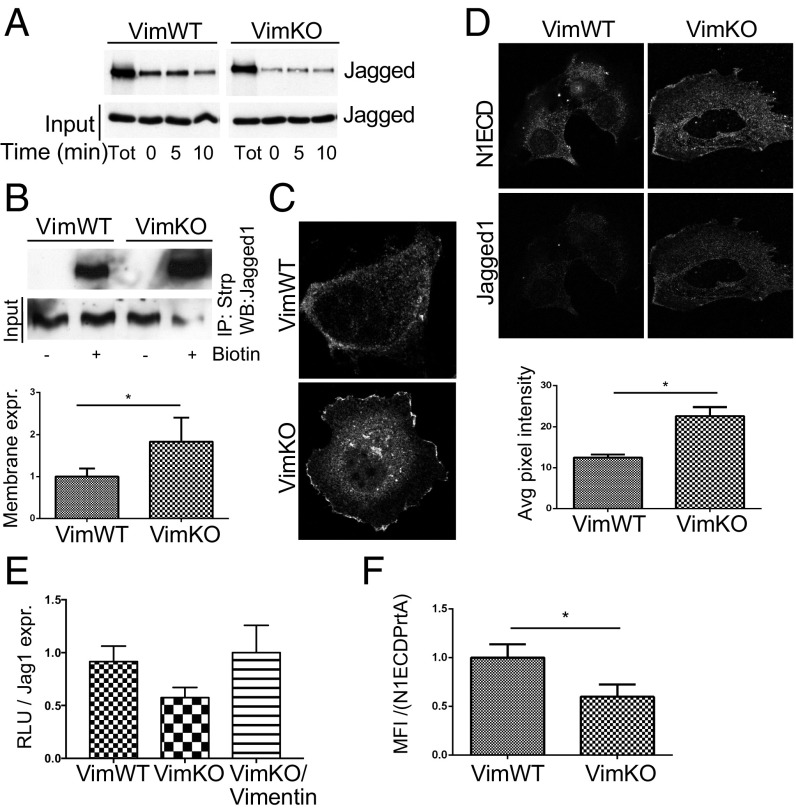
Fig. 2.
Vimentin alters Jagged 1 trafficking. (A) Representative immunoblot shows Jagged 1 protein levels in VimWT and VimKO MEFs. (B) Quantification of ligand endocytosis VimWT and VimKO cells using fluorescently labeled recombinant peptides mimicking the Notch 1 extracellular domain conjugated to a fluorescent probe (N1ECD) to label the ligands. Values represent means ± SEM. Statistical significance was determined using one-way ANOVA and Bonferroni post hoc test, *P < 0.05. MFI, mean fluorescence intensity. (C) Representative images of N1ECD localization in VimWT and VimKO cells. (Scale bar, 10 µm.) (D) VimWT and VimKO cells were live labeled with N1ECD–Alexa-488 and vesicles with green fluorescence were followed for 1 min. Projection of N1ECD–Alexa-488 positive vesicle movement in VimWT and VimKO MEFs during 1 min of vesicle tracking. Vesicles were segmented and tracked with CellProfiler. Graphs were created using R from information produced with CellProfiler. One color represents one vesicle. (E) Analysis of N1ECD–Alexa-488 labeled vesicle track linearity in VimWT and VimKO cells. Values represent means ± SEM. Statistical significance was determined using Student’s t test, P < 0.05. (F) Analysis of N1ECD–Alexa-488 labeled vesicle speed in VimWT and VimKO cells. (Scale bar, 10 µm.) (G) Ligand localization into different endosomal compartments was analyzed by evaluating the colocalization between Jagged 1-stained vesicles and Rab4- or Rab11-linked recycling endosomes. Jagged 1 colocalization with Rab4 and Rab11 was analyzed by Manders’ colocalization coefficient. Confocal microscopy images of 30 cells from three experiments described above were quantified using Fiji ImageJ. P < 0.05.
Endocytosis of Notch ligands is important for Notch activity (41–43). As vimentin has been shown to regulate protein trafficking (3), we assessed whether vimentin affects Jagged routing. To this end, we tracked the ligand with fluorophore-conjugated recombinant peptides, mimicking the extracellular domain of Notch 1 (N1ECDF). Flow cytometry demonstrated enhanced binding and internalization of N1ECDF in VimKO compared with VimWT cells (Fig. 2B). Confocal microscopy revealed that the N1ECDF-positive vesicles were differently distributed in VimKO cells compared with VimWT MEFs (Fig. 2C).
To further investigate intracellular routing of ligands, we tracked the movement of N1ECDF vesicles in VimWT and VimKO cells. In VimKO cells, the average linearity of the vesicle tracks was significantly enhanced compared with WT cells, implying that vimentin decreases directional mobility of N1ECDF vesicles (Fig. 2 D and E). No significant differences were detected in movement speed of the vesicles (Fig. 2 D and F). We next analyzed the occurrence of Jagged in different endosomal compartments. The colocalization of Jagged with Rab4 or Rab11, markers for fast and slow recycling endosomes, respectively (54), was reduced in VimKO cells compared with VimWT (Fig. 2G).
We then used a biotin cell surface labeling and stripping protocol to analyze recycling of Jagged 1 in VimWT and VimKO MEFs. The results indicated that fairly small amounts of Jagged were recycled at steady state in both VimWT and VimKO MEFs. Recycling of ligands appeared faster in VimKO cells (Fig. 3A), which is in agreement with the reduced presence of Jagged in recycling endosomes (Fig. 2G). We subsequently used a biotinylation assay to analyze Jagged surface levels and demonstrated that Jagged was accumulated at the cell membrane in VimKO MEFs (Fig. 3B). Confocal microscopy of cells immunolabeled for Jagged corroborated that the surface levels were enhanced (Fig. 3C). Importantly, surface levels were also enhanced in vimentin-negative SW13 cells, suggesting that surface accumulation is directly linked to vimentin depletion (Fig. S2 E and F) and not related to total Jagged levels (Fig. 2A and Fig. S2B).
Fig. 3.
Vimentin regulates Jagged recycling and Notch activation. (A) Analysis of Jagged 1 recycling in VimWT and VimKO cells using a biotin cell surface labeling and stripping protocol. (B) Cell surface proteins were labeled with biotin and immunoprecipitated using streptavidin agarose beads. Immunoblotting was performed with an antibody detecting Jagged 1. (C) Representative confocal microscopy images showing Jagged 1 immunofluorescence in VimWT and VimKO cells. (D) Representative confocal microscopy image shows Jagged 1 cell surface localization and N1ECD binding in VimWT and VimKO cells. (Scale bar, 10 µm.) The graph shows quantification of pixel intensity. Values represent means ± SEM. Statistical significance was determined using Student’s t test, P < 0.05. (E) Jagged signal sending potential measured by coculturing VimWT and VimKO cells and VimKO cells reexpressing vimentin with 293HEK cells expressing the Notch 1 receptor (293HEK-FLN1) using a luciferase-based reporter system. Jagged signal sending potential as related to surface levels of Jagged. Values represent means ± SEM. Statistical significance was determined using Student’s t test, P < 0.05. RLU, relative light unit. (F) The ability of VimKO and VimWT to internalize N1ECD–Alexa-488 coupled to protein A agarose beads (N1ECDPrtA). Internalization of N1ECDPrtA in VimKO and VimWT cells as related to surface levels of Jagged. Values represent means ± SEM. Statistical significance was determined using Student’s t test, P < 0.05. MFI, mean fluorescence intensity.
We next assessed the ability of Jagged to bind the extracellular domain of Notch using a Jagged 1 fingerprint assay. VimWT and VimKO MEFS were cultured on coverslips coated with peptides corresponding to the extracellular domain of Notch (N1ECD). After protein cross-linking, we extracted the cells in a harsh detergent and the Jagged 1 cross-linked to N1ECD was detected by immunolabeling. More Jagged 1 was found bound to the coated N1ECD in VimKO cells compared with VimWT cells in line with enhanced surface accumulation of Jagged (Fig. 3D). The data demonstrated that the Jagged ligands on the surface of VimKO cells efficiently bound the Notch receptor. These data may explain the high binding and internalization of N1ECDF demonstrated in Fig. 2B.
Vimentin Potentiates Jagged-Mediated Force-Dependent Notch-Activating Endocytosis.
Notch signaling is linear and tightly regulated by the levels of receptors and ligands present on the surface of the signal-receiving and signal-sending cell. However, our data suggest that vimentin depletion reduces Jagged-mediated Notch activation (Fig. 1E), despite enhancing Jagged surface levels (Fig. 3 B and C). We assessed the signal potential of VimKO and VimWT MEFs expressing endogenous levels of Jagged, by coculturing the cells with reporter cells. Despite elevated Jagged levels and enhanced receptor binding in VimKO MEFs, Notch activation in reporter cells was not enhanced. On the contrary, when signal activity was related to ligand levels, the Notch activation potential was significantly reduced in VimKO. This effect could be rescued by the reintroduction of vimentin (Fig. 3E). Vimentin-negative SW13 cells also demonstrated reduced signaling potential compared with vimentin-expressing SW13 cells (Fig. S2G). During active signaling, Notch transendocytosis has been proposed to create a force on the receptor, known as the “pulling force,” which is thought to reveal the cleavage site and promote receptor activation (42). A recent publication demonstrated that ligand-mediated NECD transendocytosis during signal activation is mechanistically distinct from steady-state ligand endocytosis (55). We attached a fluorescently labeled Notch1ECD to Protein A beads (N1ECDPrtA) to resemble the mechanical strain raised during receptor transendocytosis (42) and assessed internalization of N1ECDPrtA by flow cytometry. N1ECPrtA internalization was reduced in VimKO cells (Fig. 3F), in contrast to the enhanced internalization of N1ECDF (Fig. 2B). Internalization of N1ECPrtA was also decreased in vimentin-negative SW13 fibroblasts, a phenotype that was rescued by the reintroduction of vimentin (Fig. S3A). On the contrary, basal endocytosis of Jagged as measured by internalization of N1ECDF was enhanced in vimentin-negative SW13 cells (Fig. S3B). Taken together, the data show that despite increased surface levels of Jagged and efficient receptor binding, the signal activation potential of Jagged is reduced in the absence of vimentin.
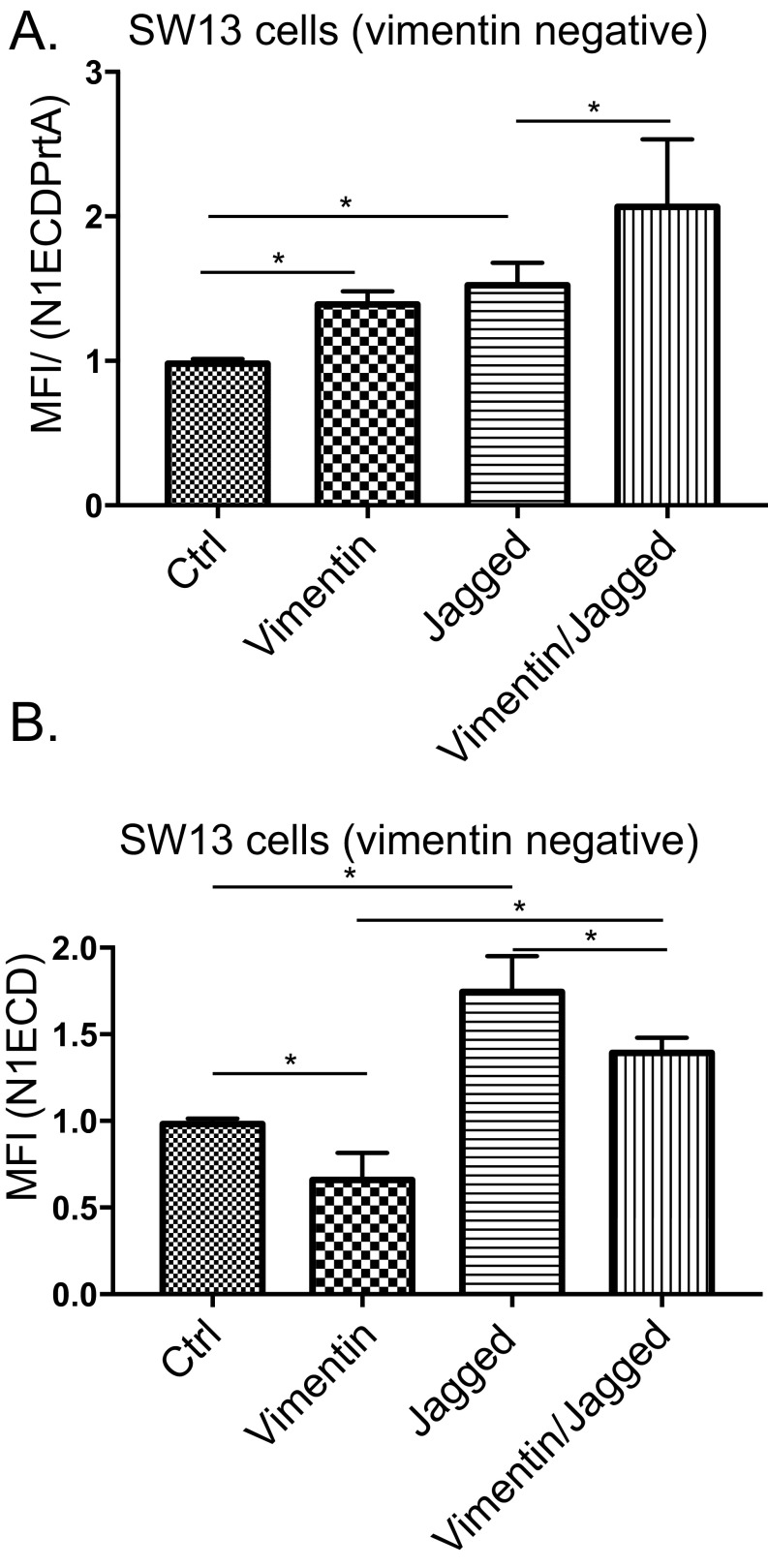
Fig. S3.
Vimentin regulates Jagged endocytosis, Notch receptor transendocytosis, and Notch activity in vimentin-negative fibroblasts and epithelial breast cancer cells. (A) The ability of vimentin-negative control SW13 cells or cells transfected with vimentin, Jagged, or vimentin and Jagged to internalize N1ECD–Alexa-488 coupled to protein A agarose beads (N1ECDPrtA). (B) Endocytosis of Jagged in vimentin-negative SW13 cells or in cells transfected with vimentin, Jagged, or vimentin together with Jagged as measured by a fluorescently labeled N1ECD peptide. Values represent means ± SEM. Statistical significance was determined using Student's t test, P < 0.05. Values represent means ± SEM. Statistical significance was determined using Student’s t test, P < 0.05. MFI, mean fluorescence intensity.
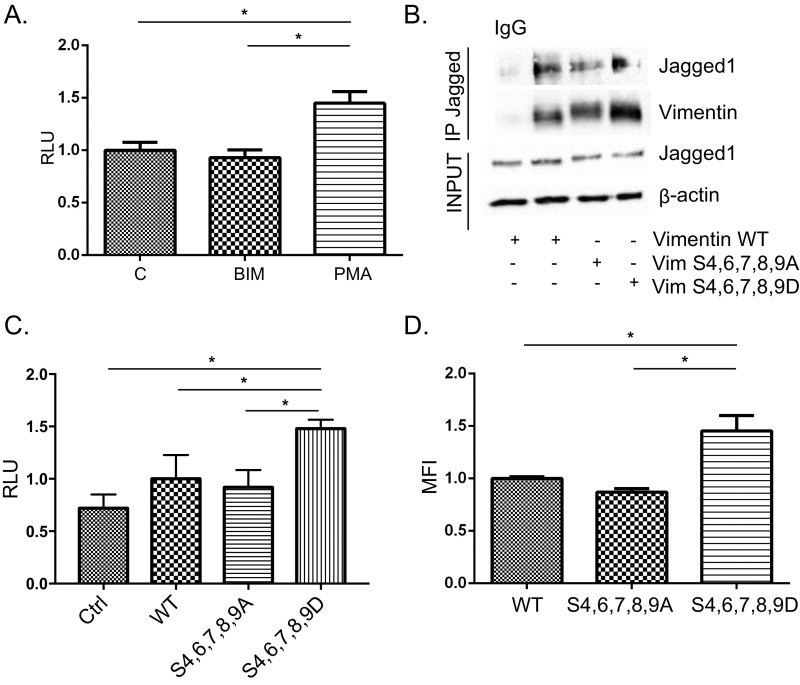
IFs are phosphoproteins and phosphorylation of vimentin regulates organization of the IF network and vimentin–protein interactions. Phamacological activation of the vimentin kinase PKC enhanced Notch activation in cocultured reporter cells. (Fig. S4A). We next tested whether phosphorylation on serines 4, 6, 7, 8, and 9, known PKC targeted sites in the N terminus of vimentin, influenced Jagged interactions and signaling using phosphomutant forms of vimentin. Immunoprecipitation assays demonstrated that Jagged interacted with both wild-type vimentin and phosphomutants, but the interaction with the phosphomimicking mutant was slightly enhanced (Fig. S4B). Furthermore, both the signal activation potential (Fig. S4C) and receptor transendocytosis (Fig. S4D) were enhanced by expression of the phosphomimicking mutant, indicating that vimentin-mediated Jagged regulation is dependent on the phosphorylation status of vimentin.
Fig. S4.
Vimentin-mediated regulation of Jagged is vimentin phosphorylation dependent. (A) Analysis of Notch signaling potential after pretreatment with the PKC inhibitor bis-indolylmaleimide I (BIM) or the PKC activator Phorbol-12-Myristate-13-Acetate (PMA). The ability of the treated cells to activate Notch in cocultured 293 FLN cells was measured using a luciferase-based reporter system (12xCSL-luc). Values represent means ± SEM. Statistical significance was determined using one-way ANOVA and Bonferroni post hoc test, P < 0.05. (B) Immunoprecipitation of Jagged from vimentin-negative SW13 cells expressing wild-type vimentin, phosphomimicking (VimS4,6,7,8,9D), or phosphodeficient (VimS4,6,7,8,9A) forms of vimentin. (C) Notch activation potential of vimentin-negative control SW13 cells or cells transfected with wild type, phosphomimicking (VimS4,6,7,8,9D), or phosphodeficient (VimS4,6,7,8,9A). Values represent means ± SEM. Statistical significance was determined using Student’s t test, P < 0.05. (D) Receptor-transendocytosis of vimentin-negative control SW13 cells or cells transfected with wild type, phosphomimicking (VimS4,6,7,8,9D), or phosphodeficient (VimS4,6,7,8,9A). Values represent means ± SEM. Statistical significance was determined using Student’s t test, P < 0.05.
Deletion of Vimentin Disrupts Sprouting Angiogenesis and Attenuates Embryonic Angiogenesis.
Our data indicate that vimentin binds to Jagged and potentiates Jagged-mediated receptor transendocytosis and Notch activation, but does not interact with Dll4. Dll4 and Jagged ligands have opposite functions during angiogenesis, which makes angiogenesis an interesting process for evaluating the physiological effects of ligand-specific regulation. Blocking Dll4 or inhibiting Notch signaling by γ-secretase inhibitors (GSIs) leads to hyperbranching, whereas Jagged inhibition reduces branching and attenuates angiogenesis (31, 38, 56). As vimentin is important for efficient Jagged signaling, loss of vimentin could disturb the equilibrium between proangiogenic Jagged and antiangiogenic Dll4 signaling.
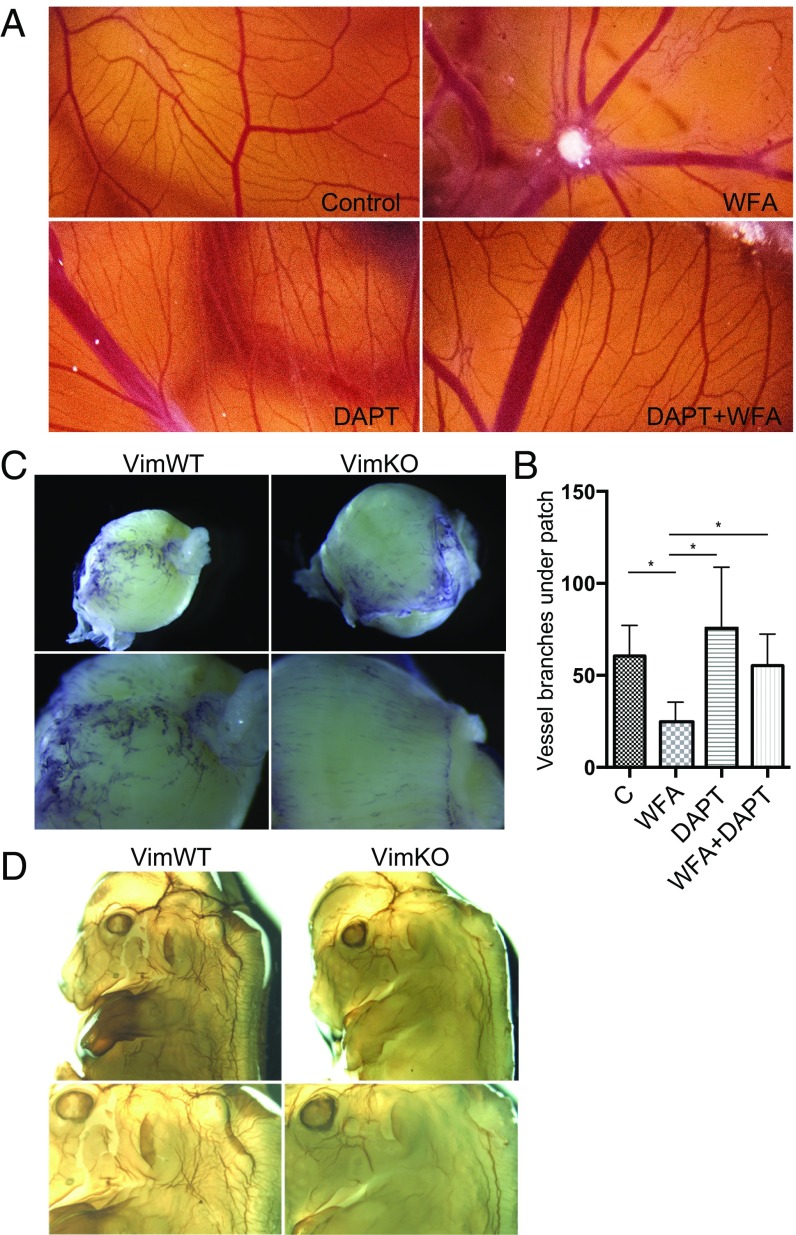
This notion was supported by the disrupted angiogenesis observed in chorioallantoic membranes of fertilized chicken embryos observed upon treatment with the vimentin-targeting drug Withaferin A (WFA). WFA inhibited branching and microvessel formation (Fig. 4 A and B). The effect of WFA was counteracted by Notch inhibition by the GSI DAPT (N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester) (Fig. 4 A and B) (35), indicating that the effect of WFA treatment was overridden by blockage of Dll4 activity.
Fig. 4.
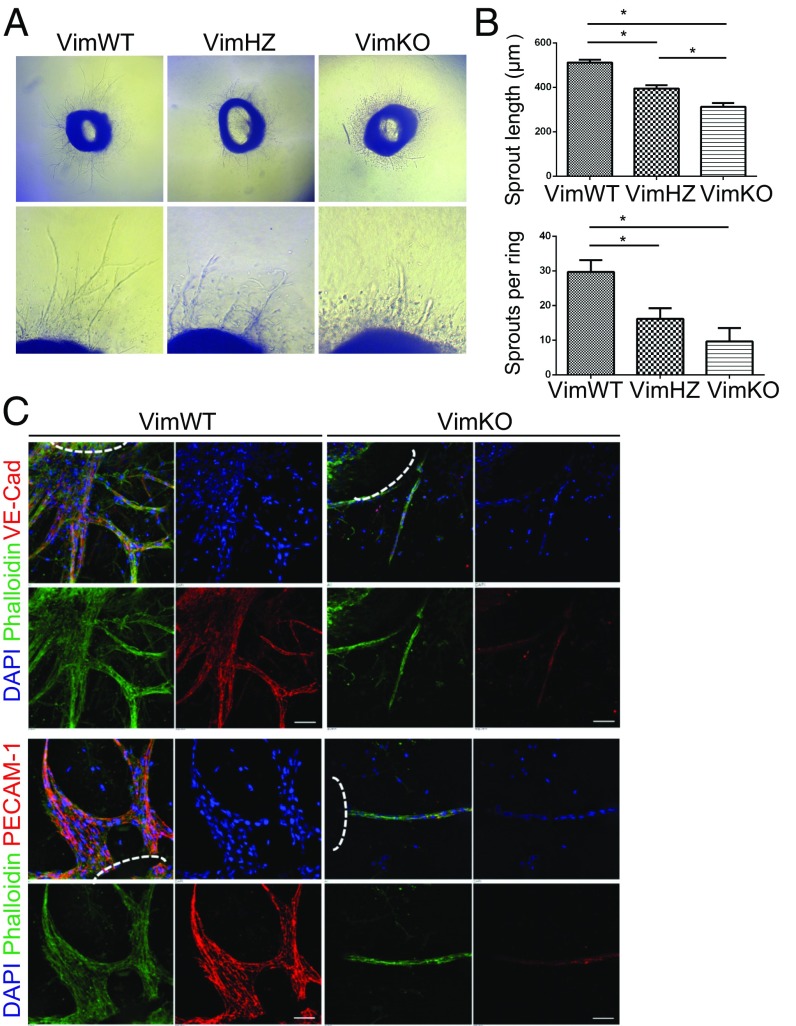
Loss of vimentin disrupts angiogenesis. (A) Notch inhibition rescues the antiangiogenic effect of WFA. The effects of the vimentin-targeted drug WFA, the γ-secretase inhibitor DAPT, and the combination of DAPT and WFA were analyzed in the CAM angiogenesis model. (B) Number of vessel branches per field of view under the patch containing the drugs in one representative experiment. (C) Whole-mount PECAM-1 immunostaining of E11.5 placental tissue from VimWT and VimKO mice. (D) Whole-mount PECAM-1 immunostaining of VimWT and VimKO embryos at E12.5.
The influence of vimentin on angiogenesis was next assessed by analyses of the developing vasculature in the placental tissue and in embryos of VimWT and VimKO mice by whole-mount immunostaining for PECAM-1. The placental tissue of VimKO at embryonic day 11.5 (E11.5) displayed disturbed vascular patterning (Fig. 4C). By E12.5 the VimKO embryos showed a less developed and a less intricate vasculature with fewer blood vessels and less branching compared with WT embryos (Fig. 4D). The difference in vascular branching was particularly noticeable in the head region and the upper parts of the embryo (Fig. 4D).
We then assessed sprouting angiogenesis in VimKO and WT mice using an aortic ring assay. Aortic rings from VimKO and WT mice were excised and embedded in collagen containing growth factors to induce sprouting (57). The assay was optimized using different growth factor supplements to ensure appropriate conditions for endothelial outgrowth of endothelial-lined tubes (Fig. S5). Endothelial sprouting in response to VEGF stimulation was significantly reduced in VimKO aortic rings compared with WT (Fig. 5 A and C). Both the length of the sprouts and sprout number was affected (Fig. 5B). Endothelial outgrowths can be identified by V-Cad and PECAM. VimKO aortic rings did not produce these sprouts in the same manner as in VimWT (Fig. 5C). Aortic rings from heterozygous (VimHZ) mice displayed an intermediate phenotype, indicating that vimentin levels are critical in this system (Fig. 5 A and B).
Fig. S5.
Aortic ring assay. To determine the most appropriate conditions during aortic ring assays for robust endothelial outgrowth, aortic rings were fed Opti-MEM containing 2.5% FBS supplemented with nothing (control, C), 40 ng/mL VEGF, 40 ng/mL FGF, or EGM2-MV.
Fig. 5.
Decreased sprouting from aortic rings from vimentin knockout mice. (A) Representative images showing endothelial sprouting in aortic rings from VimWT, VimHZ, and VimKO mice. (B) Quantification of endothelial sprout length and sprout number. Sprout length was quantified as the distance (in micrometers) from the edge of the ring to the tip of invading endothelial structure. Data represent the average number of endothelial sprouts per ring in a single plane. Error bars represent SEM. Statistical significance was determined using one-way ANOVA and Bonferroni post hoc test, P < 0.05. (C) Immunofluorescence staining was performed to determine whether sprouting structures contained endothelial and smooth muscle cells. Aortic ring assays using VimWT and VimKO aortic rings were stained with VE-cadherin and phalloidin-FITC (Upper) or PECAM-1 and phalloidin-FITC (Lower). In all panels, overlap and DAPI images are shown in Upper Left and Right images, respectively. Z stacks of 1-µm step-size compressed images of aortic rings were taken using a Nikon TI A1R inverted confocal microscope at 40× magnification. (Scale bar, 100 μm.) Dotted white line represents edge of aortic ring where outgrowth initiates.
Taken together the data above demonstrated that loss of vimentin disrupts sprouting angiogenesis and delays embryonic vascular development in line with disrupted balance between Jagged and Dll4 signaling.
Defective Sprouting of VimKO Endothelial Cells Can Be Rescued by Transactivation with Recombinant Jagged 1 Ligands.
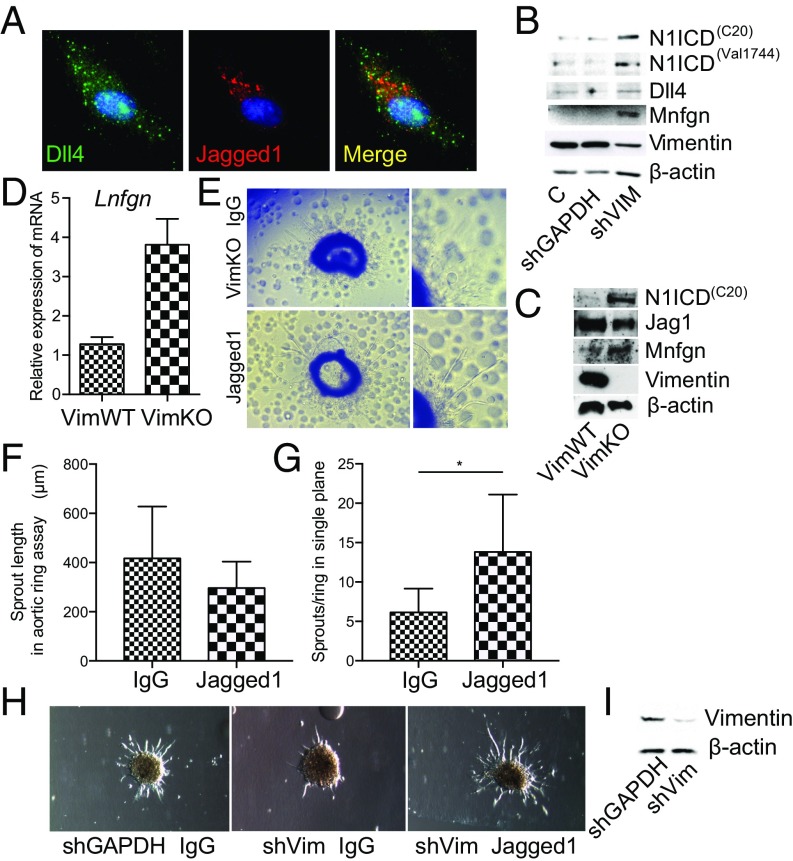
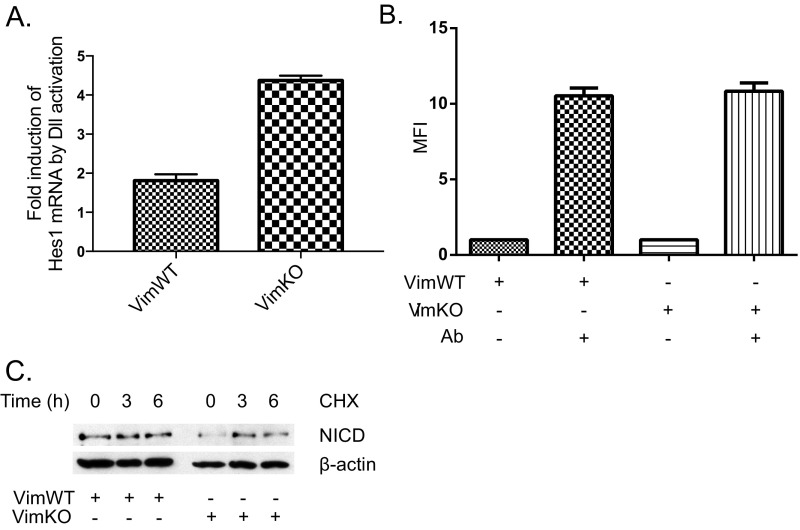
To further assess the effect of vimentin depletion on Notch signaling in endothelial cells, we down-regulated vimentin by shRNA (VimKD). Immunostaining of Dll4 and Jagged 1 (Fig. 6A) revealed that Dll4 and Jagged 1 were localized in distinct intracellular vesicles in endothelial cells, supporting the notion of selective regulation of Notch ligand endocytosis. Immunoblotting of control and VimKD cells revealed increased levels of the active form of Notch, NICD and, intriguingly increased levels of Fringe proteins in VimKD endothelial cells, whereas levels of Dll4 were unaffected (Fig. 6B). Increased levels of NICD and Fringe proteins were also observed in endothelial cells isolated from VimKO mice, whereas levels of Jagged 1 were unaffected (Fig. 6C). Fringe glycosyltransferases modify Notch receptors and enhance transactivation from Dll ligands. Increased expression of Fringe mRNA in vimentin-depleted cells was further verified by qPCR (Fig. 6D). In line with increased expression of Fringes, the expression of the Notch target gene Hes1 is increased in VimKO cells compared with VimWT cells upon activation of Notch by immobilized Dll ligands (Fig. S6A). No effects on surface levels of the Notch receptor (Fig. S6B) or NICD stability were observed (Fig. S6C).
Fig. 6.
Immobilized Notch Jagged 1 rescues sprouting. (A) Confocal image showing Dll4 and Jagged 1 immunolabeling in HUVECs. (B) Expression of Notch receptors, ligands, and regulators in control or vimentin knockdown HUVECs as determined by immunoblotting. The immunoblot shows expression of NICD by two different antibodies: C20 and Val1744, and Dll4, Manic Fringe (Mnfgn), and vimentin. β-Actin was used as a loading control. (C) Expression of Notch receptors, ligands, and regulators in endothelial cells isolated from VimWT or VimKO mice as determined by immunoblotting. Images from B and C are composites, and the bands were taken from several gels run from the same samples with equal loading. (D) The influence of vimentin on the expression of Lunatic Fringe determined by qPCR. (E) To determine whether exogenous addition of Notch ligands rescued vimentin null sprouting responses, Notch ligands or IgG control were coupled to protein A agarose beads and mixed with collagen before embedding vimentin null aortic rings. Representative images show endothelial sprouting in aortic rings from VimKO mice in the presence of immobilized Notch ligands Dll4 and Jagged1. (F) Quantification of endothelial sprout length in aortic rings from VimKO mice in the presence of IgG or immobilized Notch ligands Dll4 and Jagged 1. Sprout length was quantified as the distance (in micrometers) from the edge of the ring to the tip of the invading endothelial structure. (G) Quantification of the number of endothelial sprouts per ring in a single plane. Values represent means ± SEM. Statistical significance was determined using Student’s t test, P < 0.05. (H) Representative images of an in vitro 3D angiogenesis assay with HUVECs transfected with shRNA against vimentin. Endothelial sprouting was analyzed in the presence of IgG or immobilized Notch ligands Dll4 and Jagged 1. (I) Expression of vimentin in control or vimentin knockdown HUVECs as determined by immunoblotting.
Fig. S6.
Dll-mediated Notch activation is enhanced in VimKO cells. (A) Levels of Notch receptors on the surface of VimWT and VimKO cells were analyzed by flow cytometry (FACS) using an antibody recognizing the extracellular domain of Notch. Cells were labeled at +4 °C, before harvesting and analyzes by FACS. (B) VimWT and VimKO cells were cultured on Dll-FC ligands immobilized to G protein beads for 6 h (58). The cells were harvested, mRNA isolated, and samples subsequently analyzed for expression of the Notch target gene Hes1. The graph denotes fold induction of Hes1 expression in VimWT and VimKO cells as related to expression in cells grown on FC immobilized to protein G beads (58). (C) Cyclohexamide treatment of VimWT and VimKO cells demonstrated that NICD in VimWT and VimKO remains stable for 6 h, excluding an effect on Hes1 expression by differences in NICD turnover in WT and KO cells.
Taken together, our data indicate that in the absence of vimentin, Jagged function is compromised and cells express enhanced levels of Fringe proteins. This may shift the balance between Jagged and Dll4 signaling toward Dll4 signaling, giving rise to the antiangiogenic phenotype. To explore this hypothesis, we reactivated Jagged-mediated Notch signaling in aortic rings from VimKO mice and in an in vitro 3D Matrigel sprouting assay using recombinant ligands. Activation of Notch signaling using immobilized Jagged 1 (58) significantly enhanced the sprouting from VimKO aortic rings (Fig. 6 E and G). By contrast, sprout length was not affected by activation of Notch signaling by Jagged 1 (Fig. 6F). An in vitro 3D angiogenesis assay with human umbilical vein endothelial cells (HUVECs) showed similar results. Vimentin knockdown in HUVECs prevented sprouting in 3D, an effect rescued by Jagged 1 (Fig. 6H).
Discussion
We show that VimKO mice display disturbed Notch signaling and vascular patterning during embryogenesis. Depletion of vimentin restricts Jagged-mediated transactivation and enhances expression of Fringe proteins and disrupts sprouting angiogenesis. Aortic rings from mice lacking vimentin produce fewer sprouts compared with WT mice and sprouting is rescued by reactivating Jagged-mediated Notch signaling by recombinant ligands immobilized within the collagen matrix in which the aortic rings are embedded. These results add to the current view of the effect of these ligands on angiogenesis (31, 59). Jagged has been suggested to counteract Dll4 signaling through cis-inhibition by direct binding to the Notch receptors and inhibition of Dll4-mediated activation in the signal receiving cell (35, 60, 61). By contrast, our data show that the phenotype can be rescued by externally presented recombinant Jagged ligands, which support the existence of transsignaling mechanisms in the proangiogenic functions of Jagged.
There is mounting evidence that the vimentin filaments form a dynamic protein scaffold to integrate cellular processes during stages when tissues are undergoing development or remodeling. We have previously shown that vimentin promotes endothelial cell invasion by reinforcing a complex between RACK1 and focal adhesion kinase (FAK) to control FAK activity (62). Further, vimentin promotes endothelial invasion of 3D collagen matrices by complexing with and promoting membrane translocation of the metalloproteinase MT1-MMP, which is required for successful sprouting responses (2). Like Notch receptors, MT1-MMP is a transmembrane receptor that can be internalized to the endolysosomal compartment (63), raising the possibility that loss of vimentin also negatively regulates MT1-MMP stability and endothelial sprouting through enhanced lysosomal degradation. Here, we demonstrate an important role of vimentin in balancing Notch signaling in sprouting angiogenesis, indicating that vimentin performs several vital functions to promote the various steps of angiogenesis.
The signal sending capacity of Jagged is impaired in the absence of vimentin. Ligand-mediated transendocytosis of the receptor produce a strain on the receptor, a process that requires assembly of actin filaments at the membrane (42). Vimentin binds actin (64), while also regulating cytoskeletal tension (65) and actin filament contractility at the membrane (60). Hence, vimentin might link Jagged to the actin cytoskeleton. In this respect, it is attractive to speculate that the interaction between Notch and vimentin would serve as a hub to coordinate cytoskeletal and developmental signaling in the regulation of angiogenesis. Vimentin is required for efficient Jagged-Notch signaling and provides dynamic control of the strength of signal activity as cellular effects of Notch signaling are highly dose sensitive (36, 53, 66). Such control is important, because Jagged recycling and surface accumulation is enhanced, but Jagged surface levels are decoupled from signaling strength in vimentin-depleted cells. The data imply that the maturation model and the pulling force model might not be mutually exclusive but rather coexist, maybe to produce different Notch activity levels with different cellular outcomes.
Our data demonstrate that vimentin interacts with Jagged. Intriguingly swapping the ICD and ECD of Jagged and Dll4 indicates that the Jagged ICD is a more potent signal activator, and vimentin enhances signaling. The ECD of Dll4 has a high affinity for Notch receptors (67) and may be a more important determinant of Dll4 signaling strength. Ligand-specific control of signaling strength may be obtained by posttranslational modifications (68), protein clustering, and localization (69). It was recently demonstrated that different force requirements are needed to activate Notch signaling by different ligands and the authors suggest that this may be a mechanism for Notch-expressing cells to tune their sensitivity to discriminate between different ligands (70). It is possible that a binding between Jagged and vimentin acts as a force-generating mechanism to ensure efficient Jagged-Notch activation. Taken together, our data reveal that Dll4 and Jagged ligands are differently regulated and vimentin contributes to Jagged signaling strength. The molecular basis requires further investigation but might involve specific sets of interacting proteins. Although the Jagged and Dll interactomes remain to be elucidated, the different ligands may have distinct links to the cytoskeleton (51, 71). Vimentin interacts with membrane tethered proteins through PDZ motifs (48) and Jagged and Dll ligands carry distinct PDZ sequences in their intracellular domains (49–51). Clathrin-mediated endocytosis (CME) of Dll depends on Epsin 1 and 2, together with the alternative adaptor protein clathrin assembly lymphoid myeloid leukemia protein (CALM), rather than AP2, which is central to CME of most proteins (42). Vimentin interacts with the AP adaptor complexes to regulate endocytosis (18, 72) and therefore might not affect Dll signaling. The view that vimentin potentiates Jagged function is corroborated by the data showing that Jagged expression correlates with vimentin expression in several tissues and in cancer, in particular. Elevated expression of both vimentin and Jagged has been implicated in tumor progression (6, 8, 10, 16, 73–76). Furthermore, observations in vimentin knockout mice links vimentin to defects in wound healing, fibrosis, inflammation, epidermal aging, and epithelial mesenchymal transition in cancer (4, 7, 8). These are all processes critically regulated by Jagged (77).
Vimentin may also specify ligand-specific signaling by modulating expression of Fringe proteins (31), as vimentin depletion increased Fringe gene expression. At present, we do not know the exact mechanisms through which vimentin regulates Fringe transcription. However, vimentin has been shown to affect gene expression (16), and increasing evidence points to a role for vimentin as a signaling hub (10, 74) and thus loss of vimentin may affect specific signaling pathways regulating expression.
Taken together, our data show that vimentin balances Notch signaling activities and they implicate a complex but distinct role for Jagged in angiogenesis, which relates to the control of signaling strength. Vimentin selectively promotes Jagged activity. Therefore, understanding the regulation of the vimentin–Jagged axis in angiogenesis may unravel new strategies to target the Notch pathway in angiogenesis, but also it has implications for the importance of this pathway in other cell systems known to coexpress these molecules, including differentiating stem cells and cancer.
Acknowledgments
We thank Helena Saarento for technical assistance, Rob Driessen for his help with immunostaining of endothelial cells, Oscar Stassen and Mika Linden for fruitful discussions and insightful comments on the manuscript, and the Cell Imaging Core at the Turku Center of Biotechnology for access to infrastructure and technical assistance. This work was supported by the Academy of Finland Grant 218062 (to C.M.S.) and the Sigrid Juselius Foundation and the Cancer Society of Finland (C.M.S. and C.A.). C.M.S. is supported by a Marie Curie CIG grant. M.S. and D.A. are supported by the Graduate Programme of Molecular Biosciences at Åbo Akademi University. J.E.E. is supported by the Academy of Finland, the Sigrid Juselius Foundation, the Cancer Society of Finland, and the Magnus Ehrnrooth Foundation. In addition this work was supported by the Swedish Cultural Foundation of Finland (M.S., S.L., and R.N.), the Magnus Ehrnrooth Foundation (M.S., S.L., and D.A.), the Waldemar von Frenckell Foundation (M.S., S.L., and D.A.), Svensk-Österbottniska Samfundet (D.A., M.S., and R.N.), the Finnish Cultural Foundation (D.A.), the K. Albin Johansson Foundation (M.S., D.A., S.L., and R.N.), the Aarne Koskelo Foundation (D.A.), and the Einar and Karin Stroem Foundation (M.S. and D.A.). C.M.S. and S.L. were supported by the Swedish Research Council, European Union [Marie Curie–International Training Network (MC-ITN) NotchIT]. F.C. was supported by the Academy of Finland. Research conducted by K.J.B. and C.L.D. was supported by the National Heart, Lung, and Blood Institute, NIH, Grant R01HL095786.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703057114/-/DCSupplemental.
References
- 1.Schiffers PM, et al. Altered flow-induced arterial remodeling in vimentin-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:611–616. doi: 10.1161/01.atv.20.3.611. [DOI] [PubMed] [Google Scholar]
- 2.Kwak HI, et al. Calpain-mediated vimentin cleavage occurs upstream of MT1-MMP membrane translocation to facilitate endothelial sprout initiation. Angiogenesis. 2012;15:287–303. doi: 10.1007/s10456-012-9262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ivaska J, et al. PKCepsilon-mediated phosphorylation of vimentin controls integrin recycling and motility. EMBO J. 2005;24:3834–3845. doi: 10.1038/sj.emboj.7600847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieminen M, et al. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–162. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 5.Pallari HM, Eriksson JE. Intermediate filaments as signaling platforms. Sci STKE. 2006;2006:pe53. doi: 10.1126/stke.3662006pe53. [DOI] [PubMed] [Google Scholar]
- 6.Hyder CL, Isoniemi KO, Torvaldson ES, Eriksson JE. Insights into intermediate filament regulation from development to ageing. J Cell Sci. 2011;124:1363–1372. doi: 10.1242/jcs.041244. [DOI] [PubMed] [Google Scholar]
- 7.dos Santos G, et al. Vimentin regulates activation of the NLRP3 inflammasome. Nat Commun. 2015;6:6574. doi: 10.1038/ncomms7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virtakoivu R, et al. Vimentin-ERK signaling uncouples Slug gene regulatory function. Cancer Res. 2015;75:2349–2362. doi: 10.1158/0008-5472.CAN-14-2842. [DOI] [PubMed] [Google Scholar]
- 9.Cheng F, et al. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling. Proc Natl Acad Sci USA. 2016;113:E4320–E4327. doi: 10.1073/pnas.1519197113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivaska J, Pallari HM, Nevo J, Eriksson JE. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res. 2007;313:2050–2062. doi: 10.1016/j.yexcr.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 11.Toivola DM, Boor P, Alam C, Strnad P. Keratins in health and disease. Curr Opin Cell Biol. 2015;32:73–81. doi: 10.1016/j.ceb.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Hobbs RP, Jacob JT, Coulombe PA. Keratins are going nuclear. Dev Cell. 2016;38:227–233. doi: 10.1016/j.devcel.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilhelmsson U, et al. Astrocytes negatively regulate neurogenesis through the Jagged1-mediated Notch pathway. Stem Cells. 2012;30:2320–2329. doi: 10.1002/stem.1196. [DOI] [PubMed] [Google Scholar]
- 14.Hobbs RP, et al. Keratin-dependent regulation of Aire and gene expression in skin tumor keratinocytes. Nat Genet. 2015;47:933–938. doi: 10.1038/ng.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiu Y, et al. Bidirectional interplay between vimentin intermediate filaments and contractile actin stress fibers. Cell Reports. 2015;11:1511–1518. doi: 10.1016/j.celrep.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Vuoriluoto K, et al. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene. 2011;30:1436–1448. doi: 10.1038/onc.2010.509. [DOI] [PubMed] [Google Scholar]
- 17.Dave JM, Bayless KJ. Vimentin as an integral regulator of cell adhesion and endothelial sprouting. Microcirculation. 2014;21:333–344. doi: 10.1111/micc.12111. [DOI] [PubMed] [Google Scholar]
- 18.Styers ML, et al. The endo-lysosomal sorting machinery interacts with the intermediate filament cytoskeleton. Mol Biol Cell. 2004;15:5369–5382. doi: 10.1091/mbc.E04-03-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue Y, et al. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet. 1999;8:723–730. doi: 10.1093/hmg/8.5.723. [DOI] [PubMed] [Google Scholar]
- 20.Krebs LT, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 21.Limbourg FP, et al. Essential role of endothelial Notch1 in angiogenesis. Circulation. 2005;111:1826–1832. doi: 10.1161/01.CIR.0000160870.93058.DD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phng LK, Gerhardt H. Angiogenesis: A team effort coordinated by notch. Dev Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Hansson EM, et al. Control of Notch-ligand endocytosis by ligand-receptor interaction. J Cell Sci. 2010;123:2931–2942. doi: 10.1242/jcs.073239. [DOI] [PubMed] [Google Scholar]
- 24.Gale NW, et al. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci USA. 2004;101:15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trindade A, et al. Overexpression of delta-like 4 induces arterialization and attenuates vessel formation in developing mouse embryos. Blood. 2008;112:1720–1729. doi: 10.1182/blood-2007-09-112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindner V, et al. Members of the Jagged/Notch gene families are expressed in injured arteries and regulate cell phenotype via alterations in cell matrix and cell-cell interaction. Am J Pathol. 2001;159:875–883. doi: 10.1016/S0002-9440(10)61763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guarani V, et al. Acetylation-dependent regulation of endothelial Notch signalling by the SIRT1 deacetylase. Nature. 2011;473:234–238. doi: 10.1038/nature09917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kangsamaksin T, et al. NOTCH decoys that selectively block DLL/NOTCH or JAG/NOTCH disrupt angiogenesis by unique mechanisms to inhibit tumor growth. Cancer Discov. 2015;5:182–197. doi: 10.1158/2159-8290.CD-14-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon CH, et al. High glucose-induced jagged 1 in endothelial cells disturbs notch signaling for angiogenesis: A novel mechanism of diabetic vasculopathy. J Mol Cell Cardiol. 2014;69:52–66. doi: 10.1016/j.yjmcc.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Yamamura H, et al. Activation of Notch signaling by short-term treatment with Jagged-1 enhances store-operated Ca(2+) entry in human pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol. 2014;306:C871–C878. doi: 10.1152/ajpcell.00221.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benedito R, et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 32.Jakobsson L, et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 33.Hellström M, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 34.Suchting S, et al. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci USA. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cordle J, et al. A conserved face of the Jagged/Serrate DSL domain is involved in Notch trans-activation and cis-inhibition. Nat Struct Mol Biol. 2008;15:849–857. doi: 10.1038/nsmb.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gama-Norton L, et al. Notch signal strength controls cell fate in the haemogenic endothelium. Nat Commun. 2015;6:8510. doi: 10.1038/ncomms9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hori K, Sen A, Artavanis-Tsakonas S. Notch signaling at a glance. J Cell Sci. 2013;126:2135–2140. doi: 10.1242/jcs.127308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedrosa AR, et al. Endothelial Jagged1 antagonizes Dll4 regulation of endothelial branching and promotes vascular maturation downstream of Dll4/Notch1. Arterioscler Thromb Vasc Biol. 2015;35:1134–1146. doi: 10.1161/ATVBAHA.114.304741. [DOI] [PubMed] [Google Scholar]
- 39.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 40.Andersson ER, Sandberg R, Lendahl U. Notch signaling: Simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 41.Shergill B, Meloty-Kapella L, Musse AA, Weinmaster G, Botvinick E. Optical tweezers studies on Notch: Single-molecule interaction strength is independent of ligand endocytosis. Dev Cell. 2012;22:1313–1320. doi: 10.1016/j.devcel.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meloty-Kapella L, Shergill B, Kuon J, Botvinick E, Weinmaster G. Notch ligand endocytosis generates mechanical pulling force dependent on dynamin, epsins, and actin. Dev Cell. 2012;22:1299–1312. doi: 10.1016/j.devcel.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sjöqvist M, et al. PKCζ regulates Notch receptor routing and activity in a Notch signaling-dependent manner. Cell Res. 2014;24:433–450. doi: 10.1038/cr.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Ha T. Defining single molecular forces required to activate integrin and notch signaling. Science. 2013;340:991–994. doi: 10.1126/science.1231041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chowdhury F, et al. Defining single molecular forces required for Notch activation using Nano Yoyo. Nano Lett. 2016;16:3892–3897. doi: 10.1021/acs.nanolett.6b01403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee HK, Hsu AK, Sajdak J, Qin J, Pavlidis P. Coexpression analysis of human genes across many microarray data sets. Genome Res. 2004;14:1085–1094. doi: 10.1101/gr.1910904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kilpinen S, et al. Systematic bioinformatic analysis of expression levels of 17,330 human genes across 9,783 samples from 175 types of healthy and pathological tissues. Genome Biol. 2008;9:R139. doi: 10.1186/gb-2008-9-9-r139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phua DC, Humbert PO, Hunziker W. Vimentin regulates scribble activity by protecting it from proteasomal degradation. Mol Biol Cell. 2009;20:2841–2855. doi: 10.1091/mbc.E08-02-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adam MG, et al. Synaptojanin-2 binding protein stabilizes the Notch ligands DLL1 and DLL4 and inhibits sprouting angiogenesis. Circ Res. 2013;113:1206–1218. doi: 10.1161/CIRCRESAHA.113.301686. [DOI] [PubMed] [Google Scholar]
- 50.Hock B, et al. PDZ-domain-mediated interaction of the Eph-related receptor tyrosine kinase EphB3 and the ras-binding protein AF6 depends on the kinase activity of the receptor. Proc Natl Acad Sci USA. 1998;95:9779–9784. doi: 10.1073/pnas.95.17.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mizuhara E, et al. MAGI1 recruits Dll1 to cadherin-based adherens junctions and stabilizes it on the cell surface. J Biol Chem. 2005;280:26499–26507. doi: 10.1074/jbc.M500375200. [DOI] [PubMed] [Google Scholar]
- 52.Kopan R, Ilagan MXG. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Z, et al. The intracellular domains of Notch1 and Notch2 are functionally equivalent during development and carcinogenesis. Development. 2015;142:2452–2463. doi: 10.1242/dev.125492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones MC, Caswell PT, Norman JC. Endocytic recycling pathways: Emerging regulators of cell migration. Curr Opin Cell Biol. 2006;18:549–557. doi: 10.1016/j.ceb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Musse AA, Meloty-Kapella L, Weinmaster G. Notch ligand endocytosis: Mechanistic basis of signaling activity. Semin Cell Dev Biol. 2012;23:429–436. doi: 10.1016/j.semcdb.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kume T. Novel insights into the differential functions of Notch ligands in vascular formation. J Angiogenes Res. 2009;1:8-2384-1-8. doi: 10.1186/2040-2384-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baker M, et al. Use of the mouse aortic ring assay to study angiogenesis. Nat Protoc. 2011;7:89–104. doi: 10.1038/nprot.2011.435. [DOI] [PubMed] [Google Scholar]
- 58.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA. 2008;105:6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Napp LC, et al. Extrinsic Notch ligand Delta-like 1 regulates tip cell selection and vascular branching morphogenesis. Circ Res. 2012;110:530–535. doi: 10.1161/CIRCRESAHA.111.263319. [DOI] [PubMed] [Google Scholar]
- 60.Glittenberg M, Pitsouli C, Garvey C, Delidakis C, Bray S. Role of conserved intracellular motifs in Serrate signalling, cis-inhibition and endocytosis. EMBO J. 2006;25:4697–4706. doi: 10.1038/sj.emboj.7601337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sprinzak D, et al. Cis-interactions between Notch and Delta generate mutually exclusive signalling states. Nature. 2010;465:86–90. doi: 10.1038/nature08959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dave JM, Kang H, Abbey CA, Maxwell SA, Bayless KJ. Proteomic profiling of endothelial invasion revealed receptor for activated C kinase 1 (RACK1) complexed with vimentin to regulate focal adhesion kinase (FAK) J Biol Chem. 2013;288:30720–30733. doi: 10.1074/jbc.M113.512467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zucker S, Hymowitz M, Conner CE, DiYanni EA, Cao J. Rapid trafficking of membrane type 1-matrix metalloproteinase to the cell surface regulates progelatinase a activation. Lab Invest. 2002;82:1673–1684. doi: 10.1097/01.lab.0000041713.74852.2a. [DOI] [PubMed] [Google Scholar]
- 64.Esue O, Carson AA, Tseng Y, Wirtz D. A direct interaction between actin and vimentin filaments mediated by the tail domain of vimentin. J Biol Chem. 2006;281:30393–30399. doi: 10.1074/jbc.M605452200. [DOI] [PubMed] [Google Scholar]
- 65.Gregor M, et al. Mechanosensing through focal adhesion-anchored intermediate filaments. FASEB J. 2014;28:715–729. doi: 10.1096/fj.13-231829. [DOI] [PubMed] [Google Scholar]
- 66.Mazzone M, et al. Dose-dependent induction of distinct phenotypic responses to Notch pathway activation in mammary epithelial cells. Proc Natl Acad Sci USA. 2010;107:5012–5017. doi: 10.1073/pnas.1000896107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andrawes MB, et al. Intrinsic selectivity of Notch 1 for Delta-like 4 over Delta-like 1. J Biol Chem. 2013;288:25477–25489. doi: 10.1074/jbc.M113.454850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.LeBon L, Lee TV, Sprinzak D, Jafar-Nejad H, Elowitz MB. Fringe proteins modulate Notch-ligand cis and trans interactions to specify signaling states. eLife. 2014;3:e02950. doi: 10.7554/eLife.02950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chitnis A, Balle-Cuif L. The Notch meeting: An odyssey from structure to function. Development. 2016;143:547–553. doi: 10.1242/dev.131086. [DOI] [PubMed] [Google Scholar]
- 70.Luca VC, et al. Notch-Jagged complex structure implicates a catch bond in tuning ligand sensitivity. Science. 2017;355:1320–1324. doi: 10.1126/science.aaf9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 72.Margiotta A, Bucci C. Role of intermediate filaments in vesicular traffic. Cells. 2016;5:E20. doi: 10.3390/cells5020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi JH, et al. Jagged-1 and Notch3 juxtacrine loop regulates ovarian tumor growth and adhesion. Cancer Res. 2008;68:5716–5723. doi: 10.1158/0008-5472.CAN-08-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ivaska J. Vimentin: Central hub in EMT induction? Small GTPases. 2011;2:51–53. doi: 10.4161/sgtp.2.1.15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim MH, et al. Colon cancer progression is driven by APEX1-mediated upregulation of Jagged. J Clin Invest. 2013:pii: 65521. doi: 10.1172/JCI65521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang S, Chung WC, Miele L, Xu K. Targeting Met and Notch in the Lfng-deficient, Met-amplified triple-negative breast cancer. Cancer Biol Ther. 2014;15:633–642. doi: 10.4161/cbt.28180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leong KG, et al. Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J Exp Med. 2007;204:2935–2948. doi: 10.1084/jem.20071082. [DOI] [PMC free article] [PubMed] [Google Scholar]