Significance
Our paper addresses an important question about the brain circuits for visual spatial attention: what are the mechanisms that make it possible to focus on some visual objects and ignore others? Recent work suggests that cortical and subcortical areas might make distinct contributions to attention: some cortical areas may be responsible for changes in perceptual sensitivity whereas other cortical and subcortical areas may control how subjects select between visual objects. Contrary to this suggestion, our results demonstrate that activity from a subcortical structure in the primate, the superior colliculus, is also necessary to produce changes in perceptual sensitivity in an attention-cueing paradigm. Thus, attention-related changes in perceptual sensitivity are not accomplished by cortical areas alone but also depend on subcortical signals.
Keywords: attention, perception, sensitivity, superior colliculus, cortex
Abstract
Spatial cues allow animals to selectively attend to relevant visual stimuli while ignoring distracters. This process depends on a distributed neuronal network, and an important current challenge is to understand the functional contributions made by individual brain regions within this network and how these contributions interact. Recent findings point to a possible anatomical segregation, with cortical and subcortical brain regions contributing to different functional components of selective attention. Cortical areas, especially visual cortex, may be responsible for implementing changes in perceptual sensitivity by changing the signal-to-noise ratio, whereas other regions, such as the superior colliculus, may be involved in processes that influence selection between competing stimuli without regulating perceptual sensitivity. Such a segregation of function would predict that when activity in the superior colliculus is suppressed by reversible inactivation, animals should still show changes in perceptual sensitivity mediated by the intact cortical circuits. Contrary to this prediction, here we report that inactivation of the primate superior colliculus eliminates the changes in perceptual sensitivity made possible by spatial cues. These findings demonstrate changes in perceptual sensitivity depend not only on neuronal activity in cortex but also require interaction with signals from the superior colliculus.
Spatial cues are known to improve perceptual sensitivity on visual discrimination and detection tasks, and these behavioral effects seem to originate from changes in neural activity across several brain regions (1). In monkey visual cortex, spatial cues change the statistics of neuronal activity, and these changes are thought to reflect an increase in signal-to-noise synonymous with the spatially restricted changes in perceptual sensitivity characteristic of selective attention (2–4). The frontal and parietal cortex are also strongly implicated in the control of spatial attention (2, 3). Electrical microstimulation of neurons in frontal cortex of monkeys leads to shifts in selective attention mimicking the effects of visual cues (5); conversely, suppression of neural activity in frontal or parietal cortex leads to deficits in performance on attention-demanding tasks (6–8).
These and similar observations have led to an explanatory framework in which the fidelity of sensory processing in visual cortex is regulated by a network of frontal and parietal cortical areas (9–12). This framework predicts that suppression of activity in one or more of these areas should impair the ability of an animal to use cues to improve its perceptual sensitivity. This prediction has not been directly tested in animals, although clinical cases (13) and experiments in humans using transcranial magnetic stimulation (14, 15) corroborate the idea that these cortical areas are important for the orienting of attention and the use of spatial cues.
In addition to this network of cortical areas, selective attention also involves subcortical brain regions, including the superior colliculus (SC) and thalamus (16, 17). Thalamic nuclei such as the pulvinar seem to be an important extension of the cortical network, regulating signal transmission across cortical areas (18). In contrast, the SC seems to lie functionally downstream of, or parallel to, the visual cortex. The SC plays a causal role in the control of selective attention, as shown by the effects of electrical microstimulation (19, 20) and the neglect-like deficits caused by reversible inactivation (21). However, during the deficits in selective attention induced by SC inactivation, neurons in visual cortex still display cue-related changes in activity normally associated with improvements in perceptual sensitivity (22); this indicates that SC activity is not necessary for regulating sensory signals in visual cortex but instead is necessary for regulation of a subsequent stage of processing, in cortex or elsewhere.
Recent studies have generated a specific suggestion about what this subsequent stage of processing might be. Luo and Maunsell (23) adopted a classic signal detection framework (24) and showed that attention-related modulation in visual cortical area V4 was related to changes in perceptual sensitivity, but not to changes in selection criterion; in their discussion, they suggest the complementary possibility that criterion shifts are mediated by subcortical structures such as the SC. This suggestion is consistent with other physiological results implicating the SC in criterion changes (25) and stimulus selection (26), and with recent models of attention that draw distinctions between changes in perceptual sensitivity and changes in choice bias (27). If the SC were associated with criterion shifts and not perceptual sensitivity, then when activity in the SC is suppressed by reversible inactivation animals should still show changes in perceptual sensitivity, because this aspect of their behavior would be mediated by cortical circuits left intact during the SC inactivation. The experiments reported in this paper directly test this hypothesis.
Results
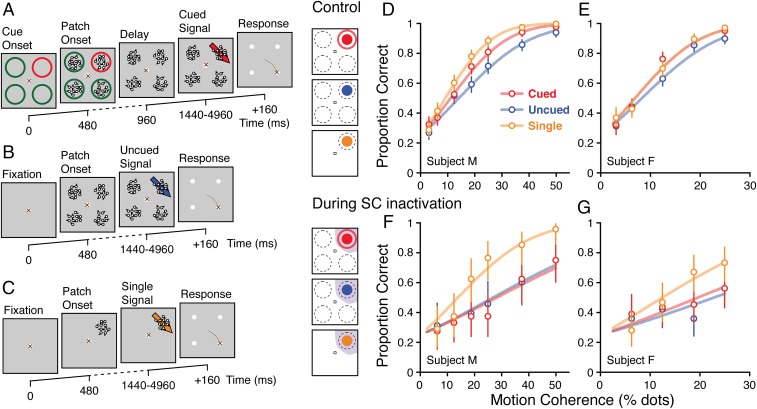
To investigate the possible causal role of the SC in spatial cueing, we adapted the task used in Lovejoy and Krauzlis (21) so that the measure of selective attention was the difference in perceptual sensitivity in the animal’s behavior, with and without spatial cues. The task was a four-alternative, forced-choice motion discrimination task in which the motion stimulus appeared in one of four quadrants of the visual field. In the “cued” condition (Fig. 1A), a visual cue indicated the upcoming location of the coherent motion; the cue was always valid, and no foil stimuli appeared in competition with the motion pulse. Monkeys reported the discriminated direction of motion with a saccade in the same direction as the motion. In the “uncued” condition (Fig. 1B), the motion pulse could appear in any of the four locations. At the time that the 160-ms motion pulse occurred, the stimuli were visually identical between the two conditions and the only difference was the certainty about stimulus location conveyed by the preceding cue. Similar trial conditions are associated with cue-related changes in firing rate in extrastriate cortex (28), frontal eye fields (8), and SC (29). We assessed the impact of distracters on performance by including a “single” condition in which there was only one motion stimulus on the screen (Fig. 1C). All three conditions were presented in blocks of 44 trials, with the four possible directions of the motion pulse randomly interleaved within each block. In blocks of cued and single trials the motion stimulus appeared in the same quadrant each time, whereas in a block of uncued trials the stimulus appeared in any of the four locations at random. We measured choice behavior during sessions in which the SC was inactivated by injection of muscimol, as well as during controls sessions without SC inactivation, and measured psychometric curves by varying motion coherence according to the method of constant stimuli.
Fig. 1.
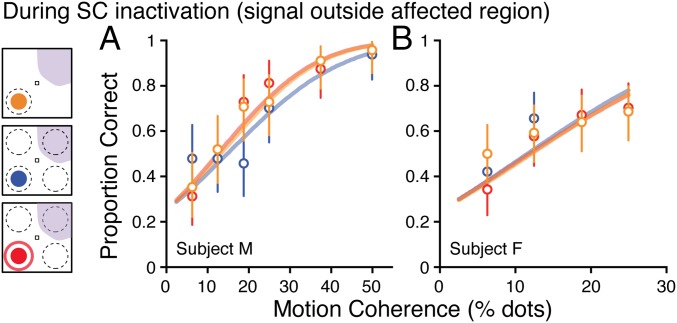
Task and performance. (A) Cued motion discrimination task. After monkeys established and maintained fixation to start the trial, a spatial cue appeared (color oddball indicated cued location). Stochastic motion stimuli next appeared in the areas encircled by the cue rings. After cue rings disappeared and following a variable duration delay, incoherent motion transitioned into coherent motion at the previously cued location (denoted by red arrow). Monkeys received a fluid reward for reporting the direction of motion at the cued location by producing a saccadic eye movement to a target in the same direction, from fixation, as the discriminated direction of motion. (B) Uncued motion discrimination task. Task proceeded in the same sequence as cued version except that no cue appeared. The motion stimulus could appear in any one of the four possible locations. (C) Single-stimulus motion discrimination task. Similar timing as uncued task, but only a single motion stimulus was presented. (D and E) Proportion correct responses as a function of motion coherence. Red symbols indicate cued trials, blue symbols indicate uncued trials, and orange symbols denote single-stimulus trials. Error bars are 95% confidence intervals on the point estimate of the proportion under a binomial distribution. Plots in D and E show data from two different subjects. (F and G) Proportion responses as function of motion coherence when the motion stimulus appeared within the affected region of visual space during SC inactivation.
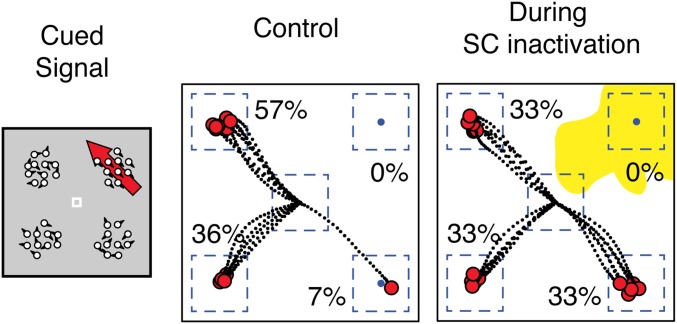
Discrimination performance in control sessions was greater on trials in which the monkey received a visual cue than on those without, demonstrating a behavioral cueing effect (Fig. 1 D and E) and consistent with previous neuronal recordings (8, 28, 29). The cues elevated performance close to that in the single condition—the reduction in spatial uncertainty provided by the cues largely eliminated the effects of increased sensory noise. During SC inactivation, performance was impaired for motion stimuli presented in the affected region of visual space in all three trial types (Fig. 1 F and G). Specifically, monkeys were markedly limited in their ability to use the spatial cue to improve performance when distracters were present; performance in the cued condition was nearly identical to that in the uncued condition, and lower than that in the single condition. Thus, SC inactivation eliminated the effects of spatial cues in this task when they were presented in the affected region of visual space (also illustrated in Fig. S1).
Fig. S1.
Dashed lines show trajectories of saccade responses on individual trials and red circles denote saccade endpoints. Blue dashed lines indicate virtual windows for detecting eye position. Percentages are based on fraction of total responses that landed within each response window. Yellow shaded region indicates extent of inactivated region of visual space. Data are from subject M.
To quantify the changes in both perceptual sensitivity and response bias caused by SC inactivation, we developed and fitted a Bayesian decision model to the behavioral data. As in the familiar signal detection theory (SDT) model often used for two alternative discrimination tasks, perceptual sensitivity was reflected by the separability of evidence distributions when signals were present or absent, and response bias was reflected by the relative criteria by which one option must exceed another for it to be preferred (Methods for more detailed description and Tables S1 and S2).
Table S1.
Parameter estimates by maximum likelihood estimation and 95% confidence intervals by Monte Carlo Markov chain simulation for subject F
| Sensitivity () | Up-right bias () | Down-right bias () | Up-left bias () |
| Control | |||
| 6.468 (6.082, 6.854) | −5.800 (−6.856, −4.660) | −3.468 (−4.367, −2.524) | −2.714 (−3.513, −1.814) |
| 5.052 (4.800, 5.355) | 0.683 (0.007, 1.423) | 0.576 (−0.096, 1.286) | 0.646 (−0.043, 1.421) |
| 6.125 (5.790, 6.508) | −0.834 (−1.726, 0.060) | −2.187 (−3.164, −1.280) | −0.103 (−1.005, 0.725) |
| Signal in affected region | |||
| 4.795 (4.117, 5.900) | −12.125 (−18.200, −8.928) | −5.332 (−8.375, −3.503) | −1.292 (−2.737, −0.048) |
| 2.296 (1.788, 3.134) | −3.737 (−6.294, −2.397) | −2.540 (−4.305, −1.662) | −1.153 (−2.201, −0.501) |
| 2.732 (2.232, 3.513) | −4.567 (−6.970, −3.271) | −2.769 (−4.351, −1.839) | −0.243 (−0.999, 0.457) |
| Signal in unaffected region | |||
| 4.442 (3.783, 5.389) | −5.637 (−8.698, −3.775) | −4.127 (−6.601, −2.545) | 1.510 (0.326, 2.856) |
| 5.165 (4.429, 6.332) | −10.905 (−16.754, −7.805) | −6.585 (−10.575, −4.342) | −0.426 (−2.182, 1.333) |
| 4.960 (4.293, 6.032) | −11.174 (−16.617, −8.192) | −5.399 (−8.323, −3.606) | −0.218 (−1.619, 1.088) |
Bias terms reflect preference for the respective response option over the down-left choice. Bias of zero indicates no excess preference, and negative bias indicates that monkeys preferred the reference choice (down-left) over the corresponding response option. Even under control conditions monkeys tended to be biased away from the signal location when a single stimulus appeared on the screen, but not in the uncued or cued conditions. SC inactivation produced significant bias away from response targets within the affected region of visual space.
Table S2.
Parameter estimates by maximum likelihood estimation and 95% confidence intervals by Monte Carlo Markov chain simulation for subject M
| Sensitivity () | Up-right bias () | Down-right bias () | Up-left bias () |
| Control | |||
| 4.907 (4.647, 5.159) | −3.955 (−4.731, −3.194) | −0.021 (−0.554, 0.518) | −1.962 (−2.580, −1.351) |
| 2.993 (2.849, 3.126) | 0.730 (0.369, 1.099) | 0.472 (0.103, 0.845) | −0.053 (−0.414, 0.322) |
| 3.836 (3.650, 4.033) | 0.632 (0.201, 1.032) | −0.704 (−1.183, −0.269) | −0.905 (−1.353, −0.454) |
| Signal in affected region | |||
| 3.577 (3.128, 4.227) | −5.875 (−8.851, −4.012) | −2.024 (−3.605, −0.886) | −1.001 (−2.215, 0.035) |
| 1.673 (1.408, 2.083) | −1.327 (−2.218, −0.732) | −1.147 (−1.974, −0.563) | −0.397 (−0.984, 0.103) |
| 1.676 (1.402, 2.087) | −1.325 (−2.274, −0.763) | −1.217 (−2.074, −0.658) | 0.345 (−0.093, 0.821) |
| Signal in unaffected region | |||
| 4.091 (3.595, 4.815) | −2.051 (−4.267, −0.251) | 0.571 (−1.050, 2.316) | 4.626 (3.161, 6.783) |
| 3.314 (2.891, 3.936) | −4.490 (−6.949, −2.973) | −2.774 (−4.588, −1.524) | −1.087 (−2.379, 0.013) |
| 3.940 (3.467, 4.651) | −3.815 (−6.076, −2.335) | −4.577 (−7.125, −2.992) | −0.418 (−1.649, 0.727) |
Bias terms reflect preference for the respective response option over the down-left choice. Bias of zero indicates no excess preference, and negative bias indicates that monkeys preferred the reference choice (down-left) over the corresponding response option. Even under control conditions monkeys tended to be biased away from the signal location when a single stimulus appeared on the screen, but not in the uncued or cued conditions. SC inactivation produced significant bias away from response targets within the affected region of visual space.
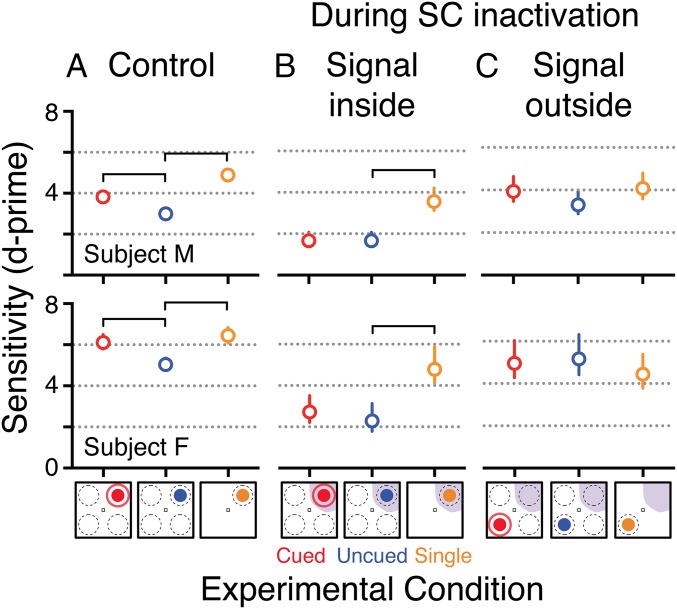
Both subjects displayed changes in perceptual sensitivity commensurate with changes in performance. During control sessions, perceptual sensitivity was highest for the single-stimulus case, decreased in the presence of distracters, and increased with spatial cues (Fig. 2A). During SC inactivation sessions, when the motion stimulus appeared inside the affected region of the visual field, there was a nearly complete loss of the normal increase in sensitivity associated with spatial cues (Fig. 2B and Fig. S2). There was also a generalized decrease in perceptual sensitivity consistent with previous results (21), perhaps due to disruption of the normal weighting of motion signals or decreases in motivation during SC inactivation associated with a decrease in reward rate. When motion stimuli appeared outside the affected region, the spatial cueing effect was again absent but for potentially very different reasons: first, because performance on the uncued condition was already saturated, similar to that on the single conditions, there was little room for detectable increases in perceptual sensitivity with spatial cues (Fig. 2C and Fig. S3); second, because one of the distracters was inside the affected region, it may have been less distracting, consistent with the neglect-like effects seen in ref. 21; and third, because the animals may have been biased to attend to the stimulus in the unaffected region.
Fig. 2.
Summary of effects on perceptual sensitivity. (A) Control data. A spatial cueing effect was demonstrated by the higher sensitivity in the cued (red) than uncued (blue) condition, which were both lower than the sensitivity in the single-stimulus condition (orange). (B) Performance when the motion stimulus was inside the visual field region affected by SC inactivation. The spatial cueing effect was absent—sensitivity was not different between cued and uncued conditions, and both were lower than the single-stimulus condition. (C) Performance when the motion stimulus was outside the region affected by SC inactivation. Sensitivity was again not different between cued and uncued conditions, but both were comparable to the single-stimulus condition. Sensitivity describes the efficiency with which signal energy is transformed into evidence and so would have units reflecting rate of change of evidence with change in coherence. Bars indicate sensitivity values that were significantly different (P < 0.05) by likelihood ratio tests for composite hypotheses. Error bars are 95% confidence intervals on the parameter estimates found via Monte Carlo Markov chain simulation.
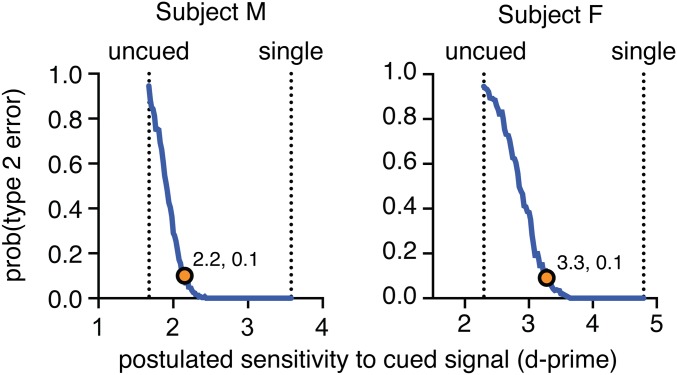
Fig. S2.
Probability of a type 2 error plotted as a function of hypothesized sensitivity to the cued signal when placed in the affected region of the visual field. Vertical dashed lines indicate the sensitivity measured for the uncued and single-patch conditions. Orange circles indicate the point at which type 2 errors cross 0.1, which provides an estimate of the largest d-prime that could have been present in the data and not been detected.
Fig. S3.
Proportion correct responses as a function of motion coherence. Red symbols indicate cued trials, blue symbols indicate uncued trials, and orange symbols denote single stimulus trials. Error bars are 95% confidence intervals on the estimate of the proportion under a binomial distribution. Plots in A and B show data from the two different subjects.
Discussion
Many studies of visual attention over the past three decades are built on the basic observation that telling subjects where the visual stimulus will appear leads to more accurate performance with shorter reaction times compared with when subjects are obliged to attend to multiple locations (1). By combining this visual task design with pharmacologic manipulation in monkeys, our results show that neuronal activity in the primate SC is necessary for the changes in perceptual sensitivity made possible by visual spatial cues.
These results provide mechanistic insights into how cortical and subcortical circuits contribute to the control of visual spatial attention. Several areas in the cerebral cortex are known to play an important role in attention-related changes in the perception of visual objects (4, 11, 30). Activity from the SC may act like a set of spatial weights or biases (31, 32) on the output from cortex, facilitating some (e.g., associated with stimuli at the cued location) at the expense of others—reminiscent of the “attention field” in the normalization model of attention (10). The site of this interaction is not known, although the results from Zénon and Krauzlis (22) suggest it takes place downstream of the visual cortex, perhaps in other attention-related cortical areas, or at the level of the thalamus or basal ganglia (16).
Our results show that this interaction seems to affect both perceptual sensitivity and stimulus selection. The effects on stimulus selection are consistent with models of selective attention based on noise exclusion (33)—during SC inactivation, the changes in the spatial pattern of activity from the SC might have led to an artificial identification of stimuli in the affected region as noise and stimuli in the unaffected region as behaviorally relevant. If the SC’s role in attention were limited to stimulus selection, we might have found evidence of cortically mediated changes in perceptual sensitivity preserved and superimposed on the deficits caused by suppression of SC activity. Instead, we did not. The cueing effects were eliminated, indicating that cortical mechanisms alone are not sufficient to generate attention-related changes in perceptual sensitivity. Changes in perceptual sensitivity seem to be implemented by circuits distributed across cortical and subcortical brain regions, and specifically require SC activity.
Methods
Animal Preparation.
We performed reversible inactivation of the intermediate and deep layers of the SC in two adult rhesus monkeys (subjects F and M) that were 10–15 y of age and weighed 12–15 kg. The monkeys were prepared using standard surgical techniques described in detail previously (34). All experimental protocols were approved by the Institutional Animal Care and Use Committee and complied with US Public Health Service policy on the humane care and use of laboratory animals. The laboratory setup for behavioral control and monitoring was identical to that described previously (34).
Visual Tasks.
The visual tasks required that the monkeys maintain fixation while attending to a stochastic motion stimulus (Fig. 1 A–C). In all three conditions, each trial began as the monkeys fixated a small, stationary spot presented at eye level directly in front of them at the center of a cathode ray tube display with a refresh rate of 75 Hz. The fixation spot consisted of a single pixel of background luminance surrounded by a one-pixel-thick white square. With our display geometry, three pixels subtended nine minutes of arc of visual angle.
In the cued condition, four colored circles appeared for 480 ms. The odd-colored ring indicated the cued location. Stochastic motion stimuli appeared within the circles, and after an additional 480 ms the cue circles disappeared. Stimuli (0% coherent motion) remained on the screen for 480 ms plus a geometrically distributed delay of mean 1,000 ms. The hazard function was flat for nearly the full duration of the trial to minimize temporal predictability. Coherent motion pulses (160 ms) occurred at the cued location, and the monkeys’ task was to report the direction of motion at the cued location by making a saccade to a choice target corresponding to that direction of motion. As previously (21), the direction of motion at the cued location was drawn randomly from four possible diagonal directions; consequently, response direction was independent of the cue location.
In the uncued condition, the task proceeded in the same sequence but no cue was presented, and the coherent motion pulse could occur in any of the four motion patches.
This cued versus uncued design not only allowed us to balance the frequency of the different trial types, making more efficient use of the limited number of trials available during inactivation, but also maintained compatibility with the “cues versus foil” design that we used in a previous study (21).
In the single condition, the timing was again similar, but only a single motion patch was presented. Other task details were the same as used previously (21).
SC Inactivation.
As described previously (34), we injected muscimol (0.5 μL, 5 μg/μL), a GABA agonist, into the intermediate and deep layers of the SC using an injection cannula with an electrode threaded down its barrel. We localized the cannula tip within the intermediate and deep layers of the SC before injection using three criteria (21). First, we advanced the cannula to a depth (1.5–3 mm below the SC surface) corresponding to the intermediate and deep layers. Second, we recorded activity during saccades consistent with known responses in the SC, thus confirming the depth in the SC. The location of the units’ movement fields also indicated our placement within the SC’s retinotopic map. Third, we evoked saccades with microstimulation. The current required to evoke saccades (typically 10 mA in the intermediate and deep layers) provided an additional indication of depth, and the direction and amplitude of the evoked saccades indicated the position within the map. Muscimol injection produced changes in saccade metrics consistent with targeting the intermediate and deep layers of the SC (Fig. S4), namely, that saccades directed into the affected quadrant were hypometric with decreased peak velocity and increased error in their endpoints. Nevertheless, we cannot be certain that our effects are due solely to inactivation of neurons in these layers of the SC, because some drug may have diffused vertically through the layers or tracked up the shaft of the injection cannula to affect neurons in the overlying superficial layers. We performed a total of eight SC inactivation experiments (four for subject M and four for subject F) and pooled the data across those sessions separately for each subject. To properly pool data based on inactivation location and cued location we reflected responses along the horizontal and vertical axes. As a result, the relationship between the choice directions and cued locations were consistent and the relationship between choice directions within the same hemisphere was preserved. We reflected inactivation data such that the inactivated region was in the upper right quadrant with respect to the choice directions. We reflected control data so that the discriminandum was in the upper right quadrant.
Fig. S4.
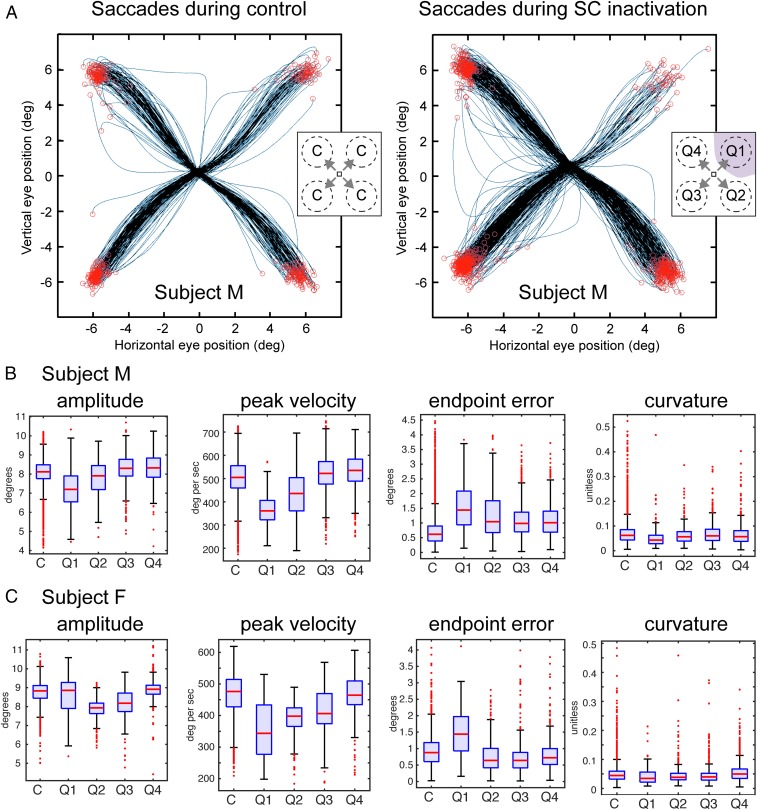
Effects of SC inactivation on saccade metrics. (A) Sample trajectories from subject M from a control session during noninactivation trials (Left) and during SC-inactivation trials (Right); thin blue lines indicate trajectories and red circles indicate saccade endpoints. (Insets) Identity of quadrants and relative directions used in pooling of saccade data. (B and C) Summary of metrics of all saccades made during the attention task for subjects M (B) and F (C). We report four metrics: (i) amplitude of the response saccade, (ii) peak velocity of the saccade, (iii) endpoint error, defined as the absolute value of the distance between eye position at the end of the saccade the center position of the response target, and (iv) curvature, defined as the maximum deviation of the saccade trajectory from a straight line connecting the end-points divided by the amplitude of the saccade (36). Box-and-whisker diagrams display the median of the sample (red line), the upper and lower quartiles of the sample (extent of blue box), 1.5 times the interquartile range (black whiskers), and outliers, or data lying outside the 1.5 interquartile range (red dots). Data are shown separately for saccades during control sessions (C), and saccades during SC inactivation, according to quadrant of saccade endpoint (Q1, Q2, Q3, or Q4) as defined in the insets in A.
Bayesian Decision Model.
We separately characterized perceptual sensitivity and response bias from the behavioral choice data with a multiple alternative version of a traditional SDT model. SDT-based models are often used to describe both detection and two-alternative forced-choice tasks (24). Extensions of this framework to multiple alternatives have been described in detail previously (27, 35). Our model is derived in a manner similar to that of Sridharan et al. (27) and we therefore superficially recapitulate its derivation.
The model accounts for the discrimination as a decision process based on latent (or only indirectly observable) evidence variables. Each of the four response options is associated with an evidence variable. The evidence variables together form a multivariate evidence vector, and the decision is made based on where the vector falls within a decision space. As in the traditional SDT model (24), the perceptual sensitivity of the observer is derived from the separability of the evidence distributions when the signal is present and when the signal is absent. The model makes no assumptions about the specific mechanisms that give rise to these evidence distributions except for the usual assumption that these distributions are Gaussian. Similarly, response bias is reflected by the decision criterion, but instead of a single scalar value as in the SDT model, criteria are conceptualized as planes separating the different regions of the decision space (27).
First we describe the representation of perceptual sensitivity. We index the response options as and refer to the evidence for the corresponding response option as Evidence for a response direction is related to the stimulus configuration in terms of the motion coherence, which we denote as and the direction of the motion, which we denote as We take noise to be normally distributed with zero mean and unit variance: Therefore, the signal model is
| [1] |
The perceptual sensitivity is because it is the expected difference between the evidence at maximum coherence and at no coherence:
Second, we describe the representation of response bias. We model response bias as related to the expected value of the response options. According to Bayesian decision theory, the optimal decision is that which minimizes the total expected risk (rather than the decision with the maximum evidence). The total expected risk (Rij) for response is the sum of the conditional risks for choosing option when the correct response may be . It is specified in terms of the loss function (Lij, the loss associated with choosing option i when the correct response is option j) and posterior probability: The total expected loss for option is the sum over all conditional losses: As Sridharan et al. (27) observed, all decision regions share at least a point as a boundary within the decision space, meaning that planes of equal risk, or risk “isosurfaces,” separate each decision region from every other decision region. Within each region, the conditional loss for the corresponding choice is less than that of each of the alternatives. Therefore, the response option that minimizes total expected loss also has lower conditional risk than all alternatives.
Because conditional risk is equal on decision boundaries, the plane separating the region corresponding to response option from that corresponding to response option can be defined by the ratio of conditional risks. If response option is preferred over response option , then the following inequality holds:
Without much loss of generality, we assume that the cost of any given response option is independent of the possible alternatives. As a result, we can express the inequality in terms of the expected value of a response, :
| [2] |
Thus, the decision boundary is the plane in evidence space for which the likelihood ratio is equal to a ratio of expected value for each response. Furthermore, because we have assumed that the evidence variable is normally distributed, we can more easily work with the relationship as the log-likelihood ratio:
| [3] |
Here we define bias for response , , as the log of the expected value of that response. Response option is preferred to option if the following rule is satisfied:
| [4] |
This decision rule allows us to fully specify the region of evidence space for which the corresponding response, is :
| [5] |
Finally, we can find the probability of response given a motion direction by finding the probability of the event described in Eq. 5:
Substituting the signal model in Eq. 1 for the evidence variables gives the event in terms of the Gaussian distributed noise variables:
Here is the Kronecker delta function, which is zero everywhere except where its argument is zero, in which case it is one. Calculating the probability of response requires first finding the conditional probability of response given a particular value of the noise variable, :
| [6] |
However, the right-hand side of Eq. 6 can be expressed as the product of cumulative normal distributions:
With this result we are prepared to find the probability of response given a stimulus configuration in terms of perceptual sensitivity and response bias:
Because response probabilities depend on the difference in bias terms, all four terms cannot be specified independently. We therefore chose one of the response directions to be a reference (analogous to the pivot in multinomial logistic regression). The bias terms are then defined with respect to this direction. For convenience, we chose the direction always pointing away from the motion stimulus or from the inactivated region (response option indexed as 3). Therefore, bias is reparameterized as and Proportion correct is then
| [7] |
Examination of Eq. 7 reveals that as either sensitivity or motion coherence increases, choice behavior becomes less dependent on response bias, as expected. Conversely, as bias toward a response option increases, the likelihood of choosing that option increases regardless of motion coherence or direction. Furthermore, Eq. 7 is reminiscent of the probit model in that the argument to the cumulative normal function is a linear combination of signal strength and bias; in fact, were there only two options, Eq. 7 would reduce to this model.
We estimated the perceptual sensitivity and response bias terms using maximum likelihood estimation. Because Eq. 7 has no closed form solution, for the purposes of estimating parameters we used Monte Carlo integration with a sample size of . Given a Monte Carlo sample and we approximate Eq. 7 as
We formed the log-likelihood function based on the number of correct responses () and trials () for each of the four directions () and each of the coherences used () for binomially distributed data:
We minimized the negative log likelihood function using the constrained minimization function in MATLAB (we constrained the sensitivity to be always positive). In addition, we performed hypothesis testing on the alternative models using a likelihood ratio test based on this log-likelihood function. We calculated independent 95% confidence intervals as the limits of a distribution of the parameters generated with Monte Carlo Markov Chain simulation ().
We demonstrate application of this model to both control and inactivation data for the single patch condition in Fig. S5. The model captures the asymmetries in task performance based on response direction that are a product of response bias. Parameter estimates and confidence intervals for both subjects in all conditions are given in Tables S1 and S2.
Fig. S5.
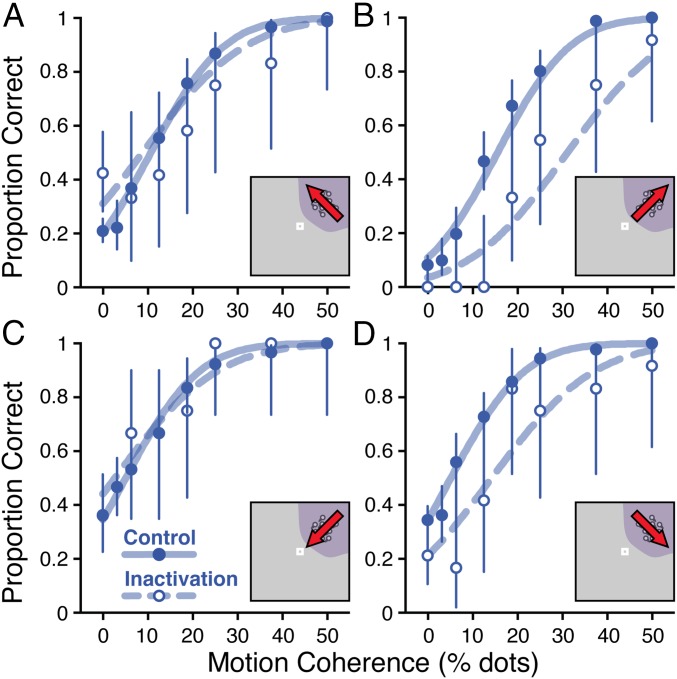
Proportion correct responses as a function of motion coherence for each of the four motion directions. Symbols show data, and curves show model fits; solid indicates control sessions, and dashed/open indicates SC inactivation sessions. Error bars are 95% confidence intervals on the estimate of the proportions. Plots show results separated according to the motion direction presented in the affected region of the visual field: up-leftward (A), up-rightward (B), down-leftward (C), and down-rightward (D). Data are from subject M.
SI Methods
Sample Results Illustrating Deficits in Task Performance Even When Responses Were Directed Outside the Region Affected by SC Inactivation.
In this trial condition from one experimental session, the cued motion instructed an eye movement directed up and to the left, away from the region affected by the SC inactivation (Fig. S1). There was a large drop in performance (from 57 to 33%) even though the eye movements were directed to the unaffected side. Conversely, there was an increase in errors directed to the lower-right quadrant (from 7 to 33%) even though this required an increase in the number of saccades directed into the affected hemifield.
Estimating the Likelihood of Failing to Detect a Cueing Effect Despite SC Inactivation (Probability of Type 2 Errors).
We have argued that monkeys cannot use visual cues in the affected region of visual space during SC inactivation. We depend on the apparent absence of the change in sensitivity that one would have normally expected with selective spatial attention. We sought to estimate how likely this finding would be even if there were, in fact, a cueing effect present. We made multiple simulations of the experiment at a range of possible sensitivities. By applying our analysis to each simulation, we estimated the likelihood of type 2 error—how likely it was that we had missed the cueing effect simply by random chance (Fig. S2).
For each simulation, we assumed that the only difference in outcome was the change in sensitivity to cued stimuli. We then used the parametric model to predict the distribution of responses at a range of sensitivities. Each of these simulations yielded an estimate of the sensitivity, and with these we could compare the degree of overlap between the distributions of estimates. We postulated that the sensitivity to single stimuli was the maximum that the animal could have for cued stimuli and that the sensitivity to uncued stimuli was the lowest. The simulation results demonstrate that it is unlikely that even a moderate cueing effect on sensitivity was missed. Dots indicate the largest possible sensitivity at which a cueing effect might have been missed with probability 0.1.
Task Performance During SC Inactivation When Motion Stimuli Appeared Outside the Region of the Visual Field Affected by the Inactivation.
In both monkeys there was also no significant difference between cued and uncued conditions when the motion stimuli appeared outside the affected region (Fig. S3). In this case, the absence of a difference seems to arise because sensitivity to uncued stimuli was already similar to that to single stimuli, leaving little room for improvement with cues.
Task Performance and Model Fits Separated According to Motion Direction, During Control and SC Inactivation Sessions.
Task performance varied based on motion direction under both control and inactivation conditions. Therefore, modeling of the behavior was required to distinguish changes in performance related to changes in response bias from those related to changes in sensitivity to the motion signal. To illustrate how the model disambiguated changes in perceptual sensitivity from changes in response bias, Fig. S5 shows fits of the decision model fits to data from the single patch case under both control and intrainactivation conditions for subject M. Because of response bias even under control conditions, performance is not chance at zero coherence for all directions—monkeys tended to prefer choices away from the signal location (i.e., lower than chance in Fig. S5B, greater than chance in Fig. S5C). After inactivation, this bias was exaggerated, leading to large deficits in performance when the motion direction was oriented toward the affected hemifield (Fig. S5 B and D). Performance was relatively unchanged when motion pointed away from the affected hemifield (Fig. S5 A and C); this results from both an increase in bias toward the unaffected hemifield and also a decrease in sensitivity to the motion signal, which is most apparent in terms of a decrease in the slope of the psychometric curves.
Effects of SC Inactivation on Saccade Metrics in both Subjects.
Fig. S4A shows all saccade trajectories obtained during one experiment before (Left) and during (Right) SC inactivation. As expected, saccades into the affected quadrant are reduced in frequency but largely on target despite some quantitative alterations in saccade metrics. In Fig. S4B (Subject M) and Fig. S4C (Subject F) we document the effects of SC inactivation on all saccades made during the attention task, pooling across inactivation sessions by assigning the inactivated region to quadrant 1, the same hemifield to quadrant 2, the diametrically opposite quadrant to 3, and the remaining quadrant to 4. Overall, saccades directed into the affected region were hypometric, as shown by the decrease in amplitude of the saccades. Peak velocities of saccades were decreased for saccades directed into the affected region and less so into the unaffected quadrant in the same hemifield, consistent with preservation of the main sequence despite SC inactivation. The accuracy of saccades was decreased for those directed into the affected region and less so for those directed into the unaffected quadrant of the same hemifield, as shown by an increase in the error between saccade endpoint and target. Saccade curvature was not systematically altered by SC inactivation, likely because the saccade target was in the affected region rather than nearby. Of note, saccades directed into the unaffected hemifield had marginally increased amplitude and peak velocity, reminiscent of the bias of the animals to attend to the unaffected hemifield during SC inactivation.
Acknowledgments
This work was supported by the National Eye Institute Intramural Research Program at the National Institutes of Health (R.J.K.), the Simons Foundation (R.J.K.), the Institute for Neural Computation (L.P.L.), an Aginsky Scholars Award (to L.P.L.), NIH Grant MH086466 (to L.P.L.), and the Leon Levy Foundation (L.P.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1609711114/-/DCSupplemental.
References
- 1.Carrasco M. Visual attention: The past 25 years. Vision Res. 2011;51:1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Squire RF, Noudoost B, Schafer RJ, Moore T. Prefrontal contributions to visual selective attention. Annu Rev Neurosci. 2013;36:451–466. doi: 10.1146/annurev-neuro-062111-150439. [DOI] [PubMed] [Google Scholar]
- 3.Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- 5.Moore T, Fallah M. Control of eye movements and spatial attention. Proc Natl Acad Sci USA. 2001;98:1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wardak C, Olivier E, Duhamel J-R. A deficit in covert attention after parietal cortex inactivation in the monkey. Neuron. 2004;42:501–508. doi: 10.1016/s0896-6273(04)00185-0. [DOI] [PubMed] [Google Scholar]
- 7.Wardak C, Ibos G, Duhamel J-R, Olivier E. Contribution of the monkey frontal eye field to covert visual attention. J Neurosci. 2006;26:4228–4235. doi: 10.1523/JNEUROSCI.3336-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monosov IE, Thompson KG. Frontal eye field activity enhances object identification during covert visual search. J Neurophysiol. 2009;102:3656–3672. doi: 10.1152/jn.00750.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bundesen C. A theory of visual attention. Psychol Rev. 1990;97:523–547. doi: 10.1037/0033-295x.97.4.523. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61:168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 12.Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- 13.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 14.Chanes L, Chica AB, Quentin R, Valero-Cabré A. Manipulation of pre-target activity on the right frontal eye field enhances conscious visual perception in humans. PLoS One. 2012;7:e36232. doi: 10.1371/journal.pone.0036232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dugué L, Roberts M, Carrasco M. Attention reorients periodically. Curr Biol. 2016;26:1595–1601. doi: 10.1016/j.cub.2016.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krauzlis RJ, Lovejoy LP, Zénon A. Superior colliculus and visual spatial attention. Annu Rev Neurosci. 2013;36:165–182. doi: 10.1146/annurev-neuro-062012-170249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saalmann YB, Kastner S. Gain control in the visual thalamus during perception and cognition. Curr Opin Neurobiol. 2009;19:408–414. doi: 10.1016/j.conb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337:753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci. 2004;24:11236–11243. doi: 10.1523/JNEUROSCI.3724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc Natl Acad Sci USA. 2005;102:524–529. doi: 10.1073/pnas.0408311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovejoy LP, Krauzlis RJ. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat Neurosci. 2010;13:261–266. doi: 10.1038/nn.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zénon A, Krauzlis RJ. Attention deficits without cortical neuronal deficits. Nature. 2012;489:434–437. doi: 10.1038/nature11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo TZ, Maunsell JHR. Neuronal modulations in visual cortex are associated with only one of multiple components of attention. Neuron. 2015;86:1182–1188. doi: 10.1016/j.neuron.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macmillan NA, Creelman CD. Detection Theory: A User’s Guide. 2nd Ed Lawrence Erlbaum Associates; Mahwah, NJ: 2005. [Google Scholar]
- 25.McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci. 2004;7:757–763. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]
- 26.Mysore SP, Knudsen EI. A shared inhibitory circuit for both exogenous and endogenous control of stimulus selection. Nat Neurosci. 2013;16:473–478. doi: 10.1038/nn.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sridharan D, Steinmetz NA, Moore T, Knudsen EI. Distinguishing bias from sensitivity effects in multialternative detection tasks. J Vis. 2014;14:16. doi: 10.1167/14.9.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayo JP, Maunsell JHR. Graded neuronal modulations related to visual spatial attention. J Neurosci. 2016;36:5353–5361. doi: 10.1523/JNEUROSCI.0192-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat Neurosci. 2004;7:56–64. doi: 10.1038/nn1169. [DOI] [PubMed] [Google Scholar]
- 30.Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eckstein MP, et al. Rethinking human visual attention: Spatial cueing effects and optimality of decisions by honeybees, monkeys and humans. Vision Res. 2013;85:5–19. doi: 10.1016/j.visres.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sridharan D, Steinmetz NA, Moore T, Knudsen EI. Does the superior colliculus control perceptual sensitivity or choice bias during attention? Evidence from a multialternative decision framework. J Neurosci. 2017;37:480–511. doi: 10.1523/JNEUROSCI.4505-14.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Z-L, Dosher BA. Characterizing observers using external noise and observer models: Assessing internal representations with external noise. Psychol Rev. 2008;115:44–82. doi: 10.1037/0033-295X.115.1.44. [DOI] [PubMed] [Google Scholar]
- 34.Hafed ZM, Goffart L, Krauzlis RJ. Superior colliculus inactivation causes stable offsets in eye position during tracking. J Neurosci. 2008;28:8124–8137. doi: 10.1523/JNEUROSCI.1317-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeCarlo LT. On a signal detection approach to m-alternative forced choice with bias, with maximum likelihood and Bayesian approaches to estimation. J Math Psychol. 2012;56:196–207. [Google Scholar]
- 36.Smit AC, Van Gisbergen JAM. An analysis of curvature in fast and slow human saccades. Exp Brain Res. 1990;81:335–345. doi: 10.1007/BF00228124. [DOI] [PubMed] [Google Scholar]