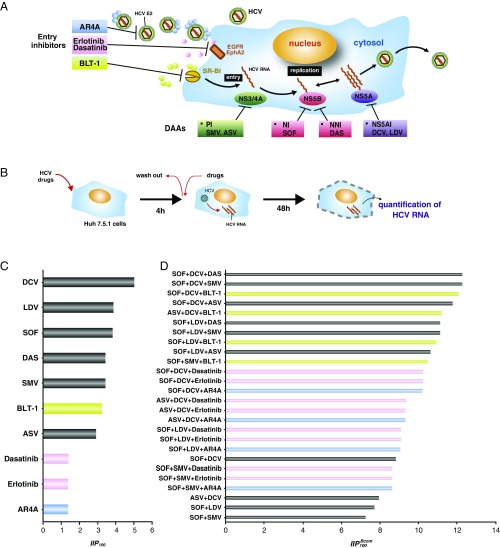
We thank Padmanabhan and Dixit for their comments (1) on our paper (2). They pointed out that entry inhibitors might form potent partners for optimal drug combinations. They analyzed previously published data on 10 hepatitis C virus (HCV) entry inhibitors that are under clinical or preclinical development and found some of these HCV entry inhibitors showed high instantaneous inhibitory potentials (IIPs) (3) compared with IIPs of direct-acting antivirals (DAAs). To analyze further the utility of combining entry inhibitors with other DAAs and to extend our original results (2), we quantified the anti-HCV effect of four different classes of entry inhibitors [AR4A (anti-HCV E2 antibody) (4), BLT-1 [scavenger receptor class B type 1 (SR-BI) inhibitor] (5), erlotinib (EGF receptor inhibitor) (6), and dasatinib (EphA2 inhibitor) (6)] singly and in combination with six DAAs studied by Padmanabhan and Dixit (1) in the HCV infectious cell culture system (Fig. 1 A and B). Single treatment of these entry inhibitors exhibited a dose-dependent reduction in HCV RNA levels. Using the median effect (1–3), we estimated , the half-maximum inhibitory concentration, and , the slope parameter, for each drug from its dose–response curve (Table 1), which enables us to calculate at (i.e., ) (Fig. 1C). We found BLT-1 shows the highest among the entry inhibitors, which is equivalent in value to DAAs. In addition, applying Bliss independence (7), we quantified the upper limits of anti-HCV activity for triple-drug treatments at () (Fig. 1D). These data clearly showed that HCV entry inhibitors augmented the antiviral effect of double DAA-based treatments. Interestingly, augmentation of antiviral effects by addition of entry inhibitors largely depended on the entry inhibitor used: Triple-drug treatments, including BLT-1, showed an especially high , which is comparable to the of triple DAA-based treatments, among the tested entry inhibitors.
Fig. 1.
Evaluation of anti-HCV drug combination with DAAs and entry inhibitors. (A) Schematic model of the targets of entry inhibitors and DAAs used in this study. NI, nucleoside-type polymerase inhibitor; NNI, nonnucleoside-type polymerase inhibitor; PI, protease inhibitor. (B) Schematic representation of the assay to evaluate the anti-HCV activity of the drugs. Upon drug treatment, Huh7.5.1 cells were inoculated with HCV JFH-1 at a multiplicity of infection of 0.5 for 4 h, and were cultured for an additional 48 h. Anti-HCV E2 antibodies were used by preincubation with HCV for 1 h and coincubation with HCV for 4 h. The infection level of HCV was quantified by measuring intracellular HCV RNA. (C) values for DAAs in our study (2) and the HCV entry inhibitors (AR3A, BLT-1, and erlotinib) at a drug concentration () determined by extrapolation. (D) Bliss-estimated of triple-drug combination, including DAAs and the entry inhibitors. ASV, asunaprevir; DAS, dasabuvir; DCV, daclatasvir; LDV, ledipasvir; SOF, sofosbuvir; SMV, simeprevir.
Table 1.
Parameter values for the HCV entry inhibitors in Huh 7.5.1 cells
| Drug | Unit | ||
| Simeprevir | 1.71 | 20.00 | nM |
| Asunaprevir | 1.46 | 17.28 | nM |
| Sofosbuvir | 1.91 | 103.19 | nM |
| Dasabuvir | 1.72 | 3318.18 | nM |
| Daclatasvir | 2.51 | 13.37 | pM |
| Ledipasvir | 1.94 | 2.81 | nM |
| Dasatinib | 0.69 | 2.90 | μM |
| Erlotinib | 0.69 | 0.84 | μM |
| BLT-1 | 1.62 | 0.96 | μM |
| AR4A | 0.68 | 14.72 | μg/mL |
Entry inhibitors are primarily aimed at preventing viral infection. However, because they are effective in eliminating HCV from already established infection in human liver chimeric mice and chimpanzees, HCV entry inhibitors can be candidates for an additional choice of anti-HCV treatment (8–11). In particular, host-targeting agents such as BLT-1, erlotinib, and dasatinib show less opportunity for emergence of drug-resistant virus. The results of Padmanabhan and Dixit (1), which are further supported by the work reported here, show that entry inhibitors can be combined with other classes of DAAs to provide potentially potent anti-HCV treatment that may be especially effective for difficult-to-treat patients.
Acknowledgments
This work was supported, in part, by the Research Program on Hepatitis from the Japan Agency for Medical Research and Development (T.W. and K.W.); NIH Grants R01-AI028433, R01-AI078881, and R01-OD011095 (to A.S.P.); the Japan Science and Technology Agency (JST) Precursory Research for Embryonic Science and Technology program (S.I.); Japan Society for the Promotion of Science KAKENHI Grants 17H05819, 16H04845, 16K13777, 15KT0107, and 26287025 (to S.I.) and Grants 17H04085, 16KT0111, and 26460565 (to K.W.); the Mitsui Life Social Welfare Foundation (S.I.); the Shin-Nihon of Advanced Medical Research (S.I.); GlaxoSmithKline Japan Research Grant 2016 (to S.I.); and the JST Core Research for Evolutional Science and Technology program (S.I. and K.W.).
Footnotes
Conflict of interest statement: The authors declare that they have no competing interests, except A.S.P., who has consulted for Gilead Sciences, Merck, Bristol Myers Squibb, Achillion, and Santaris Pharma.
References
- 1.Padmanabhan P, Dixit NM. Inhibitors of hepatitis C virus entry may be potent ingredients of optimal drug combinations. Proc Natl Acad Sci USA. 2017;114:E4524–E4526. doi: 10.1073/pnas.1704531114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koizumi Y, et al. Quantifying antiviral activity optimizes drug combinations against hepatitis C virus infection. Proc Natl Acad Sci USA. 2017;114:1922–1927. doi: 10.1073/pnas.1610197114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen L, et al. Dose-response curve slope sets class-specific limits on inhibitory potential of anti-HIV drugs. Nat Med. 2008;14:762–766. doi: 10.1038/nm1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Law M, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008;14:25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 5.Nieland TJ, Penman M, Dori L, Krieger M, Kirchhausen T. Discovery of chemical inhibitors of the selective transfer of lipids mediated by the HDL receptor SR-BI. Proc Natl Acad Sci USA. 2002;99:15422–15427. doi: 10.1073/pnas.222421399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lupberger J, et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17:589–595. doi: 10.1038/nm.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 8.Morin TJ, et al. Human monoclonal antibody HCV1 effectively prevents and treats HCV infection in chimpanzees. PLoS Pathog. 2012;8:e1002895. doi: 10.1371/journal.ppat.1002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jong YP, et al. Broadly neutralizing antibodies abrogate established hepatitis C virus infection. Sci Transl Med. 2014;6:254ra129. doi: 10.1126/scitranslmed.3009512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao F, et al. Synergy of entry inhibitors with direct-acting antivirals uncovers novel combinations for prevention and treatment of hepatitis C. Gut. 2015;64:483–494. doi: 10.1136/gutjnl-2013-306155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mailly L, et al. Clearance of persistent hepatitis C virus infection in humanized mice using a claudin-1-targeting monoclonal antibody. Nat Biotechnol. 2015;33:549–554. doi: 10.1038/nbt.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]