With the large number of drugs now approved for the treatment of chronic hepatitis C virus (HCV) infection and an even larger number under development, rational identification of effective drug combinations becomes necessary for better disease management and the judicious design of clinical trials. In PNAS, Koizumi et al. (1) address this issue by quantifying the anti-HCV activity of 15 drugs individually and in various combinations. They found that sofosbuvir (SOF), a current first-line drug, was among the most potent drugs and that three-drug combinations were more effective than two-drug combinations. Importantly, the study demonstrates the applicability of the instantaneous inhibitory potential (IIP), a metric developed to assess anti-HIV drugs (2), to the evaluation of anti-HCV drugs in culture, and establishes a quantitative framework for identifying optimal drug combinations.
The drugs studied by Koizumi et al. (1) belong to several classes, all of which inhibit HCV replication. Inhibitors of HCV entry into target cells were not included, possibly because the study used the HCV replicon system, which successfully recapitulates the intracellular HCV replication process but not virus entry and egress. HCV entry is a complex, multistep process, with each step providing a potential therapeutic target. Several HCV entry inhibitors (EIs), including small molecules, antibodies, natural products, and peptides, have been identified (3). EIs act synergistically with drugs of other classes and have shown activity against strains resistant to other drugs in culture and in a mouse model (3). Indeed, several EIs are in clinical trials (3). Our goal was to examine whether the potency of EIs was comparable to the potency of the drugs considered by Koizumi et al. (1), and whether EIs should therefore be included in future efforts to identify optimal drug combinations.
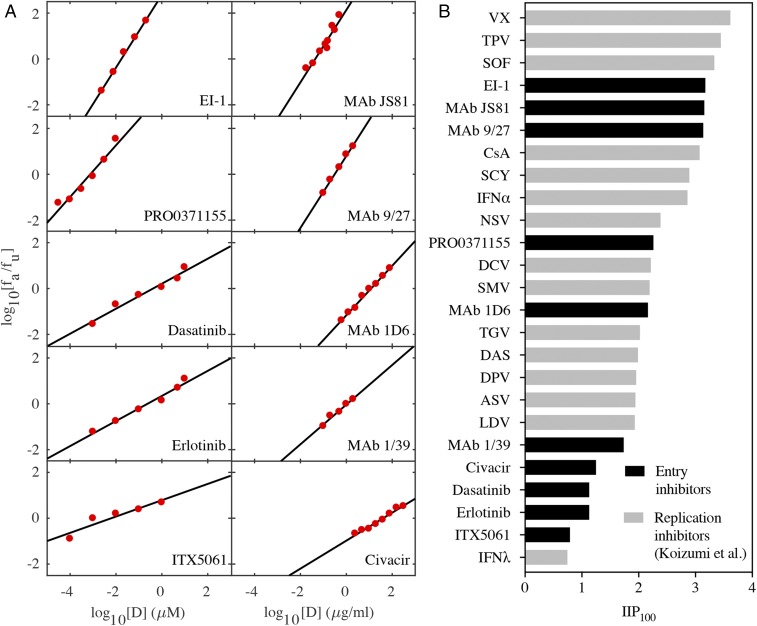
Previous studies have performed single-round infection experiments with virus particles pseudotyped with HCV envelope proteins and obtained dose–response curves to assess the efficacy of EIs (4–10). We analyzed dose–response curves of 10 EIs using the median effect equation and obtained good fits (Fig. 1A and Table 1). The resulting estimates of the half-maximal inhibitory concentration (IC50) and the slope factor, m, allowed estimation of , the log decline in single-round infectivity at drug concentration D. Following Koizumi et al. (1), we predicted IIP100 (i.e., IIP when D = 100 × IC50) of the 10 EIs. We found that the values ranged from 0.8 to 3.2 (Fig. 1B), spanning nearly the entire range reported by Koizumi et al. (1). Importantly, a few EIs had IIP100 values close to SOF (Fig. 1B), suggesting that these latter EIs may form potent ingredients of future drug combinations.
Fig. 1.
IIP of entry inhibitors compared with replication inhibitors. (A) Fits (lines) of the median effect equation, , to published experimental data (red circles) (4–10). Here, and are the fractions of entry events affected and unaffected by EIs, respectively, obtained in the experiments (investigating viral entry using virus particles pseudotyped with HCV envelope proteins) by normalizing the luciferase activity at different concentrations of EI with luciferase activity in the absence of EI. Data digitization was performed using Engauge digitizer 9.7 and fitted using the tool REGRESS in MATLAB 2016b. The best-fit parameter estimates are listed in Table 1. (B) Comparison of the IIP100 of the EIs in A with the IIP100 of the replication inhibitors studied by Koizumi et al. (1).
Table 1.
Best-fit estimates of dose–response curve parameters of EIs
| Data | Fits | |||||
| Compound | Cell line | HCV genotype | Ref. | m | IC50 | R2 |
| EI-1 | Huh-H1 | 1b | (4) | 1.59 | 1.75 × 10−2 μM | 0.997 |
| PRO0371155 | Hep3B | 1a | (5) | 1.13 | 7.67 × 10−4 μM | 0.951 |
| Dasatinib | Huh 7.5.1 | 2a (J6) | (6) | 0.55 | 0.42 μM | 0.965 |
| Erlotinib | Huh 7.5.1 | 2a (J6) | (6) | 0.55 | 0.24 μM | 0.976 |
| ITX5061 | PHH | 1b (HCV-J) | (7) | 0.36 | 6.28 × 10−3 µM | 0.88 |
| MAb 9/27 | Huh 7 | 1a | (8) | 1.57 | 0.31 μg⋅mL−1 | 0.995 |
| MAb 1/39 | Huh 7 | 1a | (8) | 0.87 | 1.1 μg⋅mL−1 | 0.971 |
| MAb JS81 | Huh 7 | N/A | (9) | 1.58 | 4.42 × 10−2 μg⋅mL−1 | 0.923 |
| MAb 1D6 | Huh 7 | N/A | (9) | 1.08 | 12.02 μg⋅mL−1 | 0.994 |
| Civacir | Huh 7.5.1 | 2a | (10) | 0.61 | 40.36 μg⋅mL−1 | 0.977 |
N/A, not available; PHH, primary human hepatocytes.
In conclusion, Koizumi et al. (1) successfully used the IIP to evaluate HCV replication inhibitors and presented a framework for identifying the best multidrug combinations in a preclinical setting. Our study suggests that the IIP can also be applied to HCV EIs and that some EIs appear nearly as potent as the best current drugs and should be considered in future efforts to identify optimal drug combinations.
Acknowledgments
This work was funded by Wellcome Trust/DBT India Alliance Senior Fellowship IA/S/14/1/501307 (to N.M.D.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Koizumi Y, et al. Quantifying antiviral activity optimizes drug combinations against hepatitis C virus infection. Proc Natl Acad Sci USA. 2017;114:1922–1927. doi: 10.1073/pnas.1610197114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jilek BL, et al. A quantitative basis for antiretroviral therapy for HIV-1 infection. Nat Med. 2012;18:446–451. doi: 10.1038/nm.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colpitts CC, Baumert TF. Hepatitis C virus cell entry: A target for novel antiviral strategies to address limitations of direct acting antivirals. Hepatol Int. 2016;10:741–748. doi: 10.1007/s12072-016-9724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldick CJ, et al. A novel small molecule inhibitor of hepatitis C virus entry. PLoS Pathog. 2010;6:e1001086. doi: 10.1371/journal.ppat.1001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coburn GA, et al. Novel small-molecule inhibitors of hepatitis C virus entry block viral spread and promote viral clearance in cell culture. PLoS One. 2012;7:e35351. doi: 10.1371/journal.pone.0035351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lupberger J, et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17:589–595. doi: 10.1038/nm.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syder AJ, et al. Small molecule scavenger receptor BI antagonists are potent HCV entry inhibitors. J Hepatol. 2011;54:48–55. doi: 10.1016/j.jhep.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Helle F, et al. The neutralizing activity of anti-hepatitis C virus antibodies is modulated by specific glycans on the E2 envelope protein. J Virol. 2007;81:8101–8111. doi: 10.1128/JVI.00127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertaux C, Dragic T. Different domains of CD81 mediate distinct stages of hepatitis C virus pseudoparticle entry. J Virol. 2006;80:4940–4948. doi: 10.1128/JVI.80.10.4940-4948.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tawar RG, et al. Broad neutralization of hepatitis C virus-resistant variants by Civacir hepatitis C immunoglobulin. Hepatology. 2016;64:1495–1506. doi: 10.1002/hep.28767. [DOI] [PMC free article] [PubMed] [Google Scholar]