Abstract
Hematopoietic stem cells (HSCs) that sustain lifelong blood production are created during embryogenesis. They emerge from a specialized endothelial population, termed hemogenic endothelium (HE), located in the ventral wall of the dorsal aorta (DA). In Xenopus, we have been studying the gene regulatory networks (GRNs) required for the formation of HSCs, and critically found that the hemogenic potential is defined at an earlier time point when precursors to the DA express hematopoietic as well as endothelial genes, in the definitive hemangioblasts (DHs). The GRN for DH programming has been constructed and, here, we show that bone morphogenetic protein (BMP) signaling is essential for the initiation of this GRN. BMP2, -4, and -7 are the principal ligands expressed in the lineage forming the HE. To investigate the requirement and timing of all BMP signaling in HSC ontogeny, we have used a transgenic line, which inducibly expresses an inhibitor of BMP signaling, Noggin, as well as a chemical inhibitor of BMP receptors, DMH1, and described the inputs from BMP signaling into the DH GRN and the HE, as well as into primitive hematopoiesis. BMP signaling is required in at least three points in DH programming: first to initiate the DH GRN through gata2 expression, then for kdr expression to enable the DH to respond to vascular endothelial growth factor A (VEGFA) ligand from the somites, and finally for gata2 expression in the DA, but is dispensable for HE specification after hemangioblasts have been formed.
Keywords: BMP, GRN, HSC, Xenopus, hematopoiesis
Hematopoietic stem cells (HSCs) reside at the top of the hematopoietic hierarchy and serve as the reservoir for all differentiated blood cells in adult life. This regenerative capacity has been harnessed to treat a range of conditions from hematological malignancies to immunodeficiency syndromes via stem cell transplantation (1). A critical factor determining transplantation success is the number of recoverable CD34+ hematopoietic stem and progenitor cells (HSPCs), which is challenging in many patients (2). Hence, de novo generation of HSCs has long been a goal to provide an ample supply of HSCs for transplantation. Transcription factor mediated reprogramming of somatic cells into induced HSCs and generation of HSCs via stepwise differentiation of pluripotent stem cells using developmental cues have both been used as methods with increasing but incomplete success (3, 4). A major caveat in both approaches is that the signaling cues and associated transcription networks required for HSC development are not yet fully understood.
HSCs are specified early during embryonic development through an intermediate stage called hemogenic endothelium (HE) from the dorsal aorta (DA) and other major arteries (5). Studies across model organisms ranging from fish and frogs to mice have shown that the hallmarks of HSC specification are conserved across species (6). In Xenopus, the cells fated to generate definitive blood are already segregated from primitive blood lineages at the 32 cell stage (7), a feature that allowed us to specifically characterize the HSC-generating endothelium of the DA and its precursors, definitive hemangioblasts (DHs) (8), at time points that are less feasible in other model organisms. Before the formation of the DA, these hemangioblasts are specified as bilateral populations in the lateral plate mesoderm, and express a cascade of transcription factors that are critical for hematopoietic and endothelial differentiation (see full list in ref. 9). In this 2013 study, we showed that vascular endothelial growth factor A (VEGFA)-dependent and -independent pathways synergize to specify this network of transcription factors culminating in a fli1+gata2+etv2+kdr+tal1+lmo2+ hemangioblast population. An analogous genetic network has recently been shown to operate in murine hematopoiesis (10). In Xenopus, fli1 resides at the top of the hierarchy and is required for gata2 and etv2 expression (11). The expression of scl/tal1 requires activation of the Kdr receptor by VEGFA in addition to the presence of the transcription factors already mentioned. It was not known whether fli1 is sufficient to activate gata2 and etv2 or how this transcription factor network is activated other than by the VEGFA input (9). Soon after the hemangioblasts are established, a subset of these cells migrate to the midline to form the DA (7, 12). Specification of the HE toward the adult hematopoietic lineage happens in the ventral wall of the DA and can be assessed by the expression of the hematopoietic markers, runx1, gfi1a, and spib/pu.1 (13, 14).
Bone morphogenetic protein (BMP) signaling has long been acknowledged to be pivotal in mesoderm induction and hematopoietic commitment (15). Specifically, BMP4 ligand is expressed in the mesenchyme around the DA across species (16–18). Addition of BMP4 ligand increases HSC output from cultured mouse aorta–gonad–mesonephros (AGM) tissue, and inhibition of BMP signaling by an antagonist ligand gremlin1 suggests that BMP is required for AGM hematopoiesis (19). In zebrafish, BMP signaling is required for the generation of runx1 positive HE and temporal loss of function analysis strongly suggests that the source of BMP is the BMP4 ligand underneath the DA (18). On the other hand, loss of function experiments in mouse embryos and embryoid body (EB) cultures did not reveal a role for BMP signaling after the specification of kdr+ mesoderm, which contains all of the precursors of blood and endothelium (20–23). Previous attempts to characterize the role of BMP signaling in primitive and definitive hematopoiesis in Xenopus lacked the temporal reagents to analyze BMP signaling specifically in the definitive blood lineage at high resolution (8). Therefore, we decided to revisit the role of BMP signaling in the definitive blood lineage to dissect the input of BMP into the updated gene regulatory network (GRN), as well as to resolve the apparent discrepancy between zebrafish and mouse with respect to BMP requirements. Here, we show that BMP signaling is required for the generation of the DH, but dispensable for the subsequent specification of HE. We identified three sequential inputs from BMP signaling into the establishment of the DH GRN, an early requirement for the expression of gata2, a transcription factor which together with Etv2 is at the top of the hierarchy controlling the establishment of definitive hematopoiesis, then for maintaining kdr expression, which is essential for tal1 expression and finally for priming the expression of gata2 after formation of the DA in the midline.
Results
BMP Signaling Is Required for Primitive and Definitive Hematopoiesis.
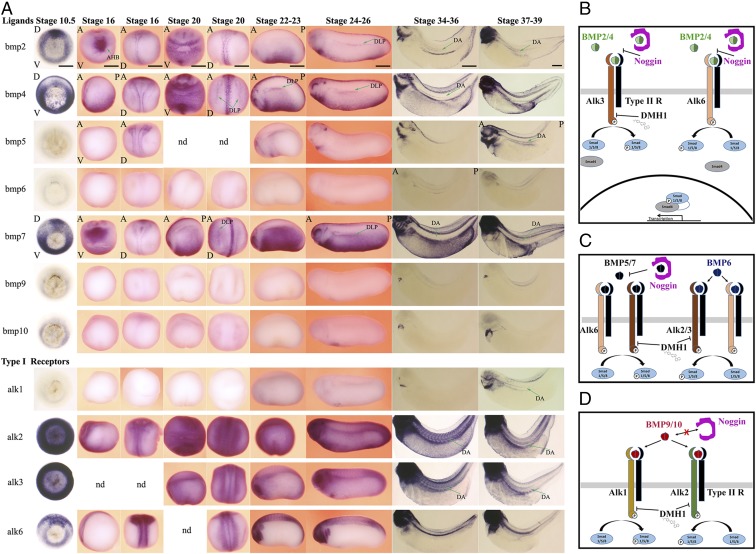
BMP signaling is required for primitive blood formation in Xenopus (8, 24). Previously, we attempted to determine the function of BMP signaling in primitive and definitive blood lineages using a truncated, dominant negative BMP type I receptor construct (tBR) (25) by injecting it as an mRNA, which led to extensive mesodermal patterning defects complicating analysis of the role of BMP signaling specifically in definitive hematopoiesis (8). In retrospect, this approach was crude because it lacked temporal control, and the complexity and redundancy in the BMP signaling cascade was not known at the time. BMP signaling can be activated by up to a dozen ligands binding to four type I receptors that form heterocomplexes with three type II receptors (26). Because the specificity of the signaling is determined by the localized expression of the ligands, we extensively analyzed the expression of all BMP ligands and their corresponding receptors to identify those that might be involved in HSC formation. We found that BMP ligands bmp2, bmp4, and bmp7 are the principal BMP signaling drivers expressed in the DA as well as in the DH precursors of the HE in the dorsal lateral plate (DLP) mesoderm (Fig. 1A). These ligands are also expressed during gastrulation and then ventrally in primitive blood and cardiac progenitors. In addition, bmp5 is expressed in the DA. Of the four type I receptors, alk2 and alk3 are ubiquitously expressed at all stages analyzed, and alk1 is expressed in the DA. Alk6 is not expressed in the DLP or DA at the times analyzed. Finally, type II receptors, acvr2a and acvr2b, are expressed broadly from gastrulation and all type II receptors including bmpr2 are expressed in the DA (Fig. S1). Hence, BMP receptors are in place at all time points starting from gastrulation to potentially have a role in the formation of the HE. Because multiple BMP type I and type II receptors are broadly expressed, a viable approach to analyzing BMP signaling input into definitive hematopoiesis would be to knock down BMP ligands bmp2, -4, and -7. However, a previous study demonstrated that at least during gastrulation, the function of these ligands are redundant and to block BMP signaling one needs to knock down all three at the same time. However, triple knockdown of bmp2, -4, and -7 in Xenopus results in disruption of ventral mesendoderm formation preventing further analysis (27). Moreover, because the ligands are expressed widely in the ventral and lateral plate mesoderm especially at earlier stages, and because they are soluble molecules, a tissue-specific knockdown approach is not feasible. To bypass the requirement for BMP signaling in mesendoderm induction and patterning, yet be able to block it completely and in a temporally controlled fashion, we used a heat shock-inducible Noggin transgenic line (28), as well as a specific chemical inhibitor of BMP signaling, DMH1 (29). Noggin is a well-characterized extracellular antagonist of BMP ligands that inhibits BMP signaling by sequestering ligands away from the receptors. Noggin inhibits signaling downstream of BMP2, -4, -5, and -7, all of the ligands expressed before the formation of the HE (Fig. 1 B and C), while leaving signaling downstream of BMP6, -9, and -10, the ligands expressed specifically in the heart and kidney tubules, intact (Fig. 1 C and D) (30, 31). Hence, noggin is an ideal tool to ablate all of the BMP signaling that might be required during HE formation. DMH1 is a second-generation chemical inhibitor of BMP signaling that inhibits signaling downstream of Alk2 and Alk3 (29), the main BMP type I receptors expressed before and during the formation of HE, as well as Alk1 (32), a DA-specific BMP type I receptor (Fig. 1A), while leaving signaling downstream of Alk4 (activin signaling), Alk5 (TGFB signaling), and Alk6 [BMP signaling in neural tissue, somites, and notochord (Fig. 1A)] intact (29, 32). Because Alk6 is not expressed in lateral mesoderm, in the DLP or in the DA, DMH1 is expected to block all signaling activated by BMP2, -4, -5, and -7 in these tissues. Therefore, DMH1 blocks BMP signaling in a similar way to Noggin in ventral mesoderm and lateral mesoderm derivatives but by blocking BMP type I receptor activity rather than sequestering BMP ligands. In summary, these two reagents are used to block all BMP signaling during HSC ontogeny.
Fig. 1.
Expression analysis of BMP ligands and receptors from midgastrulation stage to hemogenic endothelium formation and the description of reagents to inhibit all BMP signaling in blood ontogeny. Primarily, expression in tissues involved in blood stem cell lineage is described. (A) Ligands: bmp2, bmp4, and bmp7 are the principal BMP ligands expressed during gastrulation and onward. At stage 10.5, midgastrulation, bmp2 is expressed in the dorsal mesendoderm. At stage 16, early neurula, bmp2 expression is localized to anterior ventral mesoderm, which encompasses cardiac progenitors and anterior hemangioblasts (AHB). At stage 20–23, bmp2 is expressed along the ventral mesoderm and in lateral plate mesoderm, with signal tapering toward the DLP mesoderm. At stage 26, bmp2 expression is localized along the DLP as well as ventral and cardiac mesoderm. At stages 34–39, bmp2 is expressed in the DA. At stage 10.5, bmp4 is expressed in mesendoderm, with expression diminishing toward dorsal mesendoderm. At stage 16–20, bmp4 is expressed extensively in ventral and lateral mesoderm. From stage 20 onward, bmp4 expression is localized to DLP, encompassing future hemangioblast. At stages 21–26, bmp4 expression is strong along the ventral and lateral plate mesoderm, specifically delineating the DLP tissue at the dorsal most expression domain. At stage 34, bmp4 is expressed in the DA. Bmp5 is not expressed in tissues related to blood lineages, except at stage 38–39, when it is expressed in the DA. Bmp6 is not expressed in HSC lineage. The only expression detectable is transiently in kidney tubules at stage 36. Bmp7 expression at stage 10.5 is localized to ventral most mesendoderm as well as a subset of dorsal mesendoderm. At stages 16–26, bmp7 is expressed along the ventral and lateral plate mesoderm, bordering the kidney field in the dorsal most extent. Bmp7 is strongly expressed in the lateral plate mesoderm for the rest of the developmental stages analyzed. From stage 34 onward, bmp7 is also expressed in the DA. Bmp8 is not expressed during Xenopus development. Bmp9 and bmp10 are expressed in the heart from stage 26 onward. Type I receptors: alk1 is not expressed in early time points; later alk1 expression is primarily restricted to the heart and at stage 38–39 to DA. Alk2 and alk3 are ubiquitously expressed at all stages analyzed. From stage 34 onward, their expression is also observed in the DA. Alk6 is not expressed in blood stem cell lineage but is widely expressed early in dorsal mesoderm and later in neural tissues, neural crest, somites, and notochord. Embryos at stages 10.5 and 34–39 are cleared to enable visualization of the staining of the deeper tissues. For stage 10.5 embryos, D is dorsal and V is for ventral side. For stages 16–20, embryos are visualized from anterior-dorsal (A-D) and anterior-ventral (A-V) angles, except stage 16 bmp4 staining, which is documented from anterior–posterior (A-P) lateral view. From stage 21 onward, the pictures of embryos are taken from lateral view, with anterior–posterior labeled, where it is relevant, on the figure. Expression profile was not determined (nd) for some stages. (Scale bars: 0.5 mm.) (B) BMP2 and BMP4 signal through type I receptors, Alk3 and Alk6. Noggin inhibits signaling by preventing ligands from binding to receptors. DMH1 blocks signaling downstream of Alk3 but not Alk6. (C) BMP5, -6, and -7 signal through type I receptors Alk2, Alk3, and Alk6. Noggin blocks binding of BMP5 and -7 to type I receptors. Noggin does not inhibit signaling downstream of BMP6. DMH1 inhibits Alk2 and Alk3 but not Alk6. (D) BMP9 and -10 signal through type I receptors Alk1 and Alk2. Noggin does not block BMP9 or -10 from binding to receptors. DMH1 blocks Alk1 and Alk2 receptor activity.
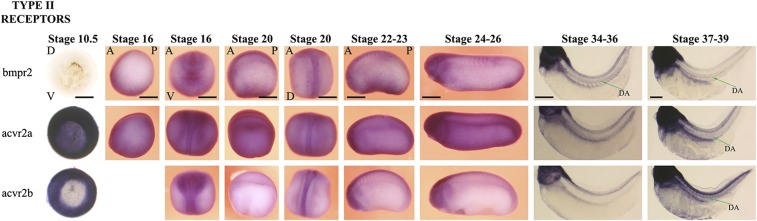
Fig. S1.
BMP type II receptors. Type II receptors complement type I receptors and can potentially interact with any type I receptor with ligands being the determinant of the nature of heterocomplexes. Bmpr2 is not expressed at gastrulation, but its expression is activated in the ventral mesoderm from stage 16, later extending to almost all tissues. At stages 34–39, Bmpr2 is also expressed in the DA. Acvr2a is expressed ubiquitously at all stages analyzed. Acvr2b is expressed ubiquitously at gastrulation but later display a restricted expression pattern, being expressed predominantly in neurons. Acvr2b is localized to DA at stage 39. Embryos at stages 10.5 and 34–39 are cleared to enable visualization of the staining in deeper tissues. For stage 10.5 embryos, D indicates dorsal and V indicates the ventral side. For stages 16–20, embryos are visualized from dorsal (D) and ventral (V) angles, with anterior (A) at the top, except stage 16 bmp4 staining, which is documented from an anterior–posterior (A-P) lateral view. From stage 21 onward, the pictures of embryos are taken from a lateral view, with anterior-posterior labeled where it is relevant, on the figure.
Because the HS:Noggin transgenic line has not been validated for the stages we aimed to analyze, to understand the dynamics of noggin induction upon heat shock, we monitored Noggin protein levels. Noggin was detectable within 30 min of heat-shock treatment, the protein level remained steady for up to 24 h (Fig. S2A), and the exogenous noggin mRNA was present throughout the embryo sections 2 h after treatment in stage 33 embryos (Fig. S2B). Furthermore, BMP-induced phosphorylation of Smad1, -5, and -8 was blocked by Noggin induction as well as DMH1 treatment (Fig. S2C). Noggin and DMH1 treatments before gastrulation led to strong dorsalization, which was avoided by starting the treatment postgastrulation at stage 14. In these embryos, lateral plate mesoderm patterning is relatively normal with some posterior patterning defects evident. Hence, we adopted a treatment regime where we heat shock or chemically treat the embryos from stage 14, with iteration of the treatment every 24 h when embryos are cultured longer than a day. As expected, inhibition of BMP signaling by Noggin and DMH1 completely blocks expression of red blood cell markers, tal1 and hba3 (hemoglobin alpha 3 subunit), further validating the reagents (Fig. 2A).
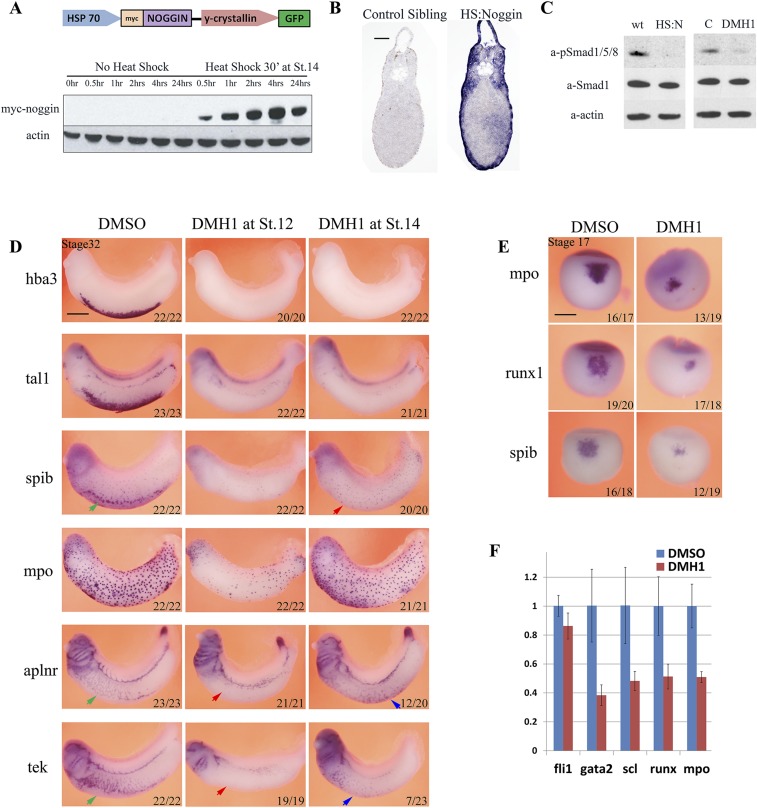
Fig. S2.
Inhibition of BMP signaling by use of HS:Noggin transgenic line and chemical inhibitor DMH1 blocks embryonic myelopoiesis, erythropoiesis, and vasculogenesis. (A) Diagram showing the transgene in HS:Noggin transgenic line. A 15-min heat shock induces myc-noggin protein expression within 30 min. Noggin levels remain steady for at least 24 h after treatment. (B) In situ hybridization on sections of stage 33 embryos shows that induced noggin is expressed across the section in HS:Noggin transgenic siblings. Embryos were heat-shocked for 15 min at 35 °C at stage 32 and fixed 2 h after the heat shock treatment. (C) Inhibition of BMP signaling in heat-shocked noggin (HS:N) and DMH1-treated embryos compared to heat-shocked wild type siblings (wt) or DMSO solvent-treated controls (C) is verified by the loss of P-Smad1/5/8 signal in Western blotting. Embryos were treated from stage14, and DLPs were explanted at stage 25, about 24 h after heat shock, further confirming that noggin protein is active a day after heat shock. Smad1 staining and actin staining were used as loading controls. (D) Timing of BMP requirement in ventral blood island. Inhibition of BMP signaling by DMH1 from stage 12 leads to a strong reduction in primitive myeloid cells originating from both first (mpo, spib) and second (ventral spib staining, red arrow) waves of myelopoiesis. DMH1 treatment blocks hematopoiesis as well as endothelial development when administered from stage 12, at the end of gastrulation. Expression of erythropoiesis markers hba3 and tal1 is absent, and expression of myelopoiesis markers spib and mpo is severely reduced. Endothelial gene expression of aplnr and tek in vitelline vessels, neighboring the ventral blood island, is also absent (red arrows). Aplnr and tek are expressed in the head and the cardinal vein precursors but at a lower level. DMH1 treatment from stage14, early neurulation, eliminates erythroid gene expression, as in treatment starting from stage 12. Spib expression in the periphery of ventral blood island are also absent (red arrow) whereas patrolling myeloid cells, marked by spib and mpo, are unaffected. DMH1 treatment from stage 14 does not block aplnr expression. In 12 of 20 embryos, aplnr expression was expanded to the most ventral tissues in the territory of blood island (blue arrow). DMH1 treatment from stage 14 reduces tek expression (23 of 23 embryos), but in 7 of 23 embryos tek expression was also observed in ventral blood island (blue arrow). (E) DMH1 treatment from stage 12 blocks myeloid gene expression of mpo, spib, and runx1 in anterior hemangioblast/aVBI at stage 16. (F) qRT-PCR analysis of dissected stage 16 aVBI tissue treated with DMH1 from stage 12 shows expression of blood genes gata2, tal1/scl, runx1, and mpo are reduced in stage 16 aVBI tissue, whereas fli1 expression is normal.
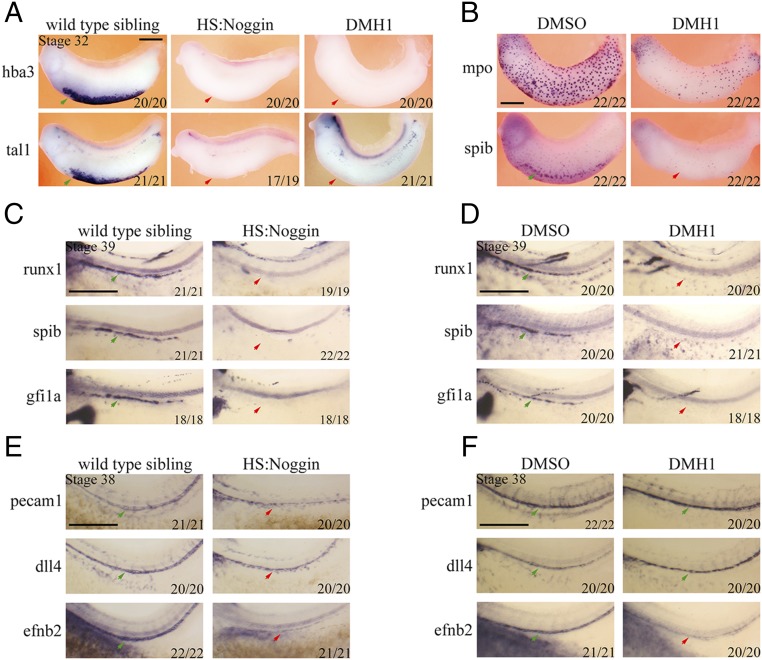
Fig. 2.
BMP signaling is required for definitive and primitive hematopoiesis in Xenopus laevis. (A) Noggin protein induced by heat-shock treatment at stage 14 leads to a complete loss of tal1 and hba3 expression in ventral blood islands (arrows) in HS:Noggin transgenic Xenopus stage 32 embryos. Similarly, DMH1 treatment starting from stage 14 blocks tal1 and hba3 expression. (B) Inhibition of BMP signaling by DMH1 from stage 14 abrogates expression of myeloid blood markers mpo and spib at stage 33 compared with DMSO-treated control. (C and D) Inhibition of BMP signaling from stage 14 by Noggin (C) or DMH1 (D) results in the loss of expression of hemogenic endothelium markers runx1, spib, and gfi1a in the DA (red arrow for absence of in treated, green arrows for normal expression in control embryos). (E and F) The endothelium marker pecam1 and arterial gene dll4 is expressed in control as well as treated samples, albeit slightly lower in HS:Noggin embryos. The arterial marker efnb2 is severely reduced in either HS:Noggin-expressing or DMH1-treated embryos (red arrows in E and F). The stages at which the in situ hybridization was performed are indicated on the top left corner. Embryos were photographed from a lateral view, with anterior on the left and dorsal at the top. Numbers in bottom right corner indicate proportion of embryos displaying the phenotype. (Scale bars: 0.5 mm.)
BMP signaling is required for primitive myelopoiesis in zebrafish (33), but a previous study in Xenopus did not detect an effect on myeloid development (34). Primitive myeloid cells are specified in two waves in Xenopus, the first one from the embryonic hemangioblast as early as the end of gastrulation at stage 13 [runx1 (35), cebpa, spib, and mpo expression profiles submitted directly to Xenbase (36) by A.C.-U.], and the second one from posterior ventral tissues around stage 30 in close association with the primitive blood islands (37). Embryonic hemangioblasts are first exposed to BMP ligands at the end of gastrulation by stage 13 (38). To probe the BMP requirement of the first myeloid wave, embryos were treated from stage 12. Indeed, DMH1 treatment resulted in a strong reduction of patrolling myeloid cells, mpo and spib, and inhibited the specification of the second wave, ventral spib staining (Fig. 2B). When BMP signaling was blocked from stage 14 onward, there wasn’t a reduction in the numbers of patrolling myeloid cells but the spib-marked second wave was still absent (Fig. S2D). In addition, we characterized embryonic hemangioblast differentiation at stage 16 by WISH and quantitative (q)PCR, and found that myeloid markers were already strongly reduced at stage 16 (Fig. S2 E and F). Hence, BMP signaling is required for primitive myelopoiesis as well as erythropoiesis in Xenopus.
BMP signaling is essential for hemangioblast formation and enhances endothelial commitment in ES cell cultures (39, 40). Embryos treated with DMH1 from stage 12 lacked vitelline vessels and displayed disorganized expression of the endothelial genes aplnr and tie2/tek in the cardinal vein. When treatment started at stage 14, this effect was largely absent, and crucially as in Myers and Krieg (34), a fraction of embryos displayed expanded expression of endothelial markers in the ventral periphery, where erythrocyte precursors would have been located (Fig. S2D), albeit in a disorganized manner. In conclusion, in addition to validating heat-shock Noggin and DMH1 as appropriate reagents to block BMP signaling, we confirmed the predicted requirement, based on zebrafish embryos and embryonic stem cell (ESC) cultures, for BMP signaling in primitive myeloid and endothelial specification in Xenopus.
Next, we investigated whether BMP signaling is required for definitive hematopoiesis. Runx1, a marker of HE and later of HSCs, is expressed along the ventral wall of the DA at stage 39. Gfi1a and spib, a Xenopus analog of pu.1, are direct downstream targets of Runx1 in the murine system (41) and are expressed in the hemogenic endothelium of the DA in Xenopus. Inhibition of BMP signaling from stage 14 by misexpression of Noggin or by treatment with DMH1 results in the loss of hemogenic endothelium marker expression in the DA of stage 39 embryos (Fig. 2 C and D). Indeed, runx1, gfi1a, and spib expression is completely eliminated in all of the treated embryos analyzed. On the other hand, the endothelium marker pecam1 and arterial marker dll4 are expressed along the trunk in treated embryos, albeit at slightly lower levels, indicating that the DA precursors are present (Fig. 2E). In addition, the arterially expressed gene efnb2 is severely reduced both in HS:Noggin misexpressed and DMH1-treated embryos (Fig. 2 E and F). Hence, we conclude that BMP signaling is required for the formation of hemogenic endothelium of the HSC lineage as well as for the proper specification of the DA.
BMP Signaling Is Required for Definitive Hemangioblast Programming but Dispensable After Their Formation.
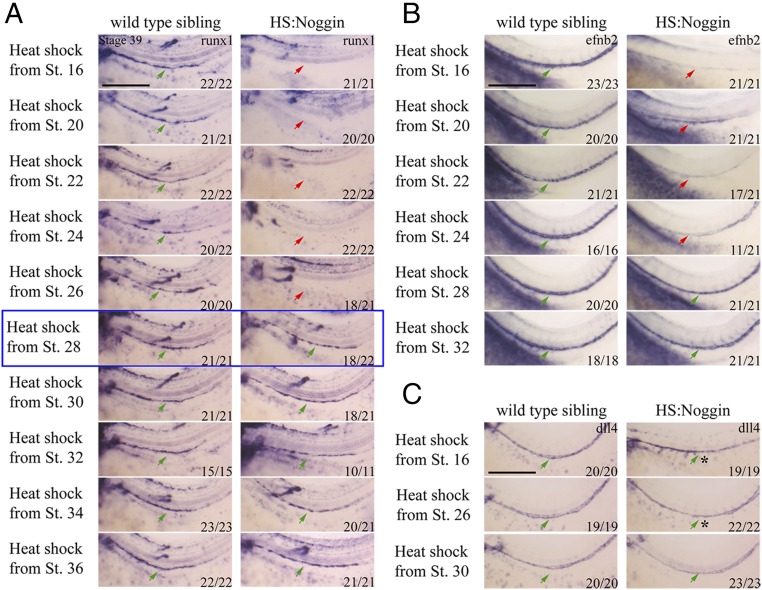
Having shown the requirement for BMP signaling for definitive hematopoiesis, we sought to dissect whether it is required at one specific stage or repetitively for key events. All HSCs in Xenopus are derived from DLP mesoderm (42, 43). The DLP first starts expressing angiogenic genes at stage 20 and is further matured into the full hemangioblast program during stages 24–27 by VEGFA signaling emanating from within the DLP as well as the somites (9). A subset of the DH starts migrating to the midline starting from stage 28, coalescing to form the DA at stage 31–32. The ventral floor of the DA becomes runx1+ at stage 36. By stage 39, HE expresses gfi1a, spib, tal1, and lmo2, as well as runx1. Runx1 expression persists until HSPCs are budding from the DA at stage 43. We therefore started Noggin and DMH1 treatment at progressively later stages to identify for which specific event BMP is necessary. Misexpression of Noggin starting from time points before stage 28 completely eliminates runx1 expression in the DA at stage 39 (Fig. 3A). Coincidentally, there was a strong reduction in the expression of DA marker efnb2 in treatments starting before stage 28 (Fig. 3B). However, another arterial marker dll4 is expressed in the DA, regardless of the time BMP signaling is blocked, showing that not all arterial programming requires BMP (Fig. 3C). A subset of stages describing the embryo-wide phenotype along with runx1 and dll4 expression, and how HS:Noggin embryos are sorted from wild-type siblings, are described in Fig. S3. In short, we observed loss of runx1 expression together with defects in the DA patterning when treatments were started before stage 28 (Fig. S3 A and B). Similarly, inhibition of BMP signaling by the drug, DMH1, starting from any time point before stage 28, results in loss of runx1 expression (Fig. S3C). Runx1 is continuously expressed in and underneath the floor of the DA until HSPCs are budding off from the DA at stages 43–44. Similar to the observation at stage 39, abolition of BMP signaling starting from stage 30, does not affect runx1 expression at stage 43 (Fig. S3 D and E). As in earlier time points (Fig. S2C), DMH1 inhibits BMP signaling in later stages effectively (Fig. S3F), and Noggin can be efficiently induced by heat shock in stage 32 embryos (Fig. S2B), which shows that the reagents work as expected at later stages. Therefore, we conclude that BMP signaling is required for specification of the definitive blood lineage during the hemangioblast stages before the formation of the DA, but is dispensable afterward.
Fig. 3.
BMP is required for the specification of hemogenic endothelium before stage 28. (A) Runx1 WISH of heat-shock time course. Runx1 expression is abolished in HS:Noggin siblings when BMP signaling is inhibited before stage 28 (blue box). Arrows mark runx1 expression along the DA, with green arrows indicating normal expression levels and red arrows indicating absent or decreased expression. Expression of runx1 is unaffected when the treatment is started at stage 28 or afterward. Runx1 is also expressed in lateral line nerves (*) as well as in some motor neurons (*), the staining of which in some images is overlaid with runx1 staining in the DA due to embryo positioning and focus. (B) Efnb2 expression is strongly reduced in HS:Noggin siblings when BMP signaling is blocked before stage 28. (C) Dll4 expression is relatively normal in HS:Noggin siblings. However, the DA is not properly lumenized (*) when BMP signaling is inhibited before stage 28. Embryos were visualized from the lateral view, zoomed in to the trunk region, with anterior to the left and dorsal at the top. Proportion of embryos which the image represents is shown in bottom right corner. (Scale bars: 0.5 mm.)
Fig. S3.
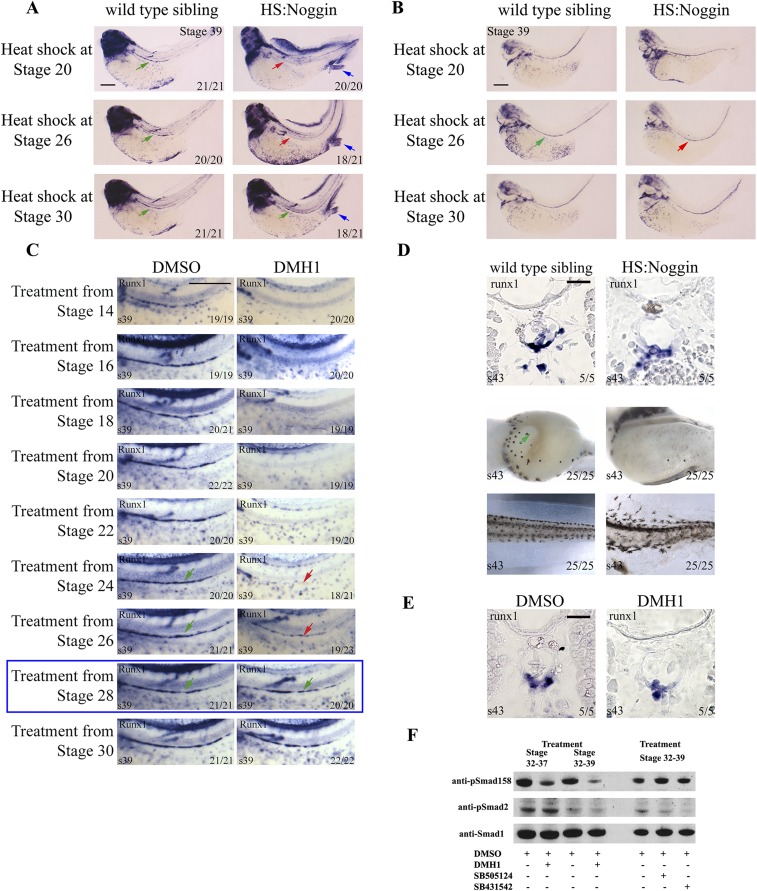
BMP is required before stage 28 for the specification hemogenic endothelium. (A) Runx1 WISH of heat-shock time course. Embryos were heat-shocked for 30 min at 35 °C at the stage indicated and were heat-shocked every 24 h until stage 39, when the embryos were collected for analysis. Runx1-expressing cells were then stained by WISH. Embryos are shown from the lateral view, with anterior to the left and dorsal to the top. Green arrows mark the position of the DA, stained for runx1 in control siblings as well as HS:Noggin embryos treated from stage 30. Red arrow shows that runx1 staining in the DA is missing when embryos are heat-shocked starting from stage 20 and 26. HS:Noggin embryos were sorted by visual inspection of shuffled fin and protruding proctodaeum phenotype (blue arrow). Proportion of embryos for which that image represents is shown in bottom right corner. (B) BMP signaling is not required for dll4 expression. Dll4 WISH of heat-shock time course. Embryos were treated as in A and stained for Dll4. The arterial marker dll4 is expressed at all stages analyzed albeit disorganized. (C) Runx1 WISH of DMH1 treatment time course. Embryos were cultured in 0.1× MBS containing 100 μM DMH1 from the stage indicated and were cultured to stage 39, when the embryos were collected for analysis. Runx1-expressing cells were then stained by WISH. Embryos are shown from the lateral view, zoomed into the trunk region, with anterior to the left and dorsal to the top. Green arrows mark the position of the DA, stained for runx1 in control siblings as well as stage 28 DMH1-treated embryos. The red arrow shows that runx1 staining in the DA is missing when embryos are treated starting from stage 24 and decreased when treated starting from stage 26. The blue box highlights stage 28; after that time point, there is no effect on runx1 expression. (D and E) BMP is not required for runx1 maintenance in Xenopus DA. BMP was inhibited from stage 28 by either 100 μM DMH1 treatment or by noggin heat-shock induction. Embryos were collected at stage 43. D, Top shows runx1-expressing cells stained by WISH on wax sections. Sections are 10-μm transversal slices taken through the trunk of the embryo. D, Middle shows embryos, visualized from the ventral aspect, with anterior to the left. The green arrow indicates normal intestinal morphogenesis, which is absent in Bmp-inhibited embryos. D, Bottom shows stage 43 embryo tails, shown from the lateral view, with anterior to the left and dorsal at the top. Bmp-inhibited embryos have an irregular tail fin, whereas control embryos have a smooth edge to the tail fin. Proportion of embryos for which that image represents is shown in bottom right corner in all figures. Images and numbers are from one experiment and are representative of at least three biological replicates except in sectioned embryos. (F) DMH1 inhibits Smad1/5/8 phosphorylation from stage 32 to, and from stage 32–39 without affecting Smad2 phosphorylation level. Treatment with TGFB inhibitors SB505124 and SB431542 from stage 32–39 reduces Smad2 phosphorylation (WB with Millipore anti-Psmad2 Ab no. 04-953) without affecting phospho Smad1/5/8 levels. Smad1 is expressed relatively uniformly between matched DMSO and chemical inhibitor treatments.
BMP Signaling Is Required for gata2 and kdr Expression in DHs.
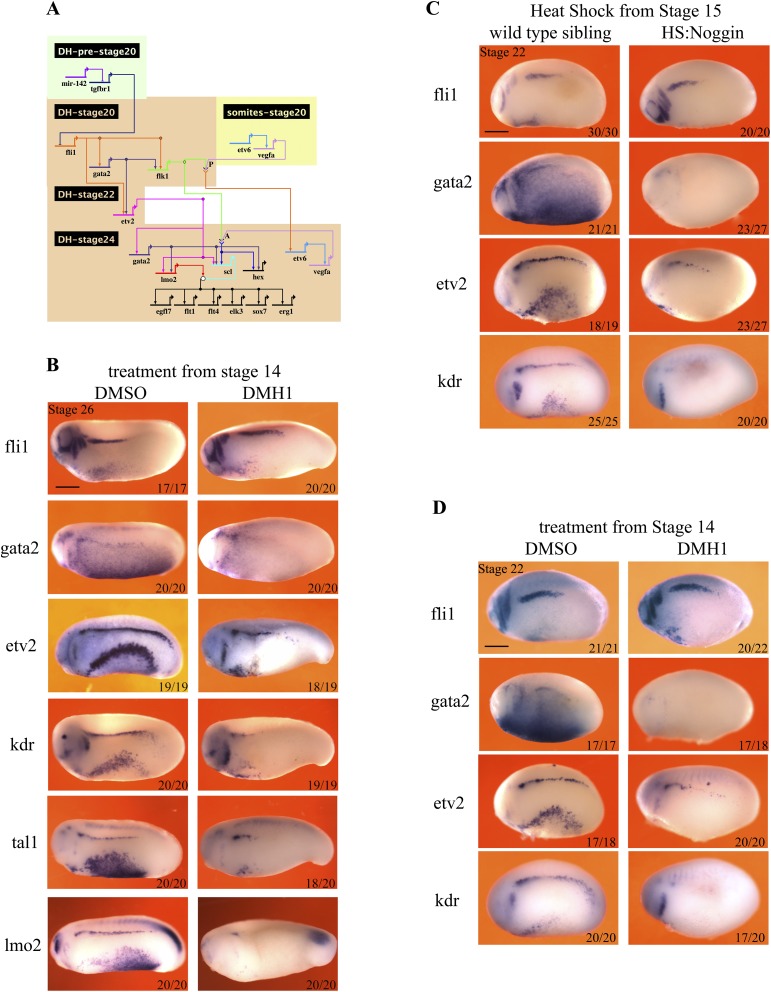
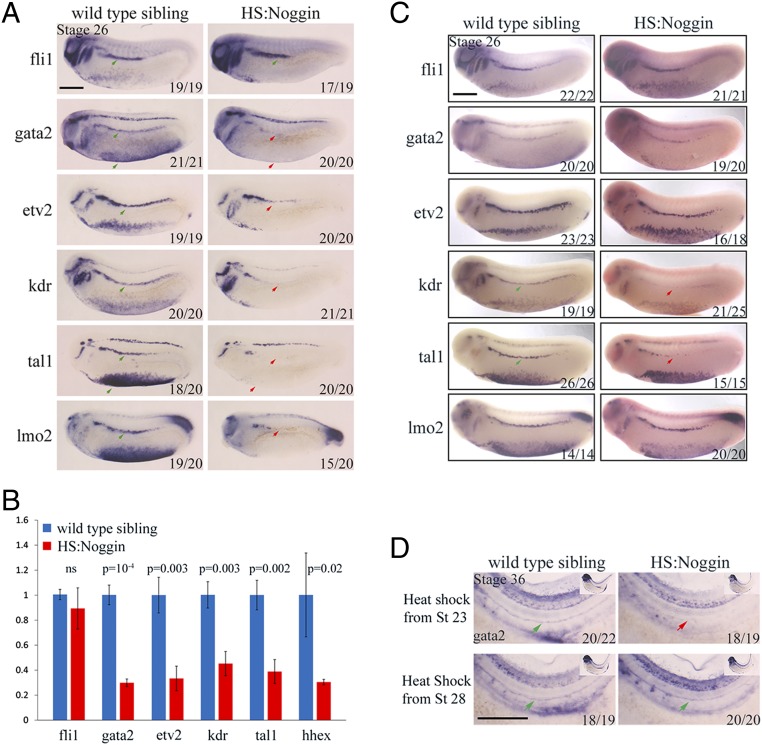
Because we observed that BMP signaling is required only during DH specification, we sought to understand its input into the GRN of the definitive hemangioblast. Previously, we described the hemangioblast transcriptional network in great detail (9). An updated version of this GRN is presented here as a guide (Fig. S4A). Importantly, it shows that fli1 and gata2 are at the top of the hierarchy, and are required early for flk1/kdr expression. Fli1 and Gata2 are later required for etv2 expression at stage 22. Crucially, the requirement for Gata2 in etv2 induction is partial (9). Once the expression of the core transcription factors, fli1, gata2 and etv2, is established, VEGFA signaling through Flk1/Kdr completes the circuit culminating in the induction of tal1/scl and hhex transcription factors. Tal1/Scl then controls the downstream gene expression cascade together with Lmo2. When BMP signaling is blocked by HS:Noggin (Fig. 4A) or DMH1 (Fig. S4B) from stage 14, there is no significant change in fli1 expression levels, but the expression of gata2 is completely abolished. The level of kdr decreases drastically whereas expression of etv2 is reduced. Furthermore, the expression of tal1/scl and lmo2 is completely abolished in the DLP. We then quantitated the levels of expression of DLP genes by qPCR using cDNA from DLP tissue dissected at stage 25. Similar to the observations in WISH, all of the hemangioblast genes except fli1 display significantly decreased expression in HS:Noggin misexpressed DLPs (Fig. 4B). Because some of the DH genes are already expressed at stage 22, we probed the effect of BMP inhibition at this stage as well. As in stage 25 embryos, gata2 and kdr are not expressed at stage 22 in treated embryos. Again, there is no effect on fli1 expression, but the level of etv2 is strongly reduced at stage 22 (Fig. S4 C and D). Thus, we conclude that BMP signaling is required to initiate the DH network through induction of gata2 expression.
Fig. S4.
Bmp signaling is required for gata2 expression in the DLP. (A) GRN summarizing the known requirements for the programming of DH in the DLP. The timing and tissue of expression is shown by the position of the gene name with gene inputs shown as arrows. Note that arrows do not necessarily signify direct interactions but indicate requirements for expression. Where two gene products physically interact for the expression of a target, their arrows converge on chevrons for receptor ligand interaction or a circle for interactions between transcription factors. Where a gene is shown twice indicates a change in its regulation (gata2 is initiated by fli1 but later requires etv2 for its maintenance). (B) Hematopoietic programming in the DLP, at stage 26, following DMH1 treatment from stage 14. Embryos were treated with 100 μM DMH1 from stage 14 and were collected for analysis at stage 26. WISH was used to stain cells expressing genes that are essential for hematopoietic programming in the DLP: fli1, gata2, kdr, etv2, tal1, lmo2, and hex. Embryos are shown in lateral view, with anterior to the left and dorsal at the top. Red arrowheads mark staining in the DLP. (C) Hematopoietic programming in the DLP at stage 22, following heat shock-induced noggin expression at stage 14. The expression of fli1 in the DLP is unchanged, whereas etv2 is expressed at reduced levels and gata2 and kdr are absent (red arrowheads). Embryos were heat-shocked for 15 min at 35 °C at stage 14 and were collected for analysis at stage 22. WISH was used to stain cells expressing genes that are essential to hematopoietic programming in the DLP: fli1, gata2, kdr, and etv2. (D) Hematopoietic programming in the DLP at stage 22, following DMH1 treatment from stage 14. The expression of fli1 in the DLP is unchanged, whereas etv2 is expressed at reduced levels and gata2 and kdr are absent. Embryos were treated with 100 μM DMH1 from stage 14 and were collected for analysis at stage 22. WISH was used to stain cells expressing genes that are essential for hematopoietic programming in the DLP: fli1, gata2, kdr, and etv2. Embryos are shown in lateral view, with anterior to the left and dorsal at the top. Numbers on individual images show the proportion of embryos that the image represents (Bottom Right). Images and numbers are from one experiment and are representative of two biological replicates.
Fig. 4.
BMP signaling is required for gata2 expression in the DLP transcriptional network. (A) Hematopoietic programming in the DLP, at stage 25, following heat shock-induced noggin expression at stage 15. Embryos were heat-shocked for 15 min at 35 °C at stage 15 and were collected for analysis at stage 25. Fli1 expression is unaffected in the DLP (green arrows), whereas gata2, kdr, tal1, and lmo2 expression is absent in the DLP as well as ventral blood island (VBI) (red arrows) of HS:Noggin transgenic siblings. Etv2 expression is decreased in the DLP and is absent in VBIs (red arrow). Numbers on individual images show the proportion of embryos for which that image represents (bottom right). (B) qPCR analysis of hemangioblast genes in stage 25 DLPs dissected from control and heat-shock noggin transgenic embryos. Fli1 is not significantly affected, whereas all of the other genes quantified show significant decreases of expression. (C) Hematopoietic programming in the DLP, at stage 26, following heat shock-induced noggin expression at stage 20. The expression of fli1, gata2, etv2, and lmo2 is unaffected. The expression of kdr and tal1 is reduced in the DLP hemangioblasts. HS:Noggin transgenic siblings were identified by the expression of Noggin transcripts (uniform Fast Red staining in HS:Noggin embryos). (D) Gata2 expression in the DA, at stage 36, following heat shock-induced noggin misexpression at stages 23 and 28. Gata2 expression is absent in the DA of HS:Noggin transgenic siblings when heat shock is done at stage 23 (red arrow), whereas gata2 is expressed in the DA of HS:Noggin siblings when heat shock is done at stage 28, compared with wild-type siblings (green arrows). The stage of the embryos are indicated in the top left corner of the top left image. Numbers on individual images shows the proportion of embryos that the image represents (Bottom Right). Images and numbers are from one experiment and are representative of two biological replicates. (Scale bars: 0.5 mm.)
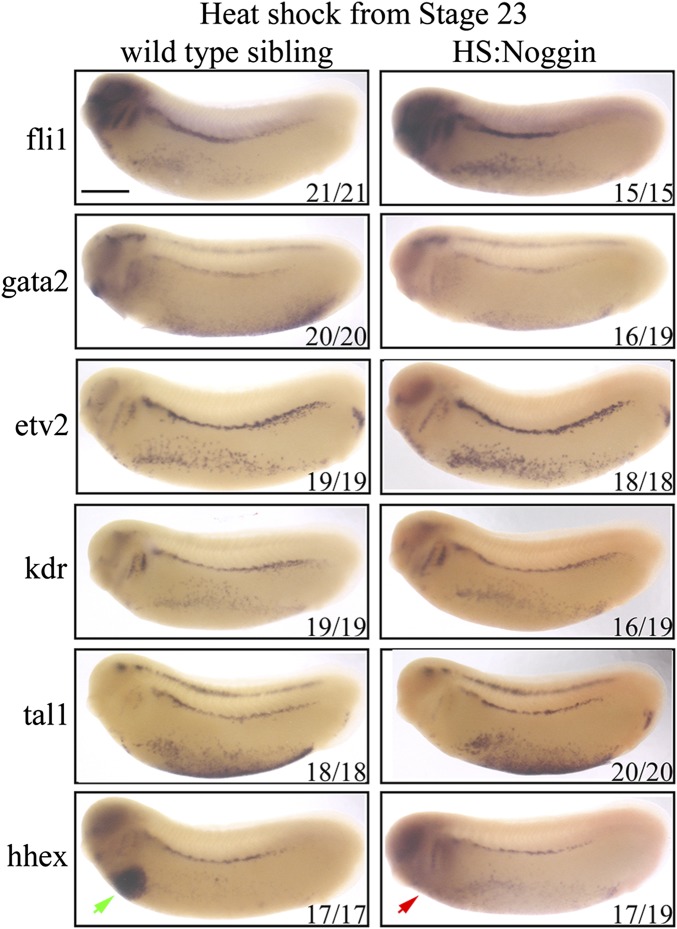
Having established that BMP signaling is required at the level of gata2 expression, we sought to determine the end point of this requirement for the DLP GRN. So we blocked signaling at stage 20 just when the first expression of fli1, gata2, and kdr commences, and analyzed expression of DH markers at stage 26. Because the HS:Noggin transgenic siblings are morphologically inseparable from wild types, we sorted transgenic siblings by counterstaining for misexpressed Noggin mRNA. In these embryos, fli1, gata2, etv2, and lmo2 are all expressed as in wild-type embryos. However, there is a strong decrease in kdr and tal1 expression (Fig. 4C). Finally, when BMP signaling is blocked from stage 23, there is no effect on the expression of hemangioblast genes at stage 26 (Fig. S5). Accordingly, we conclude that BMP signaling is required for kdr expression in the hemangioblast GRN after its requirement for gata2 expression ceases.
Fig. S5.
BMP signaling is not required for GRN of the DH after stage 23. Hematopoietic programming in the DLP, at stage 26, following heat shock-induced noggin expression at stage 23. The expression of hemangioblast genes are unaffected in the DLP. The expression of hhex gene is absent in the liver primordium (red arrow) in HS:Noggin siblings compared with wild-type siblings (green arrow). Embryos were heat-shocked for 15 min at 35 °C at the stage 23 and were collected for analysis at stage 26. WISH was used to stain cells expressing genes that are essential for hematopoietic programming in the DLP; fli1, gata2, kdr, etv2, tal1, and lmo2 together with noggin transgene transcripts. HS:Noggin transgenic siblings were identified by the expression of Noggin transcripts (Uniform Fast Red staining in HS:Noggin embryos).
When probing for the requirement for BMP signaling in HE, we concluded that BMP signaling is required until stage 28. Therefore, BMP must be instructive for the HE between stages 23 and 28 even though there is no apparent effect on gene expression in the DH after treatment at these stages. Between stages 28 and 31, precursors of the DA migrate from the DLP to the midline of the embryo, during which expression of gata2, tal1/scl and lmo2 is switched off. Gata2 is reexpressed from stage 32. So, we sought to understand whether BMP signaling has a separate requirement for gata2 expression at this stage in addition to its input during hemangioblast stages. Indeed, HS:Noggin siblings do not express gata2 in the DA, when treated from stage 23, but express gata2 like wild-type siblings when treated from stage 28 (Fig. 4D). Thus, we conclude that BMP signaling is required at two time points for gata2 expression, first to initiate gata2 expression in DH precursors, and then in DH for priming the expression of gata2 in the DA, a requirement for the emergence of HE/HSCs.
Discussion
Generation of HSCs as replacement therapy for malignancies and age-related degeneration of blood output has been a holy grail of the hematopoiesis field for decades. Achieving this goal requires an understanding of the signaling cascades and associated transcriptional networks, but this understanding has proved challenging. Indeed, the seeming complexity of signaling cascades prompted many researchers to attempt to generate HSCs using mixtures of transcription factors rather than their de novo generation from pluripotent cells, which comes with its own drawback as many of the transcription factors are oncogenic proteins. Hence, we aim to understand the signaling events in the setting up of the transcription factor networks necessary for HSC generation. To this end, we have characterized the GRN architecture before HE specification extensively, and identified positive inputs from vegfa splice isoforms into this network as well as an inhibitory effect by TGFB signaling at early stages of hemangioblast formation (9, 14, 44). The current analysis furthers our understanding by dissecting the role of BMP signaling with regard to the definitive blood transcription network. BMP signaling is required at three instances in the DH, first to initiate the DH network through gata2, second for kdr expression to enable the DH to respond to VEGFA ligand from somites, and third for gata2 expression in the DA, but is dispensable for HE gene expression after hemangioblasts have been formed (summarized in Fig. 5). Gata2 is required repetitively for the specification of the blood stem cell lineage in DHs and in the DA (9, 45, 46) and is required specifically for runx1 expression after the DA forms (47). Therefore, we propose that the main input from BMP signaling into the HSC lineage is through gata2. Additionally, inhibition of BMP signaling and consequent disruption of the DH GRN leads to a strong reduction in efnb2 expression in the DA. Efnb2 is a transmembrane ligand expressed in arterial but not venous endothelial cells before the onset of circulation and is critical for proper sorting of arterial and venous fated endothelium into distinct vascular beds but is not required for the specification of the DA (48, 49). A recent study revealed an unexpected role for Efnb2 in the DA for HE emergence (50). Hence, we conclude that BMP signaling is required for blood stem cell lineage upstream of EFNB2 signaling.
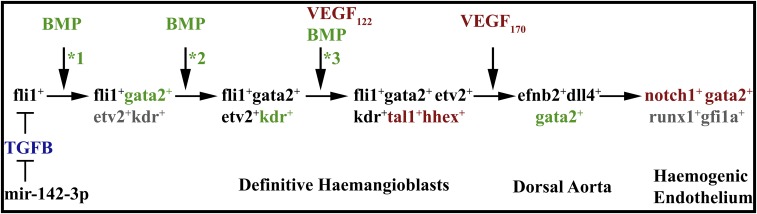
Fig. 5.
Summary of signaling events and downstream transcription factors driving specification of the blood stem cell lineage. The hemangioblast GRN commences with the expression of fli1, which is genetically upstream of gata2 and flk1/kdr (9). Meanwhile, TGFB signaling has an inhibitory effect on fli1 expression (44). BMP signaling, presumably through activation Smad transcription factors, together with Fli1, is first required for gata2 expression before stage 20 (*1) and then for kdr expression at stage 20 (*2) (current study). The genes that are affected consequentially at those stages are colored gray. VEGFA small isoform (VEGFA122) then activates Kdr receptor which lead to the expression of scl/tal1 and hhex (14). Finally, BMP signaling is still required at stage 23–26 for the initiation of gata2 expression later in the DA (*3) (current study). Later, VEGFA medium isoform (VEGFA170) from somites induces Notch1, which in turn is required for the maintenance of gata2 expression and induction of runx1 (14). The requirements for each signaling pathway are indicated by color-matching the text with the transcription factors. The three inputs from BMP signaling are shown with asterisks (*).
Previous analysis of BMP signaling requirements in zebrafish and mouse suggests that BMP signaling is required for definitive hematopoiesis in the DA, pointing to signaling emanating particularly from the mesenchyme around the aorta (16–19, 51). However, the reagents used in these papers have certain caveats. First of all, even though the truncated BMP receptor (Alk3), the main proof for a temporal requirement for BMP in the DA in Wilkinson et al., does not block activin signaling (24), because later studies showed that TGFB ligands signal through heteromeric complexes containing a BMP receptor (52), it is possible that truncated Alk3 blocked TGFB signaling as well. Second, in mouse, Gremlin1, a soluble inhibitor of BMP signaling, was used to address the requirement of BMP signaling for definitive hematopoiesis (19). In this study, the authors also noted that they didn't observe a convincing decrease in AGM hematopoiesis when Noggin was used instead of Gremlin1. Gremlin1 was later shown to be an agonist for VEGFA signaling in addition to its many other roles in other signaling pathways (53, 54). Furthermore, when the requirement for BMP signaling in ESC cultures was probed by using soluble BMP receptors, which block signaling by competing for the ligands, BMP signaling was indeed shown to be dispensable in ESC cultures once flk1/kdr+ hemangioblasts have been formed (21) as in Xenopus embryos. Moreover, mouse embryos lacking Alk3 receptor within the flk1 lineage (20), or lacking smad1 and -5 proteins within all hematopoietic precursors of the vav1 lineage (22), do not display hematopoietic defects. In addition, in chicken embryos, the DA and surrounding mesenchyme is already positive for phospho-Smad1/5 long before runx1 expression, suggesting that BMP signaling is already active at an earlier time point (55). Finally, the role of BMP signaling in the AGM has been revisited in a recent study in which the authors show that BMP signaling is down-regulated in the HSC lineage by BMP inhibitors and, unexpectedly, supplementing these precursors with BMP was inhibitory, whereas addition of noggin enhanced the production of HSCs (56). Thus, we conclude that, as in Xenopus, BMP signaling is likely to be only required for hemangioblast specification but not beyond.
Recently, in zebrafish embryos, we showed that TGFB signaling is involved in HSC generation (57). Interestingly, TGFB ligands can activate BMP signaling type Smad transcription factors such as Smad1 as well as its canonical targets such as Smad2, in a BMP receptor-dependent as well as -independent manner (52, 58). Considering that our analysis didn't reveal a role for BMP signaling after the hemangioblast stage, and the fact that TGFB ligands can trigger Smad1 phosphorylation, we suggest that the role attributed to BMP signaling in AGM hematopoiesis after the hemangioblast stages might in fact be driven by TGFB ligands, or alternatively loss of BMP signaling is being compensated by TGFB signaling in Xenopus.
In this study, while analyzing the requirements for BMP signaling in the programming of hemogenic endothelium and definitive blood, we also revisited the role of BMP signaling in primitive hematopoiesis. In addition to its well-established role in primitive erythropoiesis, we showed that BMP signaling is required for differentiation of primitive myeloid cells in Xenopus. Both in primitive and definitive hematopoiesis, the main role of BMP signaling is to induce the hemangioblast program through gata2. Here and in our previous studies, we noticed that the same set of hemangioblast markers are expressed both in primitive blood and HE precursors. However, the immediate outcomes of these two populations differ, as the first one starts differentiating to myeloid and erythroid progeny immediately, whereas the latter population holds off the hematopoietic potential until after the DA forms. Whereas BMP ligands such as bmp4 are expressed in both populations (A.C.-U., direct submission to Xenbase), vegfa is only expressed in adult hemangioblasts (13) [see extensive profile in Xenbase (36)]. Previously, Walmsley et al. (38) and Myers and Krieg (34) proposed that in the ventral blood island, sustained BMP signaling to hemangioblast precursors ensures commitment to the erythroid lineage and inhibition of endothelial cell fate. Conversely, VEGFA ligand is shown to be inhibitory to blood formation across species and, at least in the murine system, this act is through inhibition of gata1 expression (59-61). Thus, despite being induced in a similar way by BMP signaling, DHs are likely to be kept from differentiating into primitive blood by the inhibitory effect of VEGFA, most probably through inhibition of gata1, because this gene is one of the first hallmarks (9) of differential fate between the ventral blood island and the DLP DHs.
The expression of fli1, and partially etv2, is not dependent on BMP or VEGFA signaling in the DHs. Thus, an unresolved question in the initiation of the genetic program leading to the establishment of HSCs, how fli1 is activated in the DLP, remains open. Wnt signaling operates upstream of BMP in primitive hematopoiesis (62). In the same study, the authors also detected strong canonical Wnt reporter activity in the DLP DH. Therefore, Wnt signaling could be involved earlier than BMP signaling in the DLP to induce fli1 and etv2.
Materials and Methods
X. laevis Transgenic Line, Embryo Culture, and Treatments.
Xenopus embryos were obtained, cultured, and staged according to standard procedures Xenbase (xenbase.org/entry, RRID:SCR_003280). HS:Noggin transgenic line was kindly provided by Caroline Beck (28) as embryos, and raised to adulthood in house. Eggs collected from heat-shock inducible noggin transgenic frogs were fertilized using wild-type testes. Because the HS:Noggin transgenic frogs are heterozygous, this outcross results in ∼50% transgenic, 50% wild-type embryos. Wild-type siblings underwent the same heat-shock treatment to act as a control. The hsp70 promoter driving noggin expression was activated by transferring the embryos into prewarmed 35 °C 0.1× MBS and placing in a 35 °C incubator for 15 min. After this heat-shock treatment, the embryos were washed into 19 °C 0.1× MBS to continue developing before collection but heat-shocked again every 24 h if the collection point was longer than a day away. Transgenic embryos were separated from their wild-type siblings based on general morphology, or by costaining for misexpressed noggin mRNA post fixation using WISH. DMH1 Inhibitor (Sigma) was dissolved in DMSO, and the working concentration was optimized to a level that it leads to strong dorsalization, as is the case for heat-shocked HS:Noggin embryos, when treated before gastrulation. Embryos were treated with 100 μM DMH1, which needs to be titrated to this concentration from a 10mM stock solution dropwise to avoid precipitation. TFGB signaling inhibitors, SB-505124 (Sigma) and SB-431542 (Sigma) were dissolved in DMSO to make a 25 mM stock solution and used at 25 μM and 100 μM final concentration, respectively (63). All animal work was carried out according to UK Home Office regulations under the appropriate project license and approved by the University of Oxford Animal Welfare and Ethical Review Body.
In Situ Hybridization.
Whole-mount in situ hybridization (WISH) and in situ hybridization on sections (ISHS) were performed as previously described (7, 64) except when noggin probe was fluorescein labeled for double WISH, and detection was performed using Fast Red Tablets (Roche). For details of probes used see supplementary material in refs. 9 and 14. Images and numbers shown in figures are from one experiment and are representative of three experiments.
RNA Extraction and qPCR Analysis.
RNA from stage 26 excised DLPs was isolated using the RNeasy Micro Kit (74004; Qiagen) following the protocol for fibrous tissues, cDNA was synthesized using SSIV RT (ThermoFisher), and qPCR was performed using the ABI Prism 7700 with the SYBR green dye. Primers were designed to amplify both copies of the duplicated Xenopus genes (Table S1). The mRNA transcript levels were normalized to the ODC gene and changes were calculated using the Standard Curve method generated with cDNA made from a stage matched wild-type embryo as reference. Error bars represent SEM, and those treatments showing a significant change in mRNA levels relative to the controls are indicated, with their P value shown. The data shown summarize the results of three biological replicates for control and transgenic embryos.
Table S1.
List of qRT-PCR primers
| qRT-PCR primers | Forward | Reverse | Product size, bp |
| XL.fli1.sl | CCATGAGCTCACCTGTCACA | GCTGCTTGTGGGCCCTAATA | 118 |
| XL.gata2.sl | TGGAGACGTAATGCTAATGGGG | TGCATGCACCTGGAAAGTTC | 188 |
| XL.etv2.sl | CTCCTCACCATCAAGCCCTC | CTCCTTGATCCCTGTGCTGT | 101 |
| XL.kdr.sl | GGAGGAAGAGGAAGTTTGCGA | AACCACAGGCTCTACAGGAATG | 139 |
| XL.tal1.sl | TCCAGAGCTAAGCGAAGACC | ACCTCTCTCGGCTGTTGGTA | 100 |
| XL.hhex.sl | TATGCCTCCCCTCTGTACCC | TGTCCTCCTTTCCGCTTGTG | 137 |
| XL.mpo.sl | GAACAGACTCTGGACAACCAGC | ACCTGGCTGGCATCAACATAAG | 300 |
| XL.runx1.sl | GGATCCTACCACCAGTTCTCTAT | CCCGTGGAAGCGTTTGTG | 86 |
Western Blotting.
Protein extraction and Western blot analysis were done as previously described (9); phospho-Smad1/5/8 proteins were detected using an anti-PSmad158 (AB3848; Millipore) and normalized to total smad1 (6944P; Cell Signaling) and beta-actin (A3854; Sigma) protein levels. Myc-noggin was detected using myc-HRP antibody (11814150001; Roche).
Software.
Network schematics were generated using Biotapestry and figures were prepared using Photoshop. qPCR results were analyzed using Microsoft Excel.
Acknowledgments
This work was supported by the UK Medical Research Council and The Wellcome Trust.
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Gene Regulatory Networks and Network Models in Development and Evolution,” held April 12–14, 2016, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and video recordings of most presentations are available on the NAS website at www.nasonline.org/Gene_Regulatory_Networks.
This article is a PNAS Direct Submission. E.V.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1610615114/-/DCSupplemental.
References
- 1.Gratwohl A, et al. Worldwide Network of Blood and Marrow Transplantation Hematopoietic stem cell transplantation: A global perspective. JAMA. 2010;303(16):1617–1624. doi: 10.1001/jama.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giralt S, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: Consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20(3):295–308. doi: 10.1016/j.bbmt.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Sturgeon CM, Ditadi A, Awong G, Kennedy M, Keller G. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat Biotechnol. 2014;32(6):554–561. doi: 10.1038/nbt.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebina W, Rossi DJ. Transcription factor-mediated reprogramming toward hematopoietic stem cells. EMBO J. 2015;34(6):694–709. doi: 10.15252/embj.201490804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swiers G, Rode C, Azzoni E, de Bruijn MF. A short history of hemogenic endothelium. Blood Cells Mol Dis. 2013;51(4):206–212. doi: 10.1016/j.bcmd.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciau-Uitz A, Monteiro R, Kirmizitas A, Patient R. Developmental hematopoiesis: Ontogeny, genetic programming and conservation. Exp Hematol. 2014;42(8):669–683. doi: 10.1016/j.exphem.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Ciau-Uitz A, Walmsley M, Patient R. Distinct origins of adult and embryonic blood in Xenopus. Cell. 2000;102(6):787–796. doi: 10.1016/s0092-8674(00)00067-2. [DOI] [PubMed] [Google Scholar]
- 8.Walmsley M, Ciau-Uitz A, Patient R. Adult and embryonic blood and endothelium derive from distinct precursor populations which are differentially programmed by BMP in Xenopus. Development. 2002;129(24):5683–5695. doi: 10.1242/dev.00169. [DOI] [PubMed] [Google Scholar]
- 9.Ciau-Uitz A, Pinheiro P, Kirmizitas A, Zuo J, Patient R. VEGFA-dependent and -independent pathways synergise to drive Scl expression and initiate programming of the blood stem cell lineage in Xenopus. Development. 2013;140(12):2632–2642. doi: 10.1242/dev.090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu F, et al. Induction of hematopoietic and endothelial cell program orchestrated by ETS transcription factor ER71/ETV2. EMBO Rep. 2015;16(5):654–669. doi: 10.15252/embr.201439939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu F, Walmsley M, Rodaway A, Patient R. Fli1 acts at the top of the transcriptional network driving blood and endothelial development. Curr Biol. 2008;18(16):1234–1240. doi: 10.1016/j.cub.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 12.Cleaver O, Krieg PA. VEGF mediates angioblast migration during development of the dorsal aorta in Xenopus. Development. 1998;125(19):3905–3914. doi: 10.1242/dev.125.19.3905. [DOI] [PubMed] [Google Scholar]
- 13.Ciau-Uitz A, Pinheiro P, Gupta R, Enver T, Patient R. Tel1/ETV6 specifies blood stem cells through the agency of VEGF signaling. Dev Cell. 2010;18(4):569–578. doi: 10.1016/j.devcel.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Leung A, et al. Uncoupling VEGFA functions in arteriogenesis and hematopoietic stem cell specification. Dev Cell. 2013;24(2):144–158. doi: 10.1016/j.devcel.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blank U, Karlsson S. The role of Smad signaling in hematopoiesis and translational hematology. Leukemia. 2011;25(9):1379–1388. doi: 10.1038/leu.2011.95. [DOI] [PubMed] [Google Scholar]
- 16.Marshall CJ, Kinnon C, Thrasher AJ. Polarized expression of bone morphogenetic protein-4 in the human aorta-gonad-mesonephros region. Blood. 2000;96(4):1591–1593. [PubMed] [Google Scholar]
- 17.Pimanda JE, et al. The SCL transcriptional network and BMP signaling pathway interact to regulate RUNX1 activity. Proc Natl Acad Sci USA. 2007;104(3):840–845. doi: 10.1073/pnas.0607196104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkinson RN, et al. Hedgehog and Bmp polarize hematopoietic stem cell emergence in the zebrafish dorsal aorta. Dev Cell. 2009;16(6):909–916. doi: 10.1016/j.devcel.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durand C, et al. Embryonic stromal clones reveal developmental regulators of definitive hematopoietic stem cells. Proc Natl Acad Sci USA. 2007;104(52):20838–20843. doi: 10.1073/pnas.0706923105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park C, et al. Bone morphogenetic protein receptor 1A signaling is dispensable for hematopoietic development but essential for vessel and atrioventricular endocardial cushion formation. Development. 2006;133(17):3473–3484. doi: 10.1242/dev.02499. [DOI] [PubMed] [Google Scholar]
- 21.Nostro MC, Cheng X, Keller GM, Gadue P. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell. 2008;2(1):60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singbrant S, et al. Canonical BMP signaling is dispensable for hematopoietic stem cell function in both adult and fetal liver hematopoiesis, but essential to preserve colon architecture. Blood. 2010;115(23):4689–4698. doi: 10.1182/blood-2009-05-220988. [DOI] [PubMed] [Google Scholar]
- 23.Ema M, Takahashi S, Rossant J. Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood. 2006;107(1):111–117. doi: 10.1182/blood-2005-05-1970. [DOI] [PubMed] [Google Scholar]
- 24.Graff JM, Thies RS, Song JJ, Celeste AJ, Melton DA. Studies with a Xenopus BMP receptor suggest that ventral mesoderm-inducing signals override dorsal signals in vivo. Cell. 1994;79(1):169–179. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- 25.Northrop J, et al. BMP-4 regulates the dorsal-ventral differences in FGF/MAPKK-mediated mesoderm induction in Xenopus. Dev Biol. 1995;172(1):242–252. doi: 10.1006/dbio.1995.0019. [DOI] [PubMed] [Google Scholar]
- 26.Katagiri T, Watabe T. Bone morphogenetic proteins. Cold Spring Harb Perspect Biol. 2016;8(6):a021899. doi: 10.1101/cshperspect.a021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wills A, Dickinson K, Khokha M, Baker JC. Bmp signaling is necessary and sufficient for ventrolateral endoderm specification in Xenopus. Dev Dyn. 2008;237(8):2177–2186. doi: 10.1002/dvdy.21631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beck CW, Christen B, Barker D, Slack JM. Temporal requirement for bone morphogenetic proteins in regeneration of the tail and limb of Xenopus tadpoles. Mech Dev. 2006;123(9):674–688. doi: 10.1016/j.mod.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Hao J, et al. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010;5(2):245–253. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmerman LB, De Jesús-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86(4):599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 31.Krause C, Guzman A, Knaus P. Noggin. Int J Biochem Cell Biol. 2011;43(4):478–481. doi: 10.1016/j.biocel.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Cross EE, et al. Application of small organic molecules reveals cooperative TGFβ and BMP regulation of mesothelial cell behaviors. ACS Chem Biol. 2011;6(9):952–961. doi: 10.1021/cb200205z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogan BM, et al. Specification of the primitive myeloid precursor pool requires signaling through Alk8 in zebrafish. Curr Biol. 2006;16(5):506–511. doi: 10.1016/j.cub.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 34.Myers CT, Krieg PA. BMP-mediated specification of the erythroid lineage suppresses endothelial development in blood island precursors. Blood. 2013;122(24):3929–3939. doi: 10.1182/blood-2013-03-490045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tracey WD, Jr, Pepling ME, Horb ME, Thomsen GH, Gergen JP. A Xenopus homologue of aml-1 reveals unexpected patterning mechanisms leading to the formation of embryonic blood. Development. 1998;125(8):1371–1380. doi: 10.1242/dev.125.8.1371. [DOI] [PubMed] [Google Scholar]
- 36.Karpinka JB, et al. Xenbase, the Xenopus model organism database; new virtualized system, data types and genomes. Nucleic Acids Res. 2015;43(Database issue):D756–D763. doi: 10.1093/nar/gku956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciau-Uitz A, Liu F, Patient R. Genetic control of hematopoietic development in Xenopus and zebrafish. Int J Dev Biol. 2010;54(6-7):1139–1149. doi: 10.1387/ijdb.093055ac. [DOI] [PubMed] [Google Scholar]
- 38.Walmsley M, Cleaver D, Patient R. Fibroblast growth factor controls the timing of Scl, Lmo2, and Runx1 expression during embryonic blood development. Blood. 2008;111(3):1157–1166. doi: 10.1182/blood-2007-03-081323. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy M, D’Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109(7):2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldman DC, et al. BMP4 regulates the hematopoietic stem cell niche. Blood. 2009;114(20):4393–4401. doi: 10.1182/blood-2009-02-206433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka Y, et al. The transcriptional programme controlled by Runx1 during early embryonic blood development. Dev Biol. 2012;366(2):404–419. doi: 10.1016/j.ydbio.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen XD, Turpen JB. Intraembryonic origin of hepatic hematopoiesis in Xenopus laevis. J Immunol. 1995;154(6):2557–2567. [PubMed] [Google Scholar]
- 43.Maéno M, Tochinai S, Katagiri C. Differential participation of ventral and dorsolateral mesoderms in the hemopoiesis of Xenopus, as revealed in diploid-triploid or interspecific chimeras. Dev Biol. 1985;110(2):503–508. doi: 10.1016/0012-1606(85)90108-3. [DOI] [PubMed] [Google Scholar]
- 44.Nimmo R, et al. MiR-142-3p controls the specification of definitive hemangioblasts during ontogeny. Dev Cell. 2013;26(3):237–249. doi: 10.1016/j.devcel.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 45.de Pater E, et al. Gata2 is required for HSC generation and survival. J Exp Med. 2013;210(13):2843–2850. doi: 10.1084/jem.20130751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lugus JJ, et al. GATA2 functions at multiple steps in hemangioblast development and differentiation. Development. 2007;134(2):393–405. doi: 10.1242/dev.02731. [DOI] [PubMed] [Google Scholar]
- 47.Gao X, et al. Gata2 cis-element is required for hematopoietic stem cell generation in the mammalian embryo. J Exp Med. 2013;210(13):2833–2842. doi: 10.1084/jem.20130733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999;4(3):403–414. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- 49.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93(5):741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 50.Chen II, et al. EphrinB2 regulates the emergence of a hemogenic endothelium from the aorta. Sci Rep. 2016;6:27195. doi: 10.1038/srep27195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pouget C, et al. FGF signalling restricts haematopoietic stem cell specification via modulation of the BMP pathway. Nat Commun. 2014;5:5588. doi: 10.1038/ncomms6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daly AC, Randall RA, Hill CS. Transforming growth factor beta-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol Cell Biol. 2008;28(22):6889–6902. doi: 10.1128/MCB.01192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitola S, et al. Gremlin is a novel agonist of the major proangiogenic receptor VEGFR2. Blood. 2010;116(18):3677–3680. doi: 10.1182/blood-2010-06-291930. [DOI] [PubMed] [Google Scholar]
- 54.Brazil DP, Church RH, Surae S, Godson C, Martin F. BMP signalling: Agony and antagony in the family. Trends Cell Biol. 2015;25(5):249–264. doi: 10.1016/j.tcb.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Richard C, et al. Endothelio-mesenchymal interaction controls runx1 expression and modulates the notch pathway to initiate aortic hematopoiesis. Dev Cell. 2013;24(6):600–611. doi: 10.1016/j.devcel.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Souilhol C, et al. Inductive interactions mediated by interplay of asymmetric signalling underlie development of adult haematopoietic stem cells. Nat Commun. 2016;7:10784. doi: 10.1038/ncomms10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monteiro R, et al. Transforming growth factor β drives hemogenic endothelium programming and the transition to hematopoietic stem cells. Dev Cell. 2016;38(4):358–370. doi: 10.1016/j.devcel.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wrighton KH, Lin X, Yu PB, Feng XH. Transforming growth factor beta can stimulate Smad1 phosphorylation independently of bone morphogenic protein receptors. J Biol Chem. 2009;284(15):9755–9763. doi: 10.1074/jbc.M809223200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eichmann A, et al. Ligand-dependent development of the endothelial and hemopoietic lineages from embryonic mesodermal cells expressing vascular endothelial growth factor receptor 2. Proc Natl Acad Sci USA. 1997;94(10):5141–5146. doi: 10.1073/pnas.94.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koibuchi N, et al. The effect of VEGF on blood vessels and blood cells during Xenopus development. Biochem Biophys Res Commun. 2006;344(1):339–345. doi: 10.1016/j.bbrc.2006.03.140. [DOI] [PubMed] [Google Scholar]
- 61.Drogat B, et al. Vegf regulates embryonic erythroid development through Gata1 modulation. Blood. 2010;116(12):2141–2151. doi: 10.1182/blood-2010-01-264143. [DOI] [PubMed] [Google Scholar]
- 62.Tran HT, Sekkali B, Van Imschoot G, Janssens S, Vleminckx K. Wnt/beta-catenin signaling is involved in the induction and maintenance of primitive hematopoiesis in the vertebrate embryo. Proc Natl Acad Sci USA. 2010;107(37):16160–16165. doi: 10.1073/pnas.1007725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ho DM, Whitman M. TGF-beta signaling is required for multiple processes during Xenopus tail regeneration. Dev Biol. 2008;315(1):203–216. doi: 10.1016/j.ydbio.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walmsley M, Ciau-Uitz A, Patient R. Tracking and programming early hematopoietic cells in Xenopus embryos. Methods Mol Med. 2005;105:123–136. doi: 10.1385/1-59259-826-9:123. [DOI] [PubMed] [Google Scholar]