Significance
Divergent selection can lead to the evolution of distinct species, a process known as ecological speciation. Evidence for ecological speciation in the marine environment is scarce, and the few known examples have happened within a time frame of hundreds of thousands to millions of years. We present evidence that European flounders in the Baltic Sea exhibiting different breeding behaviors are a species pair arising from a recent event of ecological speciation. The two lineages diverged within less than 3,000 generations. This is the fastest event of speciation ever reported for any marine vertebrate. Extraordinarily rapid speciation driven by natural selection can therefore happen even in the marine environment.
Keywords: ecological speciation, genomics, evolution, rapid speciation, Baltic Sea
Abstract
Divergent selection may initiate ecological speciation extremely rapidly. How often and at what pace ecological speciation proceeds to yield strong reproductive isolation is more uncertain. Here, we document a case of extraordinarily rapid speciation associated with ecological selection in the postglacial Baltic Sea. European flounders (Platichthys flesus) in the Baltic exhibit two contrasting reproductive behaviors: pelagic and demersal spawning. Demersal spawning enables flounders to thrive in the low salinity of the Northern Baltic, where eggs cannot achieve neutral buoyancy. We show that demersal and pelagic flounders are a species pair arising from a recent event of speciation. Despite having a parapatric distribution with extensive overlap, the two species are reciprocally monophyletic and show strongly bimodal genotypic clustering and no evidence of contemporary migration, suggesting strong reproductive isolation. Divergence across the genome is weak but shows strong signatures of selection, a pattern suggestive of a recent ecological speciation event. We propose that spawning behavior in Baltic flounders is the trait under ecologically based selection causing reproductive isolation, directly implicating a process of ecological speciation. We evaluated different possible evolutionary scenarios under the approximate Bayesian computation framework and estimate that the speciation process started in allopatry ∼2,400 generations ago, following the colonization of the Baltic by the demersal lineage. This is faster than most known cases of ecological speciation and represents the most rapid event of speciation ever reported for any marine vertebrate.
Divergent selection may generate barriers to gene flow and ultimately lead to the evolution of distinct species—a process referred to as ecological speciation (1). Partial reproductive isolation can arise as a by-product of local adaptation within dozens to a few hundred generations, suggesting that divergent selection may initiate speciation over ecological timescales (2); more uncertain, however, is how often and at what pace divergence proceeds along the speciation continuum until strong reproductive isolation is established (2, 3). Partial reproductive isolation does not necessarily lead to the evolution of distinct species; even in cases when divergence continues, it may persists as a “quasiequilibrium state” for millions of years (4).
Ecological selection on traits that directly cause reproductive isolation favors the completion of the speciation process and influences the pace at which it proceeds (4). Such “magic traits” may play a pivotal role in facilitating speciation in the absence of impermeable geographic barriers (5). This can happen when ecological adaptations cause assortative mating, such as among sister species of Heliconius butterflies with contrasting color pattern mimicry (6), or when selection leads to habitat specialization and divergent populations mate within their preferred habitats leading to reproductive segregation (7). Selection on habitat preferences, specifically, is expected to lead to rapidly evolving barriers to gene flow (2). Early onset of reproductive isolation (through assortative mating, reproductive segregation, or an initial allopatric/parapatric phase) may thus favor rapid speciation, because it could theoretically initiate a positive-feedback loop where restricted migration enhances divergence at increasingly weakly selected loci, which in turn reduces gene flow (4).
In the marine environment, evidence for ecological speciation is scarce (8). This is somehow surprising: barriers to gene flow are rarely absolute in the sea, hence models of speciation that can operate in the presence of gene flow, such as ecological speciation, are likely to be important in explaining marine biodiversity (8). The sympatric speciation of coral-dwelling gobies of the genus Gobiodon represents the most compelling case and is estimated to have happened ∼200 kya (9). The fastest known case of speciation in a marine animal is the evolution of the sea star Cryptasterina hystera, which occurred 1–22 kya, although it is unclear whether ecological selection played any role in this speciation event (10).
Here, we present a case of extraordinarily rapid speciation in the postglacial Baltic Sea, a large body of brackish water that became connected to the North Sea at the end of the last glaciation, ∼8.5 kya (11). The narrow connection between the Baltic Proper and the North Sea presents a steep gradient in temperature and salinity: a barrier that most marine fish cannot cross and that reduces gene flow for the few that can (12). Among the few marine fish that thrive in the Baltic Sea is the European flounder (Platichthys flesus), an important fishery target. European flounders show two distinct reproductive behaviors within the Baltic Sea: offshore spawning of pelagic eggs (hereafter, “pelagic flounders”), as seen in the rest of their distribution, and coastal spawning of demersal eggs (hereafter, “demersal flounders”), a behavior observed only in the Baltic Sea (13). Pelagic spawning occurs below the 11-practical salinity unit (psu) isohaline in deep, offshore basins of the southern and central Baltic Sea where eggs can achieve neutral buoyancy (13). Demersal flounders spawn in shallow coastal waters and reproduce successfully at salinity as low as 6 psu (13), which enabled them to expand their distribution into the coastal waters of the northern Baltic Sea (Fig. 1). Demersal and pelagic spawners exhibit differences in morphological and physiological reproductive traits; demersal eggs are smaller, but with thicker chorions, a potential adaptation to the rougher conditions of shallow demersal habitats, whereas pelagic eggs are larger, a potential adaptation to increase buoyancy (13, 14). Sperm activation is salinity dependent: sperm from samples collected in pelagic spawning sites show no motility below 10–9 psu, whereas sperm activity in demersal spawners is still observed at 3 psu, hence fertilization can successfully occur at much lower salinity (13).
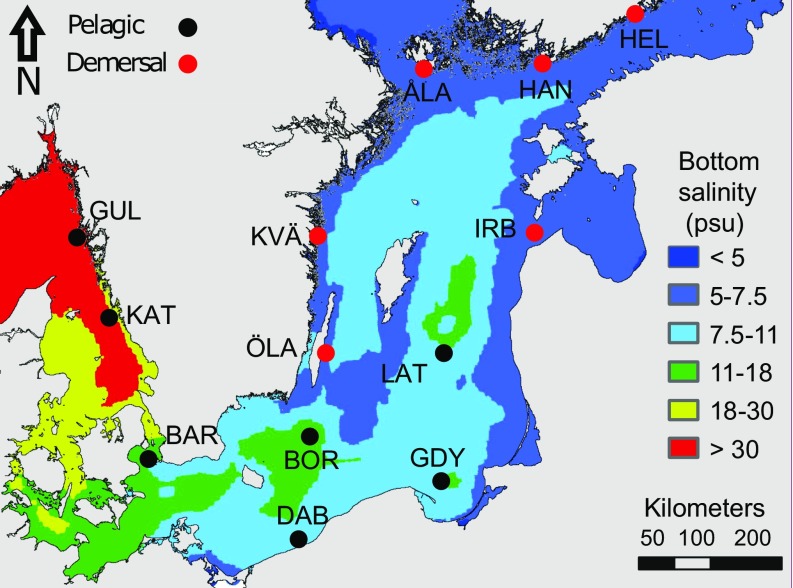
Fig. 1.
Map of the sampling locations showing modeled mean bottom salinity in the Baltic Sea from Bendtsen et al. (62). Areas theoretically suitable for pelagic spawning are colored in green, yellow, and red. Locations are color-coded based on expected spawning behaviors. ÅLA, Åland Archipelago; BAR, Barsebäck; BOR, Bornholm Basin; DAB, Dabki; GDY, Gdynia; GUL, Gullmaren; HAN, Hanko (Western Gulf of Finland); HEL, Helsinki (Gulf of Finland); IRB, Irbe Strait; KAT, Kattegat; KVÄ, Kvädöfjärden; LAT, Latvian Sea; and ÖLA, Öland Island. More than 90% (Table S1) of samples from BOR (pelagic habitat) and ÖLA (demersal habitat) were ready for spawning at the time of capture, hence these locations were used as reference pelagic and demersal populations, respectively.
There is evidence that pelagic and demersal flounders represent genetically distinct ecotypes (15, 16), although genetic differentiation among putative demersal and pelagic populations is extremely weak (FST ∼ 0.01–0.03). Weak differentiation may suggest ongoing gene flow or reflect recent divergence and large effective population sizes, depending on whether populations have reached migration-drift equilibrium (17). Even if demersal and pelagic flounders were genetically distinct taxa, gene flow could potentially still occur if males opportunistically fertilize eggs of the opposite ecotype, or if spawning behavior and/or reproductive traits show some degree of plasticity, as has been suggested for another flatfish species (18). An artificial fertilization experiment (19) suggests the two ecotypes can produce viable larvae, although it is unclear what their fitness would be under natural conditions. However, if reproductive traits such as egg buoyancy and sperm motility were under ecological selection they could act as magic traits, favoring rapid speciation via reproductive segregation (6, 20).
Here, we tested whether pelagic and demersal flounders represent two genetically divergent taxa, and investigated how far divergence has proceeded along the speciation continuum. We used some of the criteria listed by Nosil et al. (3) and Nosil and Feder (21) to determine how far speciation has progressed, and we predict that, if speciation has already resulted in strong reproductive isolation, (i) genomic divergence should be heterogeneous, showing strong signatures of divergent selection in some regions of the genome (a predicted outcome of ecological speciation) and low levels of divergence elsewhere (the latter being a reflection of recent divergence); (ii) genotypic clustering should be strongly bimodal, regardless of geographical distance; and (iii) there should be limited introgression among the two taxa, even where they co-occur, and reciprocal monophyly of the pelagic and demersal ecotypes.
Using genomic data from flounders sampled in 13 locations from the Baltic Sea, the North Sea, and the transition zone between these two regions, we show that each one of these expectations is clearly met, providing convincing evidence of selection and strong reproductive isolation. By evaluating different evolutionary scenarios within an approximate Bayesian computation (ABC) (22) framework, we demonstrate that speciation has started following an early colonization of the postglacial Baltic Sea. This represents the most rapid speciation event ever reported for any marine vertebrate.
Results
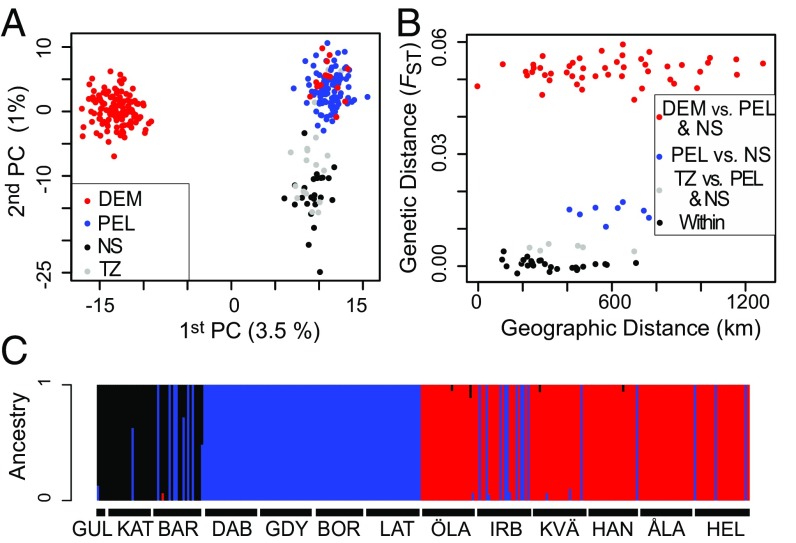
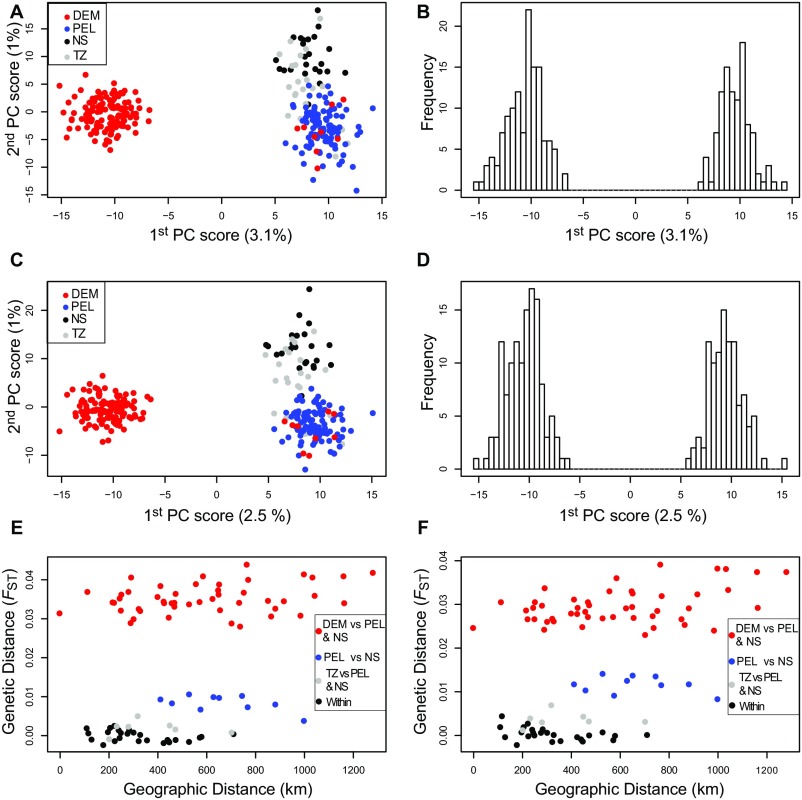
The final dataset consisted of 2,051 biallelic SNPs genotyped for 282 individuals. A principal-component analysis (PCA) performed with all loci revealed two genetic clusters along the first axis (Fig. 2A), which largely coincide with the expected distribution of demersal and pelagic spawners (Table S1 and Fig. 1). Samples from the two reference populations (Bornholm for pelagic spawners, Öland for demersal spawners) clustered as expected with the “pelagic” and “demersal” clusters, respectively (Fig. 2A). A few individuals (nine from Irbe, one from Kvädöfjärden, one from Hanko, and three from Helsinki) sampled in putative demersal locations appear to have genotypes characteristic of pelagic Baltic flounders, suggesting the two taxa co-occur in some locations (Fig. 1). PCAs run only with loci from the core FST distribution (i.e., loci unlikely to be under selection) and excluding FST outliers revealed nearly identical patterns (Fig. S1 A and C), with samples from the Baltic Sea showing a clear bimodal distribution on the first axis (Fig. S1 B and D). There was no evidence of isolation by distance, regardless of whether the dataset was analyzed as a whole or pelagic and demersal flounders were analyzed separately (Fig. 2B), and regardless of whether loci potentially under selection were excluded (Fig. S1 E and F). Genetic diversity indices were very similar for each sampling location and for the three major genetic clusters (Table S2), suggesting that no major demographic events affecting genetic diversity have occurred since the time of common ancestry.
Fig. 2.
(A) PCA of allele frequency data from all loci. Codes are as follows: DEM, putative demersal locations; NS, North Sea samples; PEL, putative pelagic locations within the Baltic; TZ, transition zone between the Baltic Sea and the North Sea (Barsebäck samples). (B) Relationship between geographic and genetic distance among sampling locations. “Within” refers to pairwise comparisons among locations belonging to the three major genetic clusters identified by PCA, fastSTRUCTURE, and K-means clustering. (C) Individual ancestries from fastSTRUCTURE analysis (K = 3).
Table S1.
Summary of the samples collected and analyzed in this study
| Location | Code | N | Coordinates | Date | % Spawning | Putative ecotype |
| Gullmaren (NS)* | GUL | 4 | 58.17N, 11.30E | February 2002 | 25 | Pelagic |
| Kattegat (NS) | KAT | 20 | 57.25N, 12.11E | September 2009 | Not recorded | Pelagic |
| Barsebäck (TZ)* | BAR | 22 | 55.45N, 12.53E | April 2003 | 4 | Pelagic |
| Dabki (BS)* | DAB | 24 | 54.45N, 16.30E | May 2003 | 4 | Pelagic |
| Gdynia (BS)* | GDY | 24 | 54.45N, 18.30E | May 2003 | 4 | Pelagic |
| Bornholm (BS)* | BOR | 22 | 55.42N, 16.11E | March 2003 | 100 | Pelagic |
| Latvian Sea (BS)* | LAT | 24 | 56.25N, 19.44E | April 2003 | 69 | Pelagic |
| Irbe (BS)* | IRB | 24 | 57.44N, 22.22E | May 2003 | 75 | Demersal |
| Öland (BS) | ÖLA | 24 | 57.14N, 17.06E | April to June 2004 | 92 | Demersal |
| Kvädöfjärden (BS)* | KVÄ | 24 | 58.01N, 16.46E | May 2003 | 58 | Demersal |
| Hanko (BS) | HAN | 22 | 59.82N, 22.99E | June 2012 | Not recorded | Demersal |
| Åland (BS)* | ÅLA | 24 | 60.09N, 19.42E | May to June 2003 | 8 | Demersal |
| Helsinki (BS)* | HEL | 24 | 60.15N, 25.30E | May 2003 | 0 | Demersal |
N, sample size; % Spawning, percentage of the sampled individuals that were ready to spawn (with running roe and sperm) at the time of collection. Locations where more than 90% of sampled individuals were ready to spawn are considered as reference demersal (Öland) and pelagic (Bornholm) populations.
Samples from these locations were originally obtained by Florin and Höglund (15).
Fig. S1.
PCA and distribution of Baltic Sea individuals along the first PC axis based on allele frequency data from loci from the core FST distribution estimated by OutFLANK (A and B) and from a dataset excluding all loci identified as outliers by any of the outlier tests performed (C and D). E and F represent the relationship between geographic and genetic distances among sampling locations from the same two “neutral” datasets. “Within” refers to pairwise comparisons among locations belonging to the three major genetic clusters identified by PCA, fastSTRUCTURE, and K-means clustering.
Table S2.
Diversity indices for each genetic cluster and location
| Group | N | S | S/n | p | D | HO | HE |
| All individuals | 282 | 2,388 | 0.017809 | 0.000702 | −2.38109 | 0.164 ± 0.001 | 0.165 ± 0.001 |
| North Sea | 39 | 1,194 | 0.008904 | 0.000682 | −2.55115 | 0.162 ± 0.003 | 0.166 ± 0.003 |
| Pelagic | 117 | 1,653 | 0.012328 | 0.000572 | −2.53157 | 0.166 ± 0.003 | 0.164 ± 0.003 |
| Demersal | 128 | 1,695 | 0.01264 | 0.000661 | −2.39161 | 0.162 ± 0.003 | 0.164 ± 0.003 |
| Kattegat | 20 | 834 | 0.006219 | 0.000652 | −2.63035 | 0.163 ± 0.003 | 0.166 ± 0.003 |
| Barsebäck | 22 | 963 | 0.007182 | 0.00071 | −2.63197 | 0.161 ± 0.003 | 0.166 ± 0.003 |
| Dabki | 24 | 898 | 0.006697 | 0.00063 | −2.62902 | 0.163 ± 0.003 | 0.164 ± 0.003 |
| Gdynia | 23 | 812 | 0.006055 | 0.000562 | −2.68268 | 0.167 ± 0.003 | 0.164 ± 0.003 |
| Bornholm | 22 | 721 | 0.005378 | 0.000458 | −2.83613 | 0.171 ± 0.004 | 0.163 ± 0.003 |
| Latvian sea | 24 | 838 | 0.00625 | 0.00054 | −2.74487 | 0.166 ± 0.003 | 0.164 ± 0.003 |
| Irbe (Pelagic) | 9 | 485 | 0.003617 | 0.000575 | −2.96216 | 0.162 ± 0.004 | 0.165 ± 0.004 |
| Irbe (Demersal) | 14 | 687 | 0.005123 | 0.000626 | −2.76915 | 0.165 ± 0.004 | 0.164 ± 0.003 |
| Öland | 24 | 960 | 0.007159 | 0.000699 | −2.57396 | 0.161 ± 0.003 | 0.164 ± 0.003 |
| Kvädöfjärden | 23 | 942 | 0.007025 | 0.000712 | −2.55571 | 0.159 ± 0.003 | 0.164 ± 0.003 |
| Helsinki | 21 | 822 | 0.00613 | 0.000582 | −2.73057 | 0.168 ± 0.004 | 0.165 ± 0.003 |
| Hanko | 22 | 882 | 0.006577 | 0.000682 | −2.55878 | 0.161 ± 0.003 | 0.164 ± 0.003 |
| Åland | 24 | 935 | 0.006972 | 0.000657 | −2.62673 | 0.166 ± 0.003 | 0.166 ± 0.003 |
D, Tajima’s D; HE, expected heterozygosity; HO, observed heterozygosity; n, total number of sites; N, number of samples; p, nucleotide diversity; S, number of segregating sites.
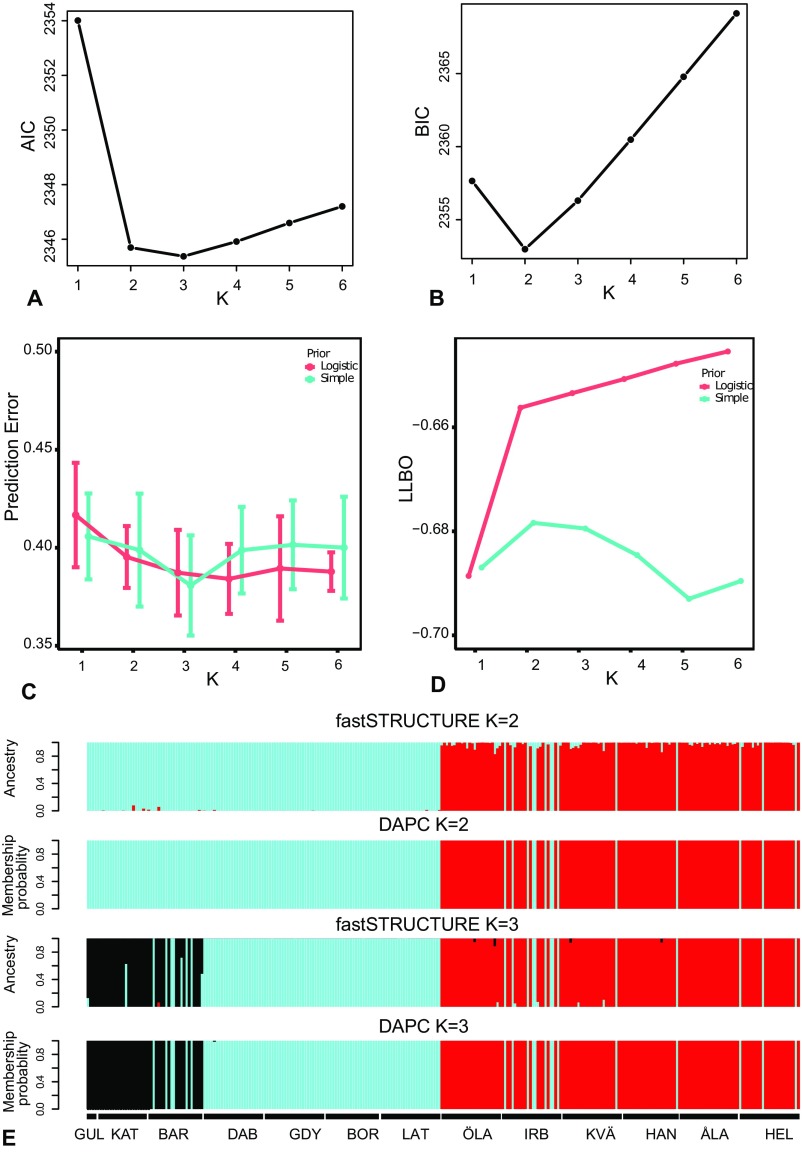
Genotypic clustering using K-means and fastSTRUCTURE suggests that values of K of 2 and 3 are useful models of the data (Fig. S2 A–D). Both fastSTRUCTURE and discriminant analysis of principal components (DAPC) identified the same genetic clusters as the PCA. At K = 2, both fastSTRUCTURE and DAPC separate the putative demersal and pelagic taxa (Fig. S2E). When K = 3, the third cluster represented the North Sea samples (Fig. 2C and Fig. S2E). Individuals from the demersal and pelagic clusters co-occur in similar proportions in one location (Irbe, Fig. 2C), and a small number of individuals with pelagic genotypes were sampled from the northern Baltic Sea coast in Finland and Sweden (Fig. 2C). We found no sign of hybridization between the two Baltic taxa as none of the 282 sampled individuals showed intermediate genotypes (Fig. 2 A and C). There was some evidence of hybridization between pelagic flounders from the Baltic Sea and the North Sea (Fig. 2C), particularly in the transition zone (Barsebäck). The private allele method (23) suggests less than one migrant per generation between each of the major genetic clusters; five independent runs of BAYESASS reported migration rates of less than 0.5% between demersal and pelagic flounders, and the 95% CI of these estimates always overlapped with zero, providing no evidence for contemporary gene flow.
Fig. S2.
Results from genotypic clustering analyses. A and B represent the Akaike information criterion (AIC) and Bayesian information criterion (BIC), respectively, for runs of K-means clustering using increasing numbers of K, and suggest K = 3 and K = 2, respectively, reflecting higher penalties of BIC for increasing model complexity. C and D represent prediction error from fivefold cross-validation for the fastSTRUCTURE analyses and plots of the log-marginal likelihood lower bound (LLBO) at increasing number of K, using both the simple and logistic models. E shows the assignment probability and estimated ancestry from DAPC and fastSTRUCTURE analyses when K = 2 and K = 3, respectively.
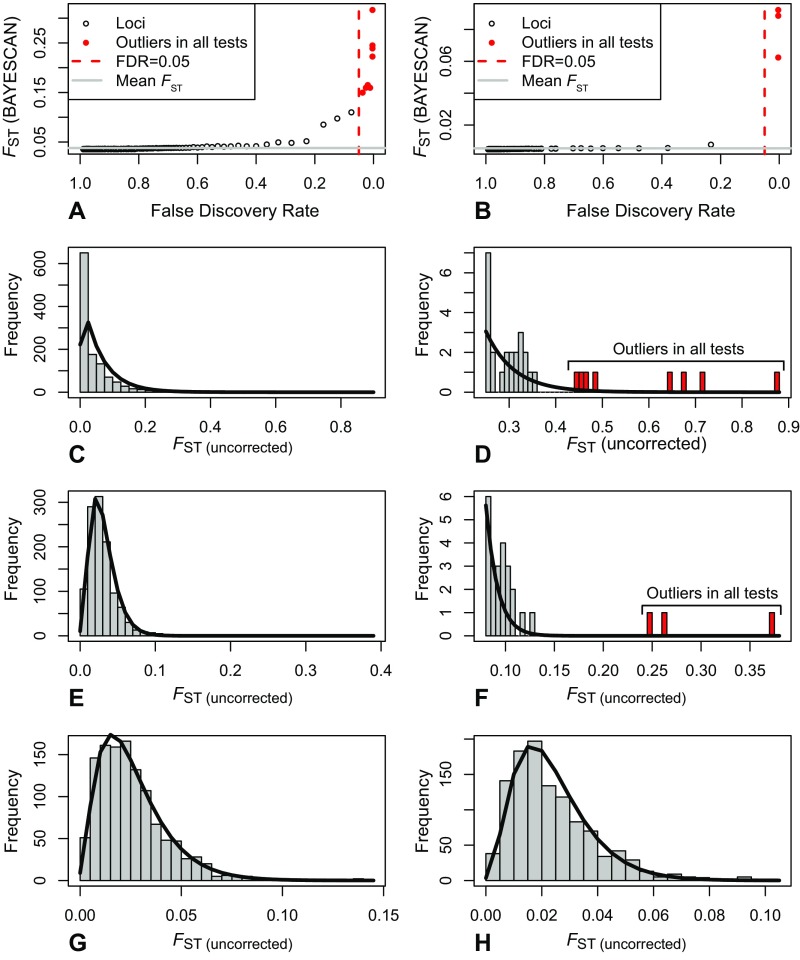
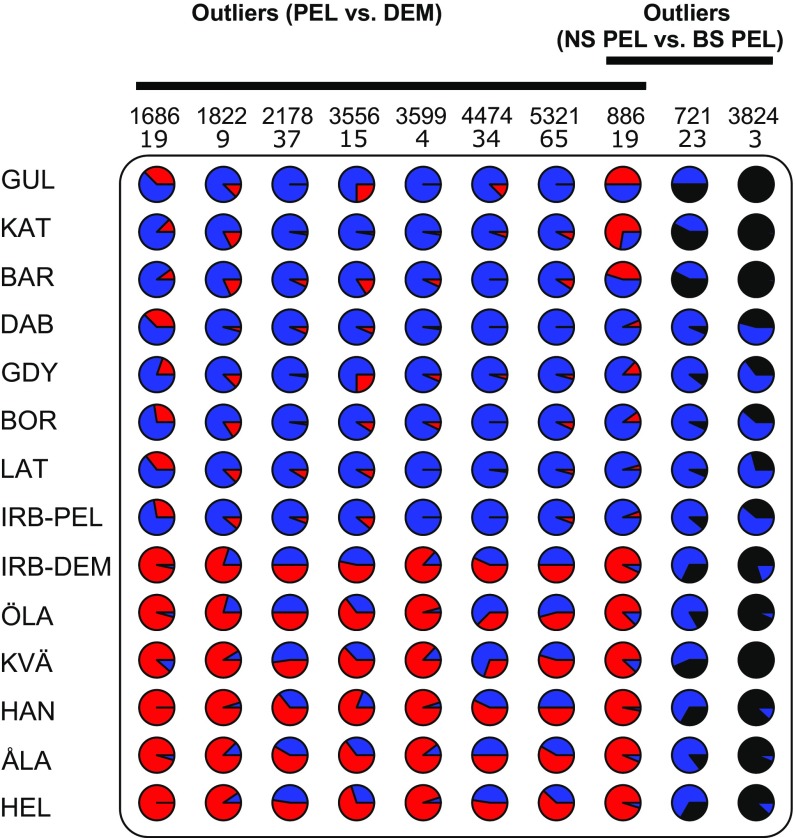
Multiple outlier tests (BAYESCAN, Arlequin, OutFLANK, and FLK) identified a set of 10 outlier loci that show higher than expected FST (Fig. S3 and Table S3). Eight of these loci show evidence of divergent selection between demersal and pelagic locations in the Baltic, whereas only three show evidence of divergent selection among locations on either side of the transition zone (Fig. 3 and Fig. S3). There was no evidence of spatially diversifying selection among populations of the same taxon within the Baltic Sea (Fig. 3 and Fig. S3 G and H). In Irbe (IRB), we present frequencies of outlier loci for putative demersal and putative pelagic individuals separately (Fig. 3), both of which show outlier frequencies similar to other demersal and pelagic populations, respectively, regardless of geographical proximity (Fig. 3). Of the six outliers for which we obtained flanking regions, four matched protein-coding genes (Table S4). One (a protocadherin FAT1-like gene) has been shown to be involved in response to salinity stress in oysters (24, 25) and teleosts (26). Locus 3599 has a nonsynonymous mutation within the dynein axonemal heavy chain 5 gene, which codes for a protein playing a central role in flagellar movement and sperm motility (27)—one of the traits that differs between the two taxa (13).
Fig. S3.
BAYESCAN analyses for outliers between pelagic and demersal flounders (A) and between North Sea and Baltic Sea pelagic locations (B). Outliers that were also identified by all other outlier tests (Fdist2, OutFLANK, and FLK) are represented by filled red circles. C–H show observed frequencies (gray histograms) of FST and expected distribution of FST under neutral expectations (black lines) as estimated by OutFLANK. (C) FST distribution between pelagic and demersal Baltic flounders; (D) right tail of the distribution, showing the eight outliers that were jointly identified by all tests. (E) FST distribution when comparing pelagic locations in the Baltic with North Sea; (F) right tail of the distribution, showing the three outliers that were jointly identified by all tests. (G) Distribution of global FST between pelagic flounders locations within the Baltic Sea and (H) comparisons among locations of demersal flounders within the Baltic Sea grouped according to sampling location. No statistically significant deviations from expected distributions were found among different sampling locations within the two Baltic populations. BAYESCAN analyses also failed to reveal any outliers in the same comparisons.
Table S3.
Results from outlier tests comparing demersal (DEM) and pelagic (PEL) flounders from the Baltic Sea, and PEL with samples from the North Sea (NS)
| Locus | Comparison | HE | FST | BAYES q value | ARL P value | OF q value | FLK P value |
| 1686_33 | PEL vs. DEM | 0.49 | 0.68 | 0 | 9.55E−78 | 2.06E−4 | NA |
| 1822_9 | PEL vs. DEM | 0.49 | 0.72 | 0 | 1.45E−130 | 1.24E−4 | NA |
| 2178_37 | PEL vs. DEM | 0.39 | 0.46 | 0.020 | 3.19E−12 | 1.12E−2 | NA |
| 3556_15 | PEL vs. DEM | 0.46 | 0.48 | 0.008 | 2.69E−10 | 8.92E−2 | NA |
| 3599_4 | PEL vs. DEM | 0.49 | 0.88 | 0 | 1.00E−07 | 7.04E−06 | NA |
| 4474_34 | PEL vs. DEM | 0.35 | 0.44 | 0.016 | 3.38E−4 | 1.33E−2 | NA |
| 5321_65 | PEL vs. DEM | 0.40 | 0.45 | 0.033 | 2.87E−10 | 1.25E−2 | NA |
| 886_19 | PEL vs. DEM | 0.50 | 0.65 | 0 | 1.74E−60 | 2.91E−4 | NA |
| 886_19 | DEM vs. NS | 0.35 | 0.35 | 0 | 5.09E−106 | 9.04E−13 | 2.82E−06 |
| 721_23 | DEM vs. NS | 0.32 | 0.25 | 0 | 1.10E−40 | 5.77E−08 | 3.71E−4 |
| 3824_3 | DEM vs. NS | 0.50 | 0.23 | 0 | 4.03E−29 | 2.84E−07 | 2.89E−05 |
ARL P value, P values from Fdist2 as implemented in Arlequin; BAYES q value, q values from BAYESCAN; FLK P value, P values from FLK; FST, uncorrected FST; HE, expected heterozygosity; OF q value, q values from OutFLANK.
Fig. 3.
Frequencies of outlier loci in each sampling location. SNPs are identified by the locus number (above) and the SNP position within the locus in base pairs (below). The first eight loci are outliers between pelagic and demersal flounders, and the last three are outliers between North Sea and Baltic pelagic locations. Individuals in Irbe were grouped separately in pelagic (above) and demersal (below) groups based on PCA, fastSTRUCTURE, and K-mean clustering results. Blue alleles are characteristic of pelagic Baltic populations; red alleles are characteristic of demersal Baltic populations. Black alleles are characteristic of North Sea populations.
Table S4.
GenBank matches of the regions flanking the outlier loci
| Locus | Fragment length, bp | SNPs | Best match | evalue | Gene | Putative function |
| 886 | 600 | 3 | XM005734944 | 7.17E-15 | Lysophosphatidic acid receptor 3 (lpar3) | Lipid signaling (69) |
| 1822 | 469 | 1 | XM018678664 | 4.51E-68 | ADAM metallopeptidase domain 15 | Cell–cell and cell–matrix adhesion, reproduction |
| 2178 | 501 | 2 | XM018703218 | 8.12E-179 | Protocadherin Fat 1-like | Cadherin-mediated adhesion and signaling |
| 3599 | 750 | 4 | XM019271703 | 3.86E-44 | Dynein axonemal heavy chain 5 | Flagellar movement, sperm motility (27) |
Best match, best match retrieved from BLAST; Fragment length, length of the fragment retrieved via I-PCR and screened; SNPs, number of SNPs within the retrieved fragment.
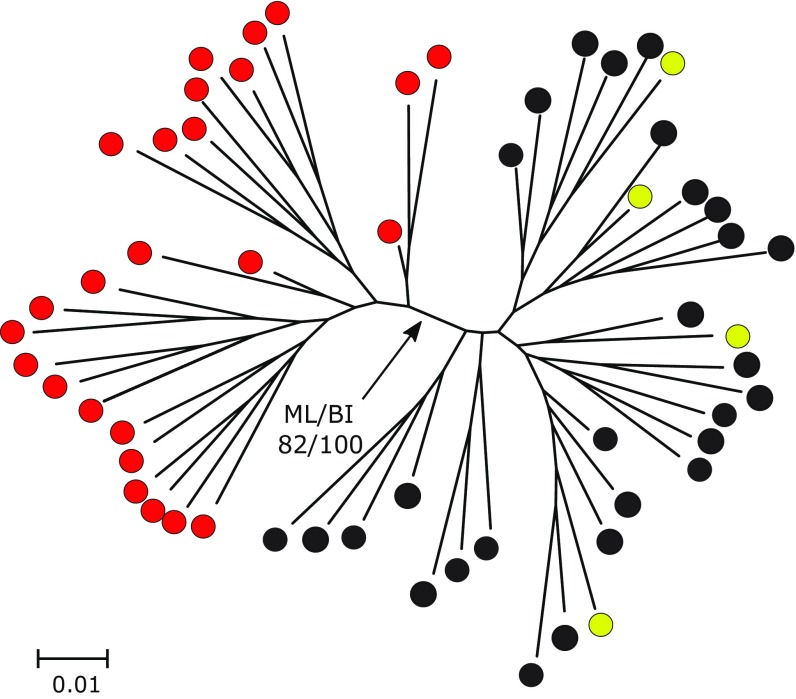
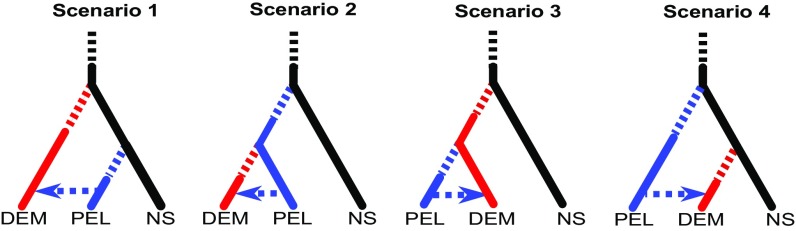
Maximum-likelihood and Bayesian phylogenetic analyses based on the sequences of 2,051 concatenated loci demonstrate that the two Baltic taxa are reciprocally monophyletic (Fig. 4). However, there is no support for a Baltic pelagic clade distinct from North Sea flounders (Fig. 4). Using an ABC–random forest (ABC-RF) approach (28, 29), we evaluated different potential evolutionary scenarios (Fig. 5 and Tables S5–S9). The demersal and pelagic flounders within the Baltic originated via two independent invasions (Fig. 5, scenario 1), which happened 2,400 (95% CI: 1,450–9,110) and 1,490 (95% CI: 596–4,780) generations ago, respectively. Evidence was also found for a demographic expansion in the ancestral population (Table S9), for a mild bottleneck in the demersal population (Table S8), and for secondary introgression—albeit weak—among the two species in the Baltic (Table S9).
Fig. 4.
Unrooted ML tree based on the concatenated sequences from 2,051 loci for a subset of 57 individuals. Black filled circles represent putative pelagic individuals. Red filled circles represent putative demersal individuals. Yellow circles represent individuals from a putative demersal location (Irbe), which clustered in all previous analyses with pelagic individuals. Branch support values represent ML bootstrap support and Bayesian clade credibility values, respectively. Only the single node that shows high support (>80) in both ML and Bayesian analyses is labeled.
Fig. 5.
Scenarios tested via ABC-RF. Scenario 1: double-colonization scenario with early invasion by demersal flounders. Scenario 2: single colonization scenario with early invasion of pelagic flounders. Scenario 3: single-colonization scenario with early invasion of demersal flounders. Scenario 4: double-colonization scenario with early invasion by pelagic flounders. Each lineage is color coded: black lines represent the North Sea lineage (NS), blue lines represent the Baltic pelagic lineage (PEL), and red lines represent the Baltic demersal lineage (DEM). Broken lines represent possible demographic changes in the ancestral population (black broken line) as well as potential bottlenecks at the time of invasion (blue and red broken lines). The blue dotted line with arrowhead represents secondary introgression among the two Baltic species. All divergence scenarios were tested against each other under an orthogonal combination of all other demographic parameters (changes in Ne and introgression). Full details of all models tested via ABC-RF are given in Supporting Information. The most likely model was scenario 1, including a demographic expansion in the ancestral population and a mild bottleneck in the demersal lineage.
Table S5.
Formulation of the four main divergence scenarios
| Scenario no. | Scenario identity |
| 1 | Independent-introductions scenario: a single split of the Baltic demersal population from the North Sea followed by another, independent single split of the Baltic pelagic population from the North Sea |
| 2 | Single-invasion scenario: a single split of the Baltic pelagic population from the North Sea, followed by a single split of the Baltic demersal from the Baltic pelagic population |
| 3 | Alternative single-invasion scenario: a single split of the Baltic demersal population from the North Sea, followed by a single split of the Baltic pelagic from the Baltic demersal population |
| 4 | Alternative independent-introductions scenario: a single split of the Baltic pelagic population from the North Sea followed by another, independent single split of the Baltic demersal population from the North Sea |
Table S9.
Results from ABC-RF model choice among scenarios with and without secondary introgression (scenario 1d-i vs. 1d-n) under both scenarios of constant ancestral Ne and a demographic expansion in the ancestral population
| Demographic parameters | Choice 1d-i | Choice 1d-i-e | Posterior probability | Prior error |
| Ancestral Ne | ||||
| Expansion | 10/10 | — | 0.611 ± 0.026 | 0.261 ± 0.0014 |
| Constant Ne | 10/10 | — | 0.542 ± 0.023 | 0.258 ± 0.0010 |
| Time of expansion | ||||
| 10,000–50,000 generations ago | 10/10 | 0.993 ± 0.005 | 0.191 ± 0.0010 |
Below, results from the final ABC-RF model choice comparing scenarios with and without demographic expansion in the ancestral population.
Table S8.
Results from ABC-RF model choice among scenarios with and without mild and strong bottlenecks at the time of Baltic colonization
| Ancestral Ne | Introgression | Choice 1d | Posterior probability ± SD | Prior error ± SD |
| Expansion | YES | 10/10 | 0.899 ± 0.017 | 0.040 ± 0.0003 |
| Expansion | NO | 10/10 | 0.430 ± 0.047 | 0.555 ± 0.0010 |
| Constant Ne | YES | 10/10 | 0.509 ± 0.023 | 0.563 ± 0.0008 |
| Constant Ne | NO | 10/10 | 0.486 ± 0.029 | 0.552 ± 0.0006 |
Scenario 1a, mild bottleneck in the pelagic lineage (Ne 1–25,000), and strong bottleneck in the demersal lineage (Ne 1–1,000); scenario 1b, mild bottleneck in the Pelagic lineage (Ne 1–25,000), and mild bottleneck in the demersal lineage (Ne 1,000–10,000); scenario 1c, no bottleneck in the pelagic lineage, and strong bottleneck in the demersal linage (Ne 1–100); scenario 1d, no bottleneck in the pelagic lineage and mild bottleneck in the demersal lineage (Ne 1,000–10,000); scenario 1e, no bottleneck.
Discussion
Ecological Speciation.
Genomic analyses revealed that pelagic and demersal spawning flounders in the Baltic Sea represent two reciprocally monophyletic taxa exhibiting limited-to-no introgression, strongly bimodal genotypic clustering, and signatures of divergent selection. Genetic differentiation among Baltic locations closely mirrored expected breeding behavior and was uncorrelated with geographic distance. Four of the six outliers for which we obtained flanking sequences matched protein-coding genes, one of which has been implicated in salinity stress response in oysters (24, 25) and fish (26). A SNP in locus 3599 causes an amino acid change in a gene governing sperm motility (27), one of the key traits that have previously been shown to differ between demersal and pelagic spawners (13), reinforcing the interpretation that the outliers identified in this study are likely targets of ecological selection. Interpreted together, these findings are indicative of strong reproductive isolation and suggestive of a well-advanced process of ecological speciation (3). Along with previous studies showing that reproductive traits are population specific (13, 30, 31), the genetic differentiation and signatures of selection between pelagic and demersal spawners uncovered here strongly suggest that breeding behavior in P. flesus is not a plastic trait, as has been previously suggested for another flatfish species (18), but rather the result of ecological adaptations to the low salinities experienced in the coastal waters of the Baltic Sea (13, 15).
Pelagic and demersal flounders have a parapatric distribution and share the same feeding and wintering grounds in the central Baltic (30). Because the two taxa can co-occur around the time of spawning in the same shallow coastal waters (for example, as they do in Irbe), it should be possible for pelagic spawners to opportunistically fertilize demersal eggs. However, a study has shown that pelagic spawners’ sperm motility is very low at salinities below 11 psu (13). Because demersal spawners lay their eggs in coastal waters where salinity rarely exceeds 7.5 psu—and never exceeds 11 psu (Fig. 1)—opportunistic fertilization by pelagic males is unlikely. Conversely, demersal spawners could remain in deeper areas after wintering and opportunistically fertilize pelagic eggs, although to what extent this may happen under natural conditions is unknown. Breeding behavior is expected to be under strong ecological selection in the northern Baltic region, and this expectation is supported by our findings of signatures of selection. Ecological selection on spawning behavior could lead to reproductive isolation via spawning segregation. As noted by Nosil (4), a trait under ecological selection causing reproductive isolation implicates a process of ecological speciation.
The extent of reproductive isolation seems to be stronger in the flounder species pair than in other cases of recent ecological speciation. Sympatric species of cichlid fish (Amphilophus citrinellus and Amphilophus zaliosus) in Lake Apoyo are not reciprocally monophyletic (the two species share many mtDNA haplotypes) and show stronger signs of introgression and less clearly bimodal genotypic clustering (32). Similarly, three-spined stickleback limnetic/benthic species pairs do occasionally mate in the wild; for example, roughly 1% of individuals in Paxton Lake are hybrids (33)—although hybrids have reduced fitness in the natural environment (34). Despite sampling throughout the Baltic Sea, we found no evidence of first-generation hybrids or of intermediate genotypes. If hybridization ever occurs under natural conditions, it must be either rare or geographically restricted.
Our data suggest the existence of another barrier to gene flow and of spatially diversifying selection between pelagic flounders from the North Sea and the Baltic Sea, coincident with a steep gradient in temperature and salinity. Reproductive isolation between the North Sea and Baltic Sea pelagic flounders is partial, as documented by a number of individuals showing admixture in the transition zone. However, it is possible that a process of speciation may have initiated between these two populations, possibly driven by selection for lower temperatures and salinity in the Baltic Sea. This hypothesis is supported by findings from reciprocal transplant experiments showing that pelagic flounders on each side of the transition zone have different expression patterns in candidate genes for osmoregulation, stress resistance, and heme protein biosynthesis (35).
Possible Evolutionary Scenarios.
The Baltic Sea became connected to the North Sea roughly 8.5 kya. Salinity remained well below the lowest salinity at which demersal flounders can reproduce until ∼7.5 kya and remained below the minimum requirements for pelagic spawning until ∼6.5 kya (11). During the period between 6.5 and 5 kya, salinity in the Baltic remained between 10 and 15 psu, creating an opportunity for colonization of the basin by pelagic spawners (11), and since has been steadily declining (11).
We propose that flounders adapted to a new ecological niche by developing a demersal spawning behavior in the early days of the Litorina Sea stage, possibly as early as 7.5 kya. Under this scenario, demersal flounders initially diverged from the pelagic flounders during a first allopatric phase lasting until ∼6 kya (11). Pelagic flounders could have invaded the Baltic Sea when conditions were suitable for pelagic spawning in most of the area (6–5 kya) and not exclusively, as today, in a few deep offshore basins. Such allopatric phase lasting 1,500/2,500 y might have prevented any homogenizing effect of gene flow in the early days of speciation. Other cases of extremely rapid ecological speciation, such as the evolution of benthic and limnetic species pairs of three-spined sticklebacks in postglacial lakes, are also thought to have started via double colonization and early divergence in allopatry (36).
This hypothesis is compatible with the double-invasion scenario identified by ABC-RF. The estimated time of divergence between demersal flounders and the North Sea population has a mode of 2,400 generations (95% CI: 1,450–9,110). Within the Baltic, flounders reach sexual maturity around 2 y of age (37), although in the northernmost locations sexual maturity in females is delayed for another 1–2 y (38). Hence the coalescence estimates suggest an early colonization of the Baltic Sea (7.2 kya, assuming a generation time of 3 y). Divergence time estimates of pelagic flounders from the ancestral population are also consistent with the hypothesis of an invasion during peak salinity 6–5 kya.
The speciation of a marine vertebrate in such a short time frame has never been reported before. Documented examples of ecological speciation in the marine environment are extremely rare (8) and have usually required much longer time frames (9). The speciation of the Baltic flounder species pair must have initiated no earlier than 8.5 kya, when the Baltic Sea first became connected to the North Sea. Other ecological speciation events have been recorded over similar time frames (<10,000 y), but such events involve species that reach sexual maturity within 1 or 2 y and none are from marine environments (32, 33). Hence, in terms of number of generations, speciation of the Baltic flounder species has likely happened faster than in those examples. To the best of our knowledge, this would place the speciation of the Baltic flounder species pair as one of the most rapid events of ecological speciation, and the fastest speciation event ever recorded for a marine vertebrate.
Conservation Implications.
The discovery that European flounders in the Baltic Sea represent a pair of closely related species calls for a reassessment of the species pair’s conservation status by the International Union for the Conservation of Nature. The fact that both species co-occur during feeding in a part of their range suggests that harvesting should be managed within the framework of a multispecies fishery.
Climate change is predicted to increase freshwater runoff in the Baltic Sea, causing a reduction in salinity and hence contracting the distribution of many marine species by hundreds of kilometers (39). At the same time, eutrophication and climate change are causing a rapid increase in hypoxia and anoxia in bottom waters where salinity is suitable for pelagic spawning (40). Consequently, the spawning habitat of pelagic flounders, which is already geographically limited, will likely contract in the future, raising concerns over a possible local extinction of this species. The endemic demersal spawners might prove more resilient, despite local declines of northern populations (41), but could experience a possible southward range shift. Young species pairs arising from recent events of ecological speciation may be prone to species collapse if anthropogenic activities alter the very factors that led to speciation, a process known as reverse speciation (42). It is possible that a decrease in salinity in the Baltic Sea linked to climate change may provide a strong selective pressure on pelagic spawners, leading to a second behavioral shift to demersal spawning and the collapse of this species pair.
Methods
Sampling.
A total of 282 samples was collected from 13 locations spanning nearly the entire distribution of European flounders in the Baltic Sea, including samples from the North Sea and the transition zone between the two regions. Sample collection took place in 2002–2004, 2009, and 2012. Details of sampling locations are given in Table S1. Most samples were collected at the time of spawning in either coastal locations (targeting demersal spawners) or offshore spawning grounds (targeting pelagic spawners). In Bornholm and Öland, almost all individuals (100% and 92%, respectively) were spawning during sampling in a pelagic (Bornholm) and coastal benthic (Öland) environment. Hence, samples from these two locations can be used as references to test for genetic differentiation among flounders exhibiting contrasting spawning behaviors. In some locations (Gdynia, Dabki, and Hanko; Fig. 1), sampling took place shortly after spawning (Table S1). Samples from Swedish waters were collected under a permit issued by the Swedish Board of Fisheries and samples from Hanko under a permit granted by Tvärminne Zoological Station (University of Helsinki). Samples from other locations were obtained from commercial fisheries. The described scientific sampling did not require ethical permission according to the Finnish Animal Conservation Law (7§ 28.6.2013/498).
SNP Genotyping.
DNA was extracted from fin clips or muscle tissues using either a standard salting out protocol or a DNeasy Blood and Tissue Kit (Qiagen). Library preparation and sequencing was carried out by Diversity Arrays Technology Pty Ltd using the standard DArTSeq. DArTSeq is a SNP genotyping-by-sequencing approach that combines genome complexity reduction via double-enzymatic digestion characteristic of DArT markers and sequencing on Illumina platforms (43). We initially tested four pairs of enzymes for complexity reduction and selected the PstI and SphI combination. Library preparation and sequencing were carried out as per Booksmythe et al. (44). Libraries were sequenced on three lanes of an Illumina Hiseq2000 platform. De novo assembly and SNP calling was performed using the pyRAD pipeline (45), using a minimum read depth of 10, a maximum read depth equal to the mean read depth plus two times the SD, a clustering threshold of 0.94, the strict quality filtering option, a minimum coverage of 95% of sampled individuals, and a maximum of five SNPs per locus. Only one random SNP per locus was retained for further analyses to avoid creating a set of tightly linked markers. We carried out the entire procedure, from library preparation to SNP calling, a second time for 60 technical replicates and retained only biallelic loci with 100% reproducibility (i.e., no genotyping errors). Loci that deviated from Hardy–Weinberg equilibrium in at least three locations (one representative of each genetic cluster: KAT from the North Sea and at least one demersal and one pelagic Baltic locations) were removed.
Analyses.
A PCA on allele frequency data was performed in the R package adegenet (46). The analysis was repeated after removing loci from the right and left 5% tails of the FST distributions obtained when comparing the main genetic clusters (North Sea, Baltic pelagic, and Baltic demersal). This “neu-tral” dataset should not include loci under strong divergent or stabilizing selection and should therefore be reflective of demographic processes such as genetic drift and migration. Genetic structure was further investigated using fastSTRUCTURE (47) and a DAPC (48). The most likely number of genetic clusters was determined by (i) running fastSTRUCTURE at multiple numbers of K using fivefold cross-validation and comparing the log-marginal likelihood lower bound (LLBO) and prediction error across increasing values of K; and (ii) running K-means clustering at multiple values of K and comparing Akaike information criterion (AIC) and Bayesian information criterion (BIC), as per Jombart et al. (48). Weir and Cockerham FST among locations and their 95% CI were estimated with 100 bootstraps in the R package diveRsity (49), and geographic distance among locations was estimated as the least cost path distance over seawater in the R package marmap (50). An estimate of number of migrants per generation was obtained using the private allele method (23). Migration rates between pelagic and demersal flounders within the Baltic were estimated using BAYESASS (51). For the latter analyses, pelagic and demersal flounders were grouped according to the results from PCA, fastSTRUCTURE, and DAPC; we ran five independent runs, each 10 million generations long, using a burn-in of 2 million generations.
We used four outlier tests to look for signatures of selection: BAYESCAN (52); OutFLANK (53); the coalescent method from Beaumont and Nichols (54) implemented in the software Arlequin, version 3.5 (55) (referred to as the Fdist method); and FLK (56). The tests were carried out first on a dataset where the samples were grouped as two populations representing the two major genetic clusters to identify outlier loci under selection among pelagic and demersal flounders. The tests were then carried out on pelagic and demersal flounders separately, grouping individuals according to geographical location to look for signatures of spatially diversifying selection. The FLK tests were only carried out for the latter analyses, using 24 individuals from the opposite taxon as outgroup population. For BAYESCAN analyses, we set prior odds to 100 to minimize chances of false positives and ran 20 pilot runs, followed by 100,000 iterations (5,000 samples, a thinning interval of 10, and a burn-in of 50,000). For the Fdist analyses, we used the island model implemented in Arlequin in cases where hierarchical genetic structure were not discovered by previous analyses, running 100 simulated demes and 20,000 coalescent simulations. For the detection of outliers between pelagic flounders in the Baltic Sea and the North Sea, we used the hierarchical, coalescent-based approach outlined by Excoffier et al. (57). In this analysis, locations were split in three groups according to PCA results: North Sea, Baltic Pelagic, and samples from the transition zone (Barsebäck). We used 10 simulated groups, 100 simulated demes for each group, and ran 20,000 coalescent simulations. OutFLANK analyses were carried out as outlined by Whitlock and Lotterhos (53). FLK analyses were carried out using the R and Python codes provided by the authors (available at https://qgsp.jouy.inra.fr/). Loci that were identified as outliers by all tests were considered as putatively under selection.
Maximum-likelihood (ML) and Bayesian phylogenies were constructed using a subsample of 57 individuals (32 putative pelagic and 25 putative demersal individuals). The alignment included the concatenated sequences of all 2,051 loci (for a total length of 135,164 bp). The ML Smart Model Selection (SMS) approach implemented in PHYML (58) was used to identify the evolutionary model (GTR+G), and ML and Bayesian phylogenetic analyses were carried out using the software PHYML (58) and MrBayes (59). ML analysis was performed using 100 bootstraps, 10 random starting trees and tree improvement by using the best of the nearest-neighbor interchange and subtree pruning and regrafting. Bayesian analyses were run for 3 million generations using two independent runs and 32 chains for each run. Convergence among runs was tested by checking SD of split frequencies fell below 0.05, and stability of each parameter was visually checked using the software TRACER (60). Convergence was reached around 2 million generations, and therefore any tree sampled before then was discarded.
We evaluated possible divergence scenarios and past demographic events under an ABC framework (see Supporting Information for a detailed description). We formulated competing scenarios describing the demographic history of P. flesus in the North Sea and the Baltic Sea (Fig. 5 and Table S5) and simulated datasets under these scenarios using the software DIYABC, version 2.1.0 (61). Model choice was performed using the recently developed ABC-RF approach (28) to evaluate the most probable scenario corresponding to our data, as well as its associated posterior probability. After ensuring the stability of model choice results for several demographic parameters (Fig. 5 and Tables S5–S9), we estimated divergence time for both demersal and pelagic flounders using the parameter estimation analysis implemented in DIYABC on the 1% closest simulated data. The adequacy of our final model was tested by performing the model-posterior checking analysis implemented in DIYABC (Fig. S4).
Fig. S4.
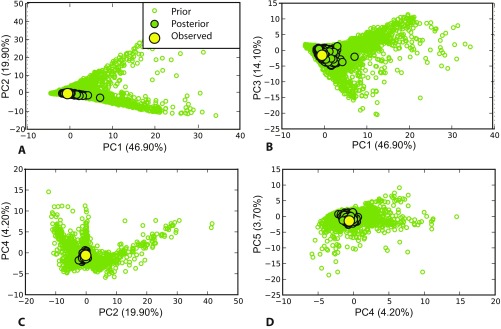
PCA in the space of summary statistics, showing datasets simulated from the prior distribution of the parameters (green open circles), from the posterior predictive distribution (green filled circles), as well as the observed dataset (yellow circle). A–D are plots of the PCA using different combinations of the first five principal components, which cumulatively represent 89% of the total variation. The observed dataset is always located within a small cluster from the posterior predictive distribution.
Inference of Platichthys flesus Evolutionary History Through Approximate Bayesian Computation–Random Forest
Formulation of Competing Scenarios.
Approximate Bayesian computation (ABC) is a methodological framework allowing to test for multiple evolutionary scenarios using molecular data (22, 29). ABC relies on the simulation of large number of datasets matching a finite set of competing scenarios and summarized with a pool of summary statistics. Simulated datasets are subsequently compared with the observed dataset to estimate approximate posterior probabilities of the most probable scenario (i.e., the most likely evolutionary history of the focal populations). ABC inference has become widely used in population genetics, spanning over diverse fields of research including anthropology (63) and invasion biology (64). Here, it was used to investigate the evolutionary history of P. flesus.
The evolutionary and demographic histories of the three flounder lineages identified in this study are potentially complex; they may have involved multiple past changes in effective population size, and a number of different divergence scenarios are thinkable. Nevertheless, these possible evolutionary scenarios are all variations of two main evolutionary models: a single-invasion scenario and a double-colonization scenario. The single-invasion scenario postulates that flounders colonized the Baltic Sea only once, and thereafter diverged in two distinct lineages within the Baltic Sea, either in sympatry or parapatry. According to the double-invasion scenario, the two Baltic Sea lineages originated via two distinct colonization events from the North Sea, and hence speciation could have started during an early allopatric phase. Within each of these two divergence scenarios, either the pelagic or the demersal flounders could have diverged first from the ancestral population. If we assume that the Baltic lineages originated from the North Sea population, we therefore have four main evolutionary scenarios, representing all possible divergence events (Fig. 5 in the main manuscript and Table S5), to test.
Within each of these four scenarios, a number of other factors should be considered and tested to reconstruct the correct demographic history of the species pair. First, the ancestral population might have been itself subject to changes in effective population size. Tajima’s D was negative and with very similar values at every location for each of the three lineages (Table S2), suggesting the ancestral population from which they originated underwent a demographic expansion. Both Baltic Sea lineages could have experienced weak or strong bottlenecks and demographic expansions at the time of colonization. Furthermore, although there is no evidence of contemporary migration between the two Baltic Sea flounder species, fastSTRUCTURE analyses suggest that, following divergence, there may have been some limited secondary introgression (specifically, from pelagic to demersal flounders; Fig. S2E). The four basic scenarios, along with all variations that are considered, are depicted in Fig. 5 in the main manuscript.
Here, we formulated competing scenarios corresponding to the most probable history of P. flesus populations in the North Sea and the Baltic Sea (Table S5) and used the software DIYABC, version 2.1.0 (61), to simulate datasets from each scenario based on uniformly distributed priors (Table S6 for detailed information on the priors).
Table S6.
Priors used for generating the reference tables used in the first ABC-RF tournament to determine which among the four divergence scenarios was the most likely
| Parameters | Parameter name | Scenario 1 | Scenario 2 | Scenario 3 | Scenario 4 |
| Population size parameters | Ne North Sea | 100–500,000 | 100–500,000 | 100–500,000 | 100–500,000 |
| Ne Pelagic | 100–200,000 | 100–200,000 | 100–200,000 | 100–200,000 | |
| Ne Demersal | 100–100,000 | 100–100,000 | 100–100,000 | 100–100,000 | |
| Ne Bottleneck pelagic* | 10–50,000 | 10–50,000 | 10–50,000 | 10–50,000 | |
| Ne Bottleneck demersal* | 10–20,000 | 10–20,000 | 10–20,000 | 10–20,000 | |
| Ancestral Ne† | 0–100,000 | 0–100,000 | 0–100,000 | 0–100,000 | |
| Times of events | Split of pelagic lineage | 100–5,000 | 500–10,000 | 100–5,000 | 500–10,000 |
| Split of demersal lineage | 500–10,000 | 100–5,000 | 500–10,000 | 100–5,000 | |
| Ancestral Ne expansion† | 10,000–50,000 | 10,000–50,000 | 10,000–50,000 | 10,000–50,000 | |
| Time of secondary admixture‡ | 0–5,000 | 0–5,000 | 0–5,000 | 0–5,000 | |
| Admixture proportion | Admixture proportion‡ | 0.01–0.2 | 0.01–0.2 | 0.01–0.2 | 0.01–0.2 |
Parameters only included in bottleneck scenarios.
Parameters only included in the scenarios including ancestral population expansion.
Parameter only included in the scenarios with introgression.
Model Choice.
An inherent drawback of ABC inference is the computational effort required to simulate sufficient amounts of datasets when multiple competing scenarios are considered (65). Recently, Pudlo et al. (28) developed a new approach called ABC–random forest (ABC-RF) to discriminate among competing models at a much less intensive computational cost—see Pudlo et al. (28) for a detailed statistical description and Fraimout et al. (64) for a case study. Briefly, ABC-RF uses the datasets simulated for different scenarios using parameter values drawn from prior distributions and their respective summary statistics (i.e., the ABC reference table) to discriminate the most probable scenario and estimate its posterior probability. We used the RF classification method implemented in the R package abcrf, version 1.1 (28), to apply ABC-RF treatment on competing evolutionary scenarios. As suggested by Pudlo et al. (28), we processed a reference table of 10,000 simulations per scenario and ran the treatments for 10 iterations to ensure model choice consistency (Tables S7–S9; values reported are means from 10 iterations).
Table S7.
Results from ABC-RF model selection, ran under eight different combinations of demographic parameters
| Ancestral Ne | Bottleneck | Introgression | Choice 1 | Posterior probability ± SD | Prior error ± SD |
| Expansion | Bottleneck | YES | 10/10 | 0.868 ± 0.012 | 0.357 ± 0.0006 |
| Expansion | Bottleneck | NO | 10/10 | 0.817 ± 0.013 | 0.233 ± 0.0007 |
| Expansion | Constant Ne | YES | 10/10 | 0.889 ± 0.015 | 0.386 ± 0.0011 |
| Expansion | Constant Ne | NO | 10/10 | 0.797 ± 0.018 | 0.275 ± 0.0008 |
| Constant Ne | Bottleneck | YES | 10/10 | 0.939 ± 0.012 | 0.356 ± 0.0005 |
| Constant Ne | Bottleneck | NO | 10/10 | 0.585 ± 0.024 | 0.252 ± 0.0006 |
| Constant Ne | Constant Ne | YES | 10/10 | 0.927 ± 0.010 | 0.354 ± 0.0001 |
| Constant Ne | Constant Ne | NO | 10/10 | 0.564 ± 0.025 | 0.290 ± 0.0010 |
Constant ancestral Ne vs. demographic expansion in the ancestral population; constant Ne vs. bottlenecks at the time of invasion, and with or without secondary introgression between the two Baltic lineages. Choice 1 represent the number of independent random forest iterations that identified model 1 as the winner.
To identify the most likely scenario, we ran a multiple-tier tournament among competing divergence and demographic models. In the first-tier competition, we ran simulations for each of the main four divergence scenarios (scenarios 1–4, Table S5) under every possible combination of the following parameters: constant ancestral Ne vs. ancestral demographic expansion, constant Ne at invasion vs. bottlenecks, and with and without secondary introgression among the two Baltic lineages (Table S6). We therefore had eight orthogonal combinations of the three above-mentioned parameters (each of which had two levels, i.e., 23). Regardless of what combination of demographic parameters was chosen, the winning scenario in every iteration of all eight random forest analyses was always scenario 1: a double-invasion scenario whereby the demersal lineage invaded the Baltic Sea first (Table S7). After identifying scenario 1 as the most likely divergence scenario, we ran further “tournaments” among versions of scenario 1 with different demographic parameters.
We ran a second-tier tournament to determine whether or not the two Baltic lineages underwent a bottleneck followed by a demographic expansion during and after the invasion of the Baltic Sea. Given the extremely low divergence of the Pelagic Baltic lineage from the North Sea population, we assumed that a very strong bottleneck of this lineage is unlikely (genetic drift is extremely low and diversity indices were very similar to other populations; Table S2); therefore, we only modeled a mild bottleneck (ancestral Ne < 25,000 with uniform prior distribution) for this population. For the demersal lineage, which shows much stronger divergence from the North Sea as well as from the Baltic pelagic population, we modeled both a strong (Ne 1–1,000, with a normal distribution—mean Ne 500 ± 250) and mild (Ne 1,000–10,000, with a normal distribution—mean Ne 5,000 ± 2,500) bottlenecks. We compared every possible combination among these parameter levels (Table S8, scenarios 1a–e) and ran simulations under all possible combinations of the remaining two still unaddressed demographic parameters (constant ancestral Ne vs. ancestral demographic expansion, and with and without secondary introgression among the two Baltic lineages). Under all combinations of ancestral Ne and introgression parameters, scenario 1d (no bottleneck in the pelagic lineage and a mild bottleneck in the demersal lineage) was always chosen as the most likely scenario in all 10 RF iterations, with posterior probabilities ranging from about 0.43 to nearly 0.9 (Table S8). Scenarios including a bottleneck in the Pelagic lineage performed consistently worse than all others.
In the final steps of model selection, we used the same approach to test whether there was any evidence of secondary introgression and of a demographic expansion in the ancestral population. Scenarios including secondary introgression (scenario 1-d-i) among Baltic lineages always performed better (they were consistently chosen in all ten RF iterations) but only marginally so, as shown by low posterior probabilities (Table S9). On the other hand, evidence for demographic expansion (scenario 1-d-i-e) in the ancestral population was very strong (mean posterior probability over 10 iterations, 0.993 ± 0.005; Table S9). Hence, the most likely scenario chosen is scenario 1-d-i-e; the colonization of the Baltic Sea lineages therefore most likely happened via a double colonization. The pelagic lineage did not undergo any major bottleneck event, whereas the demersal lineage underwent a mild bottleneck at the time of invasion. Following the invasion of the pelagic lineage, there seem to have been some secondary introgression, although evidence is not very strong. Finally, the ancestral population from which all lineages descend underwent a demographic expansion.
A reference table of 500,000 simulations was generated under scenario 1-d-i-e to estimate the posterior distribution of demographic parameters and to estimate how well the model fit the data (see next paragraphs). Because the right tail of the some of the demographic parameters (mostly Ne) was not fully captured with the set of priors used in the tournaments, we slightly modified the priors: we increased the upper bound the Ne to 500,000 for the North Sea and Pelagic Baltic lineage, and to 200,000 for the demersal Baltic lineage, and we used slightly wider priors for the time of divergence of the pelagic Baltic lineage (100–10,000 generations, normally distributed—mean, 2,000 ± 3,000) and the demersal Baltic lineage (500–20,000 generations, normally distributed—mean, 3,000 ± 3,000).
Parameter Estimation.
Through ABC inferences, the distribution of the demographic parameters’ posterior values produced under the most probable chosen scenario can be estimated. Here, a paramount aspect of our study is to estimate how recently did the ecological speciation process initiate. To specifically address this question, we estimated the posterior distribution of split time (i.e., time of divergence) from the North Sea of both pelagic and demersal flounders. We used the parameter estimation analysis implemented in DIYABC using all summary statistics proposed by the software, and a local linear regression of the 1% closest simulated datasets. We found that the Baltic demersal population diverged 2,400 generations ago (95% CI: 1,450–9,110) from the ancestral North Sea population, that is, 7,200 y before present time assuming a generation time of 3 y (38). The Baltic pelagic population's colonization time was estimated to happen 1,490 generations ago (95% CI: 596–4,780), that is, 4,470 y before present.
Model Checking.
As ABC inferences are model-based, they allow for the estimation of posterior distributions of the most probable evolutionary history from a finite set of competing scenarios. An important issue to be resolved in ABC is thus to estimate how well the most probable scenario fits with the observed dataset (i.e., matches the true evolutionary history). We used the ABC model-posterior checking method implemented in DIYABC (61) to evaluate how well our final scenario with associated parameter posterior distributions matched the observed dataset—see Cornuet et al. (66) and Fraimout et al. (64) for a detailed descriptions of the analyses. The principle of model-posterior checking is as follows: if a scenario-posterior combination fits the observed data correctly, then data simulated under this scenario with parameters drawn from associated posterior distributions should be close to the observed data. The closeness of simulated and observed datasets can be further evaluated by applying a principal-component analysis (PCA) on the total datasets (i.e., observed and simulated) and measuring the frequency at which summary statistics measured on the observed dataset are extreme with respect to the distributions of the same statistics computed from the simulated datasets (i.e., the posterior predictive distributions). Hence, too many observed summary statistics falling in the tails of distributions would cast serious doubts on the adequacy of the model-posterior combination to the observed dataset. Graphical representation of the PCA on total datasets revealed that the observed dataset points fell well within the simulated datasets produced through our final scenario (Fig. S4). In agreement with this result, none of the 36 summary statistics associated to the simulated datasets showed signs of deviation from the observed summary statistics’ distribution after applying Benjamini and Hochberg’s sequential correction for multiple comparisons. Altogether, these results show that the final scenario chosen to depict the evolutionary history of the flounder species-pair matches well the observed dataset.
Genome Walking and Outliers' Annotation
Because DArTSeq loci are fairly short (69 bp after the removal of PstI restriction site), we used an inverse-PCR (I-PCR) protocol to obtain flanking regions upstream and downstream of the eight outlier loci identified as outliers between the Baltic Sea species-pair. This was done to increase the chances of finding matches for the outlier loci in the public databases. I-PCR is a technique aimed at amplifying sequences that are flanking a known genomic region (67); it involves digestion with a restriction enzyme (usually a blunt cutter with a 4- to 6-bp-long restriction site) that does not cleave within the known region, followed by circularization of the digested product. The circular template is then amplified using PCR primers whose orientation is opposite to a standard PCR, often using a nested or heminested amplification protocol to increase specificity.
We digested genomic DNA from five individuals using, in separate experiments, three restriction enzymes that do not cleave within the known sequences: RsaI, BsaAI and HaeIII. A total of 150 ng of template DNA was digested in a 20-μL reaction at 37 °C for 2 h, following the manufacturer’s instructions. RsaI and HaeIII digestions were heat-inactivated at 80 °C for 20 min (BsaAI cannot be heat-inactivated). The target fragment was circularized via self-ligation; briefly, 5 µL of digestion product was used as template in a 25-µL ligation reaction using T4 Ligase (NEB), following the manufacturer’s instructions. RsaI and Hae III digestions were ligated at room temperature for 2 h, whereas BsaAI digestions were ligated at 4 °C overnight. The ligated products were amplified using a heminested PCR protocol and sequenced using a commercial service (MACROGEN). From the obtained sequences, we designed additional primers and screened 10 individuals (5 from demersal and 5 from pelagic flounders) to both confirm the genotypes obtained via DArTSeq and to screen for further variation in the flanking region. We were able to obtain flanking regions from six of the eight outliers; a representative sequence of each of the two alleles for each locus was submitted to GenBank (accession numbers KY933571–KY933582).
Genotypes retrieved from sequencing were always consistent with DArTSeq alleles. Four of the six outliers for which flanking regions were obtained matched protein-coding genes from the National Center for Biotechnology Information database (Table S4). Two loci (2178 and 3599) matched genes that have been implicated in salinity stress and sperm motility. Locus 2178 matched a protocadherin Fat 1-like gene; protocadherins are Ca2+-binding proteins involved in the maintenance of calcium homeostasis, cell adhesion, and signaling (68). Cadherin and protocadherin have been shown to be involved in acclimatization to salinity stress in teleosts (26) and oysters (25). Locus 3599 was found to match a dynein axonemal heavy chain 5 gene, whereby one of the SNPs identified causes an amino acid change; dyneins are a family of cytoskeletal proteins that drive the beat of eukaryotic cilia and flagella and hence have a major role in sperm motility and sperm activation (27), some of the key traits that differ between the pelagic and demersal flounders and that are considered key adaptations to the extremely low salinities of the Baltic (13).
Acknowledgments
We thank A. Stow, F. Calboli, A. Estoup, Z. Li, T. Shikano, B. Guo, and J. DeFaveri for discussion and comments. Thanks are due to the collectors of the 2003 samples in the study by Florin and Höglund (15). ABC-RF analyses were performed on the CBGP HPC and UMS 2700 OMSI computational platforms. This project was funded by the the Maj & Tor Nessling Foundation, the Academy of Finland (project 292737), and the Walter and Andrée de Nottbeck Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.S. is a guest editor invited by the Editorial Board.
Data deposition: The short sequence reads reported in this paper have been deposited in GenBank Short Read Archive (Bioproject PRJNA382467, Short Read Study SRP103564). The sequences from the outliers' flanking regions have been deposited in the GenBank database (accession nos. KY933571–KY933582). In addition, the codes for the ABC scenarios and additional datasets have been deposited in the DRYAD Digital Repository database, datadryad.org (doi: 10.5061/dryad.f6154).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1615109114/-/DCSupplemental.
References
- 1.Schluter D, Rambaut A. Ecological speciation in postglacial fishes. Philos Trans R Soc Lond B Biol Sci. 1996;351:807–814. [Google Scholar]
- 2.Hendry AP, Nosil P, Rieseberg LH. The speed of ecological speciation. Funct Ecol. 2007;21:455–464. doi: 10.1111/j.1365-2435.2006.01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nosil P, Harmon LJ, Seehausen O. Ecological explanations for (incomplete) speciation. Trends Ecol Evol. 2009;24:145–156. doi: 10.1016/j.tree.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Nosil P. Ecological Speciation. Oxford Univ Press; Oxford: 2012. [Google Scholar]
- 5.Servedio MR, Van Doorn GS, Kopp M, Frame AM, Nosil P. Magic traits in speciation: “Magic” but not rare? Trends Ecol Evol. 2011;26:389–397. doi: 10.1016/j.tree.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Jiggins CD, Naisbit RE, Coe RL, Mallet J. Reproductive isolation caused by colour pattern mimicry. Nature. 2001;411:302–305. doi: 10.1038/35077075. [DOI] [PubMed] [Google Scholar]
- 7.Ogden R, Thorpe RS. Molecular evidence for ecological speciation in tropical habitats. Proc Natl Acad Sci USA. 2002;99:13612–13615. doi: 10.1073/pnas.212248499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puebla O. Ecological speciation in marine v. freshwater fishes. J Fish Biol. 2009;75:960–996. doi: 10.1111/j.1095-8649.2009.02358.x. [DOI] [PubMed] [Google Scholar]
- 9.Munday PL, van Herwerden L, Dudgeon CL. Evidence for sympatric speciation by host shift in the sea. Curr Biol. 2004;14:1498–1504. doi: 10.1016/j.cub.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 10.Puritz JB, et al. Extraordinarily rapid life-history divergence between Cryptasterina sea star species. Proc R Soc Lond Ser B Biol Sci. 2012;279:3914–3922. doi: 10.1098/rspb.2012.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gustafsson BG, Westman P. On the causes for salinity variations in the Baltic Sea during the last 8500 years. Paleoceanography. 2002;17:12-1–12-14. [Google Scholar]
- 12.Johannesson K, André C. Life on the margin: Genetic isolation and diversity loss in a peripheral marine ecosystem, the Baltic Sea. Mol Ecol. 2006;15:2013–2029. doi: 10.1111/j.1365-294X.2006.02919.x. [DOI] [PubMed] [Google Scholar]
- 13.Nissling A, Westin L, Hjerne O. Reproductive success in relation to salinity for three flatfish species, dab (Limanda limanda), plaice (Pleuronectes platessa), and flounder (Pleuronectes flesus), in the brackish water Baltic Sea. ICES J Mar Sci. 2002;59:93–108. [Google Scholar]
- 14.Lønning S, Solemdal P. The relation between thickness of chorion and specific gravity of eggs from Norwegian and Baltic flatfish populations. Fiskeridirektoratets skrifter. Serie Havundersøkelse. 1972;16:77–88. [Google Scholar]
- 15.Florin AB, Höglund J. Population structure of flounder (Platichthys flesus) in the Baltic Sea: Differences among demersal and pelagic spawners. Heredity (Edinb) 2008;101:27–38. doi: 10.1038/hdy.2008.22. [DOI] [PubMed] [Google Scholar]
- 16.Hemmer-Hansen J, Nielsen EE, Grønkjaer P, Loeschcke V. Evolutionary mechanisms shaping the genetic population structure of marine fishes; lessons from the European flounder (Platichthys flesus L.) Mol Ecol. 2007;16:3104–3118. doi: 10.1111/j.1365-294X.2007.03367.x. [DOI] [PubMed] [Google Scholar]
- 17.Whitlock MC, McCauley DE. Indirect measures of gene flow and migration: FST not equal to 1/(4Nm + 1) Heredity (Edinb) 1999;82:117–125. doi: 10.1038/sj.hdy.6884960. [DOI] [PubMed] [Google Scholar]
- 18.Florin AB, Höglund J. Absence of population structure of turbot (Psetta maxima) in the Baltic Sea. Mol Ecol. 2007;16:115–126. doi: 10.1111/j.1365-294X.2006.03120.x. [DOI] [PubMed] [Google Scholar]
- 19.Wallin I. 2016. Opportunities for hybridization between two sympatric flounder (Platichthys flesus) ecotypes in the Baltic Sea. Master’s thesis (Uppsala University, Uppsala, Sweden)
- 20.Via S. Reproductive isolation between sympatric races of pea aphids. I. Gene flow restriction and habitat choice. Evolution. 1999;53:1446–1457. doi: 10.1111/j.1558-5646.1999.tb05409.x. [DOI] [PubMed] [Google Scholar]
- 21.Nosil P, Feder JL. Genomic divergence during speciation: Causes and consequences. Philos Trans R Soc Lond B Biol Sci. 2012;367:332–342. doi: 10.1098/rstb.2011.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beaumont MA, Zhang W, Balding DJ. Approximate Bayesian computation in population genetics. Genetics. 2002;162:2025–2035. doi: 10.1093/genetics/162.4.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slatkin M, Barton NH. A comparison of three indirect methods for estimating average levels of gene flow. Evolution. 1989;43:1349–1368. doi: 10.1111/j.1558-5646.1989.tb02587.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhang G, et al. Molecular basis for adaptation of oysters to stressful marine intertidal environments. Annu Rev Anim Biosci. 2016;4:357–381. doi: 10.1146/annurev-animal-022114-110903. [DOI] [PubMed] [Google Scholar]
- 25.Zhao X, Yu H, Kong L, Li Q. Transcriptomic responses to salinity stress in the Pacific oyster Crassostrea gigas. PLoS One. 2012;7:e46244. doi: 10.1371/journal.pone.0046244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boutet I, Long Ky CL, Bonhomme F. A transcriptomic approach of salinity response in the euryhaline teleost, Dicentrarchus labrax. Gene. 2006;379:40–50. doi: 10.1016/j.gene.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Cosson J, et al. Studying sperm motility in marine fish: An overview on the state of the art. J Appl Ichthyology. 2008;24:460–486. [Google Scholar]
- 28.Pudlo P, et al. Reliable ABC model choice via random forests. Bioinformatics. 2016;32:859–866. doi: 10.1093/bioinformatics/btv684. [DOI] [PubMed] [Google Scholar]
- 29.Cornuet J-M, et al. Inferring population history with DIY ABC: A user-friendly approach to approximate Bayesian computation. Bioinformatics. 2008;24:2713–2719. doi: 10.1093/bioinformatics/btn514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nissling A, Dahlman G. Fecundity of flounder, Pleuronectes flesus, in the Baltic Sea—reproductive strategies in two sympatric populations. J Sea Res. 2010;64:190–198. [Google Scholar]
- 31.Nissling A, Thorsen A, da Silva FF. Fecundity regulation in relation to habitat utilisation of two sympatric flounder (Platichtys flesus) populations in the brackish water Baltic Sea. J Sea Res. 2015;95:188–195. [Google Scholar]
- 32.Barluenga M, Stölting KN, Salzburger W, Muschick M, Meyer A. Sympatric speciation in Nicaraguan crater lake cichlid fish. Nature. 2006;439:719–723. doi: 10.1038/nature04325. [DOI] [PubMed] [Google Scholar]
- 33.McPhail JD. Ecology and evolution of sympatric sticklebacks (Gasterosteus): Evidence for a species-pair in Paxton Lake, Texada Island, British Columbia. Can J Zool. 1992;70:361–369. [Google Scholar]
- 34.Hatfield T, Schluter D. Ecological speciation in sticklebacks: Environment-dependent hybrid fitness. Evolution. 1999;53:866–873. doi: 10.1111/j.1558-5646.1999.tb05380.x. [DOI] [PubMed] [Google Scholar]
- 35.Larsen PF, Nielsen EE, Williams TD, Loeschcke V. Intraspecific variation in expression of candidate genes for osmoregulation, heme biosynthesis and stress resistance suggests local adaptation in European flounder (Platichthys flesus) Heredity (Edinb) 2008;101:247–259. doi: 10.1038/hdy.2008.54. [DOI] [PubMed] [Google Scholar]
- 36.Taylor EB, McPhail JD. Historical contingency and ecological determinism interact to prime speciation in sticklebacks, Gasterosteus. Proc R Soc Lond Ser B Biol Sci. 2000;267:2375–2384. doi: 10.1098/rspb.2000.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ICES . ICES WGBFAS REPORT 2016: Report of the Baltic Fisheries Assessment Working Group (WGBFAS) ICES; Copenhagen: 2016. [Google Scholar]
- 38.Erlandsson J, Östman Ö, Florin A-B, Pekcan-Hekim Z. Spatial structure of body size of European flounder (Platichthys flesus L.) in the Baltic Sea. Fish Res. 2017;189:1–9. [Google Scholar]
- 39.Vuorinen I, et al. Scenario simulations of future salinity and ecological consequences in the Baltic Sea and adjacent North Sea areas-implications for environmental monitoring. Ecol Indic. 2015;50:196–205. doi: 10.1016/j.ecolind.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carstensen J, Andersen JH, Gustafsson BG, Conley DJ. Deoxygenation of the Baltic Sea during the last century. Proc Natl Acad Sci USA. 2014;111:5628–5633. doi: 10.1073/pnas.1323156111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jokinen H, et al. Decline of flounder (Platichthys flesus (L.)) at the margin of the species’ distribution range. J Sea Res. 2015;105:1–9. [Google Scholar]
- 42.Seehausen O. Conservation: Losing biodiversity by reverse speciation. Curr Biol. 2006;16:R334–R337. doi: 10.1016/j.cub.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 43.Sansaloni C, et al. Diversity Arrays Technology (DArT) and next-generation sequencing combined: Genome-wide, high throughput, highly informative genotyping for molecular breeding of Eucalyptus. BMC Proc. 2011;5:P54. [Google Scholar]
- 44.Booksmythe I, Head ML, Keogh JS, Jennions MD. Fitness consequences of artificial selection on relative male genital size. Nat Commun. 2016;7:11597. doi: 10.1038/ncomms11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eaton DA. PyRAD: Assembly of de novo RADseq loci for phylogenetic analyses. Bioinformatics. 2014;30:1844–1849. doi: 10.1093/bioinformatics/btu121. [DOI] [PubMed] [Google Scholar]
- 46.Jombart T. adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics. 2008;24:1403–1405. doi: 10.1093/bioinformatics/btn129. [DOI] [PubMed] [Google Scholar]
- 47.Raj A, Stephens M, Pritchard JK. fastSTRUCTURE: Variational inference of population structure in large SNP data sets. Genetics. 2014;197:573–589. doi: 10.1534/genetics.114.164350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jombart T, Devillard S, Balloux F. Discriminant analysis of principal components: A new method for the analysis of genetically structured populations. BMC Genet. 2010;11:94. doi: 10.1186/1471-2156-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keenan K, McGinnity P, Cross TF, Crozier WW, Prodöhl PA. diveRsity: An R package for the estimation and exploration of population genetics parameters and their associated errors. Methods Ecol Evol. 2013;4:782–788. [Google Scholar]
- 50.Pante E, Simon-Bouhet B. marmap: A package for importing, plotting and analyzing bathymetric and topographic data in R. PLoS One. 2013;8:e73051. doi: 10.1371/journal.pone.0073051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson GA, Rannala B. Bayesian inference of recent migration rates using multilocus genotypes. Genetics. 2003;163:1177–1191. doi: 10.1093/genetics/163.3.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foll M, Gaggiotti O. A genome-scan method to identify selected loci appropriate for both dominant and codominant markers: A Bayesian perspective. Genetics. 2008;180:977–993. doi: 10.1534/genetics.108.092221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitlock MC, Lotterhos KE. Reliable detection of loci responsible for local adaptation: Inference of a null model through trimming the distribution of F ST*. Am Nat. 2015;186:S24–S36. doi: 10.1086/682949. [DOI] [PubMed] [Google Scholar]
- 54.Beaumont MA, Nichols RA. Evaluating loci for use in the genetic analysis of population structure. Proc R Soc Lond Ser B Biol Sci. 1996;263:1619. [Google Scholar]
- 55.Excoffier L, Lischer HE. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 56.Bonhomme M, et al. Detecting selection in population trees: The Lewontin and Krakauer test extended. Genetics. 2010;186:241–262. doi: 10.1534/genetics.110.117275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Excoffier L, Hofer T, Foll M. Detecting loci under selection in a hierarchically structured population. Heredity (Edinb) 2009;103:285–298. doi: 10.1038/hdy.2009.74. [DOI] [PubMed] [Google Scholar]
- 58.Guindon S, et al. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 59.Ronquist F, et al. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rambaut A, Suchard MA, Xie D, Drummon AJ. 2014 Tracer, version 1.6. Available at beast.bio.ed.ac.uk/Tracer. Accessed August 12, 2016.
- 61.Cornuet J-M, et al. DIYABC v2.0: A software to make approximate Bayesian computation inferences about population history using single nucleotide polymorphism, DNA sequence and microsatellite data. Bioinformatics. 2014;30:1187–1189. doi: 10.1093/bioinformatics/btt763. [DOI] [PubMed] [Google Scholar]
- 62.Bendtsen J, Söderkvist J, Dahl K, Hansen JL, Reker J. BALANCE Interim Report 9. BALANCE; Copenhagen: 2007. Model simulations of blue corridors in the Baltic Sea. [Google Scholar]
- 63.Verdu P, et al. Origins and genetic diversity of pygmy hunter-gatherers from Western Central Africa. Curr Biol. 2009;19:312–318. doi: 10.1016/j.cub.2008.12.049. [DOI] [PubMed] [Google Scholar]
- 64.Fraimout A, et al. Deciphering the routes of invasion of Drosophila suzukii by means of ABC random forest. Mol Biol Evol. 2017;34:980–996. doi: 10.1093/molbev/msx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lombaert E, et al. Complementarity of statistical treatments to reconstruct worldwide routes of invasion: The case of the Asian ladybird Harmonia axyridis. Mol Ecol. 2014;23:5979–5997. doi: 10.1111/mec.12989. [DOI] [PubMed] [Google Scholar]
- 66.Cornuet J-M, Ravigné V, Estoup A. Inference on population history and model checking using DNA sequence and microsatellite data with the software DIYABC (v1.0) BMC Bioinformatics. 2010;11:401. doi: 10.1186/1471-2105-11-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siebert PD, Chenchik A, Kellogg DE, Lukyanov KA, Lukyanov SA. An improved PCR method for walking in uncloned genomic DNA. Nucleic Acids Res. 1995;23:1087–1088. doi: 10.1093/nar/23.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanoue T, Takeichi M. New insights into Fat cadherins. J Cell Sci. 2005;118:2347–2353. doi: 10.1242/jcs.02398. [DOI] [PubMed] [Google Scholar]
- 69.Meyer zu Heringdorf D, Jakobs KH. Lysophospholipid receptors: Signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta. 2007;1768:923–940. doi: 10.1016/j.bbamem.2006.09.026. [DOI] [PubMed] [Google Scholar]