Abstract
Animal behavior is ultimately the product of gene regulatory networks (GRNs) for brain development and neural networks for brain function. The GRN approach has advanced the fields of genomics and development, and we identify organizational similarities between networks of genes that build the brain and networks of neurons that encode brain function. In this perspective, we engage the analogy between developmental networks and neural networks, exploring the advantages of using GRN logic to study behavior. Applying the GRN approach to the brain and behavior provides a quantitative and manipulative framework for discovery. We illustrate features of this framework using the example of social behavior and the neural circuitry of aggression.
Keywords: gene regulatory networks, social behavior network, neural networks, evolution, development
Animal development and behavior present special challenges to biologists, because these processes are complex and context-dependent (1). The idea that complicated biology can be represented as networks is at the heart of both developmental and behavioral biology: integrating techniques from mathematics, statistics, physics, and computer science to understand how the behavior of biological systems is a function of interactions between constituent elements (2). The relationship between development and behavior has been apparent for some time but tends to be underappreciated today. Writing about the genetics of Caenorhabditis elegans, Sydney Brenner (3) described a dual-encoding problem for the mapping of genes to behavior. He said that “behavior is the result of a complex and ill-understood set of computations performed by nervous systems and it seems essential to decompose the problem into two: one concerned with the question of genetic specification of nervous systems and the other with the way nervous systems work to produce behavior” (3). Genes encode developmental networks that build neurons and brains; neurons are connected in neural networks that encode behavior.
There are similarities between the developmental networks that build organ systems, called gene regulatory networks (GRNs), and the cellular networks that control behavior. One link is explicit: the development of the brain and specific neuronal subpopulations are encoded in the genome by GRNs (below) (Fig. 1A). Another link is less clear and presents an opportunity. GRN logic has transformed the study of development by systematizing a method to quantify and characterize the complexity of ontogeny. This GRN approach—focused on functional connections between relevant actors in biological processes identified through iteration of modeling and manipulation—can also be applied to neural networks.
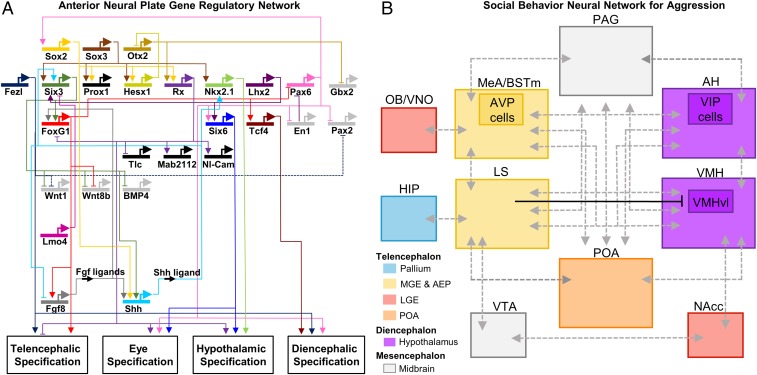
Fig. 1.
GRN and SBN circuits. (A) Circuit diagram representing the GRN controlling forebrain specification at midlate gastrula stage. The diagram shows the regulatory activity of key developmental transcription factors implicated in the specification of the different forebrain domains. Modified from ref. 11. (B) Simplified circuit diagram representing the core social behavior neural network for aggression, plus several key regions in the mesolimbic reward pathway. Some individual cell populations within these regions that play a known role in aggression are outlined within each structure. Dotted gray lines represent known physical connections between the brain regions. The black line represents one functional connection that has been recently quantified in mice, the inhibitory projection from lateral septum (LS) to the ventrolateral portion of the ventromedial nucleus of the hypothalamus (VMHvl) (67). The pallium is shaded in blue, medial ganglion eminence (MGE) and anterior entopeduncular area (AEP) are shaded in yellow, lateral ganglionic eminence (LGE) is shaded in red, the preoptic area (POA) is shaded in orange, the hypothalamus is shaded in purple, and the midbrain is shaded in gray. HIP, hippocampus; NAcc, nucleus accumbens; OB/VNO, olfactory bulb and vomeronasal organ; VTA, ventral tegmental area.
A network is defined in graph theory as a set of nodes or vertices and the edges connecting them. The topology of an entire network can be quantitatively described according to a number of features, including the number of connections that each node makes, clustering, network motifs, path lengths connecting nodes, connection density, the presence of nodes with high centrality within the network, and modularity. In addition, networks can be also modeled to produce simulations that can both reproduce empirical data and be used to make predictions. Of course, graph theoretical analyses are not new in neuroscience (4). However, to our knowledge, this approach has not yet been explicitly applied to the neural networks underpinning complex social behaviors.
The GRN framework has significantly advanced our understanding of developmental processes. Here, we argue that that the study of neural networks involved in social behavior would benefit from a similar approach. In particular, we intend to use this analogy to highlight why leveraging the rigorous methods used in the study of GRNs—and particularly, the iteration between theory and experiment—will benefit the investigation of complex neural networks. As we began to formulate this argument, it became clear that there were, in fact, significant parallels between the approach used to study GRNs and the goals outlined by the working group of prominent neuroscientists convened to discuss the United States’ Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) initiative (5, 6). Thus, we have also attempted to highlight these parallels where appropriate.
Logic of GRNs
GRN Logic in Development.
GRNs are central to the fields of genomics and developmental biology (e.g., this colloquium). The value of this research program is at least twofold. First, the complexity of developmental processes is accurately reflected in the quantitative, comprehensive nature of the approach. Indeed, the complementary “views from the genome and the nucleus” (7) effectively describe the four dimensions of development (three spatial dimensions and time). Second, the manipulative component of the GRN approach—the methodical perturbation of the system and simultaneous measurement of network outputs—enables development to be modeled as a series of regulatory circuits (8). The GRN experimental workflow can be used to identify the nodes (e.g., transcription factors or other signaling molecules) that participate in a particular developmental process and defines the edges between nodes by functional perturbation (Table 1).
Table 1.
How nodes and edges are defined in both GRNs and neural networks
| Network components | GRN | Neural network |
| Nodes | ||
| What are they? | Genes encoding TFs or molecular signals | Brain region/neuronal population |
| How are they defined? | Inclusion in the network requires that perturbation of the TF has an effect on the phenotype or another TF in the network | Inclusion in the network requires that perturbation of activity of neuronal population affects behavior or activity of another node in the network |
| Edges | ||
| What are they? | Gene regulatory regions (i.e., cis-regulatory elements, TF binding sites, promotor regions, etc.) | Direct neuronal connections (axonal projections or synapses) and neuromodulatory signals |
| How are they defined? | Evidence of a functional connection between two TFs if perturbation of one TF affects the activity of the other TF | Evidence of a functional connection between two neuronal populations if perturbation of one population affects the activity of the other population |
TF, transcription factor.
Since the pioneering studies of Davidson and coworkers (7, 8) in sea urchin embryogenesis, this approach has uncovered both shared and distinct regulatory logic among diverse organs in various species [e.g., teeth (9), limbs (10), and brain (11)]. For example, the GRN approach has aided substantially in our understanding of how the parts of the vertebrate brain are specified early in development (beginning at the blastula/gastrula transition) by graded molecular signals and region-specific transcription factors (12, 13). Specification is integrated along anterior–posterior and dorsal–ventral neuraxes by common signals [e.g., Fibroblast Growth Factor (FGF), Hedgehog (Hh), and Wingless (Wnt)] (14). Modeled as a GRN along the anterior–posterior axis, the telencephalon is specified by Foxg1 and Fez transcription factors and repressed by the eye-field factor Rx3 (11) (Fig. 1A).
Similarly, in the dorsal–ventral axis, the dorsal subdomain of the telencephalon (the pallium) is specified by Wnt signaling and downstream transcription factors Pax6/Emx, whereas the ventral subpallium is specified by Shh signal and downstream transcription factors Dlx2/Foxg1 (15). Because these dorsal and ventral telencephalic domains correspond to cortical neuron subtypes, respectively (e.g., excitatory vs. inhibitory neurons), GRN logic from development has been used to derive specific neuronal populations in vitro from stem cells (16). Conversely, the logic of subtype specification in vitro has been applied to better understand dorsal–ventral patterning of the neural tube (17).
GRN thinking also informs the study of how brains evolve. For instance, we now recognize the conservation of molecular signals and signaling centers in the developing brain across hundreds of millions of years of evolution (18). However, in contrast to theoretical expectations about which GRN components are most likely to vary (11, 19), brains can indeed diversify among closely related species, along anterior–posterior and dorsal–ventral neuraxes, via modification of “hub,” “kernel,” or input/output switches (i.e., FGF, Hh, and Wnt signals) (20–22). Widespread application of GRNs in the fields of both development and evolution has led to insights into general principles of form and function (23).
The GRN Approach to Neural Circuits.
The general framework for a network requires defining the output, nodes, edges, and directionality of the edges of the network. The output of a dynamic network is, by definition, context-dependent. In a GRN, the output of a network is typically a morphological feature at a given developmental stage, such as the brain at gastrulation in a specific organism. In the case of behavior, the output of a neural network is ultimately behavior of the organism in a specific social context, such as courtship and reproduction in the presence of an opposite sex conspecific or aggression in the presence of a rival.
In GRNs, the nodes are the genes encoding transcription factors or developmental signals, which do the work of spatially specifying gene expression patterns in the developing embryo (7). In this system, the relationships between nodes are a function of the DNA sequences of cis-regulatory elements that provide the regulatory/programmatic logic connecting genes in the network.
Defining the nodes in a neural network, however, can be a challenge. It is tempting to define a node as a single spatially defined brain region, which has proven somewhat successful in analyzing cortical networks in humans (24–26). However, these large regions are, in fact, composed of hundreds of thousands of individual neurons and many highly intermingled cell types, each with unique molecular identities and patterns of inputs and outputs. In fact, two cells that are directly adjacent to each other may have drastically different functions within the network. This lack of spatial topology of cell type, particularly in subcortical structures, has made it a challenge to use traditional electrophysiological techniques to further understand connectivity patterns of these networks at a finer scale. Indeed, it was this challenge—the need to dissect complex limbic, hypothalamic, and other subcortical circuits at the cellular level—that provided the impetus to develop optogenetic techniques (27) (Box 1).
Box 1. Enabling Technologies
These enabling technologies, alone and in conjunction, will facilitate advances in uncovering the logic of neural circuits for behavior in nonmodel species.
Immediate Early Genes (IEGs).
IEGs in the nervous system (87) exhibit a transient and rapid transcriptional response to stimuli. Such genes (e.g., c-fos) are assayed to define the spatial and quantitative activity of neuronal populations (i.e., nodes) recruited during behaviors.
Immediate Early Gene Sequencing (Ribosome Capture).
This method combines IEG and RNA-seq (88) to simultaneously identify neuronal populations activated in behavior and characterize the transcriptomes of those selected populations.
Single-Cell RNA-Seq.
This method characterizes the transcriptome of single cells rather than cellular fractions from defined (e.g., genetically, anatomically, or functionally) cell populations (89). Single-cell transcriptional dynamics can be ordered through a temporal process (e.g., neuronal differentiation and activation) by computation (90).
Assay for Transposase-Accessible Chromatin Sequencing (ATAC-Seq).
This unbiased sequencing approach allows characterization of the epigenome from small quantities of cells (91). ATAC-seq has been recently adapted for single cells (92).
CLARITY.
This method renders brains (and other tissues) optically transparent and retains the ability to image neural circuits, subcellular structures, and nucleic acid distributions in 3D (93).
Optogenetics.
This technique uses light to control the activity of defined cellular (neuronal) populations (94, 95) [typically defined genetically (86)]; it is used to perturb neural circuitry.
Activity Indicators/Optical Imaging.
This set of techniques is used to record the activity of specific cell populations or projections via fluorescence-based voltage indicators and fiber optic imaging systems; it is used to record from precise cellular populations within a neural circuit (96, 97).
We believe that, as more data become available on the specific cellular populations in the brain, the nodes of any given neural network will likely be redefined to comprise smaller, discrete populations of cells with unique molecular identities, patterns of connectivity, and computational properties. Indeed, the creation of a “census” of neuronal and glial cell types is the first major goal of the BRAIN initiative (5, 6). Immediate early gene (IEG) studies, which allow for the identification of the specific cells that are activated during any given behavior, and precisely targeted lesion studies have been and will continue to be important. The next steps, however, will require better understanding the molecular and functional identities of these active cells. These efforts will require the work of comparative neuroanatomists who can help to identify these subpopulations of neurons in many species (28) as well as new enabling technologies (Box 1).
Also, we must be able to define the edges in a neural network. The edges are ultimately the correlation in neural activity between any two molecularly and functionally distinct populations of cells. The functional connections in a neural network are encoded in the dynamic patterns of activity in the neuronal population, including both the frequency and amplitude of electrophysiological responses of neurons. Edges are typically thought of as direct physical synaptic connections between two cell populations, but connections need not be exclusively synaptic. Wiring diagrams that illustrate synaptic connections or connectomes are informative about physical edges, but most of these potential “wires” are functionally latent during any specific behavior (29). Additionally, nodes of neural networks could also be functionally connected to each other in a separate but very important way: via their mutual responsiveness to neuromodulatory or hormonal signals. The responsiveness of multiple cell populations to these long-distance chemical signals means that their activity is correlated—i.e., they are functionally connected—even if populations are not linked via direct synaptic input. Thus, defining the edges of a neural network requires obtaining simultaneous measures of activity from multiple cell populations—typically using multielectrode electrophysiology, calcium imaging, IEG activity, or other functional imaging techniques—while experimentally perturbing nodes to identify effects throughout the network. Tract-tracing studies, although enormously informative, are not sufficient for this level of analysis. The technology to precisely visualize and manipulate neural circuits in real time has only recently become widely available (and only in a small number of model species), although we anticipate that the pace of progress will rapidly increase in the next few years (Box 1).
Initial work in smaller invertebrates has shown the importance of taking a functional vs. physical approach to defining edges in the context of behavior. In these systems, the simplicity of the nervous system allows for unambiguous definition of nodes, and their genetic tractability allows for the simultaneous perturbation of individual nodes and measurement of their activity (30). Behavioral state, learning, and environment all have the potential to reconfigure the underlying circuit that processes the relevant stimuli into behavioral output. In other words, a physically connected circuit can become repurposed by neuromodulators to reveal latent functional circuits that change how information is processed (29).
A Neural Network for Social Behavior.
Perhaps the most complex and context-dependent neural circuits are those that underlie social behaviors. Social behaviors—such as reproduction, aggression, affiliation, and communication—are fundamental to an organism’s fitness. The core social behavior network (SBN) of the vertebrate brain is composed of six highly interconnected brain regions: the extended medial amygdala (the medial amygdala and the medial bed nucleus of the stria terminalis), the lateral septum (LS), the preoptic area (POA), the anterior hypothalamus (AH), the ventromedial nucleus of the hypothalamus (VMH), and the midbrain/periaqueductal gray (PAG) (31, 32). Each of these regions is active during multiple forms of social behavior. Another defining feature of the six brain regions that make up the SBN is their responsiveness to sex steroids, such as testosterone and estradiol. In addition, many other signaling molecules act in these regions, including nonapeptides (oxytocin and vasopressin), glucocorticoids, vasoactive intestinal polypeptide (VIP), prolactin, pituitary adenylate cyclase acting peptide, thyroid hormones, etc. (31). There is now extensive evidence that these brain regions and many of their functional connections are conserved across vertebrate classes, although some homologies remain unresolved (Figs. 1B and 2) (31, 33, 34). The evolutionary conservation of this core network has provided an important conceptual foundation for comparative work across species and investigations into how variation in brain function can lead to variation in behavioral phenotypes (Fig. 2).
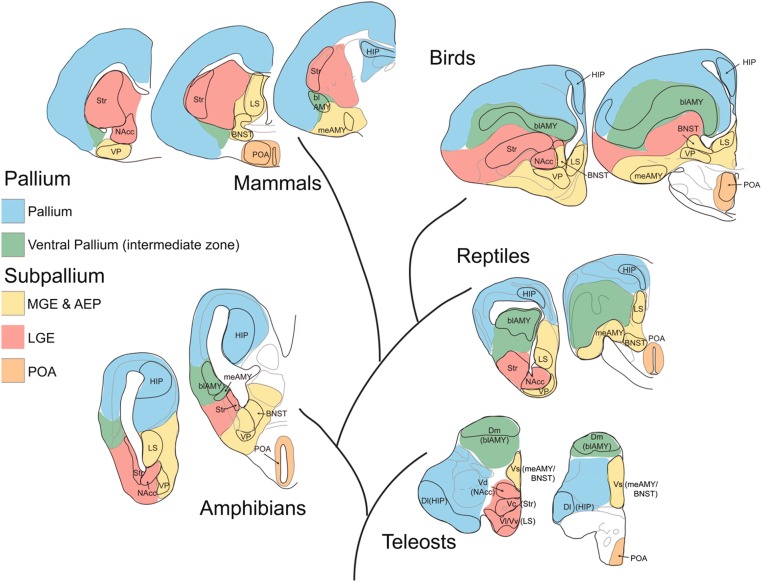
Fig. 2.
Conservation of the SBN across vertebrates. Evolutionary depiction illustrating the conservation of telencephalic brain regions within the SBN. Shown is a schematic of transverse sections from the major vertebrate groups, with colors indicating homologous developmental fields. The pallium is shaded in blue, the ventral pallium (intermediate zone) is shaded green, medial ganglion eminence (MGE) and anterior entopeduncular area (AEP) are shaded yellow, lateral ganglionic eminence (LGE) is shaded red, and the preoptic area (POA) is shaded orange. blAMY, basolateral amygdala; BNST, bed nucleus of the stria terminalis; Dl, lateral part of the dorsal telencephalon; Dm, medial part of the dorsal telencephalon; HIP, hippocampus; meAMY, medial amygdala; NAcc, nucleus accumbens; Str, striatum; Vc, central part of the ventral telencephalon; Vd, dorsal part of the ventral telencephalon; Vl, lateral part of the ventral telencephalon; Vs, supracommissural part of the ventral pallium; Vv, ventral part of the ventral telencephalon; VP, ventral pallidum (VP). Reprinted from ref. 34.
The SBN framework first proposed by Sarah Newman (32) emphasized that a given social behavior was not the product of a single brain region but rather, was an emergent property of the dynamic patterns of activity across these multiple reciprocally connected structures. At the time, this reframing was necessary to move the field away from the idea that one brain region could be responsible for performing a single specialized function. Undoubtedly, Newman’s idea (32) has encouraged the field to consider the dynamic properties of the network as a whole. However, what is needed now is a more sophisticated understanding of the specific computational properties of the cell populations within the SBN and the perturbational approach to define edges and their directions for context-dependent behavior.
It may be particularly challenging to accomplish these goals in the context of the vertebrate SBN. First, there is a lack of obvious topology of cell function and type, and cell populations with different molecular identities are highly intermingled. This intermixing of cell types has inevitably hindered our ability to determine the “correct” number of nodes in the network as well as unambiguously determine node homology. Second, although there have been extensive efforts to map the structural connections between brain regions within the SBN, we lack data on the functional connectivity of these regions. Most of these structures, which are deep within the brain, are thus relatively inaccessible and difficult to target surgically. Third, studying complex social behaviors typically requires interrogating neural activity in awake and freely moving animals as they interact.
Animals with simpler nervous systems, including invertebrates such as C. elegans or Drosophila, will undoubtedly be helpful for developing the GRN approach to study behavior, because it has already proven to be much easier to define nodes, edges, and behavioral output in these species (35–38). Indeed, studying these simpler systems has, in fact, helped researchers to define how nodes translate information (39, 40) in a context-dependent manner. Because these systems provide the ability to study neural networks in detail, they also help researchers to better understand and appreciate the space of possibilities for how neural networks can be organized or transformed (30). Issues of anatomical homology mean that we will ultimately need to study the SBN and complex behavior in vertebrates to understand the computational properties of these specific networks. However, work from these simpler systems has the potential to identify organizing principals and logic that can be used to define the nodes and edges in these larger vertebrate networks.
The SBN Aggression Circuit.
Aggression is the social behavior for which the most progress toward identifying functional connections among SBN nodes has been made. Defined as overt behavior with the intention to inflict physical damage on another individual (41), aggression has many forms and functions—including to ward off heterospecific predators, subdue prey, compete for and defend limited resources, protect offspring, and acquire nesting sites or mating opportunities.
Several converging lines of evidence have shown that multiple brain regions in vertebrates contain critical nodes in the control of aggression. Early work showed that stimulation of a part of the mediobasal hypothalamus (known as the “hypothalamic attack area”) induces aggression in both cats and rodents (42–46). Additionally, several forebrain structures that project to this region provide tonic inhibitory input to structure. Lesioning the prefrontal cortex, orbitofrontal cortices, or septal forebrain or severing the connections between the forebrain and the hypothalamus leads to the disinhibition of aggression behaviors (47–51).
More recent IEG studies in rodents confirmed that aggressive behaviors are correlated with the activity of multiple brain regions within the SBN, including the extended medial amygdala, LS, medial POA, VMH, AH, and PAG (52–54). These same brain regions also seem to be important in aggression in other species (55–58). The LS, in particular, is an important component of the aggression network. In both rodents and birds, lesions or pharmacological inactivation of the LS leads to an increase in the number of attacks on conspecifics, a phenomenon known as “septal rage” (47, 49, 50, 59–62). Conversely, electrical stimulation of neurons within the LS decreases aggressive behaviors (49, 63). Various neuromodulators within these same structures have also been shown to mediate aggression across taxa, including steroid hormones, VIP, and nonapeptides in the oxytocin/vasopressin family (64–66).
Thus far, this research has succeeded in identifying many of the relevant cell populations in the network. For example, VIP neurons in the dorsal AH potently promote territorial aggression in songbirds, suggesting that this cell population is an important node in the aggression circuit (64). However, researchers have just recently begun to explore the functional connections between these various nodes using multisite electrophysiological recordings, calcium imaging, and optogenetic manipulation. Optogenetic stimulation of cells in the ventrolateral subdivision of the ventromedial nucleus of the hypothalamus (VMHvl) causes male mice to attack other males, females, and inanimate objects (53). Optogenetic activation of the LS and also, the LS–VMH projection suppresses fighting in male mice (67). However, on further probing of the functional relationship between LS and the VMHvl cells, it was found that 80% of VMHvl cells were inhibited by input from the LS but that some cells in the VMHvl showed an increase in their firing rate in response to LS optical stimulation, suggesting that there exist both attack-inhibited and -excited cells in the VMHvl (67). These data suggest that the LS provides tonic inhibitory input to the VMH to suppress aggression (Fig. 1B). Applying GRN logic would thus allow researchers to define the functional, context-dependent edges connecting SBN nodes for the specific behavior of aggression and then, compare this neural circuitry to the networks for other social behaviors.
Identifying the relevant cellular populations and associating the activity of these neurons to a given social behavior, like aggression, may prove to be much more tractable in simpler systems (such as Drosphila or other invertebrates). The neural basis of aggression, for example, has been studied in fruit flies, and a number of relevant neuronal populations have been identified (68). Coupled with substantial advances in techniques for visualizing and manipulating specific neuronal populations, invertebrate systems can almost certainly be used to ground truth-important concepts and develop methodologies for studying more complex neural networks and behavioral phenotypes (36). Although it is possible that there are genes, molecules, or hormones in common between invertebrate and vertebrate neural circuits (and perhaps, even similar circuit wiring motifs), it is unlikely that there will be a matching between the specific neuronal populations involved in aggression because of the differences between vertebrate and invertebrate brain anatomy. Nevertheless, studies in invertebrate systems will provide an important foundation for studying more complex systems.
Discussion: What Does GRN Logic Bring to Behavior
Biologists are just beginning to systematically study the functional relationships between many relevant SBN cell populations in behavior. It is timely, then, to highlight the value of using the GRN approach to study neural networks. We see three main areas of advantage.
Modeling and Theory Synthesize Data and Enhance Rigor.
Synthesizing and constructing mathematical models have proven to be particularly valuable in the context of GRNs (69). To build an accurate model of a GRN, researchers must empirically characterize the behavior of a small and relatively simple biological process in full. They must then make explicit, quantitative hypotheses about how the different transcription factors or signaling molecules involved in that process are interconnected with each other. They can then use the output of the mathematical model to guide future experiments. We argue that this approach is sorely needed to further our understanding of the SBN.
The complexity of the SBN as well as the ever-growing body of data on its activity across organisms and social contexts highlight the need for quantitative descriptions of the neural circuitry. Mathematical theory, modeling, and simulations help to organize and synthesize large bodies of data. In particular, theory can help researchers extract general principles from those data and explain sometimes seemingly unconnected findings. Furthermore, sometimes complex networks produce unexpected outcomes that would have been difficult to predict using abstract reasoning alone. When systems become so complex that our intuitions fail us, relying on quantitative models can help to eliminate trial and error experimentation and wasted resources. Additionally, the process of describing systems in quantitative terms has an additional benefit: improving the clarity of hypotheses. Building a quantitative model forces researchers to be very explicit about any assumptions that they may have and make their hypotheses quantitative when considering the functional relationships between the components of the network. In our experience, engaging in this process—although it may be a challenge for many empirical researchers who lack advanced mathematical training—can dramatically improve the scientific process.
Mathematical thinking is urgently needed in the context of the SBN and many other neural networks. To our knowledge, there are no computational models of neural networks focused on limbic or hypothalamic regions. Indeed, a core goal of the BRAIN initiative is the integration of modeling and simulation and the explicit partnering between theorists and experimentalists (5).
Iteration Between Theory and Experiments Enhances Empirical Understanding.
Of course, the ultimate goal of engaging with theory is to build a quantitative model that can successfully recapitulate experimental data, thus showing complete understanding of a network. However, theory is much more about the journey than the destination. The GRN framework provides a guide for how to design experiments that will specifically illuminate the network topology and computational properties of neural networks, such as the SBN. The GRN approach provides a model for how to systematically study a complex biological system piece by piece. In the study of neural networks, the methodological approach will necessarily involve a combination of tracing experiments and the use of functional measures of neural activity collected from multiple network nodes simultaneously (Box 1). Additionally, experiments that methodically perturb the network nodes one by one while measuring the output at other nodes (i.e., “perturbation analysis”) would provide quantitative information about the computational contribution of each node to the network. The knockout or knockdown of nodes in neural networks can be accomplished by targeted delivery of chemicals, temporary lesion studies, cell type-specific RNA interference (RNAi) or viral vector knockdown of signaling molecules, or optogenetic activation or silencing. These methods are becoming increasingly common in model and nonmodel species as genome editing techniques facilitate construction of transgenic lines. However, it is still rare to collect quantitative data that monitor activity across multiple nodes in a neural network. These experiments may be challenging, but several decades of effort to understand and manipulate GRNs have shown the value of such patient science, especially when it is accompanied by attempts to synthesize the findings.
Mathematical models also guide research by generating specific testable predictions that can be evaluated experimentally. Each additional piece of experimental data collected about network properties can be integrated into the mathematical model, and subsequent simulations of the model activity under various conditions can be used to assist researchers in developing hypotheses about the underlying mechanisms. This iterative process allows researchers to make informed predictions and design novel, targeted, and creative experiments to test these hypotheses. In sea urchin development, building the GRN model undoubtedly guided experimental design, helping to identify parts of the system and more specifically, target experimental efforts (8). Furthermore, experimental data can guide the building of the model, providing guidance as to just how complex the model must be to predict the behavior of the network. One example of this from GRNs is whether the dynamics of the system require more computationally demanding differential equations to express the relationship between nodes or whether simpler Boolean models with “and”/“or”/“not” switches would suffice (70).
The iteration between theory and empirical research is one of the greatest advantages of using modeling to study network function. Experimental data uninformed by theory may simply not be the best data for reaching the most definitive conclusions or producing the greatest conceptual clarity. As stated in the vision document for the BRAIN initiative, the goal of theory is to “turn knowledge into understanding” (5). Mathematical modeling, by synthesizing and organizing large bodies of experimental data, can be used to reveal general principles of complex networks. Modeling certainly helped enhance our understanding in the case of GRNs, where such work revealed the importance of overrepresented network motifs (such as feedforward motifs, positive and negative autoregulation/feedback loops, and the generation of pulsatile or oscillatory patterns).
However, the ultimate payoff of this level of understanding is a predictable means to modify and manipulate behavior by making minor tweaks to network function. A better understanding of the computational roles of cell populations within a network would help researchers to identify critical nodes and edges. Which specific cell populations or functional connections between nodes play a central role in driving the output of the network, and which ones can be knocked out with minimal effect? More precise manipulation of neural networks holds great promise for the treatment of disorders resulting from dysregulation of SBN circuits in humans.
Insight into the Evolution—and Evolvability—of the SBN.
Another important contribution of the GRN approach was to provide mechanistic insight into the evolvability and conservation of gene function. Similarly, the SBN has long evoked debate and discussion about its role in behavioral evolution. The major components of the SBN are present in all vertebrates (reviewed in refs. 31, 32, and 34), and yet, subtle differences in the network also underlie remarkable species differences in behavior, such as differences in the mating system of closely related vole species or sociality in estrildid finches (71–75). Thus, which specific changes to network function are most likely to change as social behavior evolves remains an open question.
First, are neural networks likely to evolve by adding or deleting nodes in a network (i.e., by incorporating novel cell populations into the network?). The addition of new nodes in a neural network would be analogous to gene duplication events in GRNs, which make duplicated genes available to be integrated into new genetic circuits or evolve novel functions. Although there is a consensus that many components of the SBN are highly conserved (31, 32, 34), it remains to be seen whether there is, in fact, conservation at the level of the number, size, and role of the many individual cell types that make up these brain regions. Until a more complete census of the individual cell types within these networks is available for a wide range of species, just how conserved the SBN is will remain an open question.
Second, another way that networks may evolve is by changing the connections between the nodes (i.e., the edges). In the case of neural networks, changing the edges may involve removing or adding direct functional connections between nodes, changing the strength of those connections, or changing whether those connections are excitatory or inhibitory. This “rewiring” in the context of GRNs might involve the evolution of a new transcription factor binding site or other forms of regulatory control between two genes in a network. In addition, novel connections can evolve in neural networks by changes to the neuromodulatory control of neurons. An explicit demonstration of this was supplied by Bendesky et al. (76), who showed that natural variation in expression of a neuromodulator receptor in a single neuronal class was sufficient to change foraging behavior in C. elegans. Similar mechanisms might also be at play for social behaviors. For example, the gain of receptors to a hormone in a new cell population would connect two previously uncorrelated nodes. In fact, O’Connell and Hofmann (77) showed that the distribution of hormones and neuromodulator ligands within the SBN is evolutionarily flexible across vertebrates. Divergent activity of neuromodulators is paralleled in developmental GRNs by evolution of morphogen or signaling molecule gradients among species (20–22).
The robustness of a network can also provide hints as to which components are the most evolvable vs. those elements that are likely to be conserved in evolution. For example, analysis of network topology suggests that certain elements, such as those termed “differentiation gene batteries,” plugins, and input/output switches, could be changed to subtly tweak network output without completely disrupting network function (19). In contrast, evolutionary changes to the function of kernels, which are highly central nodes in the network, would lead to catastrophic breakdown of the function of the network. These views are not without criticisms [for example, they ignore modularity (78)], but there is little doubt that the framework provided by network analysis helps to advance the conversation.
Ultimately, it is an empirical question as to whether evolutionary changes to the “nodes” vs. the “edges” of neural networks, like the SBN, are more likely. Comparative work from a number of species has strongly implicated an important role for neuromodulatory signals in the evolution of SBN function, but perhaps it is just the case that more research effort has been devoted to studying the role of the hormones and other signaling systems in the SBN. Lacking a more complete accounting of the many cell populations and their functional connections, this question cannot yet be answered.
Conclusion
The GRN approach to studying neural networks, such as the SBN, would represent a unique way of thinking about network function for social behavior. As helpful as it has been to think of social behavior as a function of the dynamic patterns of activity across multiple brain regions rather than a single one, it is perhaps time to work toward a more sophisticated understanding of the specific computational properties of this neural network. Ultimately, working toward this goal will require researchers who study social behavior to enthusiastically embrace novel techniques and technologies and learn to use (or find collaborators who can use) more sophisticated quantitative methods. Fortunately, technological advances are currently revolutionizing the field of neuroscience—powerful genome editing techniques; the development of optogenetic tools; methods that render the brain transparent with all of its connections intact (i.e., CLARITY); advances in imaging; and increasingly sophisticated techniques for manipulating, tracking, and analyzing animal behavior—and this technology will facilitate unprecedented insight into the functional dynamics of the brain over the next several decades.
Furthermore, behavioral biologists, neuroendocrinologists, and neuroanatomists need to continue to champion comparative approaches and emphasize the value of studying multiple species in an evolutionary context (28). First, having such a broad perspective will ensure that the most sophisticated technologies can be used to answer important biological questions in a wider range of species. Second, one of the major insights from the GRN approach is that important insights can be gained from studying the function of biological networks in the context of evolution. In particular, we believe that researchers should strategically focus their efforts to study the microevolution of behavior in closely related species or different populations of the same species. There are a number of evolutionary systems where a strong foundation has already been laid, including territoriality and flocking in songbirds (71), schooling behavior in threespine stickleback fish (79), courtship and parental behaviors in African cichlid fish (80, 81), aggression and schooling in blind cavefish (82, 83), mating system and mating tactics in voles (84, 85), and parental behavior in Peromyscus mice (86). These systems provide insight into not only the specifics of how social behavior evolves but also, the extent to which we should expect conservation of network function across much more distantly related species. Finally, these systems offer the chance to dissect the genetic basis of fine-scale differences in neural system development, circuitry, and function.
Acknowledgments
We thank anonymous reviewers and members of the laboratories of P.T.M. and J.T.S., whose comments have improved the clarity of our manuscript. This work was supported by NIH Grants R21AG050304 (to P.T.M.), R01GM114170 (to P.T.M.), and R01GM101095 (to J.T.S.), and the Ellison Medical Foundation (P.T.M.).
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Gene Regulatory Networks and Network Models in Development and Evolution,” held April 12–14, 2016, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and video recordings of most presentations are available on the NAS website at www.nasonline.org/Gene_Regulatory_Networks.
This article is a PNAS Direct Submission. D.H.E. is a guest editor invited by the Editorial Board.
References
- 1.Streelman JT, editor. Advances in Evolutionary Developmental Biology. Wiley-Blackwell; Hoboken, NJ: 2013. [Google Scholar]
- 2.Börner K, Sanyal S, Vespignani A. Network science. Annu Rev Inform Sci Tech. 2007;41(1):537–607. [Google Scholar]
- 3.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bullmore E, Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 5.Bargmann C, et al. Medical Ventures; 2014. BRAIN 2025: A scientific vision–Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Report on New NIH Neuroscience Initiative, Neurological and Psychiatric Disorders (Natl Inst Health, Bethesda) [Google Scholar]
- 6.Jorgenson LA, et al. The BRAIN Initiative: Developing technology to catalyse neuroscience discovery. Philos Trans R Soc Lond B Biol Sci. 2015;370(1668):20140164. doi: 10.1098/rstb.2014.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson EH, et al. A genomic regulatory network for development. Science. 2002;295(5560):1669–1678. doi: 10.1126/science.1069883. [DOI] [PubMed] [Google Scholar]
- 8.Peter IS, Faure E, Davidson EH. Predictive computation of genomic logic processing functions in embryonic development. Proc Natl Acad Sci USA. 2012;109(41):16434–16442. doi: 10.1073/pnas.1207852109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connell DJ, et al. A Wnt-bmp feedback circuit controls intertissue signaling dynamics in tooth organogenesis. Sci Signal. 2012;5(206):ra4. doi: 10.1126/scisignal.2002414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuniga A. Next generation limb development and evolution: Old questions, new perspectives. Development. 2015;142(22):3810–3820. doi: 10.1242/dev.125757. [DOI] [PubMed] [Google Scholar]
- 11.Beccari L, Marco-Ferreres R, Bovolenta P. The logic of gene regulatory networks in early vertebrate forebrain patterning. Mech Dev. 2013;130(2-3):95–111. doi: 10.1016/j.mod.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Hébert JM, Fishell G. The genetics of early telencephalon patterning: Some assembly required. Nat Rev Neurosci. 2008;9(9):678–685. doi: 10.1038/nrn2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiecker C, Lumsden A. Compartments and their boundaries in vertebrate brain development. Nat Rev Neurosci. 2005;6(7):553–564. doi: 10.1038/nrn1702. [DOI] [PubMed] [Google Scholar]
- 14.Sylvester JB, Pottin K, Streelman JT. Integrated brain diversification along the early neuraxes. Brain Behav Evol. 2011;78(3):237–247. doi: 10.1159/000329840. [DOI] [PubMed] [Google Scholar]
- 15.Danesin C, et al. Integration of telencephalic Wnt and hedgehog signaling center activities by Foxg1. Dev Cell. 2009;16(4):576–587. doi: 10.1016/j.devcel.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Hansen DV, Rubenstein JLR, Kriegstein AR. Deriving excitatory neurons of the neocortex from pluripotent stem cells. Neuron. 2011;70(4):645–660. doi: 10.1016/j.neuron.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kutejova E, Sasai N, Shah A, Gouti M, Briscoe J. Neural progenitors adopt specific identities by directly repressing all alternative progenitor transcriptional programs. Dev Cell. 2016;36(6):639–653. doi: 10.1016/j.devcel.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pani AM, et al. Ancient deuterostome origins of vertebrate brain signalling centres. Nature. 2012;483(7389):289–294. doi: 10.1038/nature10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davidson EH, Erwin DH. Gene regulatory networks and the evolution of animal body plans. Science. 2006;311(5762):796–800. doi: 10.1126/science.1113832. [DOI] [PubMed] [Google Scholar]
- 20.Pottin K, Hinaux H, Rétaux S. Restoring eye size in Astyanax mexicanus blind cavefish embryos through modulation of the Shh and Fgf8 forebrain organising centres. Development. 2011;138(12):2467–2476. doi: 10.1242/dev.054106. [DOI] [PubMed] [Google Scholar]
- 21.Sylvester JB, et al. Competing signals drive telencephalon diversity. Nat Commun. 2013;4:1745. doi: 10.1038/ncomms2753. [DOI] [PubMed] [Google Scholar]
- 22.Sylvester JB, et al. Brain diversity evolves via differences in patterning. Proc Natl Acad Sci USA. 2010;107(21):9718–9723. doi: 10.1073/pnas.1000395107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidson EH. The Regulatory Genome: Gene Regulatory Networks in Development and Evolution. Academic; Burlington, MA: 2006. [Google Scholar]
- 24.Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sporns O, Zwi JD. The small world of the cerebral cortex. Neuroinformatics. 2004;2(2):145–162. doi: 10.1385/NI:2:2:145. [DOI] [PubMed] [Google Scholar]
- 26.Stam CJ, Jones BF, Nolte G, Breakspear M, Scheltens P. Small-world networks and functional connectivity in Alzheimer’s disease. Cereb Cortex. 2007;17(1):92–99. doi: 10.1093/cercor/bhj127. [DOI] [PubMed] [Google Scholar]
- 27.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450(7168):420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Striedter GF, et al. NSF workshop report: Discovering general principles of nervous system organization by comparing brain maps across species. Brain Behav Evol. 2014;83(1):1–8. doi: 10.1159/000360152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bargmann CI. Beyond the connectome: How neuromodulators shape neural circuits. BioEssays. 2012;34(6):458–465. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- 30.Bargmann CI, Marder E. From the connectome to brain function. Nat Methods. 2013;10(6):483–490. doi: 10.1038/nmeth.2451. [DOI] [PubMed] [Google Scholar]
- 31.Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav. 2005;48(1):11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877(1):242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 33.Goodson JL, Kingsbury MA. What’s in a name? Considerations of homologies and nomenclature for vertebrate social behavior networks. Horm Behav. 2013;64(1):103–112. doi: 10.1016/j.yhbeh.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. J Comp Neurol. 2011;519(18):3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- 35.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314(1165):1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 36.Jenett A, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Reports. 2012;2(4):991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierce-Shimomura JT, Morse TM, Lockery SR. The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J Neurosci. 1999;19(21):9557–9569. doi: 10.1523/JNEUROSCI.19-21-09557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berman GJ, Choi DM, Bialek W, Shaevitz JW. Mapping the stereotyped behaviour of freely moving fruit flies. J R Soc Interface. 2014;11(99):20140672. doi: 10.1098/rsif.2014.0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin X, Pokala N, Bargmann CI. Distinct circuits for the formation and retrieval of an imprinted olfactory memory. Cell. 2016;164(4):632–643. doi: 10.1016/j.cell.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gordus A, Pokala N, Levy S, Flavell SW, Bargmann CI. Feedback from network states generates variability in a probabilistic olfactory circuit. Cell. 2015;161(2):215–227. doi: 10.1016/j.cell.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moyer KE. The Psychobiology of Aggression. Harper & Row; New York: 1976. [Google Scholar]
- 42.Hess WR. Stammganglien-Reizversuche. Berichte der gesamten. Physiologie. 1928;42:554–555. [Google Scholar]
- 43.Kruk MR, et al. Discriminant analysis of the localization of aggression-inducing electrode placements in the hypothalamus of male rats. Brain Res. 1983;260(1):61–79. doi: 10.1016/0006-8993(83)90764-3. [DOI] [PubMed] [Google Scholar]
- 44.Kruk MR, van der Poel AM, de Vos-Frerichs TP. The induction of aggressive behaviour by electrical stimulation in the hypothalamus of male rats. Behaviour. 1979;70(3-4):292–322. doi: 10.1163/156853979x00106. [DOI] [PubMed] [Google Scholar]
- 45.Lammers JHCM, Kruk MR, Meelis W, van der Poel AM. Hypothalamic substrates for brain stimulation-induced attack, teeth-chattering and social grooming in the rat. Brain Res. 1988;449(1-2):311–327. doi: 10.1016/0006-8993(88)91046-3. [DOI] [PubMed] [Google Scholar]
- 46.Siegel A, Roeling TAP, Gregg TR, Kruk MR. Neuropharmacology of brain-stimulation-evoked aggression. Neurosci Biobehav Rev. 1999;23(3):359–389. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- 47.Albert DJ, Chew GL. The septal forebrain and the inhibitory modulation of attack and defense in the rat. A review. Behav Neural Biol. 1980;30(4):357–388. doi: 10.1016/s0163-1047(80)91247-9. [DOI] [PubMed] [Google Scholar]
- 48.Bard P. A diencephalic mechanism for the expression of rage with special reference to the sympathetic nervous system. Am J Physiol. 1928;84(3):490–515. [Google Scholar]
- 49.Potegal M, Blau A, Glusman M. Effects of anteroventral septal lesions on intraspecific aggression in male hamsters. Physiol Behav. 1981;26(3):407–412. doi: 10.1016/0031-9384(81)90167-0. [DOI] [PubMed] [Google Scholar]
- 50.Slotnick BM, McMullen MF, Fleischer S. Changes in emotionality following destruction of the septal area in albino mice. Brain Behav Evol. 1973;8(4):241–252. doi: 10.1159/000124357. [DOI] [PubMed] [Google Scholar]
- 51.Spiegel EA, Miller HR, Oppenheimer MJ. Forebrain and rage reactions. J Neurophysiol. 1940;3(6):538–548. [Google Scholar]
- 52.Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66(3):721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- 53.Lin D, et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470(7333):221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veening JG, et al. Do similar neural systems subserve aggressive and sexual behaviour in male rats? Insights from c-Fos and pharmacological studies. Eur J Pharmacol. 2005;526(1-3):226–239. doi: 10.1016/j.ejphar.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 55.Burmeister SS, Kailasanath V, Fernald RD. Social dominance regulates androgen and estrogen receptor gene expression. Horm Behav. 2007;51(1):164–170. doi: 10.1016/j.yhbeh.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goodson JL, Evans AK, Lindberg L, Allen CD. Neuro-evolutionary patterning of sociality. Proc Biol Sci. 2005;272(1560):227–235. doi: 10.1098/rspb.2004.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greenwood AK, Wark AR, Fernald RD, Hofmann HA. Expression of arginine vasotocin in distinct preoptic regions is associated with dominant and subordinate behaviour in an African cichlid fish. Proc Biol Sci. 2008;275(1649):2393–2402. doi: 10.1098/rspb.2008.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greenwood AK, Fernald RD. Social regulation of the electrical properties of gonadotropin-releasing hormone neurons in a cichlid fish (Astatotilapia burtoni) Biol Reprod. 2004;71(3):909–918. doi: 10.1095/biolreprod.104.030072. [DOI] [PubMed] [Google Scholar]
- 59.Goodson JL, Adkins-Regan E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata) J Neuroendocrinol. 1999;11(1):19–25. doi: 10.1046/j.1365-2826.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- 60.Lee G, Gammie SC. GABA(A) receptor signaling in the lateral septum regulates maternal aggression in mice. Behav Neurosci. 2009;123(6):1169–1177. doi: 10.1037/a0017535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDonald MM, Markham CM, Norvelle A, Albers HE, Huhman KL. GABAA receptor activation in the lateral septum reduces the expression of conditioned defeat and increases aggression in Syrian hamsters. Brain Res. 2012;1439:27–33. doi: 10.1016/j.brainres.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramirez JM, Salas C, Portavella M. Offense and defense after lateral septal lesions in Columba livia. Int J Neurosci. 1988;41(3-4):241–250. doi: 10.3109/00207458808990730. [DOI] [PubMed] [Google Scholar]
- 63.Siegel A, Skog D. Effects of electrical stimulation of the septum upon attack behavior elicited from the hypothalamus in the cat. Brain Res. 1970;23(3):371–380. doi: 10.1016/0006-8993(70)90063-6. [DOI] [PubMed] [Google Scholar]
- 64.Goodson JL, Kelly AM, Kingsbury MA, Thompson RR. An aggression-specific cell type in the anterior hypothalamus of finches. Proc Natl Acad Sci USA. 2012;109(34):13847–13852. doi: 10.1073/pnas.1207995109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kabelik D, Alix VC, Burford ER, Singh LJ. Aggression- and sex-induced neural activity across vasotocin populations in the brown anole. Horm Behav. 2013;63(3):437–446. doi: 10.1016/j.yhbeh.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 66.Trainor BC, Kyomen HH, Marler CA. Estrogenic encounters: How interactions between aromatase and the environment modulate aggression. Front Neuroendocrinol. 2006;27(2):170–179. doi: 10.1016/j.yfrne.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong LC, et al. Effective modulation of male aggression through lateral septum to medial hypothalamus projection. Curr Biol. 2016;26(5):593–604. doi: 10.1016/j.cub.2015.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoopfer ED. Neural control of aggression in Drosophila. Curr Opin Neurobiol. 2016;38:109–118. doi: 10.1016/j.conb.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 69.Hasty J, McMillen D, Isaacs F, Collins JJ. Computational studies of gene regulatory networks: In numero molecular biology. Nat Rev Genet. 2001;2(4):268–279. doi: 10.1038/35066056. [DOI] [PubMed] [Google Scholar]
- 70.Arnosti DN, Ay A. Boolean modeling of gene regulatory networks: Driesch redux. Proc Natl Acad Sci USA. 2012;109(45):18239–18240. doi: 10.1073/pnas.1215732109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goodson JL. Nonapeptides and the evolutionary patterning of sociality. In: Landgraf R, Neumann I, editors. Progress in Brain Research, Advances in Vasopressin and Oxytocin—From Genes to Behaviour to Disease. Vol 170. Elsevier; Amsterdam: 2008. pp. 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc Natl Acad Sci USA. 2006;103(45):17013–17017. doi: 10.1073/pnas.0606278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci. 1994;14(9):5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci USA. 1992;89(13):5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lim MM, et al. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429(6993):754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- 76.Bendesky A, Tsunozaki M, Rockman MV, Kruglyak L, Bargmann CI. Catecholamine receptor polymorphisms affect decision-making in C. elegans. Nature. 2011;472(7343):313–318. doi: 10.1038/nature09821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Connell LA, Hofmann HA. Evolution of a vertebrate social decision-making network. Science. 2012;336(6085):1154–1157. doi: 10.1126/science.1218889. [DOI] [PubMed] [Google Scholar]
- 78.Streelman JT, Peichel CL, Parichy DM. Developmental genetics of adaptation in fishes: The case for novelty. Annu Rev Ecol Evol Syst. 2007;38:655–681. [Google Scholar]
- 79.Greenwood AK, Wark AR, Yoshida K, Peichel CL. Genetic and neural modularity underlie the evolution of schooling behavior in threespine sticklebacks. Curr Biol. 2013;23(19):1884–1888. doi: 10.1016/j.cub.2013.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Connor CM, Marsh-Rollo SE, Ghio SC, Balshine S, Aubin-Horth N. Is there convergence in the molecular pathways underlying the repeated evolution of sociality in African cichlids? Horm Behav. 2015;75:160–168. doi: 10.1016/j.yhbeh.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 81.York RA, et al. Evolution of bower building in Lake Malawi cichlid fish: Phylogeny, morphology, and behavior. Front Ecol Evol. 2015;3:18. Available at journal.frontiersin.org/article/10.3389/fevo.2015.00018/full. [Google Scholar]
- 82.Elipot Y, Hinaux H, Callebert J, Rétaux S. Evolutionary shift from fighting to foraging in blind cavefish through changes in the serotonin network. Curr Biol. 2013;23(1):1–10. doi: 10.1016/j.cub.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 83.Kowalko JE, et al. Loss of schooling behavior in cavefish through sight-dependent and sight-independent mechanisms. Curr Biol. 2013;23(19):1874–1883. doi: 10.1016/j.cub.2013.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McGraw LA, Young LJ. The prairie vole: An emerging model organism for understanding the social brain. Trends Neurosci. 2010;33(2):103–109. doi: 10.1016/j.tins.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Okhovat M, Berrio A, Wallace G, Ophir AG, Phelps SM. Sexual fidelity trade-offs promote regulatory variation in the prairie vole brain. Science. 2015;350(6266):1371–1374. doi: 10.1126/science.aac5791. [DOI] [PubMed] [Google Scholar]
- 86.Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav. 1999;36(1):25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]
- 87.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 88.Knight ZA, et al. Molecular profiling of activated neurons by phosphorylated ribosome capture. Cell. 2012;151(5):1126–1137. doi: 10.1016/j.cell.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu AR, et al. Quantitative assessment of single-cell RNA-sequencing methods. Nat Methods. 2014;11(1):41–46. doi: 10.1038/nmeth.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trapnell C, et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol. 2014;32(4):381–386. doi: 10.1038/nbt.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10(12):1213–1218. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cusanovich DA, et al. Multiplex single cell profiling of chromatin accessibility by combinatorial cellular indexing. Science. 2015;348(6237):910–914. doi: 10.1126/science.aab1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chung K, Deisseroth K. CLARITY for mapping the nervous system. Nat Methods. 2013;10(6):508–513. doi: 10.1038/nmeth.2481. [DOI] [PubMed] [Google Scholar]
- 94.Deisseroth K. Optogenetics. Nat Methods. 2011;8(1):26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gradinaru V, et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141(1):154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miyamoto D, Murayama M. The fiber-optic imaging and manipulation of neural activity during animal behavior. Neurosci Res. 2016;103:1–9. doi: 10.1016/j.neures.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 97.Warden MR, Cardin JA, Deisseroth K. Optical neural interfaces. Annu Rev Biomed Eng. 2014;16:103–129. doi: 10.1146/annurev-bioeng-071813-104733. [DOI] [PMC free article] [PubMed] [Google Scholar]