Significance
The Dcp2 decapping enzyme targets mRNA for degradation and thereby plays a role in the regulation of gene expression. Despite numerous static crystal structures of the enzyme, it remained unclear how its catalytic activity correlates with the relative domain orientation in Dcp2. Here we used solution-state NMR spectroscopic methods and find that the active state of Dcp2 is only stably formed in the presence of the Dcp1 and Edc1 activator proteins and the mRNA cap. Importantly, our solution data provide a conclusive view of how the Dcp2 structure changes during the catalytic cycle and provide a unique example of the importance of integrated structural biology approaches to unravel the mechanism behind complex molecular machines.
Keywords: mRNA decapping, Dcp2, NMR spectroscopy, catalytic activity, protein dynamics
Abstract
Crystal structures of enzymes are indispensable to understanding their mechanisms on a molecular level. It, however, remains challenging to determine which structures are adopted in solution, especially for dynamic complexes. Here, we study the bilobed decapping enzyme Dcp2 that removes the 5′ cap structure from eukaryotic mRNA and thereby efficiently terminates gene expression. The numerous Dcp2 structures can be grouped into six states where the domain orientation between the catalytic and regulatory domains significantly differs. Despite this wealth of structural information it is not possible to correlate these states with the catalytic cycle or the activity of the enzyme. Using methyl transverse relaxation-optimized NMR spectroscopy, we demonstrate that only three of the six domain orientations are present in solution, where Dcp2 adopts an open, a closed, or a catalytically active state. We show how mRNA substrate and the activator proteins Dcp1 and Edc1 influence the dynamic equilibria between these states and how this modulates catalytic activity. Importantly, the active state of the complex is only stably formed in the presence of both activators and the mRNA substrate or the m7GDP decapping product, which we rationalize based on a crystal structure of the Dcp1:Dcp2:Edc1:m7GDP complex. Interestingly, we find that the activating mechanisms in Dcp2 also result in a shift of the substrate specificity from bacterial to eukaryotic mRNA.
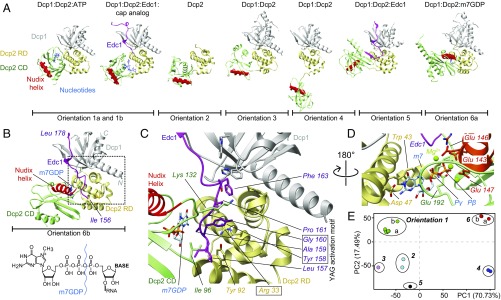
The control of mRNA degradation is essential for the regulation of gene expression and the removal of the 5′ protective cap of eukaryotic mRNA is a major control step during mRNA decay (1). The main decapping enzyme Dcp2 (2, 3) is composed of an N-terminal regulatory domain (RD) and a catalytic Nudix (4) domain (CD) that are connected by a flexible linker (5, 6). The CD shows a basal level of mRNA decapping activity, which is increased stepwise upon inclusion of the RD and upon binding of the activator proteins Dcp1 (6, 7) and Edc1 (8–10). Dcp1 binds to the RD of Dcp2 and contains a binding platform for proline-rich peptide ligands that can recruit other mRNA decay factors (9), including the intrinsically disordered decapping enhancer Edc1 that possesses a conserved YAG activation motif located N-terminal to the proline-rich Dcp1 binding sequence (8–10). This motif is essential for decapping enhancement but does not contribute to Dcp1 binding (8, 9, 11). Neither Dcp1 nor Edc1 interacts with the CD of Dcp2 and the molecular basis for their decapping activation is currently not clear (9). A large number of crystal structures have been determined for Dcp2 and include the apo enzyme (12), the Dcp1:Dcp2 complex (6, 10, 13), the Dcp1:Dcp2:Edc1 complex (10), Dcp1:Dcp2:Edc1 bound to a substrate analog (11) (Fig. 1A), and Dcp1:Dcp2 bound to the reaction product m7GDP (Fig. 1A) (13). This latter structure also contains the unrelated decapping enhancer Edc3 that binds to Dcp2 C-terminal of the CD (14) and that does not influence the Dcp2 domain orientation.
Fig. 1.
Static structures of the Dcp2 enzyme. (A) Known structures of Dcp2 display significantly different orientations of the Dcp2 RD (yellow) and CD (green). The catalytic Nudix helix in the CD is indicated in red, Dcp1 in gray, Edc1 in pink, and nucleotides in blue [orientation 1a, PDB ID code 2QKM (6); orientation 1b, 5KQ4 (11); orientation 2, 2A6T (12); orientation 3, 5LON (13); orientation 4, 2QKM (6); orientation 5, 5J3T (10); and orientation 6a, 5LOP (13)]. Note that the protein Edc3 that is present in the structure in orientation 6a is not shown for clarity. (B) Structure of the fully activated Dcp1:Dcp2:Edc1:m7GDP decapping complex (SI Appendix, Table S1). The YAG activation motif in Edc1 docks onto the C-terminal domain of Dcp2 and thereby locks Dcp2 such that the Nudix helix is in close proximity to the substrate binding site. The structure of the 5′ cap is shown and the bond that is hydrolyzed by Dcp2 is indicated. (C) Enlargement of the boxed region in B. Tyr-158 in the Edc1 YAG activation motif stacks between Arg-33 and Lys-132 in Dcp2. (D) The methylated base of the mRNA cap structure stacks onto Trp-43 in the Dcp2 RD and forms hydrogen bonds with Asp-47. Catalytically important Mg2+ ions are coordinated by Glu-143, 146, and 147 in the Dcp2 Nudix helix. (E) Based on a principle component analysis the known Dcp2 complexes can be separated into six groups.
Results and Discussion
Structure of the Dcp1:Dcp2:Edc1:m7GDP Complex.
Here, we complement the wealth of structural information on Dcp2 and determined the crystal structure of the fully activated Dcp1:Dcp2:Edc1:m7GDP complex (Fig. 1 B–D and SI Appendix, Table S1). In this structure the guanosine base of m7GDP stacks on Trp-43 of the RD and forms specific hydrogen bonds to Asp-47. Residues 189–192 of the CD contact the other side of m7GDP via van der Waals interactions and thereby sandwich m7GDP between both domains (Fig. 1 B–D). Two Mg2+ ions are complexed by the phosphate groups of m7GDP and three glutamate residues of the catalytic Nudix helix. In addition, residues 157–161 of Edc1 (including the YAG activation motif Tyr-158–Gly-160) are sandwiched between the RD and CD of Dcp2. The Tyr-158 side chain stacks between Arg-33 of the RD and Lys-132 of the CD. m7GDP and Edc1 thus stabilize the domain orientation of Dcp2 by bridging the two Dcp2 domains.
The observed domain orientation and product recognition that we observe are very similar to those displayed in the structure of the Dcp1:Dcp2:Edc3:m7GDP complex (Fig. 1A, orientation 6a) (13). This is unexpected because Edc3 is unrelated to Edc1 and binds to the C-terminal region of Dcp2 far away from the m7GDP binding site and the interface between the Dcp2 RD and CD. Edc3 is thus not able to influence the Dcp2 domain orientation. Surprisingly, the domain orientation in our Dcp1:Dcp2:Edc1:m7GDP structure differs significantly from the orientation observed in the Dcp1:Dcp2:Edc1 complex in the presence of substrate analog (11) (Fig. 1A, orientation 1b), although the base of the substrate analog makes similar contacts with the RD of Dcp2.
In summary, the known structures of Dcp2 vary significantly regarding the relative orientations of the RD and CD domains and can be divided into six unique groups (Fig. 1E). Due to the dynamic nature of Dcp2 (5) crystal packing most likely influences the observed orientations. It is thus unclear which of the Dcp2 orientations are functional and relevant in solution (15). To address this we decided to explore the conformations that Dcp2 samples in solution using a suite of methyl transverse relaxation-optimized spectroscopy (TROSY)-based NMR experiments (16–21) on complexes ranging from the isolated Dcp2 CD to the fully activated Dcp1:Dcp2:Edc1:substrate complex. Specifically, we used chemical shift perturbation (CSP) experiments to explore interaction surfaces and structural changes, NOESY, and paramagnetic relaxation enhancement (PRE) measurements (22) to gain short- and long-range structural information, respectively, and Carr–Purcell–Meiboom–Gill (CPMG) relaxation dispersion experiments (23) to extract conformational exchange rates and populations.
Dcp1 Closes the Dcp2 Enzyme in a Catalytically Incompetent State.
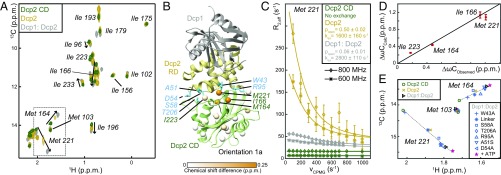
First, we focused on the Dcp2 CD and noticed that several methyl resonances in this domain (e.g., Met-164 and Met-221) undergo linearly progressing CSPs upon inclusion of the Dcp2 RD and addition of Dcp1 (Fig. 2A). This suggests a stepwise increase in the interactions between the Dcp2 RD and CD and a transition from a free (or open) toward a bound (or closed) state. In line with this, we observed NOE cross-peaks in the Dcp1:Dcp2 complex that are only compatible with orientation 1a (SI Appendix, Fig. S1). Together with the fact that the residues that experience CSPs (Fig. 2A) are located at the Dcp1:Dcp2 interface in this orientation (Fig. 2B), our data strongly suggest that Dcp1 enforces a closed Dcp2 conformation that resembles orientation 1a.
Fig. 2.
The Dcp2 domain orientation is dynamic and strongly depends on Dcp1. (A) Methyl TROSY NMR spectrum of the Dcp2 CD (green), Dcp2 (RD-CD, yellow), and the Dcp1:Dcp2 complex (gray). A number of residues (including Met-164, Ile-166, and Met-221) undergo linear and stepwise CSPs, as indicated by arrows. (B) Structure of the Dcp1:Dcp2 complex in orientation 1a. The residues that undergo CSPs (A) are colored with white to orange spheres. Residues that stabilize the interface between the Dcp2 RD and CD are indicated in blue (see E). (C) 13C CPMG relaxation dispersion profiles of Met-221 that report on the exchange rates and populations in Dcp2. The data show that Dcp1 induces a closure of the Dcp2 RD and CD. (D) Correlation between the chemical shift differences extracted from the relaxation dispersion data (C) and those observed in the NMR spectra (A). (E) Mutations at the Dcp2 RD/CD interface in orientation 1a (B) result in an opening of the Dcp1:Dcp2 complex. Plotted are the peak positions of Met-103, Met-164, and Met-221 in the methyl TROSY spectra (A). All mutations result in a collinear variation of the positions of Met-164 and Met-221, whereas Met-103, which is remote from the interface, is unaffected by the mutations. Addition of 10 mM ATP leads to a further closing of the complex in agreement with previously published small-angle X-ray scattering data (6).
To obtain insights into the dynamics of Dcp2 closure we performed relaxation dispersion experiments (Fig. 2C and SI Appendix, Fig. S2) and found that the apo Dcp2 enzyme (which contains both the RD and CD) interconverts rapidly (∼1,600 1/s) between equally populated open (e.g., orientation 4a, Fig. 1A) and closed states (orientation 1a). Addition of Dcp1 results in an almost complete shift of this equilibrium toward the closed state (Fig. 2C). Importantly, the carbon chemical shift differences between the open and closed states that we extract from these relaxation data correlate very well with the experimental CSPs (Fig. 2D). This demonstrates that the methyl chemical shifts directly report on the populations of open and closed states in Dcp2. Furthermore, we have confirmed the conformational equilibrium in Dcp2 with a large number of point mutations that destabilize the interface between the CD and RD and thereby shift the enzyme toward the open state (Fig. 2E).
To independently corroborate the Dcp2 domain orientation in the Dcp1:Dcp2 complex, we performed two complementary sets of PRE measurements. One set was obtained with a TEMPO spin label attached to position 49 of Dcp1 (Fig. 3A), and for the other set we added the short ATCUN motif (24) to the N terminus of Dcp2, which can be loaded with paramagnetic Cu2+ ions (SI Appendix, Fig. S3). In agreement with our other data, both PRE sets are only compatible with orientation 1a. In summary, our data demonstrate that the Dcp1:Dcp2 complex adopts the closed orientation 1a and that this structure transiently opens. Interestingly, this orientation is catalytically impaired because the catalytic Nudix helix is remote from the cap binding site (6, 11, 13) and the RNA binding path on the CD is partially blocked (discussed below).
Fig. 3.
PRE experiments reveal the structure of the Dcp2 enzyme in solution. (A) The correlation of PREs measured on the Dcp1:Dcp2 complex and PREs that are back-calculated based on the Dcp2 protein in orientation 1a confirm that the apo Dcp1:Dcp2 complex adopts this orientation in solution (see also SI Appendix, Fig. S3). Residues located in the Dcp2 RD are indicated in yellow and residues in the CD in green. The spin label at Dcp1-S49C is indicated in the structure with a yellow sphere; the measured PREs are displayed as red to blue spheres. (B) Same as in A, but for the Dcp1:Dcp2:Edc1 complex that adopts orientation 1a in solution (see also SI Appendix, Fig. S5). (C) Same as in A, but for Dcp1:Dcp2:Edc1:substrate complex that adopts orientation 6 in solution (see also SI Appendix, Fig. S7). (D) Path of the RNA on the structure of the Dcp1:Dcp2:Edc1:m7GDP complex (orientation 6b). The complex is colored according to the electrostatic surface potential. The nucleotides toward the 3′ end of the substrate interact with the box B region in the Dcp2 CD (see also SI Appendix, Fig. S11).
Edc1 Has No Influence on the Dcp1:Dcp2 Structure.
Edc1 is an intrinsically disordered decapping enhancer (7) that is recruited to the decapping complex by Dcp1 (8–10) (SI Appendix, Fig. S4A). The crystal structure of the Dcp1:Dcp2:Edc1 complex (10) (Fig. 1A, orientation 5) suggests that Edc1 induces a large domain reorientation in Dcp2 by inserting the YAG activation motif between Dcp1 and the Dcp2 CD. However, in solution we observe that the Edc1-induced CSPs in Dcp2 are limited to the RD (SI Appendix, Fig. S4 B and C) and that the YAG motif remains flexible (SI Appendix, Fig. S4 D–G). Moreover, the residues that directly report on the open/closed equilibrium of Dcp2 (Fig. 2E) are largely unaffected by Edc1 (SI Appendix, Fig. S4B), indicating that the domain orientation and dynamics of the Dcp1:Dcp2 complex are not influenced by Edc1 binding. Consistently, the PRE experiments on the Dcp1:Dcp2:Edc1 complex are only compatible with Dcp2 in orientation 1a (Fig. 3B and SI Appendix, Fig. S5). We thus conclude that Edc1 alone is unable to induce a conformational change in the decapping complex in solution, in contrast to previous static structural information (10).
The Dcp1:Dcp2:Edc1:Substrate Complex Forms a Stable Active Conformation.
We next added a capped RNA substrate to the Dcp1:Dcp2:Edc1 complex, such that the catalytically active state of the enzyme can form. Indeed, we observed a large number of CSPs in both the Dcp2 RD and CD (SI Appendix, Fig. S6), indicative of a large conformational change. Based on two complementary sets of PRE experiments (Fig. 3C and SI Appendix, Fig. S7), the Dcp1:Dcp2:Edc1:substrate complex adopts orientation 6 (Fig. 1 A–D) in solution. Importantly, in this orientation the Dcp2 RD and CD form a split active site around the cap structure and the Nudix helix is positioned close to the bound cap. This confirms earlier predictions based on NMR titrations (25) and is also seen in the structure solved by Charenton et al. (13) (Fig. 1A, orientation 6a).
NMR spectra of the Dcp1:Dcp2:Edc1 complex with substrate RNA are highly similar to those with the m7GDP product (SI Appendix, Fig. S8); we thus conclude that our crystal structure of the Dcp1:Dcp2:Edc1:m7GDP product represents the fully activated form of the decapping complex in solution. In that state, the Dcp2 conformation is enforced by the Edc1 YAG activation motif that interacts specifically with both the RD and CD of Dcp2 (Fig. 1 B–D and SI Appendix, Fig. S9) and thereby locks the Dcp2 domains in orientation 6.
The Dcp1:Dcp2:Substrate Complex Is Highly Dynamic.
To determine whether substrate alone is sufficient to induce this active state we assessed the conformation of the Dcp1:Dcp2:substrate complex in the absence of the activator Edc1. The NMR spectra of the complex show significant line broadening indicative of increased dynamics on the microsecond–millisecond timescale (SI Appendix, Fig. S10C). In line with this, the results of PRE measurements are not consistent with any of the orientations that Dcp2 adopts in the crystal structures (SI Appendix, Fig. S10 A and B) and PRE effects are observed on both sides of the CD. Based on this we conclude that Dcp2 adopts multiple orientations in the Dcp1:Dcp2:substrate complex. Because the Dcp1:Dcp2 complex shows decapping activity, one of these conformations must be the catalytically active form of the complex that can be stabilized by Edc1.
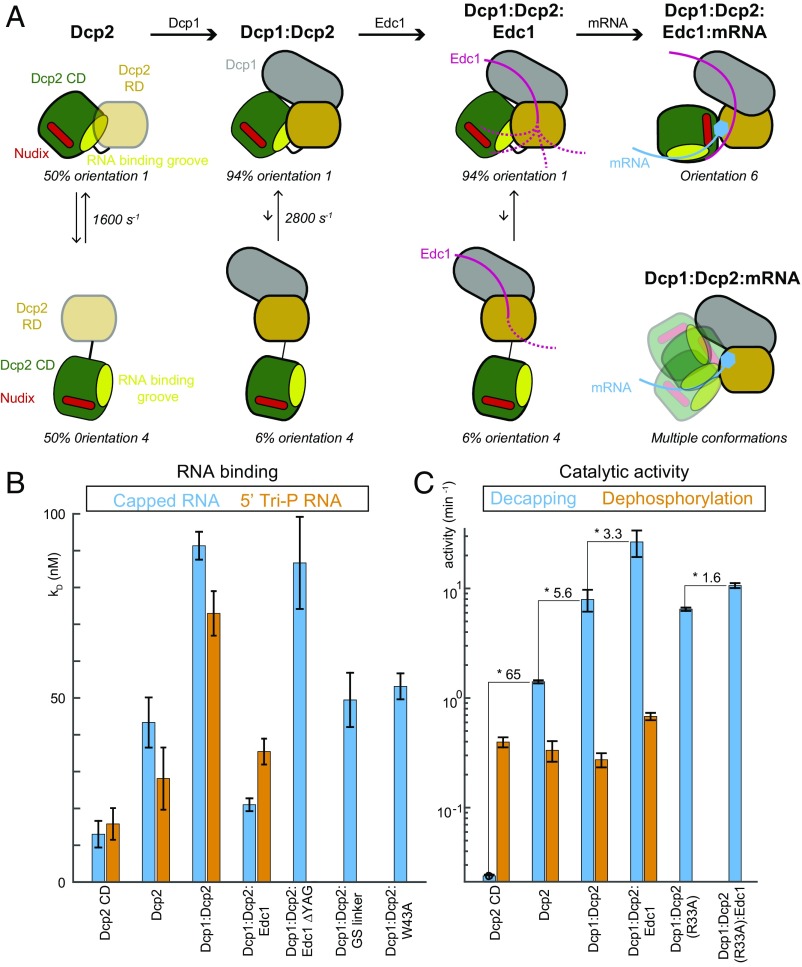
Taken together, our data elucidate the conformational space that the Dcp2 decapping enzyme samples in solution and how this is influenced by binding of Dcp1, Edc1, and capped RNA (summarized in Fig. 4A). This reveals a highly dynamic picture of the enzyme that is hidden in the static crystal structures.
Fig. 4.
Summary of the conformational changes in Dcp2. (A) Cartoon representation of the Dcp2 domain orientations in the presence and absence of Dcp1, Edc1, and/ or substrate. In the apo Dcp2 enzyme the RD and CD domains exchange between an open (orientation 4) and a closed (orientation 1) state that are equally populated. Upon formation of the Dcp1:Dcp2 complex this equilibrium is shifted toward the closed state. Recruitment of Edc1 and the formation of the Dcp1:Dcp2:Edc1 complex has no effect on the Dcp2 domain orientations. In the Dcp1:Dcp2:Edc1:RNA complex the stable active Dcp2 conformation (orientation 6) is formed. In the absence of Edc1, the Dcp1:Dcp2:RNA complex is highly dynamic (Bottom Right). Substrate is recruited to the open form of these complexes, because there the RNA binding groove (light green) is fully accessible. The colors are as indicated in Fig. 1A; the low stability of the fold of the Dcp2 RD in the absence of Dcp1 is indicated with light orange. (B) Dissociation constants of a 30mer RNA for different Dcp2 complexes, where a higher bar indicates a weaker interaction. The RNA contained either a 5′ cap structure (blue) or a 5′ triphosphate (orange). The affinity decreases upon inclusion of the Dcp2 RD and Dcp1 and increases upon recruitment of Edc1. The Dcp1:Dcp2:Edc1 complex interacts most strongly with capped RNA. Removal of the YAG activation motif in Edc1 abolishes the effect of the activator. The extension of the linker between the Dcp2 RD and CD as well as the W43A mutation open the Dcp1:Dcp2 complex (Fig. 2E) and expose the RNA binding groove, thereby increasing the substrate affinity. (C) The activity of Dcp2 on capped RNA (blue, eukaryotic mRNA) and 5′ triphosphate RNA (orange, bacterial mRNA). The decapping activity (blue) increases from the Dcp2 CD toward the Dcp1:Dcp2:Edc1 complex. The increase in catalytic efficiency is largely abolished for a version of Dcp2 that cannot interact efficiently with the Edc1 YAG activation motif (Dcp2 R33A, Fig. 1C). The activity of Dcp2 on 5′ triphosphate RNA (orange) is largely independent of the Dcp2 RD, Dcp1, and Edc1.
The mRNA Body Interacts with a Large Surface Patch on Dcp2.
Next, we sought to unravel the binding surface of the RNA body on Dcp2. To this end, we compared NMR spectra of the fully activated decapping complex in the presence of capped mRNA of increasing length (SI Appendix, Fig. S11 A–D). These data reveal that the RNA body interacts with a positively charged surface on Dcp2 that extends toward the Dcp2 box B region (2) that has previously been implicated in RNA binding (26) (Fig. 3D). Unexpectedly, we find that this RNA binding path is largely buried in the Dcp1:Dcp2 closed structure (orientation 1a, Fig. 1A and SI Appendix, Fig. S11E). This observation is confirmed by RNA binding experiments that show that the substrate affinity of the CD is decreased upon inclusion of the RD and upon addition of Dcp1. The RNA binding affinity is thus inversely correlated with the population of the closed state (Fig. 4 A and B). Upon addition of Edc1 the RNA affinity improves, which is in agreement with our structural data (Fig. 1 B–D) that show the exposure of the substrate binding groove in the Dcp1:Dcp2:Edc1:RNA complex that adopts the active state of the enzyme (Fig. 1 B–D). This conformational switch into the active state requires the Edc1 YAG activation motif (Fig. 1C) because an Edc1 variant lacking this motif is not able to improve RNA binding (Fig. 4B), in agreement with previous results (9). Likewise, a mutation in the Edc1 binding site of Dcp2 (R33A, Fig. 1C) prevents the formation of the stable catalytically active conformation in Dcp2 (SI Appendix, Fig. S12). In brief, we show that the RNA binding groove of the decapping complex is largely secluded in the absence of Edc1 and substrate, which can prevent the unspecific recruitment of RNA to the enzyme.
The Activity of Dcp2 Correlates with the RD and CD Domain Orientation.
To unravel how the different conformations in Dcp2 correlate with catalytic activity we performed mRNA decapping assays (Fig. 4C). In line with published data, we observe a stepwise increase in the decapping activity from the Dcp2 CD to Dcp2, Dcp1:Dcp2, and Dcp1:Dcp2:Edc1 (5, 9, 10) (Fig. 4C, blue bars). Our structural studies now enable us to rationalize the molecular mechanisms underlying the observed decapping activation: First, the Dcp2 RD is required as a binding platform for the mRNA cap (25) (Fig. 1 B and D). Second, Dcp1 stabilizes the fold of the Dcp2 RD, especially around the split active site, as revealed by hydrogen deuterium exchange rates (SI Appendix, Fig. S13). Finally, Edc1 enforces the active orientation in Dcp2 (Fig. 1 B–D) through specific interaction between its YAG activation motif and Dcp2.
The Dcp1:Dcp2:Edc1 Complex Is Selective for Capped mRNA.
In bacteria, mRNA degradation is initiated by the hydrolysis of the protecting triphosphorylated 5′ end (27) by the Nudix enzyme RppH (28), which is structurally homologous to the eukaryotic Dcp2 CD. Interestingly, the Dcp2 CD is also active on 5′ triphosphorylated RNA (Fig. 4C, orange bars and SI Appendix, Fig. S14) and this activity is independent of the presence of the Dcp2 RD, Dcp1, and Edc1 (Fig. 4C). This suggests that the Dcp2 RD, Dcp1, and Edc1 are factors that appeared during evolution to ensure a high and selective activity of the Dcp2 CD on eukaryotic mRNA. The mechanisms that increase eukaryotic mRNA decapping efficiency thus also switch in the substrate specificity from bacterial (5′ triphosphate) mRNA to eukaryotic (5′ capped) mRNA.
Concluding Remarks
Taken together, our data elucidate the conformational space that the Dcp2 decapping enzyme samples in solution and how this is influenced by binding of Dcp1, Edc1, and capped RNA (Fig. 4A). This reveals a highly dynamic picture of the enzyme that is hidden in the static crystal structures and prevented the conclusive interpretation of the known structural and biochemical data (15). Our data thus show an unexpected view of how conformational changes in enzymes correlate with activity and substrate selectivity. These results highlight the importance of integrative structural biology approaches (29) where static structures of molecular machines are complemented with detailed solution methods that address conformational equilibria.
Methods
Sample Preparations.
Genes coding for the Schizosaccharomyces pombe proteins were cloned into modified pET11 vectors and proteins were expressed in Escherichia coli. Proteins were subsequently purified through NiNTA, cation exchange, and size-exclusion chromatography. For NMR measurements Dcp2 was 1H3-13C–labeled at the Ile, Met, Val, Ala, and Leu methyl groups in a fully deuterated background (30). RNA was produced by standard in vitro transcription using T7 polymerase and capped with vaccinia capping enzyme (31).
X-Ray Crystallography.
The Dcp1:Dcp2:Edc1:m7GDP complex was crystallized using the sitting drop technique at a concentration of 10 mg/mL. The reservoir solution consisted of 41% MPD/PEG1000/PEG3350, 0.1 M Mops, pH 7.0, and 0.06 M MgCl2/CaCl2. Data collection and refinement statistics are given in SI Appendix, Table S1. Coordinates of the Dcp1:Dcp2:Edc1:m7GDP structure have been deposited in the Protein Data Bank (PDB) with ID code 5N2V.
NMR Spectroscopy.
Methyl TROSY- (16) based NMR spectra were recorded on 600- or 800-MHz spectrometers at 30 °C. Published methyl group assignments for isolated Dcp2 (5) were extended and transferred to Dcp2 complexes using a combination of 3D 13C-NOESY experiments and point mutations (SI Appendix, Tables S2–S4). Spin labels for PRE measurements were attached to the N teminus of Dcp2 via an ATCUN tag (24) or to position 49 of Dcp1 using iodoacetamido-TEMPO. To account for the mobility of the spin labels during back calculation of PRE values an ensemble approach was used (32). All NMR data were processed and analyzed with the NMRPipe/ NMRDraw software suite (33).
Binding and Activity Assays.
An in vitro transcribed 30mer RNA containing a single 4-thiouridine at position 15 was labeled with iodoacetamido fluorescein and used for fluorescence anisotropy measurements (9, 34). Activity assays were performed with a 21mer RNA under multiple turnover conditions (35). Reaction mixtures were analyzed by anion exchange HPLC and educt and product of the reaction were quantified based on the absorption at 260 nm. Detailed methods can be found in SI Appendix.
Supplementary Material
Acknowledgments
We thank all members of the laboratory for discussions, Silke Wiesner for constructive remarks, Vincent Truffault for maintenance of the NMR infrastructure, Andrei Lupas for hosting the crystallization facility, and Ancilla Neu for recording diffraction data. This work was supported by the Max Planck Society and the European Research Council (ERC) under the European Union’s Seventh Framework Programme (FP7/2007–2013), ERC Grant 616052.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Crystallography, atomic coordinates, and structure factors of the Dcp1:Dcp2:Edc1:m7GDP structure have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 5N2V).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704496114/-/DCSupplemental.
References
- 1.Schoenberg DR, Maquat LE. Regulation of cytoplasmic mRNA decay. Nat Rev Genet. 2012;13:246–259. doi: 10.1038/nrg3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Jiao X, Carr-Schmid A, Kiledjian M. The hDcp2 protein is a mammalian mRNA decapping enzyme. Proc Natl Acad Sci USA. 2002;99:12663–12668. doi: 10.1073/pnas.192445599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Dijk E, et al. Human Dcp2: A catalytically active mRNA decapping enzyme located in specific cytoplasmic structures. EMBO J. 2002;21:6915–6924. doi: 10.1093/emboj/cdf678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLennan AG. The Nudix hydrolase superfamily. Cell Mol Life Sci. 2006;63:123–143. doi: 10.1007/s00018-005-5386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Floor SN, Borja MS, Gross JD. Interdomain dynamics and coactivation of the mRNA decapping enzyme Dcp2 are mediated by a gatekeeper tryptophan. Proc Natl Acad Sci USA. 2012;109:2872–2877. doi: 10.1073/pnas.1113620109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.She M, et al. Structural basis of dcp2 recognition and activation by dcp1. Mol Cell. 2008;29:337–349. doi: 10.1016/j.molcel.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunckley T, Tucker M, Parker R. Two related proteins, Edc1p and Edc2p, stimulate mRNA decapping in Saccharomyces cerevisiae. Genetics. 2001;157:27–37. doi: 10.1093/genetics/157.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borja MS, Piotukh K, Freund C, Gross JD. Dcp1 links coactivators of mRNA decapping to Dcp2 by proline recognition. RNA. 2011;17:278–290. doi: 10.1261/rna.2382011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wurm JP, Overbeck J, Sprangers R. The S. pombe mRNA decapping complex recruits cofactors and an Edc1-like activator through a single dynamic surface. RNA. 2016;22:1360–1372. doi: 10.1261/rna.057315.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valkov E, et al. Structure of the Dcp2-Dcp1 mRNA-decapping complex in the activated conformation. Nat Struct Mol Biol. 2016;23:574–579. doi: 10.1038/nsmb.3232. [DOI] [PubMed] [Google Scholar]
- 11.Mugridge JS, Ziemniak M, Jemielity J, Gross JD. Structural basis of mRNA-cap recognition by Dcp1-Dcp2. Nat Struct Mol Biol. 2016;23:987–994. doi: 10.1038/nsmb.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.She M, et al. Crystal structure and functional analysis of Dcp2p from Schizosaccharomyces pombe. Nat Struct Mol Biol. 2006;13:63–70. doi: 10.1038/nsmb1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charenton C, et al. Structure of the active form of Dcp1-Dcp2 decapping enzyme bound to m(7)GDP and its Edc3 activator. Nat Struct Mol Biol. 2016;23:982–986. doi: 10.1038/nsmb.3300. [DOI] [PubMed] [Google Scholar]
- 14.Fromm SA, et al. The structural basis of Edc3- and Scd6-mediated activation of the Dcp1:Dcp2 mRNA decapping complex. EMBO J. 2012;31:279–290. doi: 10.1038/emboj.2011.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coller J. mRNA decapping in 3D. Nat Struct Mol Biol. 2016;23:954–956. doi: 10.1038/nsmb.3315. [DOI] [PubMed] [Google Scholar]
- 16.Tugarinov V, Hwang PM, Ollerenshaw JE, Kay LE. Cross-correlated relaxation enhanced 1H[bond]13C NMR spectroscopy of methyl groups in very high molecular weight proteins and protein complexes. J Am Chem Soc. 2003;125:10420–10428. doi: 10.1021/ja030153x. [DOI] [PubMed] [Google Scholar]
- 17.Audin MJ, et al. The archaeal exosome: Identification and quantification of site-specific motions that correlate with cap and RNA binding. Angew Chem Int Ed Engl. 2013;52:8312–8316. doi: 10.1002/anie.201302811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neu A, Neu U, Fuchs AL, Schlager B, Sprangers R. An excess of catalytically required motions inhibits the scavenger decapping enzyme. Nat Chem Biol. 2015;11:697–704. doi: 10.1038/nchembio.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sprangers R, Kay LE. Quantitative dynamics and binding studies of the 20S proteasome by NMR. Nature. 2007;445:618–622. doi: 10.1038/nature05512. [DOI] [PubMed] [Google Scholar]
- 20.Saio T, Guan X, Rossi P, Economou A, Kalodimos CG. Structural basis for protein antiaggregation activity of the trigger factor chaperone. Science. 2014;344:1250494. doi: 10.1126/science.1250494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Religa TL, Sprangers R, Kay LE. Dynamic regulation of archaeal proteasome gate opening as studied by TROSY NMR. Science. 2010;328:98–102. doi: 10.1126/science.1184991. [DOI] [PubMed] [Google Scholar]
- 22.Tang C, Schwieters CD, Clore GM. Open-to-closed transition in apo maltose-binding protein observed by paramagnetic NMR. Nature. 2007;449:1078–1082. doi: 10.1038/nature06232. [DOI] [PubMed] [Google Scholar]
- 23.Lundström P, Vallurupalli P, Religa TL, Dahlquist FW, Kay LE. A single-quantum methyl 13C-relaxation dispersion experiment with improved sensitivity. J Biomol NMR. 2007;38:79–88. doi: 10.1007/s10858-007-9149-7. [DOI] [PubMed] [Google Scholar]
- 24.Donaldson LW, et al. Structural characterization of proteins with an attached ATCUN motif by paramagnetic relaxation enhancement NMR spectroscopy. J Am Chem Soc. 2001;123:9843–9847. doi: 10.1021/ja011241p. [DOI] [PubMed] [Google Scholar]
- 25.Floor SN, Jones BN, Hernandez GA, Gross JD. A split active site couples cap recognition by Dcp2 to activation. Nat Struct Mol Biol. 2010;17:1096–1101. doi: 10.1038/nsmb.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piccirillo C, Khanna R, Kiledjian M. Functional characterization of the mammalian mRNA decapping enzyme hDcp2. RNA. 2003;9:1138–1147. doi: 10.1261/rna.5690503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celesnik H, Deana A, Belasco JG. Initiation of RNA decay in Escherichia coli by 5′ pyrophosphate removal. Mol Cell. 2007;27:79–90. doi: 10.1016/j.molcel.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deana A, Celesnik H, Belasco JG. The bacterial enzyme RppH triggers messenger RNA degradation by 5′ pyrophosphate removal. Nature. 2008;451:355–358. doi: 10.1038/nature06475. [DOI] [PubMed] [Google Scholar]
- 29.van den Bedem H, Fraser JS. Integrative, dynamic structural biology at atomic resolution–It’s about time. Nat Methods. 2015;12:307–318. doi: 10.1038/nmeth.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerfah R, Plevin MJ, Sounier R, Gans P, Boisbouvier J. Methyl-specific isotopic labeling: A molecular tool box for solution NMR studies of large proteins. Curr Opin Struct Biol. 2015;32:113–122. doi: 10.1016/j.sbi.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs AL, Neu A, Sprangers R. A general method for rapid and cost-efficient large-scale production of 5′ capped RNA. RNA. 2016;22:1454–1466. doi: 10.1261/rna.056614.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwahara J, Schwieters CD, Clore GM. Ensemble approach for NMR structure refinement against (1)H paramagnetic relaxation enhancement data arising from a flexible paramagnetic group attached to a macromolecule. J Am Chem Soc. 2004;126:5879–5896. doi: 10.1021/ja031580d. [DOI] [PubMed] [Google Scholar]
- 33.Delaglio F, et al. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 34.Ramos A, Varani G. A new method to detect long-range protein-RNA contacts: NMR detection of electron-proton relaxation induced by nitroxide spin-labeled RNA. J Am Chem Soc. 1998;120:10992–10993. [Google Scholar]
- 35.Audin MJ, Wurm JP, Cvetkovic MA, Sprangers R. The oligomeric architecture of the archaeal exosome is important for processive and efficient RNA degradation. Nucleic Acids Res. 2016;44:2962–2973. doi: 10.1093/nar/gkw062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.