Significance
Photoresponsive hydrogels have lately received ample attention because of their great potential in biomedical applications. However, the use of an entirely recombinant protein-based hydrogel with light-sensing abilities for stem cell applications has rarely been shown. Here, we created a B12-dependent light-sensing hydrogel by covalently stitching together the photoreceptor C-terminal adenosylcobalamin binding domain (CarHC) proteins under mild conditions. The polymeric CarHC proteins self-assemble into an elastic hydrogel in the presence of adenosylcobalamin in the dark and disassemble rapidly on light exposure. Such a photoresponsive protein hydrogel system enabled facile release/recovery of stem cells and protein molecules. This direct assembly of stimuli-responsive proteins into hydrogels represents a versatile strategy for designing “smart” materials and opens up enormous opportunities for future material biology.
Keywords: hydrogels, cell encapsulation, drug delivery, photoresponsive materials, protein engineering
Abstract
Thanks to the precise control over their structural and functional properties, genetically engineered protein-based hydrogels have emerged as a promising candidate for biomedical applications. Given the growing demand for creating stimuli-responsive “smart” hydrogels, here we show the synthesis of entirely protein-based photoresponsive hydrogels by covalently polymerizing the adenosylcobalamin (AdoB12)-dependent photoreceptor C-terminal adenosylcobalamin binding domain (CarHC) proteins using genetically encoded SpyTag-SpyCatcher chemistry under mild physiological conditions. The resulting hydrogel composed of physically self-assembled CarHC polymers exhibited a rapid gel-sol transition on light exposure, which enabled the facile release/recovery of 3T3 fibroblasts and human mesenchymal stem cells (hMSCs) from 3D cultures while maintaining their viability. A covalently cross-linked CarHC hydrogel was also designed to encapsulate and release bulky globular proteins, such as mCherry, in a light-dependent manner. The direct assembly of stimuli-responsive proteins into hydrogels represents a versatile strategy for designing dynamically tunable materials.
Hydrogels, noted for their biomimetic properties, are the leading materials for biomedical applications, such as drug delivery and stem cell therapy (1, 2). Traditional hydrogels made up of either synthetic polymers or natural biomolecules often serve as passive scaffolds for molecular or cellular species, which render these materials unable to fully recapitulate the dynamic signaling involved in biological processes, such as cell/tissue development. It is, therefore, increasingly important to design stimuli-responsive, dynamic hydrogels that can accommodate or mimic the complexity of biological systems (3, 4). Photoresponsive hydrogels are of particular interest to material scientists, because light is regarded as an ideal tool to control molecules or cell behavior with high spatiotemporal precision and little invasiveness (5–9). Thanks to the advancement of synthetic chemistry, tremendous progress has been made over the past few years in making photoresponsive hydrogels with dynamically tunable properties (10, 11). Through a combination of orthogonal click reactions and photochemistry, some of these synthetic hydrogels can be mechanically and chemically patterned in situ by light while being used for 3D cell culturing (12, 13). Diverse photoactive chemical moieties have also been incorporated into synthetic hydrogels to create photoresponsive devices for controlled therapeutic release (5, 14, 15).
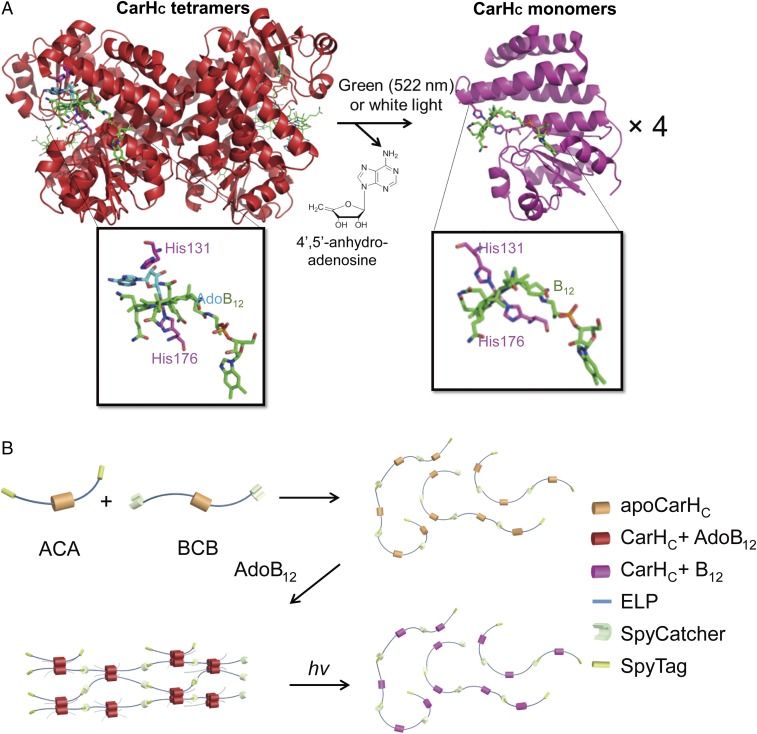
Assembling genetically engineered proteins into molecular networks represents an alternative strategy to make hydrogels with well-controlled properties (16–19). Although natural evolution has led to numerous functional protein domains that can sense and respond to a variety of environmental stimuli, such as light, oxidative stress, pH, small molecules, metal ions, etc., such ecological diversity has yet to be fully tapped to develop responsive biomaterials with dynamically tunable properties. The early work led by Murphy and coworkers (20, 21) showed the success of synthesizing calmodulin-based protein hydrogels with dynamic properties responsive to Ca2+ and the small-molecule ligand trifluoperazine. Recently, the CarH protein, a transcriptional regulator controlling bacterial carotenoid synthesis, has been shown to sense and respond to visible light through its C-terminal adenosylcobalamin binding domain (CarHC) (22–25). The CarHC domains tetramerize when binding to adenosylcobalamin (AdoB12) in the dark and can readily dissociate into monomers accompanied with a drastic protein conformational change caused by the cleavage of the C-Co bond on exposure to green (522 nm) or white light (Fig. 1A) (23, 24). We envisioned that the light-sensing CarHC domains could be used to construct protein-based photoresponsive hydrogels. To this end, the major challenge is in how to assemble these complex globular proteins into supramolecular architectures efficiently while preserving their function.
Fig. 1.
Synthesis of photoresponsive CarHC hydrogels. (A) Light exposure disassembles tetrameric CarHC accompanied by the degradation of AdoB12, the release of 4′,5′-anhydroadenosine, and the coordination of His132 to the metal center. Tetrameric CarHC (Protein Data Bank ID code 5C8A). Monomeric CarHC (Protein Data Bank ID code 5C8F). (B) The two telechelic proteins, ACA and BCB, are polymerized through SpyTag-SpyCatcher chemistry. The resulting polymers can further be assembled into a molecular network through AdoB12-induced CarHC tetramerization in the dark and disassembled on light exposure. ApoCarHC is the CarHC protein without AdoB12. hv, light.
Genetically encoded SpyTag (A)-SpyCatcher (B) chemistry, inspired by an isopeptide bond-containing bacterial adhesin, consists of a peptide/protein pair that can spontaneously form an isopeptide bond on binding under mild physiological conditions (26). Because of its high efficiency and modularity, this chemistry has led to a number of applications, including control of biomacromolecular topology, synthesis of bioactive and “living” materials, and biomolecular imaging (16, 18, 27–36). It has proven to be a powerful method for constructing complex biomolecular architectures both in vitro and in vivo. In this study, we successfully polymerized the elastin-like polypeptide (ELP)-fusion CarHC protein using SpyTag-SpyCatcher chemistry. CarHC tetramerization in the presence of AdoB12 in the dark eventually led to the formation of a hydrogel that can undergo a rapid gel-sol transition on light exposure. This result illustrates a versatile strategy for developing stimuli-responsive protein materials for biomedical applications, such as controlled therapeutic release and cell recovery, from 3D cultures.
Results and Discussion
Protein Construct Design.
To polymerize CarHC, we designed two gene constructs encoding the telechelic proteins SpyTag-elastin-like polypeptide-C-terminal adenosylcobalamin binding domain-elastin-like polypeptide-SpyTag (ACA; 41 kDa) and SpyCatcher-elastin-like polypeptide-C-terminal adenosylcobalamin binding domain-elastin-like polypeptide-SpyCatcher (BCB; 68 kDa), respectively (Fig. 1B). The ELP domain consists of repeating pentapeptides (VPGXG)15, where X represents either valine or glutamate at a ratio of 4:1, a composition that is expected to enhance the expression yield and solubility of these recombinant proteins under physiological conditions (37). We envisioned that the reaction of SpyTag and SpyCatcher should be able to covalently stitch together CarHC to form protein polymers, of which interchain interactions will be dominated by the AdoB12-induced CarHC tetramerization (Fig. 1B).
Synthesis of CarHC Hydrogels.
Using an Escherichia coli expression system, we were able to produce and isolate sufficient amounts of the ACA (∼98 mg/L) and BCB (∼66 mg/L) proteins with high purity (SI Appendix, Figs. S1–S3). After extensive dialysis against distilled water and lyophilization, the resulting protein powders can be readily dissolved in PBS. The two protein solutions, ACA and BCB (10 wt % in PBS), were mixed at an equimolar ratio immediately followed by addition of a stoichiometric amount of AdoB12 to initiate gelation at room temperature in the dark. A red gel-like material was formed within 5 min; the gelation typically continued for at least 7 h in the dark before any subsequent analysis. The resulting gel was sensitive to light and converted to liquid on exposure to white LED light (30 klux) within 20 min (Fig. 2B). The observed gel-sol transition can be explained by the light-induced disassembly of CarHC tetramers (Fig. 1). A subsequent size-exclusion chromatography (SEC) analysis showed that the major elution peak of the light-degraded products as well as that of the reaction products of ACA + BCB in the absence of AdoB12 appeared at the void volume (V0; 8.7 mL), corresponding to a molecular weight that exceeds the exclusion limit (∼2 × 106) (SI Appendix, Fig. S4). This SEC result further confirmed a sufficient polymerization of the CarHC proteins enabled by the SpyTag-SpyCatcher reaction during gelation.
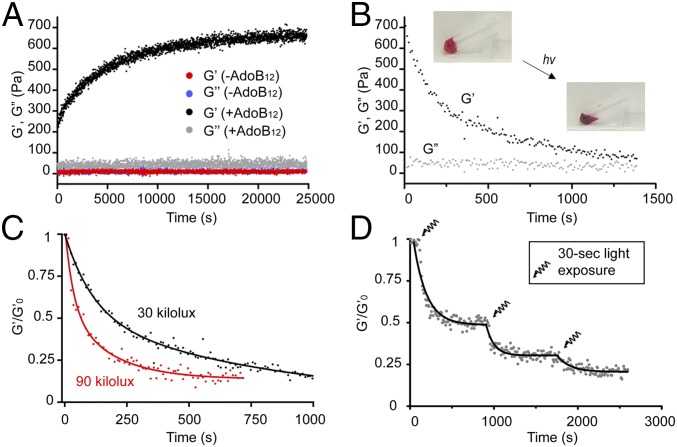
Fig. 2.
AdoB12-dependent photoresponsiveness of CarHC hydrogels. (A) Evolution of the storage modulus G′ and loss modulus G″ of ACA + BCB in the presence and absence of AdoB12 at room temperature (25 °C) in the dark as a function of time. (B) Gel-sol transition induced by light. On white LED light exposure (30 klux), the G′ and G″ of the CarHC hydrogel were monitored at a fixed shear frequency of 1 rad/s and strain of 5%. (C) Gel-sol transition rates of the CarHC hydrogels are affected by light intensity. The normalized storage modulus (G′/G′0) of the materials exposed to the 30- and 90-klux lights is compared. The G′ values corresponding to the 30-klux light exposure were from the same measurement shown in B. (D) Response of the CarHC hydrogel toward pulsed light (90 klux). Exponential decay curve fitting was used. hv, light.
Physical Properties of CarHC Hydrogels.
Dynamic shear rheology was performed to monitor the gelation process. Simply mixing ACA and BCB in the absence of the AdoB12 cofactor only led to a liquid-like material that exhibited a very low storage modulus G′ (Fig. 2A). However, the reaction of ACA + BCB in the presence of AdoB12 under dark conditions led to a gradually increased storage modulus G′ that reached a steady value of ∼0.66 kPa after 7 h and a much lower loss modulus G″ (∼0.04 kPa), suggesting that AdoB12-mediated CarHC tetramerization is essential for the hydrogel formation (Fig. 2A). The G′ of the hydrogel quickly dropped under constant light exposure, reflecting a typical gel-sol transition process (Fig. 2B), and the rates of the transition were affected by the light intensity (Fig. 2C). A stepwise gel-sol transition was also achieved by exposing the hydrogel to a pulsed light (Fig. 2D). Given the noncovalent nature of CarHC tetramerization, we speculated that the CarHC hydrogel composed of ACA + BCB would display frequency-dependent viscoelastic properties. However, the frequency sweep test on the CarHC hydrogel at room temperature (25 °C) showed a steady storage modulus (0.58–0.81 kPa) over an angular frequency of 0.01–100 rad/s (SI Appendix, Fig. S5A), which is indicative of static interchain interactions. One possible explanation is that the CarHC tetramers within the hydrogel network are kinetically inert, of which the disassembly or exchange takes longer than the timescale for the lowest shear frequency tested (628 s). Assuming that all of the CarHC domains form tetramers, a theoretical estimate of the G′ of the CarHC hydrogel (8.3 wt %) is ∼0.94 kPa based on G = ρRT/Mc, where the average molecular mass between cross-links Mc is ∼218 kDa (2ACA + 2BCB). The observed G′ matches well with the theoretical estimate, suggesting the sufficient assembly of the CarHC domains within the hydrogel network. The CarHC hydrogel also exhibited stability toward mechanical deformation (1–10% strain) as revealed by the strain-sweep test at room temperature (SI Appendix, Fig. S5B).
The ELP domains are known to have a unique phase transition behavior at a so-called lower critical solution temperature (LCST). The LCST of the ELP domain that we chose is 25 °C to 30 °C (37). To investigate the influence of temperature on these ELP-based hydrogel properties, we performed rheology studies on the hydrogels at different temperatures (16 °C, 25 °C, and 37 °C) and observed little change in their rheological behaviors, suggesting that the phase transition of ELPs has negligible effects on macroscopic mechanical properties of the materials.
The CarHC hydrogel composed of ACA + BCB + AdoB12 exhibited moderate swelling in PBS at room temperature, and the swelling ratio, calculated as the ratio of the wet gel weight to the dry protein weight, reached maximum (∼25) from an initial ratio of 12 within 3 d (SI Appendix, Fig. S6). The hydrogel is also stable. Only ∼6% of the gel was eroded in the presence of excess PBS at room temperature after 10 d (SI Appendix, Fig. S7).
Cell Encapsulation and Light-Induced Release/Recovery.
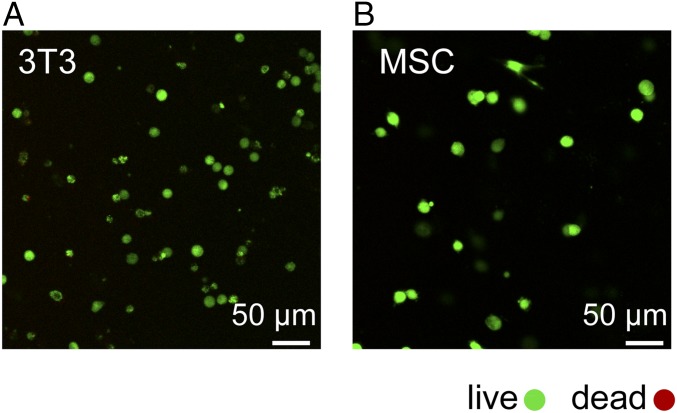
For the study of cell matrix interactions as well as tissue engineering, it is desirable to have a 3D cell culture system that allows cells to be encapsulated and readily released/recovered without resorting to complicated chemical or proteolytic degradation (38, 39). The CarHC hydrogel composed of ACA + BCB + AdoB12 that undergoes a rapid gel-sol transition on light exposure may allow us to handily recover/release cells after 3D culturing. We first examined the cytocompatibility of the CarHC hydrogel using mouse 3T3 fibroblasts and human mesenchymal stem cells (hMSCs). To encapsulate the cells, the BCB solution containing the cells was mixed thoroughly with the ACA solution at a 1:1 molar ratio followed by adding a stoichiometric amount of AdoB12 to initiate gelation in the dark. After 2 h of curing, the gels were covered with culture medium and incubated under standard conditions (37 °C at 5% CO2) away from light. After 24 h, a live/dead staining was performed to examine the cell viability. It turned out that most of the embedded fibroblasts (88 ± 3%) and mesenchymal stem cells (MSCs; 92 ± 2%) remained viable (Fig. 3), suggesting that the CarHC hydrogel is nontoxic to these cells.
Fig. 3.
Encapsulation of (A) 3T3 fibroblasts and (B) MSCs by the physically assembled CarHC hydrogels composed of ACA + BCB + AdoB12. Representative confocal fluorescence z-slice micrographs of live (green; calcein AM) and dead (red; ethidium homodimer) cells are shown.
Rapid gelation is required by cell encapsulation applications. The gelation rate of the current CarHC hydrogel system mainly depends on two events: the SpyTag-SpyCatcher reaction and AdoB12-dependent CarHC self-assembly. The SpyTag-SpyCatcher reaction is likely to be the rate-limiting step, because its second-order rate constant is ∼1.4 × 103 M−1 s−1, which is far from the diffusion limit and substantially slower than some other biomolecular interactions, such as the widely used streptavidin–biotin interaction (26). The reaction kinetics of SpyTag-SpyCatcher can be optimized through directed evolution to accelerate the gelation. Alternatively, the efficiency of CarHC self-assembly can also be improved through protein engineering, so that a hydrogel system solely based on the CarHC tetramerization can be developed to enable swift cell encapsulation.
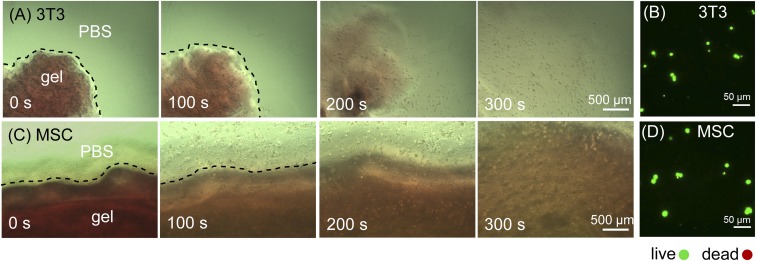
To recover these encapsulated cells, we exposed the cell-laden gels to white light. The cells gradually migrated along with the melting gel, suggesting that these cells were indeed encapsulated in a 3D manner (Fig. 4 and Movies S1 and S2). Within 5 min, the gels were completely transformed into liquid accompanied with a complete release of the cells. The live/dead staining showed that the majority of recovered cells (3T3: 90 ± 7%; MSCs: 88 ± 5%) remained viable, indicating that the photolysis of the CarHC hydrogels is amicable to the encapsulated cells (Fig. 4 B and D). The photodegradation of AdoB12 in CarH does not use a typical radical mechanism, and its photolytic product is 4′,5′-anhydroadenosine instead of more common 5′-dAdo radicals (25, 40). Although adenosine could lead to cytotoxicity at relatively high concentrations or after prolonged exposure, so far, there has been no direct evidence pointing to the cytotoxicity or any other side effect of its analog 4′,5′-anhydroadenosine on cell phenotypes (41). In addition, only trace amounts of unbound AdoB12 (∼1 µM) exists in the CarHC hydrogel (8.3 wt %) given the dissociation constant Kd for the AdoB12–CarH complex (0.8 µM) (24). Therefore, the radical species resulting from the photolysis of the free AdoB12 is negligible and can hardly cause cytotoxicity. Both the nonradical photolysis of the AdoB12 binding CarHC and the very low concentration of the free AdoB12 likely contribute to the high viability of these cells recovered from the protein hydrogels.
Fig. 4.
Release of encapsulated cells by light-induced gel-sol transition. (A) Mouse 3T3 fibroblasts and (C) hMSCs were encapsulated by CarHC hydrogels (red) and cultured for 24 h. Cell release was initiated by exposing the 3D cell culture to white light (22 klux). Representative bright-field micrographs (0, 100, 200, and 300 s after light exposure) are shown. Dashed lines indicate the gel boundaries. (B and D) Live/dead staining of recovered 3T3 fibroblasts and hMSCs.
Light-Induced Protein Release.
Protein drugs, an important category of modern therapeutics, have a short half-life time inside the body because of rapid plasma clearance and proteolytic degradation, which constitutes a fundamental challenge for their delivery (42, 43). The ELP fusion strategy has previously been shown to be able to significantly enhance pharmacokinetics and efficacy of a protein drug by prolonging its circulating half-life (44). It prompted us to examine the feasibility of using the photoresponsive CarHC-ELP hydrogel system to encapsulate and release protein molecules in a controlled manner. Inspired by the previously reported Spy network hydrogel (16), we chose two recombinant proteins, SpyTag-elastin-like polypeptide-SpyTag-elastin-like polypeptide-SpyTag (AAA) (SI Appendix, Figs. S1 and S8) and BCB, to synthesize a covalently cross-linked ELP hydrogel. The two proteins were dissolved in PBS to make 10 wt % solutions. Gelation was initiated by mixing the two components at a 2:3 molar ratio and a stoichiometric amount of AdoB12 in the dark. Dynamic shear rheology experiments in time-, frequency- and strain-sweep modes and erosion tests further confirmed the formation of a stable hydrogel (G′ = 0.96 kPa and G″ = 0.03 Pa) by AAA + BCB + AdoB12 (SI Appendix, Figs. S9 and S10).
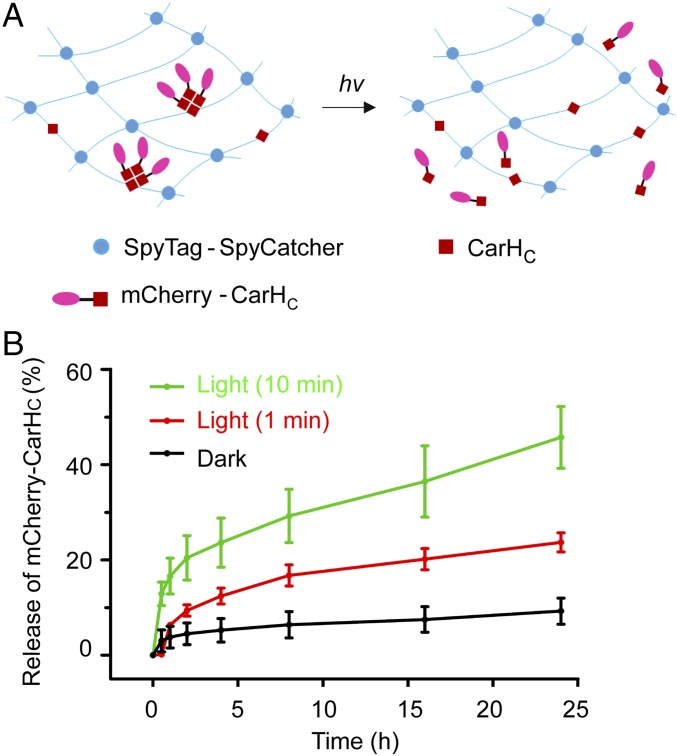
To test the feasibility of this covalently cross-linked CarHC hydrogel for controlled protein release, a recombinant CarHC-fusion mCherry protein was constructed and used as a model substrate (SI Appendix, Figs. S1 and S11). We envisioned that the mCherry-CarHC protein could be physically tethered to the hydrogel network through AdoB12-dependent CarHC self-assembly in the dark and that light exposure would disassemble the CarHC tetramers and facilitate the release of mCherry-CarHC (Fig. 5A). To test this hypothesis, three sets of mCherry-CarHC–decorated hydrogels were synthesized and immersed in PBS; two were exposed to the white LED light (90 klux) for 1 and 10 min before being moved to the dark, and the third one was kept in the dark all of the time. Aliquots of the supernatant were taken to analyze the release of mCherry over 24 h. Fig. 5B shows that about 24 and 45% of mCherry-CarHC were released from the gels that were subjected to 1- and 10-min light exposure, respectively, after 24 h, whereas the release was substantially slower in the gel always kept in the dark with less than 10% of mCherry-CarHC released. This result suggests that controlled protein release in this system can be readily achieved by adjusting the duration of the light exposure. For the mCherry protein lacking the CarHC domain, its release from the AAA + BCB hydrogel was light-independent, because similar amounts of free mCherry (∼50%) were detected in the supernatants under the dark and bright conditions, further corroborating that the CarHC domain is essential for the light-controlled release (SI Appendix, Fig. S12). It is also noteworthy that, despite two tobacco etch virus (TEV) protease cleavage sites in the BCB construct, the covalently cross-linked hydrogels composed of AAA + BCB + AdoB12 are resistant toward TEV digestion at room temperature under both dark and bright (white LED light; 90 klux) conditions (SI Appendix, Fig. S13). These ELP-based covalent networks likely served as physical barriers to protect the encapsulated proteins/peptides from proteolysis, even after photolysis. These results together show the feasibility of using the photoresponsive CarHC hydrogel for controlled protein delivery/release.
Fig. 5.
Protein immobilization and light-induced release enabled by the covalently cross-linked CarHC hydrogels. (A) Schematic showing the immobilization and release of the mCherry-CarHC protein. The hydrogel (8.6 wt %) is composed of AAA and BCB at a 2:3 molar ratio, a stoichiometric amount of AdoB12, and the fusion protein mCherry-CarHC (50 µM). CarHC tetramerization leads to the mCherry immobilization into the hydrogel in the dark. The photolysis of AdoB12 disassembles tetrameric CarHC and facilitates the release of mCherry-CarHC. (B) Release profiles of mCherry-CarHC from the hydrogels that were subjected to 0, 1, and 10 min of white light exposure. The percentage release of mCherry was calculated based on the total amount of mCherry added into the gel. Error bars show SDs from three independent measurements. hv, light.
In summary, we have reported the creation of entirely recombinant protein-based light-sensitive hydrogels by covalently assembling the CarHC photoreceptor proteins using SpyTag-SpyCatcher chemistry. The AdoB12-dependent CarHC tetramerization has been shown to be essential for the formation of an elastic hydrogel in the dark, which can undergo a rapid gel-sol transition caused by light-induced CarHC disassembly. Such photoresponsiveness has been used for facile release/recovery of fibroblasts and MSCs from 3D cultures as well as controlled release of protein molecules. This CarHC hydrogel system represents an example of using AdoB12 photochemistry to control the material properties and thus, points to a versatile strategy for creating photoresponsive materials.
Experimental Procedures
Gene Construction and Protein Expression.
Plasmids, such as pQE80l::SpyTag-ELP-RGD-ELP-SpyTag (AA), pQE80l::SpyTag-ELP-SpyTag-ELP-SpyTag (AAA), and pQE80l::SpyCatcher-ELP-SpyCatcher (BB), were constructed as described previously (7). The carHC gene was purchased as a gBlocks gene fragment from Integrated DNA Technologies. pQE80l::SpyTag-ELP-carHC-ELP-SpyTag (ACA) and pQE80l::SpyCatcher-ELP-carHC-ELP-SpyCatcher (BCB) were constructed by replacing the middle RGD site with carHC in pQE80l::AA and pQE80l::BB, respectively. SacI and SpeI restriction sites were used. pET22b::mCherry-carHC was constructed by inserting the mCherry gene into a pET22b-derived plasmid carrying a carHC gene, and NdeI and SacI restriction sites were used. E. coli strain DH5α was used for plasmid amplification.
E. coli strain BL21 (DE3) was the bacterial host for protein expression. The bacterial cells were grown at 37 °C in LB to the midlog phase (OD at 600 nm, ∼0.6–0.8) followed by adding 1 mM isopropyl β-d-1-thiogalactopyranoside (Sangon Biotech) to induce protein expression at 37 °C. After 4 h, cells were harvested and flash-frozen in liquid N2 before protein purification. The proteins were purified on HisTrap columns (GE Healthcare, Inc.) following the column manufacturer’s recommendations. The purified proteins were dialyzed against Milli-Q water (5 L × 4) at 4 °C and lyophilized at −100 °C. Lyophilized proteins were stored at −80 °C before use.
Hydrogel Preparation.
The ACA and BCB proteins were dissolved in PBS to yield 10 wt % solutions. AdoB12 (Shaanxi Pioneer Biotech) was dissolved in PBS to a final concentration of 9.2 mM. ACA and BCB were mixed at an equimolar ratio followed by addition of a stoichiometric amount of AdoB12 in the dark at room temperature.
Dynamic Shear Rheology.
Dynamic time-, strain-, and frequency-sweep experiments were performed on a TA Instruments ARES-RFS strain-controlled rheometer with a standard steel parallel-plate geometry (8-mm diameter). Gelation of the mixture of 31 µL BCB (10 wt % in PBS), 19 µL ACA (10 wt % in PBS), and 10 µL AdoB12 (9.2 mM in PBS) was monitored by time-sweep measurement, with strain and frequency fixed at 5% and 1 rad/s, respectively, at 25 °C for 7 h. Strain sweep was performed from 0.1 to 10% at a fixed frequency of 1 rad/s at 25 °C. Frequency sweep was performed from 100 to 0.01 rad/s by holding the strain at 5% at the temperature indicated (16, 25, or 37 °C). All of the samples were wrapped with aluminum foil to avoid light during the rheological measurements. Photolysis was conducted by exposing the CarHC hydrogel to white LED light (30 or 90 klux), and dynamic time sweep was used to monitor the gel-sol transition.
Protein Immobilization and Light-Induced Release.
The AAA and BCB proteins and the AdoB12 cofactor were dissolved in the PBS solution containing mCherry-CarHC or mCherry (50 µM) to yield 10 wt % protein solutions for AAA and BCB and a 9.2 mM solution for AdoB12, respectively. Hydrogels were prepared by mixing the three solutions 36 µL BCB, 7.5 µL AAA, and 7 µL AdoB12 and cured in the dark for 24 h. To examine the effect of light on the protein release, the gels were either exposed to white LED light (90 klux) for 10 min or constantly kept in the dark as a control. Both groups of samples were immersed with 500 µL PBS and transferred to the dark. To determine the amounts of protein released into the supernatant, aliquots (100 µL) were taken for fluorescence measurements (excitation at 587 nm and emission at 610 nm) with a Varioskan LUX multimode microplate reader (Thermo Scientific) at different time points, and 100 µL fresh PBS was added back to keep the supernatant volume constant.
Encapsulation and Light-Induced Release of 3T3 Fibroblasts and hMSCs.
NIH/3T3 mouse embryonic fibroblasts were cultured in high-glucose DMEM (Sangon Biotech) supplemented with 10% (vol/vol) FBS (Sangon Biotech), 1% (vol/vol) penicillin-streptomycin (Sangon Biotech), and 1% (vol/vol) nonessential amino acids (Sangon Biotech) in a 5% CO2 atmosphere at 37 °C and passaged every 3 d. At 70–80% confluence, cells were detached with 1 mL trypsin solution (Sangon Biotech) followed by addition of 2 mL DMEM to neutralize the trypsin. Around 60,000 cells were pelleted and resuspended with 31 µL BCB in DMEM (10 wt %) and then placed on a cell culture dish with a coverslip bottom. Gelation was initiated by adding 19 µL ACA in PBS (10 wt %) and 10 µL AdoB12 in PBS (9.2 mM). The gel was cured in the dark for 2 h before being covered with the culture medium. After 24 h of incubation and washing with Dulbecco's PBS (Sangon Biotech), live/dead cell viability assays (Thermo Fisher Scientific) were conducted. Fluorescence images were obtained on a Laser Scanning Confocal Microscope [LSM7 DUO (710 + LIVE); Zeiss].
hMSCs were provided as a gift from Chao Wan, Chinese University of Hong Kong, Hong Kong. The cells were cultured at 37 °C with 5% CO2 in MEM Alpha (Gibco) supplemented with 10% (vol/vol) FBS (Gibco), 2 mM l-glutamine, and 1% (vol/vol) penicillin-streptomycin. Cells were passaged every 5–6 d using 0.25% trypsin (Sangon Biotech) solution. The encapsulation procedure was similar to that of 3T3 fibroblasts as described above.
For cell release/recovery experiments, cell-laden gels were rinsed and immersed by PBS before exposure to white light (halogen lamp in the microscope, ∼22 klux). Cell release was monitored and recorded with an optical microscope equipped with a camera (Olympus CKX41). The live/dead staining assay was performed to examine the viability of recovered cells. Quantification of cell viability was done by counting live and dead cells in the micrographs (n ≥ 4) that were randomly chosen.
Supplementary Material
Acknowledgments
Funding support from Research Grants Council of Hong Kong Special Administrative Region Government Collaborative Research Fund Grant 1013-15G (to I.-M.H.), Early Career Scheme Grant 26103915 (to F.S.), and Grant AoE/M-09/12 (to F.S.); the Germany/Hong Kong Joint Research Scheme sponsored by the Research Grants Council of Hong Kong; and German Academic Exchange Service of Germany Grant G-HKUST603/16 (to F.S.) is acknowledged. J.L. is a recipient of the Asian Future Leaders Scholarship with the support from the Bai Xian Education Foundation. F.S. thanks the Department of Chemical and Biomolecular Engineering, The Hong Kong University of Science and Technology for the faculty start-up fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1621350114/-/DCSupplemental.
References
- 1.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 2.DeForest CA, Anseth KS. Advances in bioactive hydrogels to probe and direct cell fate. Annu Rev Chem Biomol Eng. 2012;3:421–444. doi: 10.1146/annurev-chembioeng-062011-080945. [DOI] [PubMed] [Google Scholar]
- 3.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 4.Burdick JA, Murphy WL. Moving from static to dynamic complexity in hydrogel design. Nat Commun. 2012;3:1269. doi: 10.1038/ncomms2271. [DOI] [PubMed] [Google Scholar]
- 5.DeForest CA, Anseth KS. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat Chem. 2011;3:925–931. doi: 10.1038/nchem.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomatsu I, Peng K, Kros A. Photoresponsive hydrogels for biomedical applications. Adv Drug Deliv Rev. 2011;63:1257–1266. doi: 10.1016/j.addr.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Katz JS, Burdick JA. Light-responsive biomaterials: Development and applications. Macromol Biosci. 2010;10:339–348. doi: 10.1002/mabi.200900297. [DOI] [PubMed] [Google Scholar]
- 8.Yagai S, Kitamura A. Recent advances in photoresponsive supramolecular self-assemblies. Chem Soc Rev. 2008;37:1520–1529. doi: 10.1039/b703092b. [DOI] [PubMed] [Google Scholar]
- 9.Deisseroth K. Optogenetics. Nat Methods. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ercole F, Davis TP, Evans RA. Photo-responsive systems and biomaterials: Photochromic polymers, light-triggered self-assembly, surface modification, fluorescence modulation and beyond. Polym Chem. 2010;1:37–54. [Google Scholar]
- 11.Fairbanks BD, Scott TF, Kloxin CJ, Anseth KS, Bowman CN. Thiol-Yne photopolymerizations: Novel mechanism, kinetics, and step-growth formation of highly cross-linked networks. Macromolecules. 2009;42:211–217. doi: 10.1021/ma801903w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeForest CA, Polizzotti BD, Anseth KS. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat Mater. 2009;8:659–664. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeForest CA, Tirrell DA. A photoreversible protein-patterning approach for guiding stem cell fate in three-dimensional gels. Nat Mater. 2015;14:523–531. doi: 10.1038/nmat4219. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez-Lorenzo C, Bromberg L, Concheiro A. Light-sensitive intelligent drug delivery systems. Photochem Photobiol. 2009;85:848–860. doi: 10.1111/j.1751-1097.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 15.Fairbanks BD, et al. A versatile synthetic extracellular matrix mimic via thiol-norbornene photopolymerization. Adv Mater. 2009;21:5005–5010. doi: 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun F, Zhang WB, Mahdavi A, Arnold FH, Tirrell DA. Synthesis of bioactive protein hydrogels by genetically encoded SpyTag-SpyCatcher chemistry. Proc Natl Acad Sci USA. 2014;111:11269–11274. doi: 10.1073/pnas.1401291111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banta S, Wheeldon IR, Blenner M. Protein engineering in the development of functional hydrogels. Annu Rev Biomed Eng. 2010;12:167–186. doi: 10.1146/annurev-bioeng-070909-105334. [DOI] [PubMed] [Google Scholar]
- 18.Gao X, Fang J, Xue B, Fu L, Li H. Engineering protein hydrogels using SpyCatcher-SpyTag chemistry. Biomacromolecules. 2016;17:2812–2819. doi: 10.1021/acs.biomac.6b00566. [DOI] [PubMed] [Google Scholar]
- 19.Fang J, et al. Forced protein unfolding leads to highly elastic and tough protein hydrogels. Nat Commun. 2013;4:2974. doi: 10.1038/ncomms3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy WL, Dillmore WS, Modica J, Mrksich M. Dynamic hydrogels: Translating a protein conformational change into macroscopic motion. Angew Chem Int Ed Engl. 2007;46:3066–3069. doi: 10.1002/anie.200604808. [DOI] [PubMed] [Google Scholar]
- 21.Sui ZJ, King WJ, Murphy WL. Protein-based hydrogels with tunable dynamic responses. Adv Funct Mater. 2008;18:1824–1831. [Google Scholar]
- 22.Kutta RJ, et al. The photochemical mechanism of a B12-dependent photoreceptor protein. Nat Commun. 2015;6:7907. doi: 10.1038/ncomms8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jost M, et al. Structural basis for gene regulation by a B12-dependent photoreceptor. Nature. 2015;526:536–541. doi: 10.1038/nature14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortiz-Guerrero JM, Polanco MC, Murillo FJ, Padmanabhan S, Elías-Arnanz M. Light-dependent gene regulation by a coenzyme B12-based photoreceptor. Proc Natl Acad Sci USA. 2011;108:7565–7570. doi: 10.1073/pnas.1018972108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jost M, Simpson JH, Drennan CL. The transcription factor CarH safeguards use of adenosylcobalamin as a light sensor by altering the photolysis products. Biochemistry. 2015;54:3231–3234. doi: 10.1021/acs.biochem.5b00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zakeri B, et al. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc Natl Acad Sci USA. 2012;109:E690–E697. doi: 10.1073/pnas.1115485109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoene C, Fierer JO, Bennett SP, Howarth M. SpyTag/SpyCatcher cyclization confers resilience to boiling on a mesophilic enzyme. Angew Chem Int Ed Engl. 2014;53:6101–6104. doi: 10.1002/anie.201402519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veggiani G, et al. Programmable polyproteams built using twin peptide superglues. Proc Natl Acad Sci USA. 2016;113:1202–1207. doi: 10.1073/pnas.1519214113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddington SC, Howarth M. Secrets of a covalent interaction for biomaterials and biotechnology: SpyTag and SpyCatcher. Curr Opin Chem Biol. 2015;29:94–99. doi: 10.1016/j.cbpa.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Chen AY, et al. Synthesis and patterning of tunable multiscale materials with engineered cells. Nat Mater. 2014;13:515–523. doi: 10.1038/nmat3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Botyanszki Z, Tay PKR, Nguyen PQ, Nussbaumer MG, Joshi NS. Engineered catalytic biofilms: Site-specific enzyme immobilization onto E. coli curli nanofibers. Biotechnol Bioeng. 2015;112:2016–2024. doi: 10.1002/bit.25638. [DOI] [PubMed] [Google Scholar]
- 32.Zhang WB, Sun F, Tirrell DA, Arnold FH. Controlling macromolecular topology with genetically encoded SpyTag-SpyCatcher chemistry. J Am Chem Soc. 2013;135:13988–13997. doi: 10.1021/ja4076452. [DOI] [PubMed] [Google Scholar]
- 33.Matsunaga R, Yanaka S, Nagatoishi S, Tsumoto K. Hyperthin nanochains composed of self-polymerizing protein shackles. Nat Commun. 2013;4:2211. doi: 10.1038/ncomms3211. [DOI] [PubMed] [Google Scholar]
- 34.Fairhead M, et al. SpyAvidin hubs enable precise and ultrastable orthogonal nanoassembly. J Am Chem Soc. 2014;136:12355–12363. doi: 10.1021/ja505584f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu ZD, et al. A novel method for synthetic vaccine construction based on protein assembly. Sci Rep UK. 2014;4:7266. doi: 10.1038/srep07266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bedbrook CN, et al. Genetically encoded spy peptide fusion system to detect plasma membrane-localized proteins in vivo. Chem Biol. 2015;22:1108–1121. doi: 10.1016/j.chembiol.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urry DW. Physical chemistry of biological free energy transduction as demonstrated by elastic protein-based polymers. J Phys Chem B. 1997;101:11007–11028. [Google Scholar]
- 38.Santoro M, Tatara AM, Mikos AG. Gelatin carriers for drug and cell delivery in tissue engineering. J Control Release. 2014;190:210–218. doi: 10.1016/j.jconrel.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vo TN, Kasper FK, Mikos AG. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev. 2012;64:1292–1309. doi: 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sension RJ, Harris DA, Cole AG. Time-resolved spectroscopic studies of B12 coenzymes: Comparison of the influence of solvent on the primary photolysis mechanism and geminate recombination of methyl-, ethyl-, n-propyl-, and 5′-deoxyadenosylcobalamin. J Phys Chem B. 2005;109:21954–21962. doi: 10.1021/jp053202w. [DOI] [PubMed] [Google Scholar]
- 41.Seetulsingh-Goorah SP. Mechanisms of adenosine-induced cytotoxicity and their clinical and physiological implications. Biofactors. 2006;27:213–230. doi: 10.1002/biof.5520270119. [DOI] [PubMed] [Google Scholar]
- 42.Tessmar JK, Göpferich AM. Matrices and scaffolds for protein delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:274–291. doi: 10.1016/j.addr.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 43.Shah NJ, et al. Adaptive growth factor delivery from a polyelectrolyte coating promotes synergistic bone tissue repair and reconstruction. Proc Natl Acad Sci USA. 2014;111:12847–12852. doi: 10.1073/pnas.1408035111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu J, Wang G, Liu X, Gao W. Enhancing pharmacokinetics, tumor accumulation, and antitumor efficacy by elastin-like polypeptide fusion of interferon alpha. Adv Mater. 2015;27:7320–7324. doi: 10.1002/adma.201503440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.