Significance
Familial esophageal squamous cell carcinoma (ESCC) often shows early onset and worse prognosis. Little is known about the genetic basis underlying the pathogenesis of familial ESCC. To identify the genetic alterations associated with familial ESCC susceptibility, we compared the gene-expression profiles of familial and sporadic ESCCs. We found that A-to-I RNA editing–mediated downregulation of SLC22A3 is almost exclusively present in familial ESCC and may serve as a potential biomarker for familial ESCC patients. Molecular mechanism study further revealed that a single mutation at the RNA level could change the protein structure of SLC22A3, leading to a loss of inhibitory capability for the metastasis-promoting protein ACTN4. Our findings provide insights that may lead to more effective clinical management of individuals at high risk of familial ESCC with SLC22A3 deregulation.
Keywords: RNA editing, metastasis suppressor, familial ESCC, SLC22A3, ADAR2
Abstract
Like many complex human diseases, esophageal squamous cell carcinoma (ESCC) is known to cluster in families. Familial ESCC cases often show early onset and worse prognosis than the sporadic cases. However, the molecular genetic basis underlying the development of familial ESCC is mostly unknown. We reported that SLC22A3 is significantly down-regulated in nontumor esophageal tissues from patients with familial ESCC compared with tissues from patients with sporadic ESCCs. A-to-I RNA editing of the SLC22A3 gene results in its reduced expression in the nontumor esophageal tissues of familial ESCCs and is significantly correlated with lymph node metastasis. The RNA-editing enzyme ADAR2, a familial ESCC susceptibility gene identified by our post hoc genome-wide association study, is positively correlated with the editing level of SLC22A3. Moreover, functional studies showed that SLC22A3 is a metastasis suppressor in ESCC, and deregulation of SLC22A3 facilitates cell invasion and filopodia formation by reducing its direct association with α-actinin-4 (ACTN4), leading to the increased actin-binding activity of ACTN4 in normal esophageal cells. Collectively, we now show that A-to-I RNA editing of SLC22A3 contributes to the early development and progression of familial esophageal cancer in high-risk individuals.
Esophageal carcinoma (EC) is ranked as the sixth leading cause of death from cancer worldwide (1). Esophageal squamous cell carcinoma (ESCC) is the major histological subtype of EC and is characterized by its remarkable geographic distribution internationally and within China (2–6). ESCC occurs with the highest frequency among the Chinese, especially the high-risk Northern Chinese, where the incidence rate is 121 per 100,000 people, more than 20 times higher than in low-risk regions worldwide (7). Within these high-risk regions, studies have shown a strong tendency toward familial aggregation, suggesting that host genetic susceptibility, in conjunction with potential environmental exposures, may be involved in the etiology of this cancer (8, 9).
The conventional approach for the identification of familial susceptibility gene is to identify its chromosomal localization by linkage analysis and subsequently to isolate the target susceptibility gene by positional cloning. A successful linkage analysis depends on the collection of satisfactory familial samples. However, it is almost impossible to collect satisfactory familial samples, because most of ESCC-affected members in the family had died when a proband was found. Therefore, alternative strategies should be used to identify the susceptibility gene in familial ESCC. Several studies have concentrated on elucidating the high frequency of familial ESCC in Shanxi, China (10–14). These earlier studies using genome-wide allelotyping of familial ESCC cases showed that 13q and 17p loss was significantly associated with ESCC development (10–12). Gene chip and SNP arrays were also used for studying the molecular mechanisms underlying familial ESCC (13, 14). Moreover, previous clinical study suggested that familial esophageal cancer may develop earlier and have a poorer prognosis than sporadic esophageal cancer (15). However, the molecular genetic basis underlying the early development and progression of familial ESCC is mostly unknown.
To identify potential genes that contribute to the development and progression of familial ESCC, we compared gene-expression profiles of tumor and adjacent nontumor (NT) tissues from five familial ESCC specimens using the Affymetrix human genome U133 Plus 2.0 GeneChip. The present study identified a number of differentially expressed genes that were related to cell development, cell motility, and immunity in familial ESCC, compared with both NT and previously reported sporadic ESCC (16). One gene, SLC22A3 (or organic cation transporter 3, OCT3), caught our attention because it was previously reported to be one of the important risk loci for prostate (17) and colon cancer (18, 19). The SLC22A3 gene encodes a cation transport protein belonging to the SLC22A family (SLC22A1-3 or OCT1-3), which is critical for drug transportation and cellular detoxification (20, 21). Moreover, it has been reported that aberrant methylation contributes to the reduced expression of SLC22A3 in prostate cancer (22), indicating a tumor-suppressive role for SLC22A3 in human cancers.
In the present work, we addressed the role of SLC22A3 in the molecular pathogenesis of familial ESCC. We reported that SLC22A3 is significantly down-regulated in NT esophageal tissues from familial ESCC patients compared with those from sporadic ESCCs. A-to-I RNA editing of the SLC22A3 gene is found to be significantly associated with the reduced SLC22A3 transcription and lymph node metastasis in the familial ESCCs. The editing level of SLC22A3 is regulated by the RNA-editing enzyme ADAR2. We also used molecular genetic studies to characterize the SLC22A3 gene and investigated the mechanism of alteration.
Results
Identification of Differentially Expressed Genes in Familial ESCC.
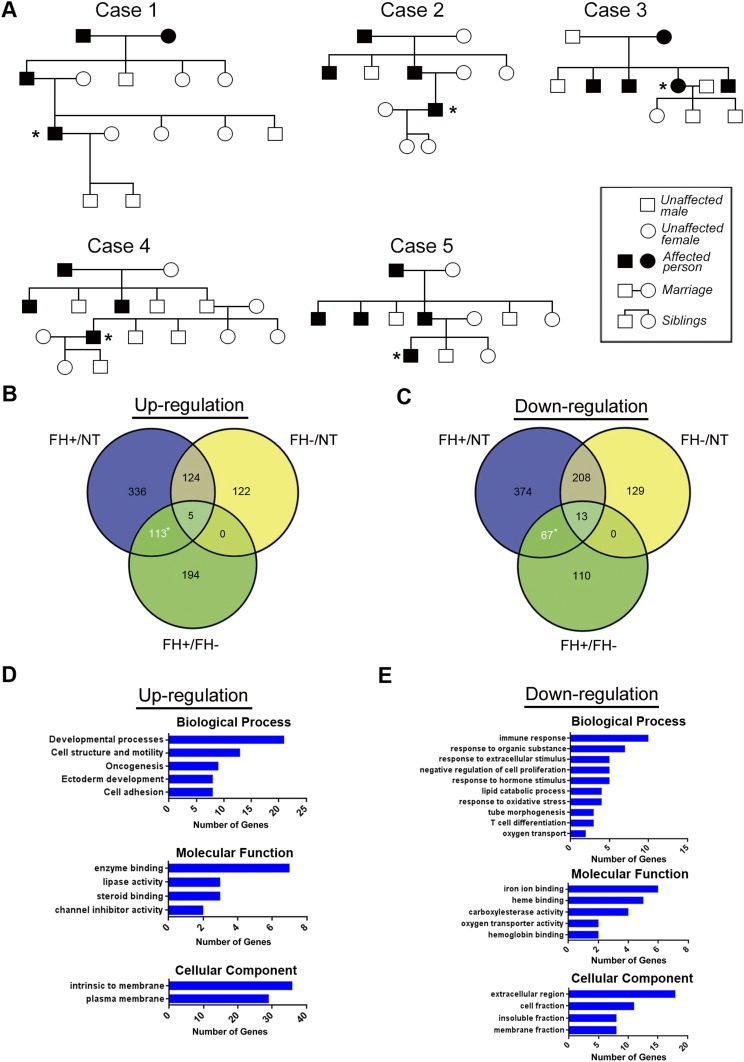
Gene-expression profiles of five familial ESCCs and their normal counterparts were obtained by microarray analysis. The five familial ESCC cases were from an ESCC high-risk area (Henan province), and were selected when the family had at least four ESCC cases within three generations or at least five patients in two generations (Fig. S1A). To identify further the genes differentially expressed in familial and sporadic ESCC, we compared the expression profiles of familial tumors with both NT tissue and previously reported sporadic tumors (16). Using an arbitrary cutoff of signal log ratio ≥2.0, we found that 180 genes with known function were significantly deregulated in familial tumor (113 up-regulated and 67 down-regulated) compared with both NT and sporadic tumors (Fig. S1 B and C and Tables S1 and S2). To study associated biological annotation, Database for Annotation, Visualization and Integrated Discovery (DAVID) gene ontology (GO) analysis was performed on the 180 differentially expressed genes (23). A selection of significant GO terms is shown for biological processes, molecular functions, and cellular component of 113 up-regulated (Fig. S1D) and 67 down-regulated genes (Fig. S1E).
Fig. S1.
(Related to Introduction and Results) cDNA microarray gene-expression profiling of esophageal cancer. (A) Family pedigrees of five familial ESCC cases (marked with an asterisk) selected for the cDNA microarray assay. (B and C) Venn diagram of differentially regulated genes based on gene fold changes ≥2.0 for three sample sets: FH+/NT, FH−/NT, and FH+/FH−. FH+ or FH−, ESCC tumors with (FH+) or without (FH−) a family history; NT, matched adjacent nontumor tissues from FH+ or FH− tumors. A total of 113 genes were up-regulated (B), and 67 genes were down-regulated (C) in FH+ tumors compared with both NT tissue and FH− tumors. (D and E) A selection of the top enriched GO biological processes, GO molecular functions, and cellular components for the 113 up-regulated genes (D) and 67 down-regulated genes (E).
Frequent Down-Regulation of SLC22A3 in Familial ESCC.
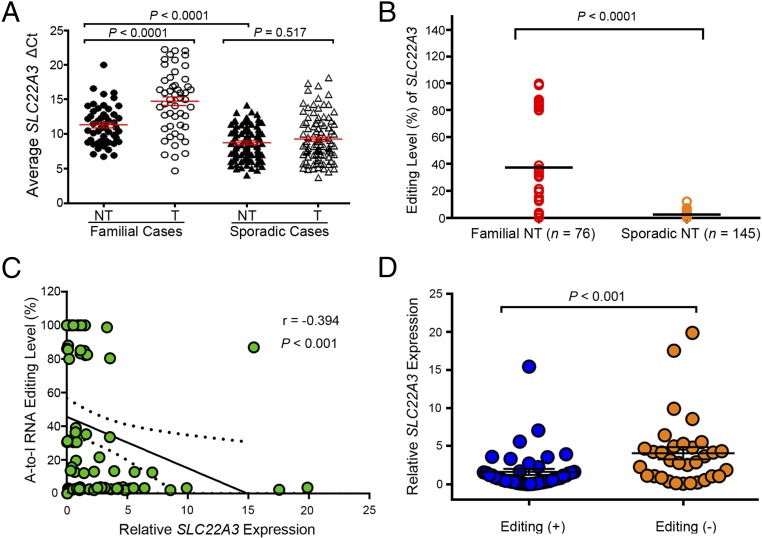
As one of many potentially relevant genes on the down-regulated gene list, SLC22A3 drew our attention because it appears in two of the top three enriched GO biological processes: response to organic substance and response to extracellular stimulus (Fig. S1E). Several lines of evidence demonstrated in the Introduction also suggested that SLC22A3 might be an epigenetically regulated cancer-related gene. We therefore selected this gene for further study in familial ESCC. Initially, quantitative PCR (qPCR) was performed to validate SLC22A3 expression levels in familial (n = 50) and sporadic (n = 100) ESCC cohorts. As shown in Fig. 1A, the mean level of SLC22A3 expression was reduced significantly in familial tumors compared with the corresponding NT tissues [change in cycle threshold (ΔCt) value 14.65 ± 0.64 vs. 11.26 ± 0.39, respectively; P < 0.0001) (higher ΔCt values correspond to lower expression). However, no significant difference in the mean level of SLC22A3 expression was observed between ESCC tumors and their counterparts in the sporadic cohorts (9.17 ± 0.32 vs. 8.67 ± 0.23, respectively; P = 0.517). Notably, of the two ESCC cohorts, the mean expression level of SLC22A3 was significantly lower in familial than in sporadic NT tissues (P < 0.0001) (Fig. 1A). These observations not only confirmed our previous array data but also suggest the involvement of SLC22A3 in the early development of ESCC in individuals at high risk of familial ESCC.
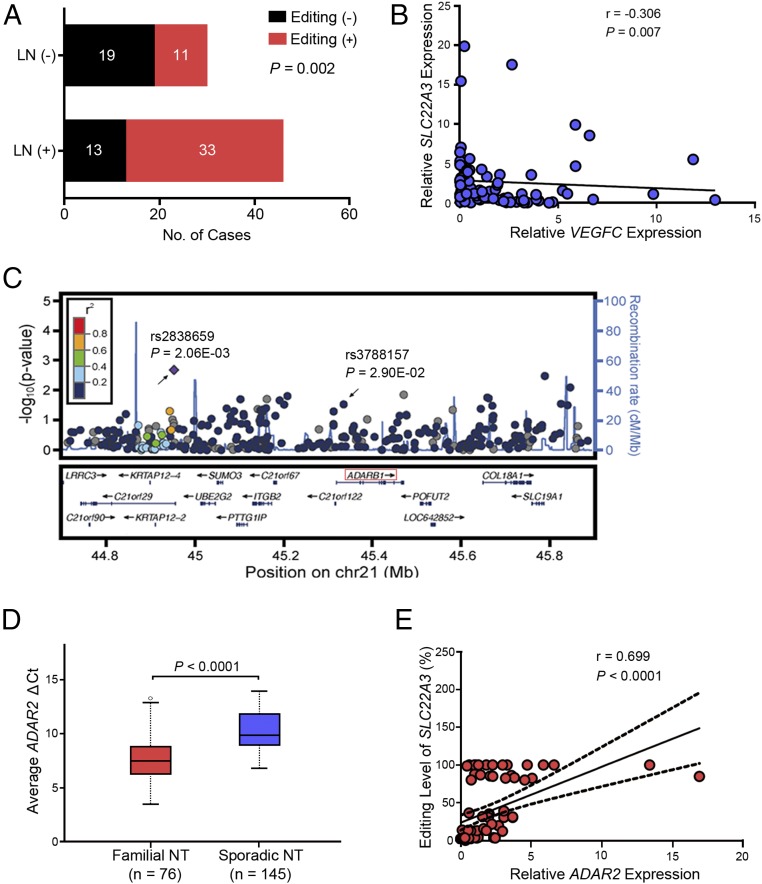
Fig. 1.
A-to-I RNA editing contributes to the down-regulation of SLC22A3 in familial ESCC. (A) qPCR was used to detect SLC22A3 transcripts in the familial (n = 50) and sporadic (n = 100) cohorts. Each cohort contains primary ESCC tumors (T) and matched adjacent NT tissues. Dot plots represent the ΔCt values of SLC22A3 (higher ΔCt values correspond to lower expression; mean ± SEM; Mann–Whitney U test. (B) Dot plots represent the SLC22A3 editing levels, determined by pyrosequencing, detected in the NT cDNA samples from patients with familial (n = 76) and sporadic (n = 145) ESCC. Horizontal black lines indicate the mean value. P < 0.0001 by the Mann–Whitney U test. (C) Negative correlation between the relative expression level of SLC22A3 and A-to-I RNA editing level in NT specimens from 76 patients with familial ESCC, expressed using Spearman’s correlation coefficients. The linear regression is shown as a solid line, and95% CIs are shown by dotted lines. (D) Dot plots represent the relative expression of SLC22A3 in familial NT cDNA samples stratified by editing status, with (Editing +) and without (Editing −) A-to-I RNA editing. Data are presented as the mean ± SEM; P < 0.001 by the Mann–Whitney U test.
Frequent A-to-I SLC22A3 RNA Editing in Familial ESCC.
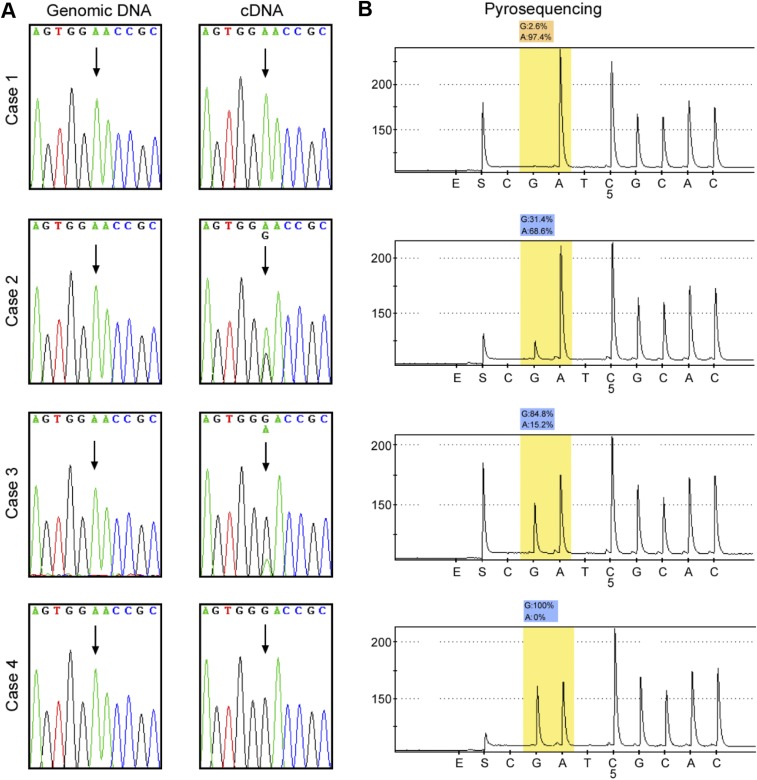
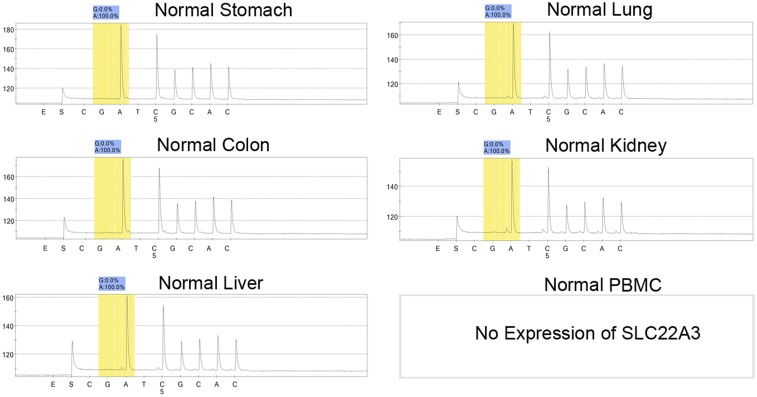
To look for a possible gene-regulation mechanism of SLC22A3, we initially determined its coding region in 20 pairs of cDNA samples from familial ESCC tumors and their adjacent NT tissues by Sanger sequencing. Although we found no evidence of mutations in the SLC22A3 coding region, an A-to-I transcript-editing event (A261 at exon 1), leading to an asparagine (Asn)-to-aspartic acid (Asp) amino acid substitution, was identified in the cDNA but not in the matched genomic DNA samples (Fig. S2A). To quantify the editing level of SLC22A3, pyrosequencing was applied, and the results were consistent with the Sanger sequencing data from the 20 familial ESCCs (Fig. S2B). We thus extended the pyrosequencing study to examine the SLC22A3 editing level in normal esophageal epithelia from 76 familial and 145 sporadic ESCCs. SLC22A3-positive editing (defined as the percentage of G signal ≥5%) was detected in 44/76 (57.9%) familial and 3/145 (2.1%) sporadic NT samples. The mean editing level of SLC22A3 was significantly higher in familial than in sporadic NT samples (37.6 vs. 2.3, respectively; P < 0.0001) (Fig. 1B). SLC22A3 editing was not detected in peripheral blood mononuclear cells and other normal human tissues (Fig. S3). These findings indicated that A-to-I SLC22A3 RNA editing is prominently associated with familial ESCC and predisposes these high-risk individuals to develop esophageal cancer.
Fig. S2.
(Related to Fig. 1) Identification of A-to-I SLC22A3 RNA editing in ESCC. (A) Representative Sanger sequencing showing matched SLC22A3 genomic DNA and cDNA sequences in the NT esophageal epithelia from patients with ESCC. The chromatograms demonstrate that the A-to-I editing is characterized by a varying degree of guanosine (G) signal in the cDNA sequence, whereas the DNA sequence exhibits only adenosine (A) signals. The arrows indicate the editing site. (B) Quantitative pyrosequencing analyses of A-to-I editing frequencies for the matched cDNA samples described in A.
Fig. S3.
(Related to Fig. 1) SLC22A3 editing levels in different normal human tissues. The percentage of wild-type (A) and edited (G) transcripts of SLC22A3 was detected by pyrosequencing.
SLC22A3 RNA Editing Contributes to Its Down-Regulation in Familial ESCC.
To determine whether down-regulation of SLC22A3 is regulated by the RNA editing, the correlation between SLC22A3 editing and transcription level was analyzed. SLC22A3 RNA editing showed a significantly negative correlation with its mRNA level in the 76 familial NT tissues (Spearman’s r = −0.394, P < 0.001) (Fig. 1C). Moreover, we compared the mean SLC22A3 expression levels in familial NT samples with and without editing. The results showed that SLC22A3 transcript was significantly reduced in the positive-editing group compared with the no editing-group, indicating that form of A-to-I SLC22A3 RNA may have decreased stability in familial cases (P < 0.001) (Fig. 1D).
Deregulated SLC22A3 Is an Early Risk Factor for Progression of Familial ESCC.
Furthermore, we investigated the clinical associations with SLC22A3 editing status obtained from NT samples in this familial ESCC cohort. As shown in Fig. 2A, edited SLC22A3 was significantly correlated with lymph node metastasis (P = 0.002) but not with other clinicopathological factors (e.g., age, sex, T stage, differentiation). Because vascular endothelial growth factor C (VEGFC) is a factor known to cause lymphangiogenesis (24), we examined the mRNA level of VEGFC in the same familial cohort. The results showed a reverse correlation between SLC22A3 and VEGFC expression in familial NT tissues (Spearman’s r = −0.306, P = 0.007) (Fig. 2B), indicating that deregulated SLC22A3 is an early event leading to the malignant development and progression of familial ESCCs.
Fig. 2.
ADAR2-mediated RNA editing of SLC22A3 in familial ESCC. (A) The bar chart represents the correlation between the editing status of SLC22A3 and lymph node (LN) metastasis in 76 cases of familial ESCC (P = 0.002, χ2 test). (B) Negative correlation between the relative expression level of SLC22A3 and the lymphangiogenesis inducer VEGFC in NT specimens from 76 cases of familial ESCC, expressed using Spearman’s correlation coefficients. The solid line indicates the linear regression. (C) Regional association plots for the ADAR2 gene at 21q22.3. Shown are the P values (−log10 scale) of the association tests for genotyped (diamond) and imputed (circles) SNPs in the discovery sample. Genetic recombination rates are represented by light-blue lines, and genes within the regions are indicated by arrows. (D) Tukey boxplots represent the ΔCt values of ADAR2 detected by qPCR in the NT specimens from familial (n = 76) and sporadic (n = 145) ESCCs. The horizontal black line indicates the median value; open circles indicate outliers; P < 0.0001 by the Mann–Whitney U test. (E) Positive correlation between the relative expression of ADAR2 and SLC22A3 editing levels in NT specimens from 76 patients with familial ESCC, expressed using Spearman’s correlation coefficients The solid line shows the linear regression; dotted lines indicate the 95% CI.
RNA-Editing Enzyme ADAR2 Contributes to SLC22A3 Editing in Familial ESCC.
A-to-I RNA editing is known to be catalyzed by members of the RNA-editing enzyme ADAR (adenosine deaminase acting on RNA) family (25). Interestingly, we found that one of the human ADAR members, ADAR2 (also known as “ADARB1”), is located at 21q22.3, a newly identified ESCC susceptibility locus by genome-wide association study (GWAS) (26). We thus performed a post hoc GWAS analysis to investigate the influence of common genetic variants around the ADAR2 gene region on ESCC risk. We selected 291 genetic variants around the ADAR2 gene region from the dataset of a previously reported GWAS in 496 ESCC cases with a family history of upper gastrointestinal tract cancers and 1,056 healthy controls (27). The results showed that one SNP (rs3788157) within intron 1 of ADAR2 is significantly associated with ESCC risk in the familial cases, suggesting that ADAR2 could be a susceptibility gene for familial ESCC (P = 2.90E-02, odds ratio = 1.247, 95% CI 1.239–1.255) (Fig. 2C). To investigate whether ADAR2 could regulate SLC22A3 editing, we first examined the ADAR2 expression levels in the NT tissues from familial (n = 76) and sporadic (n = 145) ESCC cohorts. As shown in Fig. 2D, the average ΔCt value was significantly lower in the familial NT samples than in the sporadic samples (7.59 vs. 10.05, P < 0.0001), suggesting that the ADAR2 transcripts were specifically increased in the familial NT samples. Moreover, the correlation analyses demonstrated that the ADAR2 expression level was positively correlated with the SLC22A3 editing level (Spearman’s r = 0.699, P < 0.0001) (Fig. 2E), suggesting that ADAR2 is responsible for the regulation of SLC22A3 editing in familial ESCC.
SLC22A3 Is a Metastasis Suppressor in ESCC.
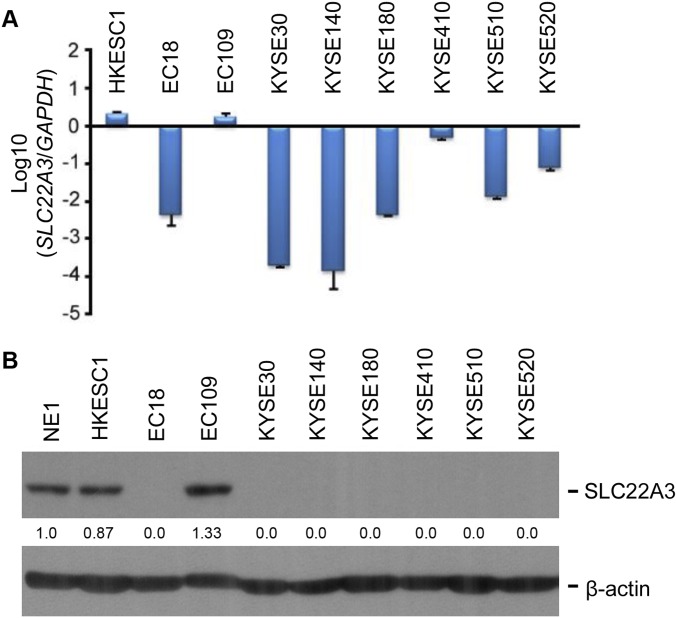
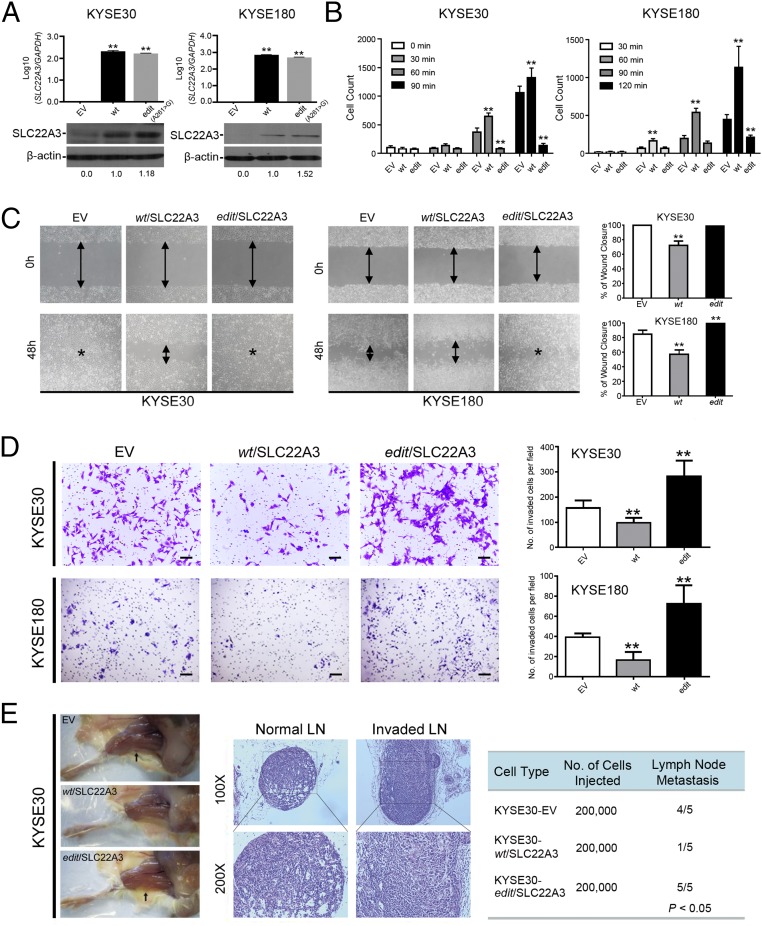
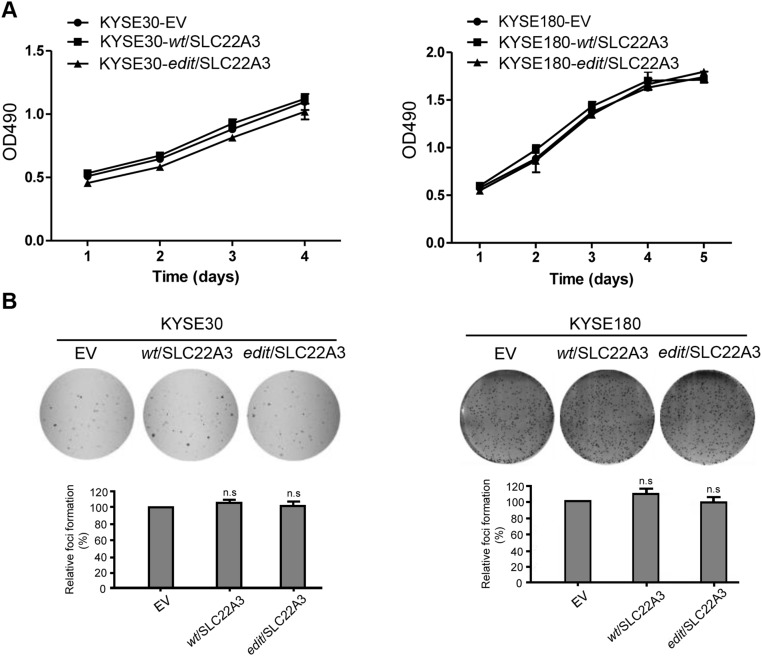
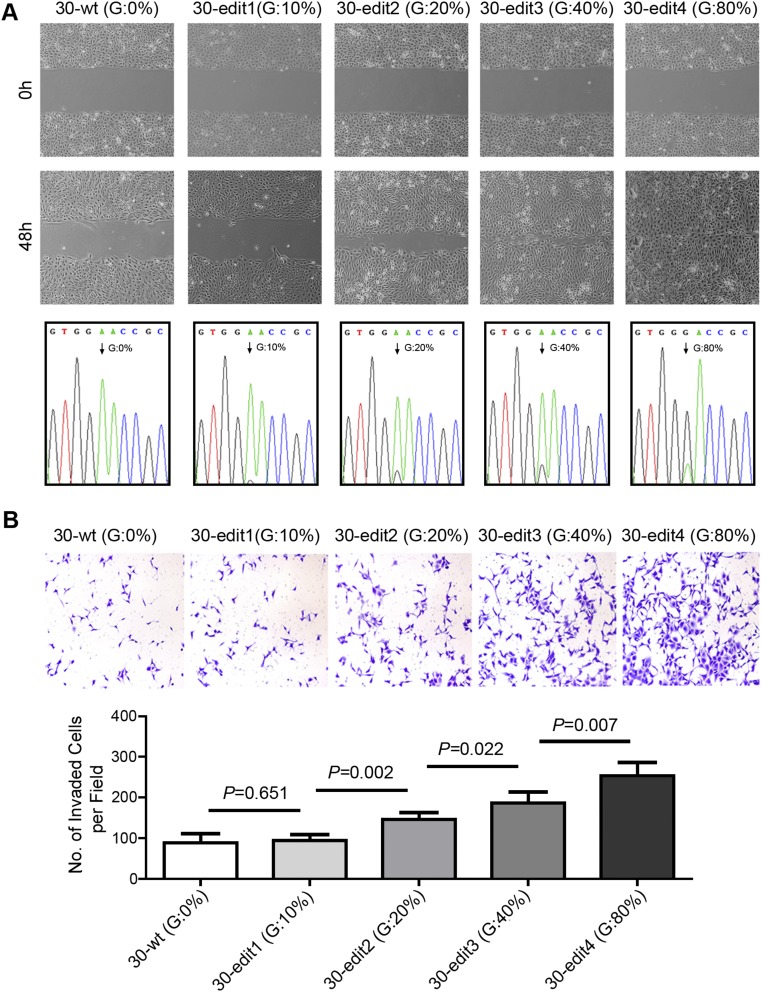
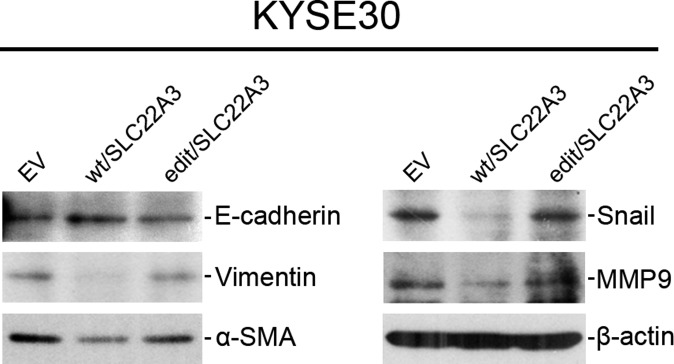
To explore the functional consequences of depleted or edited SLC22A3 in ESCC, we initially examined SLC22A3 expression in 10 esophageal cell lines. Both qPCR and immunoblotting showed that SLC22A3 expression was significantly down-regulated in seven of nine (77.8%) ESCC cell lines compared with the immortalized normal esophageal epithelial cell line NE1 (Fig. S4). We then established KYSE30 and KYSE180 cells lacking endogenous SLC22A3 expression with stable overexpression of wild-type/edited (A261 > G) SLC22A3 (KYSE30/KYSE180-wt/SLC22A3 or -edit/SLC22A3) by lentiviral transduction. Empty-vector–transfected (KYSE30-EV) cells were used as controls. The transduction efficiency was confirmed using qPCR and Western blotting (Fig. 3A). Ectopic SLC22A3 expression in KYSE30/KYSE180 cells (KYSE30/KYSE180-wt/SLC22A3 or -edit/SLC22A3) had no effect on cell growth (Fig. S5A) or on the efficiency of foci formation (Fig. S5B) compared with control cells. However, cell-adhesion assays demonstrated that wild-type SLC22A3 had increased but edited SLC22A3 had decreased cell-adhesion ability compared with control cells (Fig. 3B). Additionally, both wound-healing and invasion assays showed that wild-type SLC22A3 had inhibitory effects on ESCC cell migration and invasion, whereas edited SLC22A3 displayed loss of inhibitory function compared with control cells (Fig. 3 C and D). To analyze whether the loss-of-function phenotypes are regulated in an edited SLC22A3–dependent manner, we mixed the KYSE30-wt/SLC22A3 cells in five different percentages (0, 10, 20, 40, and 80%, respectively) of KYSE30-edit/SLC22A3 cells (30-wt, 30-edit1, 30-edit2, 30-edit3, and 30-edit4, respectively). Compared with 30-wt cells, cell migration and invasion abilities were progressively increased from 30-edit2 cells harboring 20% edited SLC22A3 transcripts to 30-edit4 cells harboring 80% edited SLC22A3 transcripts, demonstrating that tumorigenic properties correlate with SLC22A3 editing level (Fig. S6 A and B). To determine further whether wild-type SLC22A3 inhibits cell migration/invasion by suppressing the epithelial–mesenchymal transition (EMT), the expressions of epithelial marker (E-cadherin), mesenchymal markers (Vimentin and α-SMA), and EMT regulators (Snail and MMP9) were examined in KYSE30-wt/SLC22A3, KYSE30-edit/SLC22A3, and control cells by Western blotting. The results showed that E-cadherin was significantly up-regulated, whereas Vimentin, α-SMA, Snail, and MMP9 were down-regulated in KYSE30-wt/SLC22A3 cells compared with KYSE30-edit/SLC22A3 and control cells, suggesting that wild-type SLC22A3 could inhibit ESCC cell migration/invasion via a reversal of EMT process (Fig. S7). Furthermore, using a lymph node metastatic mouse model, we found strong metastasis-suppressing ability in wild-type KYSE30 cells overexpressing SLC22A3 but not in the edited clones overexpressing SLC22A3 compared with control cells (P = 0.02) (Fig. 3E). These results demonstrated that SLC22A3 has a strong metastasis-suppressive ability in ESCC.
Fig. S4.
(Related to Fig. 3) Expression of SLC22A3 in esophageal cells. (A) SLC22A3 mRNA levels measured by qPCR in 10 esophageal cell lines, including nine ESCC cell lines and one immortalized normal esophageal epithelial cell line, NE1. The log fold changes of SLC22A3 in the ESCC cell lines were expressed relative to the normal cell line NE1. (B) SLC22A3 protein levels measured by Western blotting in 10 esophageal cell lines. β-Actin was used as a loading control.
Fig. 3.
SLC22A3 has a strong metastasis-suppressive role. (A) Overexpression of wild-type and edited SLC22A3 in KYSE30 (KYSE30-wt/SLC22A3 and KYSE30-edit/SLC22A3) or KYSE180 (KYSE180-wt/SLC22A3 and KYSE180-edit/SLC22A3) clones was confirmed by qPCR (Upper) and Western blotting (Lower). Empty-vector–transfected (KYSE30/KYSE180-EV) cells were used as controls. (B) An adhesion assay was used to detect cell-adhesion ability in wild-type or edited KYSE30 and KYSE180 cells overexpressing SLC22A3 in the indicated time periods. Empty-vector–transfected cells were used as control. The cell numbers were counted from three independent experiments. **P < 0.001, ANOVA with post hoc test. (C) The effect of SLC22A3 on cell migration was determined using the wound-healing assay. Representative images show that wild-type SLC22A3-transfected cells migrated more slowly along the wound edge than the edited SLC22A3-transfected cells and control cells. The percentage of wound closure is shown as mean ± SD of three independent experiments; **P < 0.01, ANOVA with post hoc test. (D) Representative images (Left) and quantification of cells (Right) from the indicated KYSE30 and KYSE180 clones that invaded through Matrigel-coated membrane. Results are shown as the mean ± SD of three independent experiments. (Scale bar: 200 μm.) **P < 0.01, ANOVA with post hoc test. (E, Left) Representative images of popliteal lymph nodes (LNs) in SCID mice following s.c. footpad injection of the indicated KYSE30 clones. Black arrows point to the popliteal LN. (Center) Representative H&E staining of normal LNs and tumor cell-invaded LNs from tested animals (original magnification: 100×). (Right) The numbers of metastatic popliteal LNs in each group of animals were summarized (P < 0.05, χ2 test).
Fig. S5.
(Related to Fig. 3) SLC22A3 has no effect on cell growth and clonogenicity in ESCC cells. (A) Relative growth rates of wild-type and edited clones overexpressing SLC22A3 were compared with control cells by XTT assay. The results are expressed as the mean ± SD of at least three independent experiments. P > 0.05 by ANOVA. (B, Upper) Representative images of the foci formation assay in wild-type or edited clones overexpressing SLC22A3 compared with their controls. (Lower) Summary of the relative percentages of foci formed. Values are the mean ± SD of three independent experiments. n.s., not significant: P > 0.05 by ANOVA.
Fig. S6.
(Related to Fig. 3) Loss of suppressor function is regulated in an edited SLC22A3–dependent manner. (A) The dose effect of different SLC22A3 editing levels on cell migration was determined using the wound-healing assay. Representative images show that cell migration abilities are progressively increased from 30-edit2 cells harboring 20% edited SLC22A3 transcripts to 30-edit4 cells harboring 80% edited SLC22A3 transcripts compared with control cells. 30-wt, 30-edit1, 30-edit2, 30-edit3, and 30-edit4 are KYSE30-wt/SLC22A3 cells mixed with five different percentages (0, 10, 20, 40, and 80%, respectively) of KYSE30-edit/SLC22A3 cells. The percentage of edited SLC22A3 transcripts was determined by direct sequencing from the indicated cells. The arrow indicates the editing position. (B) Quantification of cells from the indicated stable cells that invaded through Matrigel-coated membrane. All data are shown as the mean ± SD of three independent experiments, and statistical was significance determined by unpaired Student’s t test throughout.
Fig. S7.
(Related to Fig. 3) Wild-type SLC22A3 inhibits cell migration and invasion by suppressing EMT process. Expressions of the epithelial marker E-cadherin, the mesenchymal markers Vimentin and α-SMA, and the EMT regulators Snail and MMP9 were compared by Western blotting analysis in KYSE30-wt/SLC22A3, KYSE30-edit/SLC22A3, and control cells. β-Actin was used as loading control.
Depletion of SLC22A3 Promotes Normal Esophageal Cell Motility.
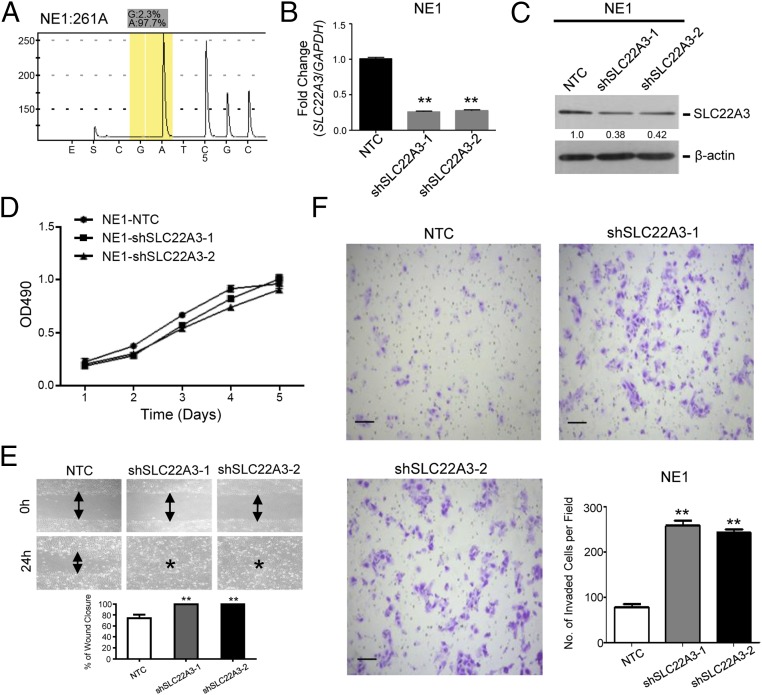
Moreover, we established NE1 cells showing wild-type SLC22A3 with SLC22A3 stable knockdown (NE1-shSLC22A3-1/-2) by lentiviral transduction (Fig. 4 A–C). Nontemplate shRNA-transfected (NE1-NTC) cells were used as controls. ShRNA-mediated depletion of wild-type SLC22A3 in NE1 had no effect on cell growth (Fig. 4D) compared with control cells. However, both wound-healing and invasion assays showed that SLC22A3 knockdown had strong promoting effects on NE1 cell migration and invasion compared with control cells (Fig. 4 E and F), further supporting the notion that deregulated SLC22A3 may contribute to the early development and progression of ESCC.
Fig. 4.
SLC22A3 knockdown increases normal esophageal cell motility. (A) The percentage of edited SLC22A3 transcripts was determined by pyrosequencing in normal esophageal NE1cells. (B and C) Expression of SLC22A3 in shSLC22A3 stably transfected NE1 clones (NE1-shSLC22A3-1 and -2) was confirmed by qPCR (B) and Western blotting (C). Nontemplate shRNA-transfected cells (NE1-NTC) were used as controls. **P < 0.001, ANOVA with post hoc test. (D) Relative growth rates of SLC22A3-repressed NE1 cells were compared with control cells by XTT assay. The results are expressed as the mean ± SD of at least three independent experiments. P > 0.05; ANOVA. (E) The effect of depleted SLC22A3 on cell migration was determined using the wound-healing assay. Representative images show that SLC22A3-repressed NE1 cells migrated faster along the wound edge than the control cells. (F) The representative images (Left and Upper Right) and quantification of cells (Lower Right) from the indicated NE1 cells that invaded through Matrigel-coated membrane. Results are shown as mean ± SD of three independent experiments. (Scale bars: 200 μm.) **P < 0.001, ANOVA with post hoc test.
SLC22A3 Interacts with ACTN4 and Inhibits ACTN4-Induced Cell Invasion.
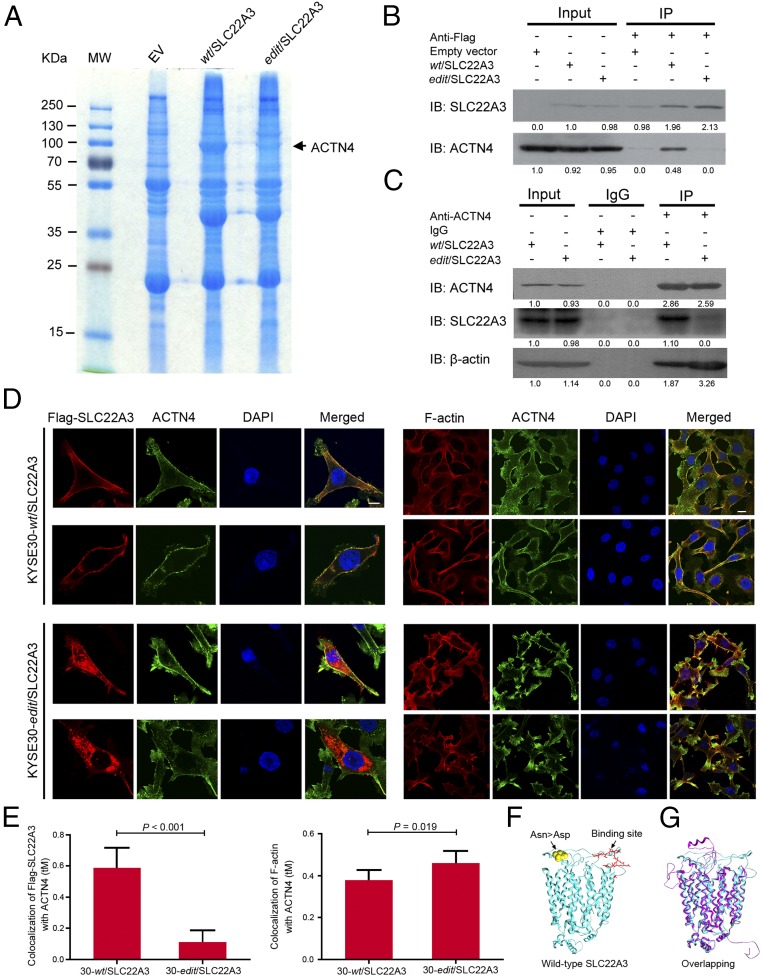
To look for the molecular mechanisms underlying the opposite effects on cell migration, invasion, and metastasis conferred by wild-type and edited SLC22A3, we initially performed coimmunoprecipitation (co-IP) and mass spectrometry using Flag-tagged wild-type or edited SLC22A3-transfected KYSE30 cells (Fig. 5A) and identified 27 proteins potentially interacting with wild-type SLC22A3 but not with the edited form (Table S3). Notably, ACTN4 is the highest-ranked SLC22A3-interacting protein involved in cell motility (Fig. 5A) (28). To confirm the interactions between SLC22A3 and ACTN4 in wild-type or edited KYSE30 cells overexpressing SLC22A3, the whole-cell lysates from these two clones were subjected first to immunoprecipitation (IP) with anti-Flag antibody bound to protein G-Sepharose beads. Then, the immunoprecipitates were subjected to Western blotting analysis using anti-SLC22A3 and anti-ACTN4 antibodies. The amount of endogenous ACTN4 precipitated by anti-Flag antibody was remarkably reduced in edited clones overexpressing SLC22A3 compared with wild-type clones overexpressing SLC22A3 (Fig. 5B). The specific interaction between SLC22A3 and ACTN4 was also detected by reverse co-IP experiments. When the cell lysates were subjected to IP with anti-ACTN4 antibody, wild-type SLC22A3 was specifically precipitated with ACTN4, but the edited form of SLC22A3 showed less binding to ACTN4 (Fig. 5C). ACTN4 is an actin-binding protein that cross-links actin filaments into bundles to form filopodia (29). The amount of actin in the IP fraction precipitated by anti-ACTN4 antibody was also significantly reduced in wild-type clones overexpressing SLC22A3 as compared with edited clones overexpressing SLC22A3, suggesting that ACTN4-mediated actin crosslinking was inhibited by wild-type SLC22A3 (Fig. 5C). Moreover, double-immunolabeling was performed with anti-Flag/anti–F-actin and anti-ACTN4 antibodies. Wild-type SLC22A3 and ACTN4 were colocalized primarily at the cell membrane, whereas edited SLC22A3 was presented mainly in the cytoplasm and showed significantly reduced colocalization with ACTN4 (P < 0.001) (Fig. 5 D and E). In contrast, colocalization between F-actin and ACTN4 was significantly increased in edited clones overexpressing SLC22A3 as compared with wild-type clones overexpressing SLC22A3 (P = 0.019) (Fig. 5 D and E). Furthermore, we predicted the protein conformation of SLC22A3 by building a homology model with the Rebetta tool from David Baker’s group [robetta.bakerlab.org/]. As shown in Fig. 5F, wild-type SLC22A3 is a transmembrane protein (cyan). We also build the structure model for the mutation form (by A-to-I editing) of SLC22A3-N72D (purple) (Fig. 5G). The main part of the protein does not change except for the N-terminal and the coils in the extracellular region (30). Although the N-terminal difference may be caused by thermodynamic fluctuations, the changes in the extracellular region are likely caused by the mutation of residue N72, which is at the center of the structure changes (yellow spheres in Fig. 5F). As we know, ACTN4 is a key element of filopodia, which form focal adhesions with the substratum at the cell surface. We did predict a potential binding interface for wild-type SLC22A3 in the extracellular region (red sticks in Fig. 5F) (31). As can be seen from the comparison of the two models (Fig. 5G), this interface is disrupted in the mutant form, indicating that the single point mutation by A-to-I editing could affect the physical interaction between SLC22A3 and ACTN4. These results suggest that wild-type SLC22A3 is directly associated with ACTN4 and inhibits ACTN4-mediated actin crosslinking.
Fig. 5.
SLC22A3 interacts with ACTN4 and inhibits ACTN4-mediated actin crosslinking. (A) Identification of SLC22A3-associated proteins from Flag-tagged wild-type or edited SLC22A3 (wt/SLC22A3 or edit/SLC22A3) transfected KYSE30 cells by IP and mass spectrometry. The gel was stained with Coomassie blue. Parallel IP from empty-vector (EV)–transfected KYSE30 was performed as a negative control. (B) Whole-cell extracts from KYSE30-wt/SLC22A3 and -edit/SLC22A3 were immunoprecipitated with anti-Flag–conjugated beads. Immunoprecipitants were subjected to Western blotting for SLC22A3 or ACTN4. Parallel IP from empty-vector–transfected KYSE30 was performed as a negative control. (C) Whole-cell extracts from KYSE30-wt/SLC22A3 and -edit/SLC22A3 were immunoprecipitated with ACTN4 antibody, followed by immunoblotting for ACTN4 or SLC22A3, respectively. Mouse IgG was used as a negative control. (D) KYSE30-wt/SLC22A3 and -edit/SLC22A3 cells were double immunolabeled with anti-Flag (red)/anti-ACTN4 (green) (Left) and anti–F-actin (red)/anti-ACTN4 (green) (Right) antibodies, respectively. Nuclei were counterstained with DAPI (blue). (Magnification: 400×.) (Scale bars: 20 µm.) (E) Quantification of colocalization between Flag-SLC22A3 (Left) or F-actin (Right) and ACTN4 in KYSE30-wt/SLC22A3 and -edit/SLC22A3 cells, performed by ZEN 2012 software. Each column represents the mean ± SD for tM (the sum of the colocalized red pixels divided by the sum of the total red pixels), with ∼50–100 cells analyzed per colocalization replicate. (F) Structural model of the wild-type form of protein SLC22A3 (cyan). The edited residue Asn > Asp is highlighted as yellow spheres, and a predicted protein interface with ACTN4 is shown as red sticks. (G) The wild-type (cyan) and the edited (purple) protein structures of SLC22A3 were superimposed to show their differences.
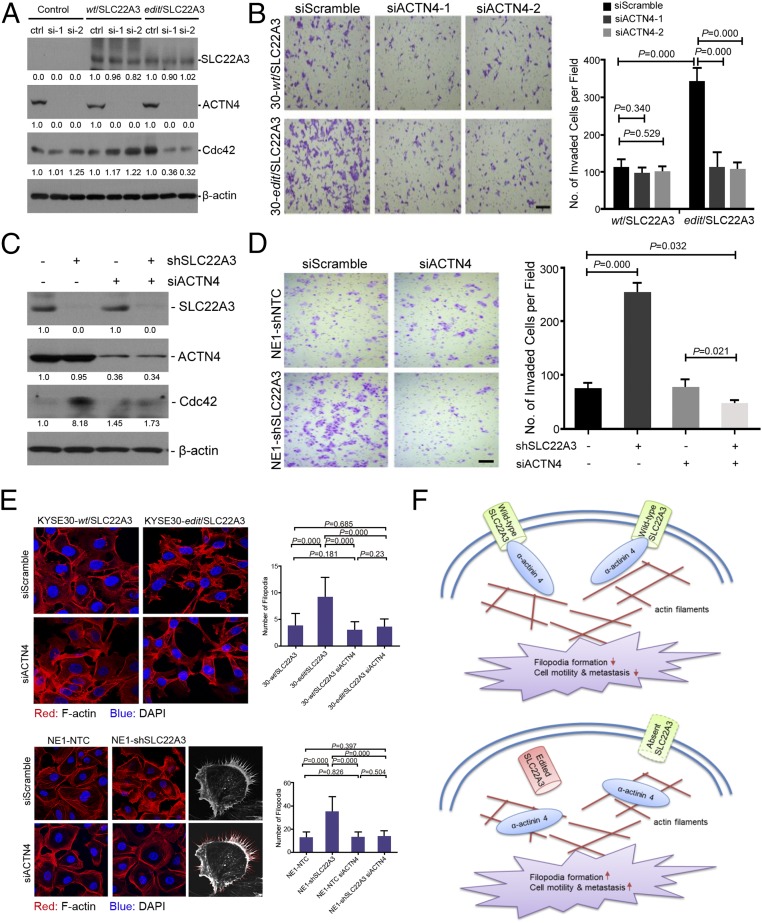
We next tested whether wild-type SLC22A3 attenuated ACTN4-mediated cell invasion. RNAi was used to knock down endogenous ACTN4 expression in wild-type or edited KYSE30 cells overexpressing SLC22A3. Western blotting results showed that siRNA against ACTN4 could significantly reduce ACTN4 expression in wild-type or edited cells overexpressing SLC22A3 and in empty-vector control cells (Fig. 6A). The cell invasion assay found that depletion of ACTN4 in edited cells overexpressing SLC22A3 resulted in reduced invasiveness compared with control cells, whereas depletion of ACTN4 in wild-type cells overexpressing SLC22A3 had no effects on cell invasion (Fig. 6B). Similarly, silencing endogenous ACTN4 expression in wild-type SLC22A3-repressed NE1 cells also showed decreased invasiveness compared with control cells (Fig. 6 C and D). ACTN4 induces cell motility by cross-linking actin filaments into bundles to form filopodia, activity controlled by the Rho family GTPase Cdc42 at the cellular periphery (32). Considering this effect, we assessed the expression of Cdc42 by Western blotting and filopodia formation by phalloidin staining in wild-type or edited KYSE30 clones overexpressing SLC22A3 and SLC22A3-repressed NE1 clones 48 h after transfection of ACTN4 siRNA or control siRNA. We found that depletion of ACTN4 in edited KYSE30 cells overexpressing SLC22A3 or SLC22A3-repressed NE1 cells displayed reduced Cdc42 expression (Fig. 6 A and C) and filopodia formation compared with control cells (Fig. 6E). All these data strongly suggest that wild-type SLC22A3 may act as a metastasis suppressor by directly inhibiting ACTN4.
Fig. 6.
SLC22A3 inhibits cell motility by suppressing ACTN4-mediated filopodia formation. (A) Expressions of SLC22A3, ACTN4, and Cdc42 were determined by Western blotting in clones overexpressing SLC22A3 (KYSE30-wt/SLC22A3 or -edit/SLC22A3) transiently transfected with Scrambled (siScramble) or ACTN4-specific (siACTN4-1 and -2), siRNAs, respectively. (B) Representative images (Left) and quantification of cells (Right) from the indicated KYSE30 clones that invaded through the Matrigel-coated membrane. (Scale bar: 200 μm.) Statistical significance was determined by ANOVA with post hoc test. (C) Western blotting results of SLC22A3 and ACTN4 expression in SLC22A3-repressed clones (NE1-shSLC22A3 or shSLC22A3+) and their control cells (NE1-shNTC or shSLC22A3−) transiently transfected with Scrambled (siACTN4−) or ACTN4-specific (siACTN4+) siRNAs, respectively. (D) Representative images (Left) and quantification of invaded cells (Right) from the indicated NE1 clones. All data are shown as the mean ± SD of three independent experiments, and statistical significance was determined by ANOVA with post hoc test. (E, Left) Phalloidin staining was performed to examine filopodia formation in wild-type or edited KYSE30 clones overexpressing SLC22A3 (Upper) and SLC22A3-repressed NE1 clones (Lower) 48 h after transfection of ACTN4 siRNA or control siRNA. (Right) Numbers of filopodia (indicated as red lines) were quantified as mean ± SD. Statistical significance was determined by ANOVA with post hoc test. (F) Proposed model illustrating that wild-type SLC22A3 inhibits cell motility and metastasis by suppressing ACTN4-mediated crosslinking of F-actin and filopodia formation (Upper), whereas depleted or edited SLC22A3 leads to the loss-of-function phenotype and promotes cell motility and metastasis (Lower).
Collectively, we propose a disease model in which the deregulation of SLC22A3, predisposed in individuals at high risk of familial ESCC, could accelerate the cell motility of esophageal epithelial cells by reducing the inhibition of the actin-binding protein ACTN4, leading to the early malignant progression of ESCC (Fig. 6F).
Discussion
Cancer is a complex disease characterized by both genetic and epigenetic alterations. Here, we provide evidence that SLC22A3 transcription is dramatically lower in NT esophageal epithelia from familial ESCCs than in those from sporadic ESCCs. A-to-I RNA editing at codon 72 (Asn→Asp) of SLC22A3 may serve as an additional epigenetic mechanism relevant to esophageal cancer susceptibility. A link between A-to-I RNA editing and cancer has been established in the past few years (33–38). Until now, only a few proteins with amino acid substitutions that are caused by a single site-specific RNA-editing event have been reported with validity, and there is no apparent causal relationship between altered RNA-editing levels and the initiation of cancer progression. Our group reported that an A-to-I RNA–editing event at codon 367 (Ser→Gly) of antizyme inhibitor 1 (AZIN1) is a gain-of-function event and may be a potential driver in the pathogenesis of HCC (34). Intriguingly, our current study found that A-to-I SLC22A3 RNA editing occurs almost exclusively in familial high-risk individuals but not in sporadic ESCC, indicating a strong genetic association between SLC22A3 RNA editing and esophageal cancer susceptibility. SLC22A3-positive editing in familial NT tissues is also well correlated with a decreased level of SLC22A3 mRNA. This observation is in line with previous reports that RNA editing may influence transcript stability, as characterized by altered transcription levels of the editing targets (39, 40).
Taking advantage of previously reported GWASs in ESCC (26, 27), we found ADAR2 (or ADARB1), one of the three RNA editing enzymes (ADAR1, ADAR2, and ADAR3) identified as catalyzing A-to-I RNA editing, to be a susceptibility gene on 21q22.3 for familial ESCC. In our study, ADAR2 is overexpressed and positively correlated with SLC22A3 editing in familial NT esophageal tissues. However, ADAR1 and ADAR3 are barely expressed in NT esophageal tissues as previously reported (41). These data suggested that aberrant expression of ADAR2 may specifically change the coding sequence of SLC22A3 in high-risk individuals with a family history of ESCC.
Functionally, we found that SLC22A3 is a metastasis-suppressor gene in esophageal cancer. A metastasis-suppressor gene is defined as a gene in which loss of function specifically enhances metastasis without affecting primary tumor growth (42). A series of in vitro and in vivo assays showed that SLC22A3 could suppress cell migration/invasion and EMT process in esophageal cancer cells and inhibit lymph node metastasis in severe combined immunodeficient (SCID) mice but has no influence on cell growth and clonogenicity. Separately from the studies in esophageal cancer cells, we also looked for a role of SLC22A3 in normal esophageal epithelial cells. Reduced SLC22A3 could promote cell motility in normal esophageal cells, strongly suggesting that SLC22A3 may drive early cell migration and invasion in the pathogenesis of ESCC. Further study revealed that membrane localization of wild-type SLC22A3 was able to inhibit cell motility by binding directly to ACTN4 and suppressing ACTN4-mediated actin crosslinking and filopodia formation. ACTN4 belongs to the α-actinin family of actin-binding and crosslinking proteins. Assembly of these cell structural proteins is believed to play a crucial role in stress fiber formation, promoting cell adhesion and regulating cell shape and motility (43, 44). Emerging evidence shows that ACTN4 is implicated in cancer cell migration/invasion, metastasis, and the EMT process (28, 45–47). Intriguingly, our previous study also found that increased expression of ACTN4 is significantly correlated with lymph node metastasis and may serve as a stage-specific marker for esophageal cancer (48). In contrast, edited or reduced SLC22A3 could accelerate cell motility by decreasing the capability to bind ACTN4. These findings revealed molecular basis underlying the early development and poor prognosis of familial ESCC.
In summary, our study demonstrates that A-to-I RNA editing renders a loss-of-function phenotype for the metastasis suppressor SLC22A3 in individuals at high risk of familial esophageal cancer. Our study also illustrates one mechanism by which wild-type SLC22A3 attenuates cell motility by binding directly to the metastasis-promoting protein ACTN4; this observation may provide insights into the early development and progression of familial ESCC and lead to more effective management of high-risk individuals with SLC22A3 deregulation.
Materials and Methods
Additional details about the experimental procedures, including descriptions of RNA/DNA extraction, qPCR, microarray analysis, PCR cloning, lentiviral transduction, in vitro functional assays, coimmunoprecipitation, mass spectrometry, immunofluorescence staining, and Western blotting, are provided in SI Materials and Methods.
Ethics Statement.
The study was approved by the Institutional Review Board (IRB) of the University of Hong Kong. Written informed consents were obtained for the original human work that produced the tissue samples. Animal experiments were approved by the Committee on the Use of Live Animals in Teaching and Research (CLATR) of the University of Hong Kong.
Clinical Specimens.
Specimens from familial and sporadic ESCC cohorts from the Linzhou People’s Hospital and Anyang Cancer Hospital in Henan and the Sun Yat-Sen University Cancer Center in Guangzhou were collected between 2005 and 2013. Snap-frozen specimens of tumors and surrounding NT esophageal tissues were collected from patients with familial and sporadic ESCC at the Linzhou People’s Hospital (n = 104 cases; including 54 familial and 50 sporadic cases), Anyang Cancer Hospital (n = 72 cases; 27 familial and 45 sporadic cases), and the Sun Yat-Sen University Cancer Center (n = 50 cases, all sporadic). A familial case was defined as a patient having at least one blood relative with ESCC within three generations, as detailed in Table S4.
Pyrosequencing Analyses.
RT-PCR was first performed using the primers for pyrosequencing listed in Table S5. The reverse primer was labeled with biotin. The single-stranded biotinylated PCR products then were processed for pyrosequencing analysis, as described previously (34), using the sequencing primers listed in Table S5. The template-sequencing primer mixture was transferred into a PSQ 96 Plate (Biotage), heated to 90 °C for 2 min, and cooled to room temperature. Sequencing reactions were performed with a PSQ 96 SNP Reagent Kit (Biotage) using a PSQ 96MA instrument (Biotage) according to the manufacturer’s instructions. Experiments were performed in triplicate. The sample genotype and percent heteroplasmy were determined using the allele frequency quantification (AQ) function in the SNP software (Biotage). The sensitivity of pyrosequencing is usually 5%. Therefore, we designate samples with a G signal >5% as positive editing in our study.
Post Hoc GWAS Study.
Based on the dataset of a previously reported esophageal cancer GWAS (27), we conducted genotyping data analyses by selecting 291 SNPs in an ∼1.2-Mb region covering the ADAR2 gene on 21q22 from 496 cases with a family history of upper gastrointestinal tract cancers and 1,056 healthy controls. We then examined potential genetic relatedness based on pairwise identity by state for all of the successfully genotyped samples using PLINK 1.07 software. The original script from EIGENSTRAT was modified to extract principal components for plotting, as described previously (49). The association results for selected 21q SNPs are summarized in Table S6.
In Vivo Lymph Node Metastasis Assay.
The study protocol for the lymph node metastasis assay was approved by and performed in accordance with the Committee on the Use of Live Animals in Teaching and Research at The University of Hong Kong. Four- to six-week-old SCID mice were injected s.c. through the footpad with 2 × 105 cells in PBS. Animals were killed 45 d later. The popliteal lymph nodes were isolated and fixed in 10% formalin, followed by H&E staining.
Prediction of Protein Structure and Interface.
Structural models of the wild-type and RNA-edited proteins were built with Rebetta [robetta.bakerlab.org/]. The main part of the model was based on a homolog structure, rat GLUT5 [Protein Data Bank (PDB) ID code 4YBQ, www.rcsb.org/] (50), and the rest of the fragments were constructed ab initio. Structural comparison was done using GRASP2 (30) to superimpose the wild-type and the edited proteins. The potential binding interfaces of the wild-type protein were predicted by using cons-PPISP (31).
Statistical Analysis.
The statistical analyses were performed with SPSS standard version 16.0 (SPSS, Inc.). The SLC22A3 expression levels in the tumors and their matched NT tissues or in groups stratified by editing status were compared using the Mann–Whitney U test. The independent Student’s t test was used to compare the values of the colocalization threshold Manders’ (tM) coefficients in wild-type and edited SLC22A3 transfected cells. ANOVA with post hoc test was used to compare in vitro cell function and relative expression levels of the target genes between different groups. A χ2 test was used to compare the frequencies of editing, clinicopathological features in patients, and numbers of mice with lymph node metastasis in the different groups. The correlation between the mRNA level of SLC22A3/ADAR2 and the editing level of SLC22A3 was analyzed using Spearman’s correlation. A P value less than 0.05 was considered statistically significant.
SI Materials and Methods
ESCC Cell Lines.
The Chinese ESCC cell line HKESC1 was kindly provided by Professor Srivastava of the Department of Pathology, The University of Hong Kong, Hong Kong. Two Chinese ESCC cell lines (EC18 and EC109) and one immortalized normal esophageal epithelial cell line (NE1) were kindly provided by Professor Tsao of the Department of Anatomy, The University of Hong Kong, Hong Kong. Six Japanese ESCC cell lines (KYSE30, KYSE140, KYSE180, KYSE410, KYSE510, and KYSE520) were obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen, GmbH (DSMZ), the German Resource Centre for Biological Material (51).
RNA Extraction and qPCR.
Total RNA from ESCC tissue samples was extracted by TRIzol (Invitrogen). Briefly, 2 μg of total RNA was used to synthesize cDNA with the PrimeScript RT Master Mix (Takara), following the standard protocols provided by the manufacturer. cDNA products were amplified using a SYBR Green PCR Kit (Applied Biosystems). The amplification protocol consisted of incubations at 95 °C for 15 s, 60 °C for 1 min, and 72 °C for 1 min for 40 cycles. Quantification was performed using the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). All gene-expression values were normalized using the housekeeping gene GAPDH and calculated using the comparative Ct (ΔΔCt) method (52). Primer sequences for qPCR are listed in Table S5.
Microarray Analysis.
Affymetrix human genome U133 Plus 2.0 GeneChip (Affymetrix), covering 47,000 transcripts and variants, was used to identify genes differentially expressed in tumors from patients with a family history of ESCC and in adjacent NT tissues. Microarray reaction was done according to the manufacturer’s instructions as described previously (16). Probe-set intensities were calculated using the Microarray Analysis Suite (MAS v5.0, Affymetrix) software and were normalized against 100 housekeeping genes to a mean intensity of 2,000 in mask files of U133 Plus 2.0 before further statistical analysis. Then normalized expression values in tumor and adjacent NT tissues were compared. Fold-change differences were calculated to identify up-regulated and down-regulated genes. Transcripts with more than a 2.0-fold difference in expression level were defined as differentially expressed. DAVID functional annotation tools (23) were used to classify the biological process, molecular functions, and cellular component of the identified differentially expressed genes. cDNA microarray data have been submitted to Gene Expression Omnibus (GEO) (www.ncbi.nlm.nih.gov/geo/) under accession number GSE33810.
PCR Cloning, Mutagenesis, and Lentiviral Transduction.
Wild-type SLC22A3 cDNA was PCR amplified, sequence-verified, and cloned into a pCDH-CMV-MCS-EF1-GFP-T2A-Puro vector (System Biosciences) to construct the wild-type SLC22A3 (wt/SLC22A3) lentiviral overexpression plasmid. PCR mutagenesis was performed using the wt/SLC22A3 plasmid as a template to generate the edited SLC22A3 (edit/SLC22A3) lentiviral overexpression plasmid. The forward and reverse oligonucleotide primers containing the single nucleotide alteration are listed in Table S5. The wt/SLC22A3 or edit/SLC22A3 lentiviral overexpression plasmid or an empty-vector control plasmid was packaged in the 293FT cell line using an HIV-based packaging mix (GeneCopoeia) and used to infect esophageal cell lines (KYSE30 and KYSE180) to establish cells constitutively expressing wt/SLC22A3 or edit/SLC22A3. SLC22A3 shRNA lentiviral knockdowns (shSLC22A3-1: CCGGGCACCAAACTTCCCTGTGTTTCTCGAGAAACACAGG GAAGTTTGGTGCTTTTTG; shSLC22A3-2:CCGGGCAGTGGTGTATCAAGGACTTCTCGAG AAGTCCTTGATACACCACTGCTTTTTG) (NM_021977; Sigma Aldrich) or NT cell shRNA was packaged using a Mission Lentiviral Packaging Mix (Sigma-Aldrich) and used to infect NE1 cell line to establish cells constitutively repressing SLC22A3. Stable clones were selected using puromycin. Cells were infected with lentiviral medium in the presence of 8 mg/mL Polybrene (Sigma-Aldrich) overnight in an incubator at 37 °C. cDNA from stable wt/SLC22A3- or edit/SLC22A3-expressing clones was resequenced to confirm the mutation.
In Vitro Functional Assays.
Cells were seeded into a 96-well plate at a density of 1 × 103 cells per well and were incubated for 4 d. The cell growth rate was detected using a cell-proliferation XTT kit (Roche) according to the manufacturer’s instructions. For the foci formation assay, cells were seeded into a six-well plate. After 1–2 wk of culture, the surviving colonies (>50 cells per colony) were counted with Giemsa staining and are represented as the mean ± SD of triplicates within the same experiment. Cell-adhesion ability was measured by seeding 5,000 cells into 96-well plates precoated with 10 g/mL collagen I (Invitrogen). After incubation for 30–120 min, the cells were washed gently with 1×PBS. The numbers of adherent cells were counted by 0.1% crystal violet staining and are presented as the mean ± SD. The experiment was performed three times in replicates of six wells. For the wound-healing assay, cells were grown to confluence and then were mechanically scratched with a sterile pipette tip. Cells were rinsed with PBS and grown in serum-free DMEM for an additional 48 h. The cell motility, in terms of wound closure, was measured by photographing three random fields at the 0-, 24-, and 48-h time points. For the invasion assay, cells were starved with serum-free medium for 24 h before the assay. Cells (5 × 104) were suspended in 0.5 mL serum-free medium and loaded on the upper compartment of a commercially available invasion chamber coated with Matrigel (BD Biosciences). Medium containing 10% FBS in the lower chamber served as the chemoattractant. After 48 h, invasive cells were fixed, stained, and counted under a microscope. For both the migration and invasion assays three independent experiments were performed in triplicate.
Co-IP.
Cells were lysed with radioimmunoprecipitation assay (RIPA) buffers (Invitrogen) supplemented with protease inhibitor mixture (Roche). Approximately 400–600 mg of whole-cell lysates were incubated overnight at 4 °C with anti-FLAG M2 Affinity Gel (Sigma-Aldrich) or anti-ACTN4 antibody (Abcam) followed by precipitation of the antibody–protein complex using protein G-agarose beads (Santa Cruz). A matched isotype antibody (IgG) was used as a negative control in the IP experiment. After being washed four times with lysis buffer, precipitated protein complexes were extracted with 2× SDS sample buffer for Western blotting.
SDS/PAGE and Mass Spectrometry.
Immunoprecipitations were resolved on 12.5% polyacrylamide gels. Gels then were minimally stained with Coomassie brilliant blue to differentiate IgG bands. The bands differentially expressed in wild-type or edited samples overexpressing SLC22A3 at a mass of ∼70–130 KDa were cut from gels. These bands were digested overnight with 100 ng of trypsin, extracted twice with 100% acetonitrile, and dried in a Speed-Vac (Labconco). Peptides then were resuspended in 5% methanol and loaded onto a BioBasic C18 column. The LTQ Velos linear ion trap LC-MS system (Thermo Fisher Scientific) was run in a data-dependent mode. Spectral data were searched with MASCOT software.
Immunofluorescence Staining.
Cells were cultured onto cover slides to 60–80% confluence in six-well plates. Cells were fixed, permeabilized, and blocked and then were incubated with primary antibodies targeting Flag (Sigma-Aldrich), ACTN4 (Abcam), and F-actin (Rhodamine Phalloidin; Molecular Probes) and with Alexa Fluor dye-conjugated secondary antibodies, followed by counterstaining with DAPI (Invitrogen). Images were acquired using an LSM 700 confocal microscope (Carl Zeiss) with a 40× oil immersion objective (NA = 1.3). ZEN 2012 software (Carl Zeiss) was used for image analysis and quantification of colocalization; the locations of Flag-SLC22A3 (or F-actin) and ACTN4 proteins were determined from measurement statistics associated with individual pixel intensities. The fraction of Flag-SLC22A3 or F-actin (red pixels) that colocalized with ACTN4 (green pixels) was analyzed by calculation of the tM coefficients, which represent the sum of the colocalized red pixels divided by the sum of the total red pixels. tM values ranged from 0 (no colocalization) to 1 (complete colocalization of red pixels with green pixels). ZEN 2012 software automatically determined minimum values for red and green intensities, and these minimum values were set as the threshold to distinguish signal from background. Fifty to one hundred cells were analyzed for each sample.
F-Actin Staining.
Cells were fixed, stained with Rhodamine Phalloidin Conjugate (Molecular Probes), and counterstained with DAPI (Invitrogen). Images were acquired using an LSM 700 confocal microscope (Carl Zeiss) with a 40× oil immersion objective (NA = 1.3). Filopodia were manually annotated using ImageJ software (National Institutes of Health) in 10 random fields at 400× magnification. At least 50 cells were counted for each sample.
Western Blotting.
Western blotting was performed according to the standard protocol with antibodies for SLC22A3 (Abmart), E-cadherin (Abcam), Vimentin (ImmunoWay), α-SMA (Cell Signaling), Snail (Cell Signaling), MMP9 (Cell Signaling), ACTN4 (Abcam), Cdc42 (Cell Signaling), and β-actin (Santa Cruz). The blots were developed with New Clarity Western ECL Substrate (Bio-Rad) and were imaged and analyzed with a ChemiDoc XRS system and Quantity One software (Bio-Rad).
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China (NSFC) Grants 81372583, 81472250, 81572359, U1601229, 81272416, and 81272227; Science and Technology Foundation of Shenzhen Grants CXZZ20150430092951135, KQTD20140630100658078, and JCYJ20150422150029094; Natural Science Foundation of SZU Grant 201573; Key Laboratory Project of Shenzhen Grant ZDSY20130329101130496; the Hong Kong Research Grant Council (RGC) Grants GRF 767313, 17143716, and CUHK/766613 and Grants RGC CRF C7038-14G and C7027-14G; Health and Medical Research Fund 02131876; Theme-based Research Scheme Fund Grant T12-704/16-R; China National Key Projects of Research and Development Grants 2016YFC0904600 and 2016YFC1302305; China National Basic Research Program Grant 012CB967001; and Macau Science and Technology Development Fund Grant 087/2013/A3. X.-Y.G. is a Sophie YM Chan Professor of Cancer Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: cDNA microarray data have been submitted to Gene Expression Omnibus (GEO), (https://www.ncbi.nlm.nih.gov/geo/) (accession no. GSE33810).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703178114/-/DCSupplemental.
References
- 1.Torre LA, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Pickens A, Orringer MB. Geographical distribution and racial disparity in esophageal cancer. Ann Thorac Surg. 2003;76:S1367–S1369. doi: 10.1016/s0003-4975(03)01202-5. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler JB, Reed CE. Epidemiology of esophageal cancer. Surg Clin North Am. 2012;92:1077–1087. doi: 10.1016/j.suc.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Thrift AP, et al. Australian Cancer Study Clinical Follow-Up Study The influence of prediagnostic demographic and lifestyle factors on esophageal squamous cell carcinoma survival. Int J Cancer. 2012;131:E759–E768. doi: 10.1002/ijc.27420. [DOI] [PubMed] [Google Scholar]
- 5.Zhao P, Dai M, Chen W, Li N. Cancer trends in China. Jpn J Clin Oncol. 2010;40:281–285. doi: 10.1093/jjco/hyp187. [DOI] [PubMed] [Google Scholar]
- 6.Castellsagué X, et al. Influence of mate drinking, hot beverages and diet on esophageal cancer risk in South America. Int J Cancer. 2000;88:658–664. doi: 10.1002/1097-0215(20001115)88:4<658::aid-ijc22>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Ke L. Mortality and incidence trends from esophagus cancer in selected geographic areas of China circa 1970-90. Int J Cancer. 2002;102:271–274. doi: 10.1002/ijc.10706. [DOI] [PubMed] [Google Scholar]
- 8.Carter CL, et al. Segregation analysis of esophageal cancer in 221 high-risk Chinese families. J Natl Cancer Inst. 1992;84:771–776. doi: 10.1093/jnci/84.10.771. [DOI] [PubMed] [Google Scholar]
- 9.Hu N, et al. Familial aggregation of oesophageal cancer in Yangcheng County, Shanxi Province, China. Int J Epidemiol. 1992;21:877–882. doi: 10.1093/ije/21.5.877. [DOI] [PubMed] [Google Scholar]
- 10.Hu N, et al. Evidence for a familial esophageal cancer susceptibility gene on chromosome 13. Cancer Epidemiol Biomarkers Prev. 2003;12:1112–1115. [PubMed] [Google Scholar]
- 11.Hu N, et al. Allelotyping of esophageal squamous-cell carcinoma on chromosome 13 defines deletions related to family history. Genes Chromosomes Cancer. 2005;44:271–278. doi: 10.1002/gcc.20242. [DOI] [PubMed] [Google Scholar]
- 12.Huang J, et al. High frequency allelic loss on chromosome 17p13.3-p11.1 in esophageal squamous cell carcinomas from a high incidence area in northern China. Carcinogenesis. 2000;21:2019–2026. doi: 10.1093/carcin/21.11.2019. [DOI] [PubMed] [Google Scholar]
- 13.Su H, et al. Gene expression analysis of esophageal squamous cell carcinoma reveals consistent molecular profiles related to a family history of upper gastrointestinal cancer. Cancer Res. 2003;63:3872–3876. [PubMed] [Google Scholar]
- 14.Hu N, et al. Genomic characterization of esophageal squamous cell carcinoma from a high-risk population in China. Cancer Res. 2009;69:5908–5917. doi: 10.1158/0008-5472.CAN-08-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen D, et al. Early onset, multiple primary malignancies, and poor prognosis are indicative of an inherited predisposition to esophageal squamous cell carcinoma for the familial as opposed to the sporadic cases–an update on over 14-year survival. Eur J Med Genet. 2009;52:381–385. doi: 10.1016/j.ejmg.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Lu P, et al. Genome-wide gene expression profile analyses identify CTTN as a potential prognostic marker in esophageal cancer. PLoS One. 2014;9:e88918. doi: 10.1371/journal.pone.0088918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eeles RA, et al. UK Genetic Prostate Cancer Study Collaborators British Association of Urological Surgeons’ Section of Oncology UK ProtecT Study Collaborators Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet. 2008;40:316–321. doi: 10.1038/ng.90. [DOI] [PubMed] [Google Scholar]
- 18.Cui R, et al. Common variant in 6q26-q27 is associated with distal colon cancer in an Asian population. Gut. 2011;60:799–805. doi: 10.1136/gut.2010.215947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grisanzio C, et al. Genetic and functional analyses implicate the NUDT11, HNF1B, and SLC22A3 genes in prostate cancer pathogenesis. Proc Natl Acad Sci USA. 2012;109:11252–11257. doi: 10.1073/pnas.1200853109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu X, et al. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J Biol Chem. 1998;273:32776–32786. doi: 10.1074/jbc.273.49.32776. [DOI] [PubMed] [Google Scholar]
- 21.Koepsell H. Polyspecific organic cation transporters: Their functions and interactions with drugs. Trends Pharmacol Sci. 2004;25:375–381. doi: 10.1016/j.tips.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, et al. Genetic and epigenetic regulation of the organic cation transporter 3, SLC22A3. Pharmacogenomics J. 2013;13:110–120. doi: 10.1038/tpj.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 24.Skobe M, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 25.Nishikura K. Functions and regulation of RNA editing by ADAR deaminases. Annu Rev Biochem. 2010;79:321–349. doi: 10.1146/annurev-biochem-060208-105251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu C, et al. Genome-wide association study identifies three new susceptibility loci for esophageal squamous-cell carcinoma in Chinese populations. Nat Genet. 2011;43:679–684. doi: 10.1038/ng.849. [DOI] [PubMed] [Google Scholar]
- 27.Wang LD, et al. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet. 2010;42:759–763. doi: 10.1038/ng.648. [DOI] [PubMed] [Google Scholar]
- 28.Barbolina MV, et al. Motility-related actinin alpha-4 is associated with advanced and metastatic ovarian carcinoma. Lab Invest. 2008;88:602–614. doi: 10.1038/labinvest.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weins A, et al. Disease-associated mutant alpha-actinin-4 reveals a mechanism for regulating its F-actin-binding affinity. Proc Natl Acad Sci USA. 2007;104:16080–16085. doi: 10.1073/pnas.0702451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrey D, Honig B. GRASP2: Visualization, surface properties, and electrostatics of macromolecular structures and sequences. Methods Enzymol. 2003;374:492–509. doi: 10.1016/S0076-6879(03)74021-X. [DOI] [PubMed] [Google Scholar]
- 31.Chen H, Zhou HX. Prediction of interface residues in protein-protein complexes by a consensus neural network method: Test against NMR data. Proteins. 2005;61:21–35. doi: 10.1002/prot.20514. [DOI] [PubMed] [Google Scholar]
- 32.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 33.Paz N, et al. Altered adenosine-to-inosine RNA editing in human cancer. Genome Res. 2007;17:1586–1595. doi: 10.1101/gr.6493107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L, et al. Recoding RNA editing of AZIN1 predisposes to hepatocellular carcinoma. Nat Med. 2013;19:209–216. doi: 10.1038/nm.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han SW, et al. RNA editing in RHOQ promotes invasion potential in colorectal cancer. J Exp Med. 2014;211:613–621. doi: 10.1084/jem.20132209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han L, et al. The genomic landscape and clinical relevance of A-to-I RNA editing in human cancers. Cancer Cell. 2015;28:515–528. doi: 10.1016/j.ccell.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paz-Yaacov N, et al. Elevated RNA editing activity is a major contributor to transcriptomic diversity in tumors. Cell Reports. 2015;13:267–276. doi: 10.1016/j.celrep.2015.08.080. [DOI] [PubMed] [Google Scholar]
- 38.Nakano M, Fukami T, Gotoh S, Nakajima M. A-to-I RNA editing up-regulates human dihydrofolate reductase in breast cancer. J Biol Chem. 2017;292:4873–4884. doi: 10.1074/jbc.M117.775684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim DD, et al. Widespread RNA editing of embedded alu elements in the human transcriptome. Genome Res. 2004;14:1719–1725. doi: 10.1101/gr.2855504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dominissini D, Moshitch-Moshkovitz S, Amariglio N, Rechavi G. Adenosine-to-inosine RNA editing meets cancer. Carcinogenesis. 2011;32:1569–1577. doi: 10.1093/carcin/bgr124. [DOI] [PubMed] [Google Scholar]
- 41.Qin YR, et al. Adenosine-to-inosine RNA editing mediated by ADARs in esophageal squamous cell carcinoma. Cancer Res. 2014;74:840–851. doi: 10.1158/0008-5472.CAN-13-2545. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen DX, Massagué J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8:341–352. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- 43.Honda K, et al. Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J Cell Biol. 1998;140:1383–1393. doi: 10.1083/jcb.140.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao H, Wang JH, Pollak MR, Wells A. α-actinin-4 is essential for maintaining the spreading, motility and contractility of fibroblasts. PLoS One. 2010;5:e13921. doi: 10.1371/journal.pone.0013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Honda K, et al. Actinin-4 increases cell motility and promotes lymph node metastasis of colorectal cancer. Gastroenterology. 2005;128:51–62. doi: 10.1053/j.gastro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Kikuchi S, et al. Expression and gene amplification of actinin-4 in invasive ductal carcinoma of the pancreas. Clin Cancer Res. 2008;14:5348–5356. doi: 10.1158/1078-0432.CCR-08-0075. [DOI] [PubMed] [Google Scholar]
- 47.An HT, Yoo S, Ko J. α-Actinin-4 induces the epithelial-to-mesenchymal transition and tumorigenesis via regulation of Snail expression and β-catenin stabilization in cervical cancer. Oncogene. 2016;35:5893–5904. doi: 10.1038/onc.2016.117. [DOI] [PubMed] [Google Scholar]
- 48.Fu L, et al. Identification of alpha-actinin 4 and 67 kDa laminin receptor as stage-specific markers in esophageal cancer via proteomic approaches. Cancer. 2007;110:2672–2681. doi: 10.1002/cncr.23110. [DOI] [PubMed] [Google Scholar]
- 49.Deng M, et al. Genome-wide association analyses in Han Chinese identify two new susceptibility loci for amyotrophic lateral sclerosis. Nat Genet. 2013;45:697–700. doi: 10.1038/ng.2627. [DOI] [PubMed] [Google Scholar]
- 50.Berman HM, et al. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimada Y, Imamura M, Wagata T, Yamaguchi N, Tobe T. Characterization of 21 newly established esophageal cancer cell lines. Cancer. 1992;69:277–284. doi: 10.1002/1097-0142(19920115)69:2<277::aid-cncr2820690202>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 52.Zhang C, et al. Fibroblast growth factor receptor 2-positive fibroblasts provide a suitable microenvironment for tumor development and progression in esophageal carcinoma. Clin Cancer Res. 2009;15:4017–4027. doi: 10.1158/1078-0432.CCR-08-2824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.