Significance
Sensory neurons encode environmental stimuli in their electrical activity and alter behavior and physiology by transmitting this information to downstream circuits. Their response properties can be characterized by tuning curves that relate stimulus parameters to neural responses. Tuning curves identify the response threshold, the stimulus features at the tuning curve peak, and high-slope regions that give maximum stimulus discrimination. Here we show that two antagonistically acting molecular oxygen sensors, a neuroglobin and a soluble guanylate cyclase, sculpt a sharp sigmoidal tuning curve in the URX oxygen sensing neurons of Caenorhabditis elegans. By combining experiments with computational modelling, we show that these changes in stimulus-encoding properties broaden C. elegans’s O2 preference.
Keywords: sensory neuron tuning, neural coding, oxygen sensing, neuroglobin, computational model
Abstract
Sensory receptor neurons match their dynamic range to ecologically relevant stimulus intensities. How this tuning is achieved is poorly understood in most receptors. The roundworm Caenorhabditis elegans avoids 21% O2 and hypoxia and prefers intermediate O2 concentrations. We show how this O2 preference is sculpted by the antagonistic action of a neuroglobin and an O2-binding soluble guanylate cyclase. These putative molecular O2 sensors confer a sigmoidal O2 response curve in the URX neurons that has highest slope between 15 and 19% O2 and approaches saturation when O2 reaches 21%. In the absence of the neuroglobin, the response curve is shifted to lower O2 values and approaches saturation at 14% O2. In behavioral terms, neuroglobin signaling broadens the O2 preference of Caenorhabditis elegans while maintaining avoidance of 21% O2. A computational model of aerotaxis suggests the relationship between GLB-5–modulated URX responses and reversal behavior is sufficient to broaden O2 preference. In summary, we show that a neuroglobin can shift neural information coding leading to altered behavior. Antagonistically acting molecular sensors may represent a common mechanism to sharpen tuning of sensory neurons.
The response properties of sensory neurons can be characterized by tuning curves that relate stimulus parameters to the evoked response (1, 2). Some sensory neurons show dynamic ranges that span several orders of stimulus magnitude (e.g., odor concentration), whereas others show remarkably narrow tuning curves. For example, glomus cells of the carotid body show oxygen-evoked responses that are tuned to a twofold to threefold change in O2 levels at the physiologically appropriate O2 concentration range (3, 4). How such sharp tuning is achieved is poorly understood.
Neuroglobins are members of the globin family of heme-binding proteins expressed mainly in neurons (5). They have been described throughout metazoa, from cnidarians to man (6). Their physiological functions are unclear. They have been proposed to metabolize reactive oxygen species (ROS), signal redox state, store O2, control apoptosis, and negatively regulate Gi/o signaling (7). The genome of Caenorhabditis elegans encodes an unusually large family of globins, and many are expressed in neurons (8). One of these, GLOBIN-5 (GLB-5), is expressed in the oxygen sensing neurons URX, AQR, PQR, and BAG, where it accumulates at dendritic endings (9, 10). C. elegans avoids both normoxia (21% O2) and hypoxia (11). Avoidance of 21% O2 enables the animal to escape the surface and is mediated by O2 receptors, most importantly the glb-5–expressing URX, AQR, and PQR neurons (12). Like vertebrate neuroglobins, GLB-5 has the spectroscopic fingerprints of a hexa-coordinated heme iron and rapidly oxidizes to the ferric state in normoxia (9). The glb-5 gene is defective in the domesticated reference strain of C. elegans, N2 (Bristol), but natural isolates encode a functional allele, glb-5(Haw) (9, 10) (Haw refers to Hawaii, the geographical origin of the natural isolate in which this allele was first described). Behavioral and Ca2+ imaging studies suggest that functional GLB-5 alters the properties of the O2 receptors and C. elegans’ O2 responses (9, 10). However, how GLB-5 alters the representation of environmental information in these neurons leading to behavioral change is unknown.
The URX O2 receptors exhibit phasic–tonic signaling properties and, in response to changes in O2 concentration, evoke both transient behavioral responses that are coupled to the rate of change of O2, dO2/dt, and more persistent behavioral responses coupled to O2 levels (8, 13). The transient responses are reversals and turns that allow C. elegans to navigate O2 gradients. The sustained responses involve persistent changes in the rate of movement that enable feeding animals to escape 21% O2 or to accumulate in preferred lower O2 environments. Besides GLB-5, the URX neurons express another putative molecular O2 sensor, a soluble guanylate cyclase composed of GCY-35 and GCY-36 (guanylate cyclase) subunits (11, 13, 14). These soluble guanylate cyclases have a heme–nitric oxide/oxygen (H-NOX) binding domain that appears to stimulate cGMP production upon binding molecular O2 (10, 11). Recent work suggests mammalian soluble guanylate cyclases also mediate O2 sensing in glomus cells of the carotid body, although they do not bind O2 (15). Here we show that the GLB-5 neuroglobin and soluble guanylate cyclases work antagonistically to confer on URX a sigmoidal O2 stimulus–response curve that has its steepest slope between 15 and 19% O2 and begins to plateau as O2 levels approach 21%. By tuning URX, GLB-5 broadens the range of O2 environments preferred by C. elegans. Using computer modeling we show that this altered preference can be explained by changes in how URX evokes reversals in response to O2 stimuli.
Results
The GLB-5 Allele in Natural Isolates Broadens C. elegans’s O2 Preference.
Previous studies of glb-5 used a loss-of-function allele that arose in the N2 laboratory strain during domestication (9, 10, 16). This allele, glb-5(N2), is a partial duplication that generates multiple splice isoforms, and it is unclear if it abolishes glb-5 function. We therefore compared animals bearing the glb-5(Haw) functional allele to mutants carrying a predicted null mutation, glb-5(tm5440), which deletes part of the globin domain and introduces a premature stop codon. To analyze how the GLB-5 neuroglobin alters O2 preference we compared the distribution of glb-5(Haw) and glb-5(tm5440) animals in a shallow 0–21% O2 gradient in the presence of food. Unless otherwise indicated, in our experiments we used strains defective in the neuropeptide receptor npr-1, because besides harboring a defective glb-5 allele, the N2 laboratory strain has acquired a gain-of-function mutation in npr-1 that confers O2-sensing defects (11). glb-5(tm5440); npr-1 animals accumulated in a narrow range of O2 concentrations, between 7 and 10% O2 (Fig. 1 A and B). By contrast, animals bearing the natural glb-5(Haw) allele distributed over a broader range of O2 concentrations, between 17 and 5% O2, but still avoided 21% O2 and hypoxia (Fig. 1 A and B). These behavioral data imply that the GLB-5(Haw) neuroglobin changes how O2-sensing neurons respond in O2 gradients.
Fig. 1.
The GLB-5 neuroglobin broadens C. elegans’s O2 preference. (A) Aerotaxis behavior. Distribution of animals in a 0–21% O2 gradient. n = 6. Plots show mean ± SEM. (B) The glb-5(Haw) allele broadens C. elegans’s O2 preference. High O2 avoidance index = (fraction of animals in 7–14% O2) − (fraction of animals in 14–21% O2)/(fraction of animals in 7–21% O2). O2 preference (7–10% O2) = (fraction of animals in 7–10% O2)/(fraction of animals in 7– 21% O2). **P < 0.01, Mann–Whitney u test. Data are from A. (C) Mean Ca2+ responses of URX neurons to the indicated O2 ramp stimulus. n = 9–12. Shading represents SEM. The Ca2+ sensor is GCaMP6s coexpressed with mCherry (URX). Also shown are the responses of gcy-35; glb-5(tm5440) mutants; GCY-35 is required for measurable O2-evoked Ca2+ responses. The color bars represent the average response for each genotype at time point indicated by the black bar. Data show mean ± SEM. **P < 0.01, ANOVA with Tukey’s post hoc test. The O2 ramp stimulus (Top) shows the mean of four measurements ± SEM.
GLB-5 Changes the Dynamic Range of the URX O2 Sensor.
We used the GCaMP6s Ca2+ sensor to examine how functional glb-5 alters neural coding of O2 levels in the URX O2 sensors (17). The dynamics of the Ca2+ responses evoked in URX by a 7–21% O2 step stimulus did not differ significantly between glb-5(tm5440) and glb-5(Haw) animals (Fig. S1). However, the Ca2+ responses to a 7–19% O2 exponential ramp stimulus differed markedly between these strains (Fig. 1C). In animals expressing glb-5(Haw), Ca2+ in URX increased continuously as O2 levels rose from 7 to 19%. By contrast, the Ca2+ responses of glb-5(tm5440) mutants appeared to plateau at ∼14% O2. As expected, the URX neurons did not respond to the O2 stimulus in animals defective in both glb-5 and the gcy-35 soluble guanylate cyclase (Fig. 1C). This defect could be rescued by expressing wild-type gcy-35 and glb-5(Haw) cDNA selectively in URX (Fig. S2 A and B). Selectively expressing wild-type gcy-35 cDNA but glb-5(Haw) cDNA that contained a stop codon conferred URX responses that plateaued at ∼14% O2 (Fig. S2 A and B). These differences suggest that the GLB-5 neuroglobin changes the dynamic range of URX (Fig. 1C). The effect of glb-5 alleles on the URX Ca2+ response was similar whether we imaged animals in the presence (Fig. 1C) or absence of food (Fig. S2 C and D).
Fig. S1.
The URX Ca2+ response to strong stimulation. (A) Mean URX Ca2+ responses to a 7–21% O2 stimulus in glb-5(Haw) (red trace) and glb-5(tm5440) (blue trace) animals. n = 8. Shading represents SEM. (B) Average ΔR/R0 ± SEM evoked by the O2 stimulus during the time intervals indicated in A. NS, not significant (Mann–Whitney u test).
Fig. S2.
URX Ca2+ responses to an exponential ramp O2 stimulus. (A and C) Ca2+ responses in URX evoked by an exponential ramp O2 stimulus in animals of the indicated genotype. Animals in C were imaged in the absence of food. n = 10. Shading represents SEM. The Ca2+ sensor was GCaMP6s (coexpressed with mCherry). The O2 stimulus used in both A and C is shown above the traces in A, plotted as the mean O2 concentration ± SEM. n = 4. (B and D) Average responses compared at time points indicated by the black bar in A and C, respectively. *P < 0.05, **P < 0.01 (Mann–Whitney u test).
To investigate further how the GLB-5 neuroglobin alters neural coding, we delivered different patterns of O2 stimuli and imaged Ca2+ responses in URX. We focused on URX because these sensory neurons are sufficient for several O2-coupled behaviors including aerotaxis and aggregation (18, 19) (Fig. S3). To plot the relationship between stimulus intensity and URX Ca2+ responses we sequentially increased the O2 stimulus given to the same animal in 2% increments, returning to 7% O2 between stimuli (Fig. 2A). In glb-5(Haw) animals URX neurons showed a higher O2 response threshold than in glb-5(tm5440) mutants, as well as a steep sigmoidal O2 response curve whose half maximum was at much higher O2 concentrations (Fig. 2 A and B). Selectively expressing a glb-5(Haw) transgene in URX in glb-5(tm5440) mutants conferred a URX stimulus–response profile that closely resembled that of glb-5(Haw) animals (Fig. 2 A and B). Thus, GLB-5(Haw) cell-autonomously shifts the URX stimulus–response curve toward higher O2 concentrations (Fig. 2B).
Fig. S3.
Selective expression of glb-5(Haw) in URX broadens C. elegans’s O2 preference. (A) Aerotaxis of C. elegans strains of the indicated genotypes that express gcy-35 or gcy-35 and glb-5(Haw) selectively in URX. glb-5(tm5440) is a negative control; the same dbExpflp-8::gcy-35 transgene was used with or without the pURX::glb-5(Haw) transgene. n = 10–12. Data plotted are mean ± SEM. (B and C) High O2 avoidance index = (fraction of animals in 7–14% O2) − (fraction of animals in 14–21% O2)/(fraction of animals in 7–21% O2). O2 preference (7–10% O2) = (fraction of animals in 7–10% O2)/(fraction of animals in 7–21% O2). *P < 0.05, **P < 0.01, ANOVA with Tukey’s post hoc test. Data are from A.
Fig. 2.
GLB-5 changes the stimulus–response curve of O2-evoked Ca2+ responses in URX. (A) Ca2+ responses evoked in URX by a graded series of O2 steps that start from a 7% O2 baseline (n = 10). Data show mean ± SEM. Responses are normalized to the GCaMP6s/mCherry ratio averaged over the 10 s before delivery of the stimulus train. (B) Maximum amplitude of the responses from A plotted against O2 stimulus intensity. Bars represent SEM. *P < 0.05; **P < 0.01; glb-5(Haw) vs. glb-5(tm5440). ++P < 0.01; glb-5(tm5440) URXp::glb-5(Haw) vs. glb-5(tm5440). ANOVA with Dunnett’s post hoc test.
To extend these observations, we examined URX responses to a different set of stimuli in which we increased O2 levels in 2% steps but varied the starting O2 concentration and delivered only one stimulus per animal. Again, we observed that the glb-5(Haw) allele shifted the tuning curve of URX such that both the tonic Ca2+ levels at the new O2 concentration and the change in Ca2+ normalized to the prestimulus Ca2+ level, ΔR/R0 (which is a measure of the response amplitude), gradually increased as O2 approached 19–21% (Fig. 3 A and B). By contrast, in glb-5(tm5440) mutants, ΔR/R0 was at a maximum when animals experienced an 11 → 13% O2 stimulus (Fig. 3 A and B). Expressing glb-5(Haw) cDNA selectively in URX in glb-5(tm5440) animals was sufficient to confer a glb-5(Haw)–like dose–response curve to this neuron under these stimulation conditions (Fig. 3 A and B).
Fig. 3.
Response properties of URX sensory neurons with and without GLB-5(Haw). (A) Averaged traces of the Ca2+ response of URX to different 2% O2 step stimuli in the genotypes indicated. n = 12–18. (B) Maximum amplitudes of the responses shown in A plotted against stimulus intensity. Data show mean ± SEM. (C) Averaged traces of the Ca2+ response of URX to different 2% O2 ramp stimuli in glb-5(Haw) and glb-5(tm5440) animals. n = 13–16. (D) Maximum amplitude of the responses shown in C plotted against stimulus intensity. Data show mean and SEM. The O2 plots of a step and a ramp stimulus at the top of A and C show the mean of 9 (A) and 10 (C) measurements ± SEM. *P < 0.05; **P < 0.01; glb-5(Haw) vs. glb-5(tm5440). +P < 0.05; ++P < 0.01; glb-5(tm5440) URXp::glb-5(Haw) vs. glb-5(tm5440). ANOVA with Dunnett’s post hoc test (B). Mann–Whitney u test (D).
Step stimulation is used widely to study the properties of sensory neurons, but in their natural environment, C. elegans likely also encounter slowly varying O2 levels, similar to those encountered by animals in the aerotaxis assay (Fig. 1A). We therefore measured the Ca2+ responses evoked in URX by a set of 2% O2 exponential ramp stimuli. Our results revealed a response pattern similar to that observed for the corresponding step stimulus (Fig. 3 C and D). In animals expressing the glb-5(Haw) allele, URX responses to ramp stimuli increased gradually as O2 levels increased. By contrast, in glb-5(tm5440) mutants the URX response amplitudes, measured as ΔR/R0, showed a peak response to the 13 → 15% O2 stimulus and were otherwise similar across the different ramp stimuli we delivered (Fig. 3 C and D). The response property changes conferred by GLB-5(Haw) are therefore robust to different O2 stimulation patterns. Together, our Ca2+ imaging experiments suggest that GLB-5(Haw) alters neural encoding of O2 stimuli in URX, shifting the dynamic range to higher O2 concentrations and making it more sharply tuned.
cGMP Signaling in URX.
In previous work we used the genetically encoded cGMP sensor cGi500 (20) to visualize cGMP dynamics in the PQR O2-sensing neuron (14). We showed that a rise in O2 stimulates a tonic rise in cGMP that requires the GCY-35 soluble guanylate cyclase and that the Ca2+ influx resulting from gating of cGMP channels feeds back to limit O2-evoked rises in cGMP by stimulating cGMP hydrolysis (14) (Fig. S4A). We used the cGi500 sensor to examine if GLB-5(Haw) can modulate cGMP dynamics in URX. We could not detect O2-evoked cGMP responses in the cell body of URX neurons unless we disrupted cng-1, which encodes a cGMP-gated channel subunit required for O2-evoked Ca2+ responses in URX (Fig. S4 A and B). This suggests that URX and PQR have similar negative feedback control of cGMP accumulation. The cGMP responses evoked in URX by an exponential ramp O2 stimulus were comparable in glb-5(tm5440) cng-1 and glb-5(Haw) cng-1 animals under our experimental conditions (Fig. 4 B and C). These results would suggest that GLB-5(Haw) does not alter URX neural coding by modulating cGMP levels, However, we cannot exclude the possibility that measuring cGMP in the cell body does not adequately report cGMP changes in the dendritic ending, where GLB-5, GCY-35/GCY-36, and the cGMP channels are localized.
Fig. S4.
O2-evoked cGMP responses in URX. (A) Model of sensory transduction of O2 stimuli in URX. (B) cGMP responses evoked in URX by an exponential O2 ramp stimulus. Data show mean ± SEM. (Middle) Traces from glb-5(tm5440) (blue) and glb-5(Haw) (red) animals. (Bottom) Traces from cng-1(db111); glb-5(tm5440) (blue) and cng-1(db111); glb-5(Haw) (red) animals. n = 10–11. (Top) The O2 stimulus used. Data show mean O2 ± SEM. n = 4. (C) Average responses compared at time points indicated by the black bar (5 min) in B, Bottom. Data show mean ± SEM. NS, not significant (Mann–Whitney u test).
Fig. 4.
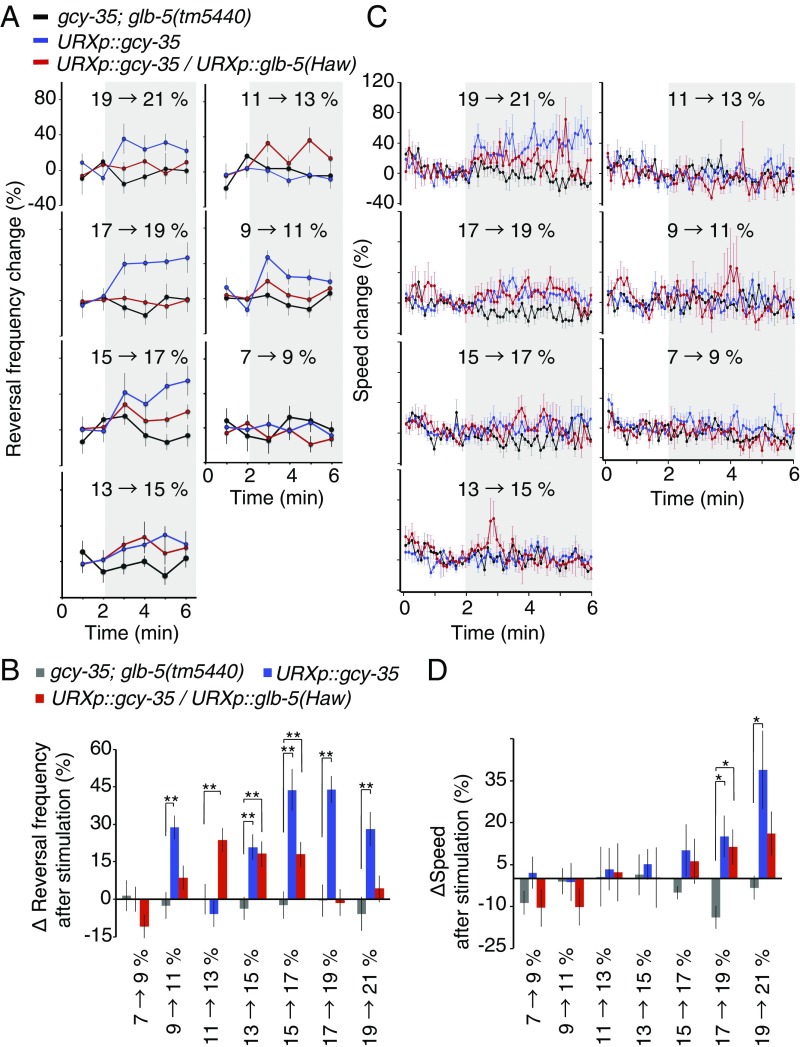
The relationship between O2 stimulus intensity and URX-dependent behavioral outputs. (A) Frequency of reversal behavior evoked by O2 step stimuli in animals of the genotypes indicated. Reversal frequencies were quantified every minute. n = 15–30 animals. Bars represent SEM. (B) Reversal frequency evoked by step O2 stimuli averaged over 4 min after the stimulus. Data represent mean ± SEM. (C and D) Instantaneous speed in response to step O2 stimuli. n = 30–60 animals. Plots show mean ± SEM. (D) Mean speed ± SEM calculated for a 4-min interval beginning 30 s after the step stimulus. The behavior of gcy-35(ok769); glb-5(tm5440) animals was used as a negative control and is shown as black traces or gray bars. *P < 0.05, **P < 0.01, ANOVA with Dunnett’s post hoc test.
GLB-5 Effects on URX Behavioral Outputs.
How do the changes in URX information coding mediated by GLB-5(Haw) alter motor responses to O2 stimuli? To address this question, we quantified behavioral responses to a range of O2 stimuli, focusing on reversal in the direction of movement and changes in speed, both important features of O2-evoked behaviors (18, 19). Avoidance of high O2 levels is mediated principally by three sensory neurons, URX, PQR, and AQR, each of which expresses the GCY-35/36 O2 receptor and GLB-5 (11–13, 18). To selectively study how URX output alters behavior we studied gcy-35(ok769); glb-5(tm5440) mutants that expressed glb-5(Haw) and/or gcy-35 cDNA in URX but not AQR or PQR (Methods). Unexpectedly, only O2 stimuli that evoked intermediate URX Ca2+ responses evoked strong reversals in these animals (Figs. 3 A and B and 4 A and B). O2 stimuli that evoked either small or large URX Ca2+ responses failed to evoke reversals (Figs. 3 A and B and 4 A and B). We also observed this relationship between the Ca2+ response magnitude and reversals when we expressed glb-5(Haw) selectively in URX, although the O2 range that evoked the reversals most strongly was different (Figs. 3 A and B and 4 A and B). These data suggest that URX associated circuits include a filter that prevents strong stimulation of URX from inducing reversals. Consistent with this, a 13 → 21% O2 step stimulus that evoked a large URX Ca2+ response did not evoke reversals in animals expressing gcy-35 selectively in URX (Fig. S5).
Fig. S5.
Strong stimulation of URX does not induce reversals. (A) Mean URX Ca2+ response evoked by a 13 → 21% step O2 stimulus in genotypes indicated. Color shadings represent SEM (n = 8–11). (B) Reversal frequencies of C. elegans in response to a 13 → 21% step O2 stimulus. Reversal frequency was quantified every 1 min (n = 14–20 animals). Bars represent SEM.
One important output for the URX O2 sensors is the RMG interneurons, which are connected to URX by both synapses and gap junctions (21–23). O2-evoked responses in RMG correlated well with those in URX when we stimulated animals expressing gcy-35 selectively in URX with 13 → 15% and 13 → 21% O2 steps (Fig. S6). This suggests information transfer from URX to RMG does not explain the nonlinearity in the relationship between URX Ca2+ responses and reversals. URX output also stimulated locomotory speed when O2 levels rose above 17% O2 in both glb-5(tm5440) and glb-5(Haw) strains (Fig. 4 C and D). Together, our results suggest that information from URX is transmitted to both reversal and speed circuits; however, reversals can be evoked by changes in URX activity that evoke modest or no changes in speed.
Fig. S6.
The relationship between RMG and O2 stimuli. (A) Mean RMG Ca2+ response evoked by a 13 → 21% or 13 → 15% step O2 stimulus in gcy-35(ok769); glb-5(tm5440); npr-1(ad609) animals expressing gcy-35 in URX. Color shadings represent SEM (n = 15–16). (B) Average peak ΔF/F0 ± SEM evoked by the O2 stimulus in A. *P < 0.05 (Mann–Whitney u test).
A Computational Model for Aerotaxis.
Using our detailed analyses of how URX responds to different O2 stimuli, we carried out computational modeling experiments to ask if the relationship between URX activity and reversal behavior was sufficient to explain the altered O2 preference of animals expressing glb-5(Haw). To build a model for aerotaxis we incorporated the Ca2+ imaging data in Fig. 3A and the behavioral data in Fig. 4C (Methods). For simplicity, and because URX is sufficient for C. elegans to show an O2 preference (Fig. S3), we excluded other O2-sensing neurons, including PQR and AQR, in the computational model. The model included a command neuron that randomly generates a reversal; a URX model neuron transmitted a signal to this command interneuron via an interneuron that could act as a differentiator to promote reversal (Fig. 5A and Methods). In the model, URX Ca2+ responses to O2 stimuli were approximated by a nonlinear–linear–nonlinear (NLN) model (Fig. 5A). The parameters for the NLN model were estimated from imaging URX responses to 2% step O2 stimuli in glb-5(tm5440) and glb-5(Haw) (Fig. S7). A single model reproduced URX Ca2+ responses to a variety of O2 changes. As a result of modeling the URX responses, we acquired two sets of parameters, one for glb-5 (tm5440) and the other one for glb-5 (Haw). The parameters for steps downstream of URX were common for glb-5(tm5440) and glb-5(Haw) virtual animals.
Fig. 5.
A computational model that links O2-evoked Ca2+ responses in URX to behavioral output. (A) Schematic of the computational model. (B) Heat map representing the location of 10000 fictive glb-5(tm5440) or glb-5(Haw) animals in a 7–21% O2 gradient. Locations are plotted every second. (C) Histograms of the existence frequency of glb-5(tm5440) and glb-5(Haw) in 7–21% O2 gradient during the last 100 s of the computational experiments shown in B. The fictive URX responses and reversal frequency of these model worms during aerotaxis are shown in Fig. S8. (D) The modified aerotaxis results from Fig. 1A are shown to compare results of computational experiments and those of aerotaxis experimental data.
Fig. S7.
NLN model: reconstruction of URX responses. Reconstruction by the NLN model of Ca2+ responses evoked in URX by 2% O2 step stimuli in (Top) glb-5(Haw) and (Bottom) glb-5(tm5440) animals. Solid and dashed lines indicate reconstructed data and experimental data, respectively. The experimental data used for comparison are taken from Fig. 3A.
Having set up our model, we ran in silico aerotaxis experiments in which the position of a worm was represented as a single point (Fig. S8). These experiments showed that worms for which the URX NLN model used parameters obtained for glb-5(tm5440) preferred 7–10% O2 (Fig. 5 B and C), whereas those using glb-5(Haw) parameters showed broader O2 preference, with the majority of worms preferring 7–16% O2 (Fig. 5 B and C). The simulations made by our computational model mirrored the results of aerotaxis experiments (Figs. 1A and 5 B–D). In our initial computational model, the values for constants in the model interneuron () and command neuron () were selected arbitrarily (Methods). We therefore examined how changing these parameters over a wide range altered the results of our simulated aerotaxis experiments. For almost all parameter values we tested, the glb-5(tm5440) virtual animals preferred lower and narrower O2 concentrations than the glb-5(Haw) virtual animals (Fig. S9). The distinct O2 preferences of the two strains are thus not strongly influenced by the values of these parameters.
Fig. S8.
URX responses and reversal frequency of model worms in Fig. 5. Histograms of fictive URX responses of (A) glb-5(tm5440) and (B) glb-5(Haw) model worms over the 1,800 s of the in silico experiment shown in Fig. 5. (C) Simulated tracks of three worms navigating the O2 gradient in our computer simulations. Shown are 1,801 steps. The x and y axes are the dimensions of the fictive aerotaxis chambers. Colors code local O2 concentration. (D) Reversal frequency at each O2 concentration of the model worms during the 1,800 s. Red and blue traces indicate fictive glb-5(Haw) and glb-5(tm5440) animals, respectively.
Fig. S9.
Varying parameters and only weakly alters simulated aerotaxis. (A and B) The parameters of and were varied for 35 and 40 steps, respectively. Thus, 1,400 combinations (35 × 40) of the parameters were attempted. In each simulation, 1,000 fictive worms were run for 30 min, and O2 concentrations acquired from the positions of fictive worms during the last 100 s of the computational experiments averaged. The average values are plotted in the heat maps. The red squares indicate the parameters used in Fig. 5 and Fig. S11 ( ). (C) The average preferred O2 concentrations of glb-5(tm5440) were subtracted from those of glb-5(Haw) and plotted in the heat map shown. For most values of and the average O2 concentration preferred by glb-5(Haw) worms was higher than that of glb-5(tm5440) worms. (D and E) Standard deviation (SD) of O2 concentrations preferred by the fictive worms during the last 100 s of the computational experiments is plotted in the heat maps. The SD is nearly equal to the extent of worms’ distribution on the virtual O2 gradient. (F) SDs of preferred O2 concentrations of glb-5(tm5440) were subtracted from those of glb-5(Haw) and plotted in the heat map.
To extend our model, we incorporated data on O2-evoked changes in speed (Fig. 4C and Fig. S10), in addition to O2-evoked changes in reversals. We found this did not substantially change the performance of glb-5(tm5440) and glb-5(Haw) in virtual aerotaxis assays. Model worms that modulated both reversals and speed in response to O2 changes distributed similarly to animals that modulated only reversal (Fig. S11). By contrast, model worms in which changes in O2 influenced only speed distributed almost evenly in a virtual aerotaxis chamber (Fig. S11). Thus, in our model the relationship between URX responses and reversal frequency is sufficient to account for the worm’s O2 preference in a shallow O2 gradient.
Fig. S10.
Data processing of speed data for computational experiments. (A) Detrending speed data. To incorporate experimental speed data into our model, we identified and removed a slow downward trend in an averaged time series of the speed of animals responding to O2 cues. The raw experimental speed data (from Fig. 4C) are shown in Left, with the dashed lines showing the fitted lines used for detrending. The detrended data are in Right. (B and C) Detrended average speed before (triangle) or after (circle) we delivered the O2 stimuli described in Fig. 3. We acquired parameters for the Hill equation used in the computational experiments by performing curve fitting on this data.
Fig. S11.
Modeling how O2-evoked changes in speed alter O2 preference in aerotaxis assays. Instantaneous location of 10,000 fictive glb-5(tm5440) and glb-5(Haw) animals in different aerotaxis models, plotted at 1-s intervals and represented by heat maps. (Top) Fictive animals have constant speed but show O2-evoked reversals according to our model for URX output. (Middle) Fictive animals show O2-evoked changes in speed according to our model for URX output but not in reversals. (Bottom) Fictive animals show O2-evoked changes in both reversals and speed. Histograms show the existence frequency of model worms during the last 100 s of the computational experiments.
Discussion
The neuroglobin GLB-5 changes how the URX O2-sensing neurons encode O2 concentration. URX sensory receptors enable C. elegans to avoid and escape 21% O2. We find that URX neurons combine two putative molecular O2 sensors, a soluble guanylate cyclase and a neuroglobin, to sculpt a sigmoidal O2 tuning curve in which the neurons show little Ca2+ response to stimuli below 13% O2, gradually increase their responsiveness above this O2 concentration, show a sharp increase in responsiveness between 15 and 19% O2, and approach saturation as O2 approaches 21%. The neuroglobin GLB-5 imposes the sigmoidal function by inhibiting the O2-evoked Ca2+ response in URX when O2 levels fall below 21%. When GLB-5 is defective, the URX stimulus–response curve is shifted to lower O2 levels and approaches saturation at 14% O2. At a behavioral level, the effects of GLB-5 signaling are to broaden the O2 environments preferred by C. elegans while maintaining avoidance of 21% O2. If glb-5 is defective, as in the N2 laboratory strain or the glb-5(tm5440) mutant, animals prefer a narrow O2 range, from 7 to 10%. Animals with functional glb-5 signaling distribute more broadly, from 17 to 5% O2. It will be interesting to explore if other sensory neurons that exhibit steep sigmoidal tuning curves at defined intensity intervals achieve their properties by combining antagonistic molecular sensors. Studies of O2 sensing in the glomus cells of the carotid bodies of mammals have implicated multiple O2-sensing mechanisms that could act together to sculpt O2 response features (4). Similarly, a range of CO2/pH-responsive molecules have been identified in mammals, although whether any of the numerous CO2/pH-responsive cells use a combination of transducers is unclear (24).
Unexpectedly, we find that the relationship between URX Ca2+ response (a proxy of O2 stimulus intensity) and behavioral output is nonlinear. Whereas intermediate stimulation of URX induces animals to reverse, strong stimulation is less effective. We have not investigated the neural mechanisms that underpin nonlinear control of reversals by URX. However, the neuroanatomical reconstructions reveal synapses from URX to both AVE interneurons that promote reversals and AVB interneurons that promote forward movement (21, 25) (wormwiring.org/), which could be differentially regulated according to URX stimulation.
Several computational models have been constructed to elucidate behavioral mechanisms underlying C. elegans taxis behavior (26–28). These models have been built using detailed observation of animals moving in gradients. A taxis model that incorporates quantitatively measured neural activities has not, however, been reported but is required to understand how neural signals are processed and transformed to behavior. We incorporated URX Ca2+ responses measured using GCaMP6s into a random walk model. These data can be extended into a more detailed model to study neural circuits of C. elegans at a systems level, e.g., incorporating activities of interneurons and motor neurons, to probe how encoded neural information in neural circuits are used to evoke worm behaviors.
C. elegans respond to changes in O2 by altering both their speed and their reversal behavior. Previous work has shown that worms use a klinokinesis strategy, where frequency of reversal is changed depending on the concentration of stimuli, when aerotaxing in the absence of food (29). This strategy resembles that used by worms chemotaxing to other cues such as salts and odors (26, 30). Our quantitative experiments show that the URX oxygen sensors evoke reversals in response to O2 stimuli that have only minor effects on speed. Our computational experiments can replicate the results of aerotaxis experiments without incorporating O2-evoked modulation of speed. These results imply that modulation of reversal is more important than modulation of speed when C. elegans navigates O2 gradients. The persistent stimulation of rapid movement when O2 levels approach 21% may conversely enable animals to escape from the surface when animals cannot detect an O2 gradient.
How does the GLB-5 neuroglobin alter the Ca2+ responses of neurons at a molecular level? Like mammalian neuroglobin (31), GLB-5 rapidly oxidizes to a ferric form at 21% O2 (9), suggesting it could participate in ROS or redox signaling. In URX, GLB-5 colocalizes with GCY-35/GCY-36 soluble guanylate cyclases at dendritic endings (10, 16) and could potentially regulate the function of this other heme-binding protein. Our cGMP imaging did not reveal GLB-5–dependent differences in the O2-evoked responses of URX. However, the cGMP dynamics we measured in the URX cell body were very slow compared with the Ca2+ response, which implies that we are measuring a highly filtered response compared with the cGMP dynamics pertaining at the cGMP-gated channel. Although we do not exclude a role for GLB-5 in regulating soluble guanylate cyclases, our data suggest that GLB-5 can alter neural responses independently of these molecules.
In summary, we find that a neuroglobin can participate in neural information processing. The C. elegans genome encodes a variety of other neurally expressed globins that may similarly modify neural function (8). It would be interesting to investigate whether neuroglobin alters information processing in vertebrate neural circuits.
Methods
Strains.
Animals were grown at 22–23 °C under standard conditions on Nematode Growth Medium (NGM) seeded with Escherichia coli OP50 (32). Strains used are listed in Supporting Information.
Neural Imaging.
Immobilized animals.
Animals expressing GCaMP6s or cGi500 were glued to agarose pads (2% in M9 buffer) using Dermabond tissue adhesive (Ethicon), with the nose and tail immersed in E. coli OP50 unless otherwise indicated. Glued worms were covered with a polydimethylsiloxane (PDMS) microfluidic chamber, as described previously (12), and imaged using a 40× C-Apochromat lens on an inverted microscope (Axiovert; Zeiss) equipped with a Dual View emission splitter (Photometrics) and a Cascade II 512 electron multiplying charge coupled device (EMCCD) camera (Photometrics). The filters used were as follows: GCaMP6s/mCherry, ex480/15 and 565/15 nm, di525/25 and 625/45 nm, em520/30 nm, em630/50 nm, and di565 nm; and YFP-CFP FRET, ex430/20 nm, di450 nm, em480/30 nm, em535/40 nm, and di505 nm. Fluorescent images were captured at 1 frame per second (fps) with 2 × 2 or 1 × 1 binning using MetaMorph acquisition software (Molecular Devices). Data analysis used MATLAB (MathWorks) and Igor Pro (WaveMetrics). All time-lapse imaging data were denoised using binomial smoothing (Gaussian filter).
Delivery of gas stimuli.
Humidified gas mixtures of defined composition were delivered using a PHD 2000 Infusion syringe pump (Harvard Apparatus). The flow rate was 1.0 mL/min for all ramp stimuli and 2.0 mL/min for all step stimuli. Syringes containing gas mixtures were connected to PDMS chambers via polyethylene tubing and Teflon valves (AutoMate Scientific). A custom-built frame counter switched the valves at precise time points using transistor–transistor logic pulses from the camera. To create the ramp stimulation, we used backlash air from the outlet of the PDMS chamber. O2 stimuli in chambers were measured using an O2 probe (Oxygen Sensor Spots PSt3; PreSens).
Behavioral Assays.
Aerotaxis assays were performed as described previously (11); animal positions were noted 25 min into the assay. Briefly, rectangular PDMS chambers (33 × 15 × 0.2 mm) connected at either end to syringe pumps delivering the indicated gas concentration were placed over 50–100 worms on a 9-cm NGM agar plate with food (E. coli OP50). The distribution of worms was recorded by counting animals in each of nine equal areas of the chamber.
To measure behavioral responses to step O2 stimuli, five adult hermaphrodites were placed on NGM plates seeded 36–40 h earlier with 20 μL of E. coli OP50 grown in 2× TY medium. To create a behavioral arena with a defined atmosphere, we placed a PDMS chamber (1 × 1 × 0.2 cm) on top of the worms, with inlets connected to a PHD 2000 Infusion syringe pump (Harvard Apparatus), and delivered humidified gas mixtures of defined composition at a flow rate of 3.0 mL/min. Videos were captured at 2 fps using FlyCapture software (Point Gray) on a Grasshopper camera (Point Gray) mounted on a Leica M165FC stereo microscope. Videos were analyzed using custom-written MATLAB software to calculate instantaneous speed. Instantaneous speed data were denoised by binning over 6 s. Reversal frequency was counted manually. If the posterior and anterior tips of a worm’s body moved backward until the worm stopped, this behavior was counted as one reversal; such events were often followed by turns.
Computational Experiments and Modeling.
In the computational model, a worm was represented as a single point in a virtual field that represented an O2 gradient in our experimental 18 mm (W) × 15 mm (L) aerotaxis chamber. O2 levels in the virtual chamber varied from 7% at W = 0 mm to 21% at W = 18 mm. The worm moved forward either at constant (∼0.05 mm/s) or at variable speed. For iterations when speed varied according to O2 concentration at the animal’s position we acquired parameters for speed by performing curve fitting with a Hill equation using the speed data shown in Fig. 4C (Fig. S11 B and C). The trend in an averaged time series was identified and removed based on the gradient of the time series. If the worm reached the edge of the chamber, the direction of forward movement was reflected. The model worm has three modules that correspond to the sensory neuron (URX), an interneuron, and a command neuron (Fig. 5A). The information signal about O2 level is transmitted from the sensory neuron to the command neuron through the interneuron. The activation of the command neuron causes a worm to start reversing. Reversals are expressed as a change in the direction of locomotion in the model. The locomotion direction after the reversal was randomly chosen from a uniform distribution because experimentally measured reversals contain turning events. We assumed that the relationship between URX responses and reversal frequency was approximately linear in our model. This applies because animals in the virtual O2 gradient, like those in a real-life aerotaxis assay, do not encounter large step O2 stimuli.
The dynamics of the sensory circuit were represented by an NLN model. The NLN model consisted of two nonlinear static filters and a linear temporal filter. O2 stimulation was first converted by the input nonlinear filter, processed by the temporal filter, then converted by the output nonlinear filter. The nonlinear filter was expressed using a Hill equation,
where and were the parameters that defined the range and strength of the nonlinearity of the filter, respectively. For convenience, the input and output nonlinear filter are hereafter denoted as and , respectively. The linear temporal filter has a 361 sample length () and satisfies
where and are the input and output time courses of the temporal filter, respectively. This typical expression of a temporal filter should be expanded because our dataset has multiple time courses (multidose). If O2 concentration is left as , can be written as
where corresponds to the O2 concentration at time of dth step stimulation and is replaced by . can be expressed as
where corresponds to the response of the sensory neuron at time in response to dth oxygen step stimulation. The linear temporal filter can be obtained by evaluating
For denoising, singular value decomposition was applied, and the largest 100 components were used. To find the value of parameters of nonlinear filters, the Nelder–Mead simplex optimization method was used, and the sum of the square difference between and corresponding experimental Ca2+ responses of URX were minimized. Because this optimization was done separately for glb-5(Haw) and glb-5(tm5440), we obtained two parameter sets for the NLN model.
The interneuron and command neurons were designed as described below. Because the worms show random reversals, the command neuron should be randomly activated. Furthermore, because the basal URX Ca2+ response (i.e., before stimulation of 2% change of oxygen) depends on the basal concentration of O2 but basal reversal frequency does not, the model should contain a temporal differentiation functionality. Therefore, the activity of interneuron was modeled as
where is a lag constant and was initially fixed as 11 and then varied (Fig. S9). The activity of command neuron is positive when
where is the basal reversal frequency that is computed from experimental data, is the coefficient of the effect of O2 stimulation, and is a uniformly distributed random number between 0 and 1. and c were fixed to 0.0723 (reversal frequency per 1 s before a stimulation is given) and 3, respectively; c was subsequently varied (Fig. S9). Note that the parameters of interneuron and command neuron (, , and ) are independent of the glb-5 genotype.
Strains
AX5890, glb-5(tm5440); npr-1(ad609); dbEx[gcy-32p::GCaMP6s, gcy-32p::mCherry, unc-122p::mCherry]
AX5891, glb-5(Haw); npr-1(ad609); dbEx[gcy-32p::GCaMP6s, gcy-32p::mCherry, unc-122p::mCherry]
AX6075 gcy-35(ok769); glb-5(tm5440); npr-1(ad609); dbEx[gcy-32p::GCaMP6s, gcy-32p::mCherry, unc-122p::mCherry]]
AX6088, glb-5(tm5440); npr-1(ad609); dbEx[gcy-32p::GCaMP6s, gcy-32p::mCherry, unc-122p::mCherry]; dbEx[gcy-32p::glb-5 (Haw)::sl-2::mCherry, unc-122p::GFP]
AX1891, glb-5(Haw); npr-1(ad609)
AX5935, gcy-35(ok769); glb-5(tm5440); npr-1(ad609)
AX5936, gcy-35(ok769); glb-5(tm5440); npr-1(ad609); dbEx[flp-8p::gcy-35::gfp, unc-122p::gfp]
AX6124, gcy-35(ok769); glb-5(tm5440); npr-1(ad609); dbEx[flp-8p::gcy-35::gfp, unc-122p::gfp], dbEx[flp-8p::glb-5::sl-2::mCherry, lin-44p::GFP]
AX1908, glb-5(Haw); npr-1(ad609) lin-15(n765ts); dbEx[gcy-32p::YC3.60, lin-15(+)]
AX5850, glb-5(tm5440); npr-1(ad609) lin-15(n765ts); dbEx[gcy-32p::YC3.60, lin-15(+)]
AX3535, glb-5(tm5440); npr-1(ad609)
AX2417, glb-5(Haw); cng-1(db111); npr-1(ad609); dbEx[gcy-37p::cGi500]
AX6024, glb-5(tm5440); cng-1(db111); npr-1(ad609); dbEx[gcy-37p::cGi500]
AX2084, glb-5(Haw); npr-1(ad609); dbEx[gcy-37p::cGi500]
AX6012, glb-5(tm5440); npr-1(ad609); dbEx[gcy-37p::cGi500]
AX6755 gcy-35(ok769); glb-5(tm5440); npr-1(ad609); dbEx[flp-8p::gcy-35::sl2::gfp, RMGp::GCaMP6s (ncs-1p::Cre, flp-21p::loxPstoploxP::GCaMP6s), lin-44p::GFP]
AX6838, gcy-35(ok769); glb-5(tm5440); npr-1(ad609); dbEx[gcy-32p::GCaMP6s, flp-8p::glb-5(Haw)::sl2::mCherry, flp-8p::gcy-35, lin-44p::gfp]]
AX6840, gcy-35(ok769); glb-5(tm5440); npr-1(ad609); dbEx[gcy-32p::GCaMP6s, gcy-32p::mCherry, flp-8p::gcy-35, lin-44p::gfp]]
AX6933, gcy-35(ok769); glb-5(tm5440); npr-1(ad609); dbEx[gcy-32p::GCaMP6s, flp-8p::glb-5(Haw)mut (del31A, G648A)::sl2::mCherry, flp-8p::gcy-35, lin-44p::gfp]]
Acknowledgments
We thank the National Bioresource Project (Japan) for strains; W. Schafer and Y. Iino for plasmids; and I. Beets, L. Fenk, T. Tomida, and S. Laughlin for comments on the manuscript. This work was supported by the Medical Research Council (MC_U105178786) and the European Research Council (AdG 269058) (to M.d.B.); the Uehara Memorial Foundation (S.O.); Grants-in-Aid for Young Scientists (B) (26830006 to Y.T.); and Grant-in-Aid for Scientific Research on Innovative Areas (16H01418 “Resonance Bio” and 17H05970) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to Y.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614596114/-/DCSupplemental.
References
- 1.Butts DA, Goldman MS. Tuning curves, neuronal variability, and sensory coding. PLoS Biol. 2006;4:e92. doi: 10.1371/journal.pbio.0040092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dayan P, Abbott LF. 2005. Theoretical Neuroscience: Computational and Mathematical Modeling of Neural Systems, Computational Neuroscience Series (MIT Press, Cambridge, MA)
- 3.Ward JP. Oxygen sensors in context. Biochim Biophys Acta. 2008;1777:1–14. doi: 10.1016/j.bbabio.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 4.López-Barneo J, et al. Oxygen sensing by the carotid body: Mechanisms and role in adaptation to hypoxia. Am J Physiol Cell Physiol. 2016;310:C629–C642. doi: 10.1152/ajpcell.00265.2015. [DOI] [PubMed] [Google Scholar]
- 5.Burmester T, Hankeln T. Function and evolution of vertebrate globins. Acta Physiol (Oxf) 2014;211:501–514. doi: 10.1111/apha.12312. [DOI] [PubMed] [Google Scholar]
- 6.Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- 7.Burmester T, Hankeln T. What is the function of neuroglobin? J Exp Biol. 2009;212:1423–1428. doi: 10.1242/jeb.000729. [DOI] [PubMed] [Google Scholar]
- 8.Tilleman L, et al. Globins in Caenorhabditis elegans. IUBMB Life. 2011;63:166–174. doi: 10.1002/iub.443. [DOI] [PubMed] [Google Scholar]
- 9.Persson A, et al. Natural variation in a neural globin tunes oxygen sensing in wild Caenorhabditis elegans. Nature. 2009;458:1030–1033. doi: 10.1038/nature07820. [DOI] [PubMed] [Google Scholar]
- 10.McGrath PT, et al. Quantitative mapping of a digenic behavioral trait implicates globin variation in C. elegans sensory behaviors. Neuron. 2009;61:692–699. doi: 10.1016/j.neuron.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray JM, et al. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- 12.Busch KE, et al. Tonic signaling from O2 sensors sets neural circuit activity and behavioral state. Nat Neurosci. 2012;15:581–591. doi: 10.1038/nn.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung BH, Arellano-Carbajal F, Rybicki I, de Bono M. Soluble guanylate cyclases act in neurons exposed to the body fluid to promote C. elegans aggregation behavior. Curr Biol. 2004;14:1105–1111. doi: 10.1016/j.cub.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Couto A, Oda S, Nikolaev VO, Soltesz Z, de Bono M. In vivo genetic dissection of O2-evoked cGMP dynamics in a Caenorhabditis elegans gas sensor. Proc Natl Acad Sci USA. 2013;110:E3301–E3310. doi: 10.1073/pnas.1217428110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prabhakar NR, Semenza GL. Oxygen sensing and homeostasis. Physiology (Bethesda) 2015;30:340–348. doi: 10.1152/physiol.00022.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross E, et al. GLOBIN-5-dependent O2 responses are regulated by PDL-1/PrBP that targets prenylated soluble guanylate cyclases to dendritic endings. J Neurosci. 2014;34:16726–16738. doi: 10.1523/JNEUROSCI.5368-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen TW, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung BH, Cohen M, Rogers C, Albayram O, de Bono M. Experience-dependent modulation of C. elegans behavior by ambient oxygen. Curr Biol. 2005;15:905–917. doi: 10.1016/j.cub.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Rogers C, Persson A, Cheung B, de Bono M. Behavioral motifs and neural pathways coordinating O2 responses and aggregation in C. elegans. Curr Biol. 2006;16:649–659. doi: 10.1016/j.cub.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Russwurm M, et al. Design of fluorescence resonance energy transfer (FRET)-based cGMP indicators: A systematic approach. Biochem J. 2007;407:69–77. doi: 10.1042/BJ20070348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 22.Macosko EZ, et al. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature. 2009;458:1171–1175. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurent P, et al. Decoding a neural circuit controlling global animal state in C. elegans. eLife. 2015;4:4. doi: 10.7554/eLife.04241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huckstepp RT, Dale N. Redefining the components of central CO2 chemosensitivity–towards a better understanding of mechanism. J Physiol. 2011;589:5561–5579. doi: 10.1113/jphysiol.2011.214759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chalfie M, et al. The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci. 1985;5:956–964. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierce-Shimomura JT, Morse TM, Lockery SR. The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J Neurosci. 1999;19:9557–9569. doi: 10.1523/JNEUROSCI.19-21-09557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh DD, et al. Neural architecture of hunger-dependent multisensory decision making in C. elegans. Neuron. 2016;92:1049–1062. doi: 10.1016/j.neuron.2016.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izquierdo EJ, Lockery SR. Evolution and analysis of minimal neural circuits for klinotaxis in Caenorhabditis elegans. J Neurosci. 2010;30:12908–12917. doi: 10.1523/JNEUROSCI.2606-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hums I, et al. Regulation of two motor patterns enables the gradual adjustment of locomotion strategy in Caenorhabditis elegans. eLife. 2016;5:5. doi: 10.7554/eLife.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iino Y, Yoshida K. Parallel use of two behavioral mechanisms for chemotaxis in Caenorhabditis elegans. J Neurosci. 2009;29:5370–5380. doi: 10.1523/JNEUROSCI.3633-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dewilde S, et al. Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J Biol Chem. 2001;276:38949–38955. doi: 10.1074/jbc.M106438200. [DOI] [PubMed] [Google Scholar]
- 32.Sulston J, Hodgkin J. In: The Nematode Caenorhabditis elegans. Wood WB, editor. CSHL Press; Cold Spring Harbor: 1988. pp. 587–606. [Google Scholar]