Abstract
Skeletal muscle in vertebrates is formed by two major routes, as illustrated by the mouse embryo. Somites give rise to myogenic progenitors that form all of the muscles of the trunk and limbs. The behavior of these cells and their entry into the myogenic program is controlled by gene regulatory networks, where paired box gene 3 (Pax3) plays a predominant role. Head and some neck muscles do not derive from somites, but mainly form from mesoderm in the pharyngeal region. Entry into the myogenic program also depends on the myogenic determination factor (MyoD) family of genes, but Pax3 is not expressed in these myogenic progenitors, where different gene regulatory networks function, with T-box factor 1 (Tbx1) and paired-like homeodomain factor 2 (Pitx2) as key upstream genes. The regulatory genes that underlie the formation of these muscles are also important players in cardiogenesis, expressed in the second heart field, which is a major source of myocardium and of the pharyngeal arch mesoderm that gives rise to skeletal muscles. The demonstration that both types of striated muscle derive from common progenitors comes from clonal analyses that have established a lineage tree for parts of the myocardium and different head and neck muscles. Evolutionary conservation of the two routes to skeletal muscle in vertebrates extends to chordates, to trunk muscles in the cephlochordate Amphioxus and to muscles derived from cardiopharyngeal mesoderm in the urochordate Ciona, where a related gene regulatory network determines cardiac or skeletal muscle cell fates. In conclusion, Eric Davidson’s visionary contribution to our understanding of gene regulatory networks and their evolution is acknowledged.
Keywords: skeletal myogenesis, muscle origins, second heart field, gene regulatory networks, cell lineages
Movement is a fundamental requirement for animal survival, both for finding food/feeding and for avoiding predators/locating to a propitious environment, and depends on cells with contractile properties, mainly based on actomyosin motor activity. In more sophisticated organisms, specialized contractile proteins are present in muscle cells. In vertebrates, striated muscle permits movement and also underlies the pumping activity of the heart, which, like the simpler peristaltic pumps present in the vascular system of many animals, performs a vital function in ensuring the circulation of nutrients within the body. Striated muscles contain a range of distinct and overlapping contractile protein isoforms, adapted to functional requirements of the motor activity of different skeletal muscles or of contractility in different cardiac compartments of the heart. Although the contractile apparatus is similar at the protein level, the upstream regulation of cardiac or skeletal muscle genes is different. Skeletal muscle formation depends on myogenic regulatory factors of the myogenic determination factor (MyoD) family, whereas in cardiac muscle, these basic helix–loop–helix factors do not play a role, and other families of transcription factors lead to the activation of striated muscle genes. The mesodermal progenitor cells that contribute to cardiac and skeletal muscle also have distinct embryological origins, although it has now emerged that the skeletal muscles of the head share common ancestry with the myocardium of the heart. In this review, the formation of vertebrate skeletal muscle will be discussed, with emphasis on the formation of head muscles and lineage relationships with the heart. The focus will be on amniote myogenesis, based on the mouse as a genetic model, with embryological background provided by experimental manipulations in avians.
Skeletal Muscles of the Trunk and Limbs
All of the skeletal muscles present in the trunk and limbs are derived from somites, segments of paraxial mesoderm that form progressively on either side of the body axis from the anterior to the posterior of the developing embryo (1). Muscles form after delamination of cells from the dorsal somite, the dermomyotome, which maintains an epithelial structure after cells in the ventral part of the somite have acquired a mesenchymal phenotype, forming the sclerotome, which subsequently gives rise to bone and cartilage of the vertebral column and ribs. At different axial levels, myogenic progenitors from the part of the somite adjacent to the axial structures of the neural tube and notochord will form the epaxial myotome, which contributes to back muscles, while the hypaxial myotome gives rise to body wall muscles. At limb level, myogenic progenitors migrate out into the limb bud and subsequently differentiate to form the muscles of the limb. Cell migration from cervical somites is also important for the formation of the diaphragm muscle more anteriorly. As development proceeds, the initial muscle primordia, under the action of surrounding connective tissue, become subdivided into distinct muscle masses; innervation takes place; and tendinous junctions with bones are established. In the initial waves of epaxial and hypaxial myogenesis, myogenic progenitors enter the muscle program and differentiate into muscle. It is only later, when the epithelial structure of the central dermomyotome breaks down and cells enter the underlying muscle masses, that some of these cells do not differentiate, but constitute a reserve of myogenic progenitors for subsequent muscle growth and later muscle regeneration. In the limbs, part of the population of myogenic cells is also retained as a progenitor pool.
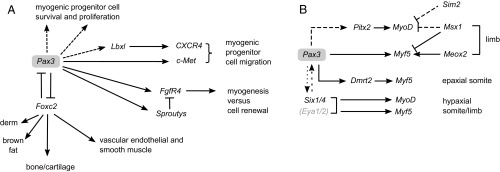
The gene regulatory network (2) that governs muscle progenitor cell behavior (Fig. 1A) and controls activation of the myogenic determination genes (Fig. 1B) is dominated by paired box factor 3 gene (Pax3) (3), which is first expressed in presomitic mesoderm immediately anterior to the first somite and then throughout the early epithelial somite, before becoming restricted to myogenic progenitors of the dermomyotome. Forkhead box protein factor c2 (Foxc2) is coexpressed with Pax3 in the early somite and then is expressed, together with Foxc1, at a high level in the sclerotome, where these Foxc transcription factors are required for the formation of bone and cartilage. Foxc2 remains expressed at a lower level in the dermomyotome, notably in the hypaxial domain. Genetic manipulations in the mouse embryo have shown that Pax3 and Foxc2 reciprocally inhibit each other (4). When the dose of Foxc2 is diminished, Pax3-dependent myogenic progenitors predominate, whereas diminution of the level of Pax3 favors nonmuscle cell fates, such as vascular smooth muscle or endothelial cells. Somitic cells are maintained in a multipotent stem cell state, until the balance is tipped toward Pax3 or Foxc2. In this model, signaling from adjacent cells can shift the equilibrium, as illustrated for Notch signaling in limb-level somites (5). In addition to its role in cell fate determination, Pax3 is required for cell survival in the somite, illustrating the point that, in the absence of a key upstream regulator, potentially dangerous unguided cells tend to die. Pax3 also contributes to control of the important choice between myogenic progenitor cell self-renewal vs. entry into the muscle differentiation program by regulating fibroblast growth factor (FGF) signaling, via the genes for Sprouty inhibitors and fibroblast growth factor receptor4 (FgfR), which is a direct Pax3 target. Myogenic progenitor cell migration also depends on Pax3, which directly activates the c-met gene. In the absence of c-Met receptor tyrosine kinase signaling, myogenic progenitors do not leave the somite, and muscles such as those in the limb are absent. The chemokine receptor type 4 (CXCR4) is also required for migration of a subset of myogenic progenitors into the limb. Activation of this gene depends on a transcription factor encoded by ladybird homeobox 1 (Lbx1), which lies genetically downstream of Pax3. Thus, Pax3 orchestrates key steps—cell fate choice, survival, self-renewal, and migration—in the progression of progenitor cells toward myogenesis.
Fig. 1.
Gene regulatory networks at the onset of myogenesis in the trunk and limbs, where Pax3 plays a central role in controlling many aspects of myogenic progenitor cell behavior (A) including choice of the myogenic cell fate, survival, proliferation, migration, and entry into the differentiation program, which depends on activation of the myogenic determination genes at different sites where skeletal muscle formation is initiated in the mouse embryo (B). derm refers to the dorsal dermis.
Pax3 also controls the onset of myogenesis (Fig. 1B). Entry into the myogenic program (2) depends on the myogenic determination factors MyoD and myogenic factor 5 (Myf5), with myogenic regulatory factor 4 (Mrf4) performing this function with Myf5 when muscle formation is initiated in the somite, before expression of MyoD. Muscle-cell differentiation depends on subsequent expression of the myogenic differentiation factor, Myogenin, which, together with other transcription factors, such as myocyte enhancer factor 2 (Mef2) family members, plays an essential role in activating downstream muscle genes. The myogenic determination factors, notably Mrf4 and MyoD, can also perform this function. Activation of Myf5 is controlled by Pax3, which directly regulates enhancer sequences that govern most of the spatiotemporal expression of this early myogenic determination gene. The early epaxial enhancer that controls Myf5 expression in the epaxial somite does not depend directly on Pax3, but is activated by wingless-related integration site (WNT) signaling molecules signaling from the neural tube and by doublesex- and mab-related transcription factor 2 (Dmrt2), which is also implicated in maintaining somite integrity. Dmrt2 is a direct Pax3 target. Later activation of MyoD is not directly dependent on Pax3, but depends on other factors acting at different sites of myogenesis, including the transcription factor encoded by the paired-like homeodomain factor 2 (Pitx2), which lies genetically downstream of Pax3, acting in part of the hypaxial somite and the limb. During the migration of myogenic progenitors to the limb, it is important to prevent the premature onset of myogenesis. Msx1 directly represses the limb enhancer of Myf5 and also delays the activation of MyoD. Sim 2 also performs this function in the limb. In this context, Meox2 plays a role in the onset of activation of the Myf5 limb enhancer (6). Signaling pathways also control the spatiotemporal activation of the myogenic determination genes. Shh signaling from the notchord and floor plate, as well as from the zone of polarizing activity in the limb, directly acts on Myf5 enhancers to up-regulate them in the epaxial somite and limb bud, respectively. WNT signaling is also promyogenic, whereas bone morphogenetic protein (BMP) signaling prevents the expression of Myf5 and MyoD. In the somite, BMP activity from the dorsal neural tube or lateral mesoderm is buffered by inhibitors such as noggin. In general, the effects of signaling pathways on the onset of myogenesis are fine-tuned by a complex combination of components that modulate the signal at precise sites where muscle will form as development proceeds (1).
Sine oculis homeobox transcription factors 1/4 (Six1/4), with their coactivators Eya1/2, are also important players in myogenesis (2). Together with Pax3, they control the enhancers responsible for hypaxial somite and limb expression of Myf5. They also directly activate enhancer elements on the MyoD gene. In Six1/4/Myf5(Mrf4) compound mutants, MyoD is not activated, and skeletal muscles do not form in the body and limbs. Premature activation of the myogenic determination genes in the limb is prevented by Dach, which is a corepressor of Six. Unlike Pax3—which, if maintained in muscle cells, inhibits differentiation—Six factors activate Myogenin and, together with Myogenin, regulate downstream muscle genes. In the Six1/4 double mutant, Pax3 expression is compromised, suggesting that these factors also play an upstream role. Reciprocally, in the Pax3/Myf5(Mrf4) compound mutant, which has a similar phenotype to the Six1/4/Myf5/(Mrf4) mutant, Six1/4 do not rescue MyoD activation, indicating that Pax3 lies genetically upstream of Six1/4. In this discussion, the focus is on Pax3; however, the sister gene, Pax7, is coexpressed in the central domain of the dermomyotome and becomes the predominant Pax factor in myogenic progenitors, because Pax3 is down-regulated during fetal development. In the perinatal/postnatal period and in satellite cells, the myogenic progenitors that permit adult muscle regeneration, Pax7 predominates (3).
Skeletal Muscles of the Head and Neck
In mesoderm anterior to the first cervical somite, the distinction between paraxial and lateral splanchnic mesoderm is not clear-cut. During development of this region, protrusions known as branchial or pharyngeal arches progressively form in the pharyngeal region on either side of the embryo. Mesoderm protrudes into these pouches, overlain by surface ectoderm with underlying pharyngeal endoderm. Many head muscles (known as branchiomeric muscles) derive from this pharyngeal mesoderm (7). The first branchial arches give rise to muscles, such as the masseter and temporalis that control jaw movements, whereas the second arches are the source of myogenic progenitors of the facial expression muscles. The mesodermal core of the more posterior arches (numbered 3, 4, and 6) contributes pharyngeal and laryngeal muscles and also some neck muscles, which are not somite-derived. The skeletal muscle of the mammalian esophagus is also of nonsomitic origin. Myogenic progenitors from the pharyngeal region colonize this structure, moving posteriorly along the smooth muscle layer into the body from the early fetal stage (8). Somitic cells that have moved anteriorly after delamination contribute to some muscles positioned in the head region, as seen for intrinsic laryngeal and tongue muscles that derive from myogenic cells in the hypoglossal chord, a structure formed from the hypaxial region of occipital somites. These cells move anteriorly as a coherent mass and begin to activate myogenic determination genes en route; however, as for myogenic progenitors that migrate to the diaphragm or limb, formation of the hypoglossal chord depends on Pax3 activation of c-met (3). The six extraocular muscles (7), which constitute the most anterior muscles in the embryo, are thought to be mainly derived from prechordal mesoderm, which, as its name implies, lies anterior to the notochord and is contiguous laterally with paraxial mesoderm. A distinguishing feature of head vs. body myogenesis is the major role played by neural crest in the development of head musculature (7). In the trunk, neural crest cells that migrate out from the dorsal neural tube transitorily affect early epaxial myogenesis (9). However, anteriorly, neural crest cells invade the branchial arches and occupy the head region, giving rise to the connective tissue, as well as to the craniofacial skeleton and smooth muscle cells of the vasculature. Connective tissue, which signals to myogenic progenitors and models maturation of the muscle masses, is therefore of neurectodermal origin in the head, whereas it is mesodermal in the body. Myogenic cells from the branchial arches or from prechordal mesoderm undergo extensive cell displacement to form the developing muscle masses, moving as coherent groups of cells that have often already activated the myogenic determination genes. This movement is in contrast to the migration of isolated progenitor cells from trunk somites to the diaphragm or limbs. Nonsomitic muscles differentiate during fetal stages, so that most head muscles are clearly distinguishable at embryonic day 14.5 (E14.5) in the mouse embryo (10), and the esophagus skeletal muscle is only laid down at E15.5 (8). This process differs from the first skeletal muscles of the trunk, the myotomes, which form immediately adjacent to the somites from E8.5 (1).
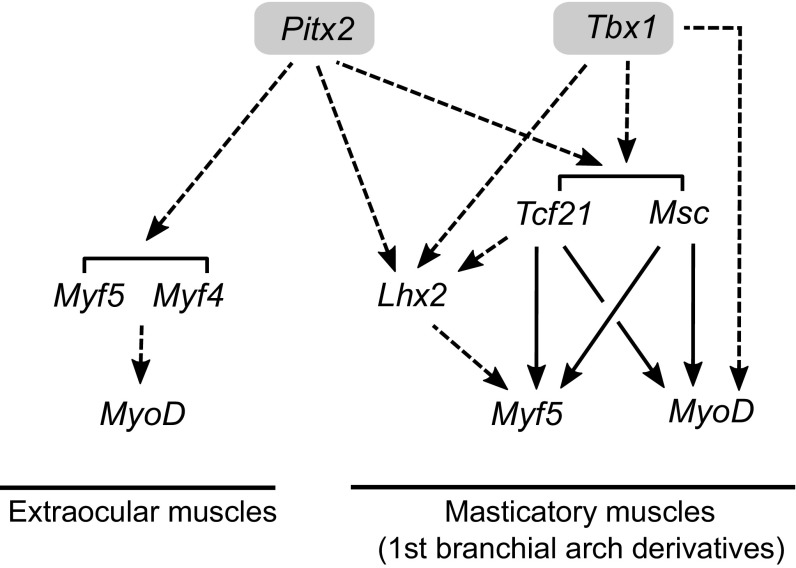
In general, the same downstream muscle genes are expressed in skeletal muscles of the head and body, although head muscles may express specific isoforms (11), exemplified by extraocular myosin heavy chain. Formation of muscles in the head, like those in the body, depends on the myogenic regulatory genes of the MyoD family. Different combinations of myogenic determination genes are expressed in cells at different sites of myogenesis (12). Activation of the extraocular muscle program depends on Mrf4 and Myf5, whereas MyoD, together with Myf5, is a driver of myogenesis in cells that will form branchiomeric muscles. However, the upstream gene regulatory network (2) that leads to activation of myogenic determination genes is different. Pax3 is not a player. Pax7 is only activated later during fetal development, when it marks the reserve cell population of myogenic progenitors and is also a regulator of adult satellite cells, as in the trunk and limbs. Six genes are expressed in nonsomitic muscle progenitors. However, the Six1/4 mutant does not have a phenotype in these muscles, possibly because of compensation by Six5, coexpressed with Six1 and Six4 in head muscle progenitors (13). Pitx2 and Tbx1 (T-box factor 1 gene) are major upstream regulators of head myogenesis (Fig. 2). In extraocular muscle progenitors, Pitx2 is required both for cell survival and for activation of the myogenic determination genes Myf5 and Mrf4. In the Pitx2 mutant, MyoD is not activated, and eye muscles do not form. Similarly, in the first branchial arch, where a mesodermal deletion of Pitx2 has been examined in detail, myogenic cell survival and growth are compromised, with failure to activate myogenic determination genes, leading to defective masticatory muscle formation. The role of Pitx2 therefore resembles that of Pax3 at sites of myogenesis in the body. The upstream regulation of Pitx2 in the head is not well understood, but it is expressed at higher levels in extraocular compared with limb muscle progenitors. In Tbx1 mutants, branchial arches posterior to the first fail to develop, and masticatory muscles derived from the first arch are hypoplastic, with sporadic unilateral formation. Tbx1, like Pitx2, is not only expressed in mesoderm; however, a mesodermal specific deletion demonstrates the direct function of Tbx1 in this tissue. In Tbx1/Myf5 double mutants, muscles derived from the first branchial arch are absent, indicating that Tbx1 lies genetically upstream of MyoD in this context where MyoD would normally determine myogenic cell fate in the absence of Myf5. In the gene regulatory network that leads to myogenesis in the arches (Fig. 2), genes encoding mesenchymal stem cell transcription factor (Msc or MyoR) and transcription factor 21 (Tcf21), basic helix–loop–helix transcription factors expressed in myogenic progenitors, also play a role. In the absence of Msc, Tbx1 is up-regulated with reduction of Myf5 and MyoD expression, consistent with maintenance of a progenitor cell pool. In the double Msc/Tcf21 mutant, major masticatory muscles are lost, and Myf5 expression is down-regulated. These factors bind regulatory sequences of both Myf5 and MyoD genes with functional consequences shown for Myf5 transcription. The LIM homeobox 2 (Lhx2) transcription factor is also an intermediate in branchiomeric myogenesis. Lhx2 acts genetically upstream of Myf5, and its mutation leads to defects in the specification of myogenic cells and abnormal head muscle patterning. It lies genetically downstream of Tbx1, Pitx2, and Tcf21, which bind to putative regulatory elements of Lhx2. In Tbx1/Lhx2 double mutants, branchiomeric muscles are absent. This finding is consistent with Lhx2 and Tbx1 activation of Myf5 and MyoD, respectively.
Fig. 2.
Gene regulatory networks that control the formation of head muscles, highlighting the role of Tbx1 and Pitx2 in extraocular and first branchial arch-derived myogenic progenitors, for which most functional information is available in the mouse embryo.
Not only are the upstream transcriptional regulators distinct, but the impact of signaling pathways on activation of the myogenic program is different from that documented for the somites. Thus, WNT signaling is inhibitory, and sonic hedgehog signaling (Shh) appears to have little effect (7). Signals are released by surrounding tissues, and notably by neural crest, which is a major component of the anterior region of the embryo where muscles form. Neural crest cells release the WNT antagonist Frzb and also noggin and gremlin to counteract BMP signaling, which, as in the trunk, inhibits myogenesis. In the pharyngeal arches, FGF signaling plays a major role in regulating progenitor cell proliferation vs. differentiation (14).
Genetic tracing also underlines the differences between head and body muscles. By using a Pax3Cre allele as a driver for reporter gene expression from a floxed Rosa26 allele, all skeletal muscles in the trunk and limbs are marked by the reporter, whereas this labeling is not seen for non-somite-derived muscles (3). Lbx1Cre is also a useful tool (15) because it marks myogenic progenitors that migrate from the somite, without the confounding effect of Pax3 expression in cranial neural crest. Comparison with a mesodermal posterior 1 Cre (Mesp1Cre) driver, expressed in head muscle progenitors, distinguishes these muscles. Many non-somite-derived muscles are also marked when the Cre is under insulin gene enhancer protein (Isl1) control (14). This labeling is seen for muscles in the neck region, as well as facial expression muscles, whereas masticatory muscles derived from the first branchial arch are partially labeled. Isl1 mutants die before these muscles form, but overexpression of this transcription factor represses myogenesis, consistent with the expression of Islet1 in myogenic progenitor cells. An enhancer of the Mef2C gene also drives reporter gene expression in some branchiomeric muscles, including those in the neck (16), whereas somite-derived muscles are not labeled. The neck constitutes a transition zone where some muscles, like those in the body, are somite-derived, whereas others arise from pharyngeal mesoderm similar to head muscles (17).
Cardiac Muscle and the Second Heart Field
Many of the genes that mark and regulate nonsomitic skeletal muscle formation are also major players in cardiogenesis (18, 19). The early heart tube forms from ∼E7.5 in the mouse embryo by fusion at the midline of a bilateral cardiac crescent containing differentiating cells. These cells derive from lateral splanchnic mesoderm. Subsequently, the tube grows by the addition of cardiac progenitor cells that initially lie medial and caudal to the crescent and then behind the developing heart tube and in adjacent pharyngeal mesoderm. These cells constitute the second heart field (SHF) (20). The initial tube will mainly contribute the myocardium of the left ventricle. This early cardiac tube and the differentiated cells of the cardiac crescent that precedes it are considered to be derivatives of the first heart field. At the arterial pole, the SHF is the major source of the right ventricle and also of all of the myocardium of the outflow tract, which will constitute the base of the pulmonary trunk and aorta. The SHF contributes the myocardium of the venous pole at the base of the pulmonary vein and superior caval veins, as well as the dorsal part of the right and left atria (21). Proliferating cardiac progenitor cells lying posterior to the heart feed into the tube over a period of several days, such that venous pole myocardium is still forming at E12.5. At the arterial pole, as the branchial arches develop, mesoderm in the core of the arches is recruited into the heart tube, exemplified by labeling of cells in the second branchial arch that contribute to the distal outflow tract at E9.5 (22). The heart tube is initially positioned adjacent to the first branchial arches as they form, subsequently moving caudally as a result of morphogenetic movements, so that it becomes aligned with the more posterior arches. The tube undergoes looping, resulting in the anterior positioning of both poles, and expansion of the cardiac chambers with their subsequent septation. This complex morphogenesis, which occurs as the heart beats, also involves neural crest cells, which invade the anterior SHF and play a major role in septation of the outflow region and valve formation.
The myogenic regulatory factors of the MyoD family are specific to skeletal muscle. The formation of cardiac muscle (23) depends on a combination of more widely expressed transcription factors—such as NK homeobox protein 2-5 (Nkx2-5), GATA DNA binding factor 4 (Gata4), and members of the Mef2 family—that activate downstream striated muscle genes, some of which encode proteins also present in skeletal muscle. In this case, regulation often depends on skeletal or cardiac-specific enhancers, as for the α-cardiac actin gene (24). Expression of cardiac or skeletal muscle-specific isoforms of the same gene family also distinguish the two striated muscles (11). In general, head vs. body muscles do not preferentially express cardiac isoforms. An exception is the expression of an α-myosin heavy chain, otherwise specific to the heart, found in tongue and some mammalian jaw muscles, where the presence of the cardiac transcription factor Nkx2-5 has also been reported. These muscles are labeled after genetic tracing with an Nkx2.5Cre (25). Nkx2-5 is expressed in cardiac progenitors of the SHF, as well as in the myocardium, and plays an important role in cardiogenesis, as evidenced by the severe developmental defects in Nkx2-5 mutant hearts (26). Other regulatory genes, discussed in the context of nonsomitic skeletal muscle progenitors, are also essential for the formation of the heart (18, 27). Genetic tracing with Mesp1Cre, expressed in precardiac mesoderm in the primitive streak, marks myocardium in all compartments of the heart and its progenitors. Mesp1 mutants have cardiac defects. Islet1 has been considered as a marker of the SHF, although it is expressed at a low level in the cardiac crescent. The Isl1 mutant has a phenotype typical of SHF function with major defects affecting the right ventricle and the atria, leading to embryonic death (28).
The SHF is a heterogeneous population of cardiac progenitors, with regional patterns of gene expression (27). Pitx2 is initially expressed in the left SHF, reflecting its response to Nodal signaling in left/right patterning. Pitx2 mutants have outflow tract defects, and left vs. right atrial specification is lost, with mutant hearts showing right atrial isomerism, due to the loss of Pitx2 repression of right atrial identity in progenitors of the left SHF. Pitx2 also represses progenitor cell proliferation in the sinus venosus. This repressive effect is in contrast to the proliferative defects in skeletal muscle progenitors reported for the Pitx2 mutant. However, the function of Pitx2, its cofactors, and targets are poorly understood in these contexts. Tbx1 expression is restricted to an anterior domain of the SHF. In its absence, outflow tract formation is defective, and pulmonary trunk myocardium is reduced, leading to a common arterial trunk at the arterial pole of the heart. TBX1 is a major candidate gene for Di-George syndrome, which is characterized by congenital heart defects, notably the arterial pole malformation known as Tetralogy of Fallot, thought to be due to reduction of subpulmonary myocardium. In addition to the cardiac phenotype, absence of Tbx1 results in craniofacial defects, including defects in branchial arch-derived skeletal muscles (29). Although its expression is limited to the anterior part of the SHF, Tbx1 is also implicated in the addition of cells to the venous pole of the heart (30), associated with an arterial pole contribution from the posterior SHF (Cell Lineage Analyses). Another marker of cells in the anterior SHF is provided by a transgene under the control of an SHF enhancer of the Mef2c gene. In this case, the complete outflow tact and right ventricular myocardium are labeled, suggesting that this element is active in a larger progenitor cell population than Tbx1. An extensive gene regulatory network is active in the SHF and its subdomains (27, 19); however, cells expressing many of these genes have not been genetically traced in head muscles, and mutant phenotypes have not been documented in this context, apart from the examples discussed here. In many cases, conditional mutants would be required because genes that are essential for heart development result in death of the embryo before head muscle formation, as seen for Foxc1/2 genes, which are expressed in the SHF and are required for arterial pole development. These transcription factors regulate Isl1 and are potential regulators of Tbx1 (18); however, any role in cardiac/skeletal muscle cell fate specification equivalent to their role in the somite context remains to be explored.
Within the mesodermal core of the branchial arches, where progenitor cells for cardiac and skeletal muscle are present, distal and proximal domains can be distinguished, in which MyoD and Myf5 have been activated or where expression of Isl1 and other SHF markers are maintained (14). Signaling pathways functioning in pharyngeal mesoderm affect the behavior of these progenitor cells. FGF signaling from surrounding ectoderm, pharyngeal endoderm, and neural crest, as well as from the SHF cells themselves, promotes proliferation and prevents premature differentiation in both skeletal and cardiac progenitors. BMP signaling, conversely, has opposite effects, inhibiting differentiation of skeletal muscle progenitors and promoting cardiac differentiation as progenitors are recruited into the cardiac tube. BMP and WNT signaling will tend to inhibit myogenesis of head muscles (7), which may be why myogenic regulatory genes are only activated in the distal domain of the arches.
The overlap between paraxial and lateral splanchnic mesoderm and the expression of SHF genes in skeletal muscle progenitors in pharyngeal mesoderm raise the question of a common progenitor for cardiac and skeletal muscle. However, these observations do not distinguish between the presence of a common progenitor or the coexistence of distinct progenitors.
Cell Lineage Analyses
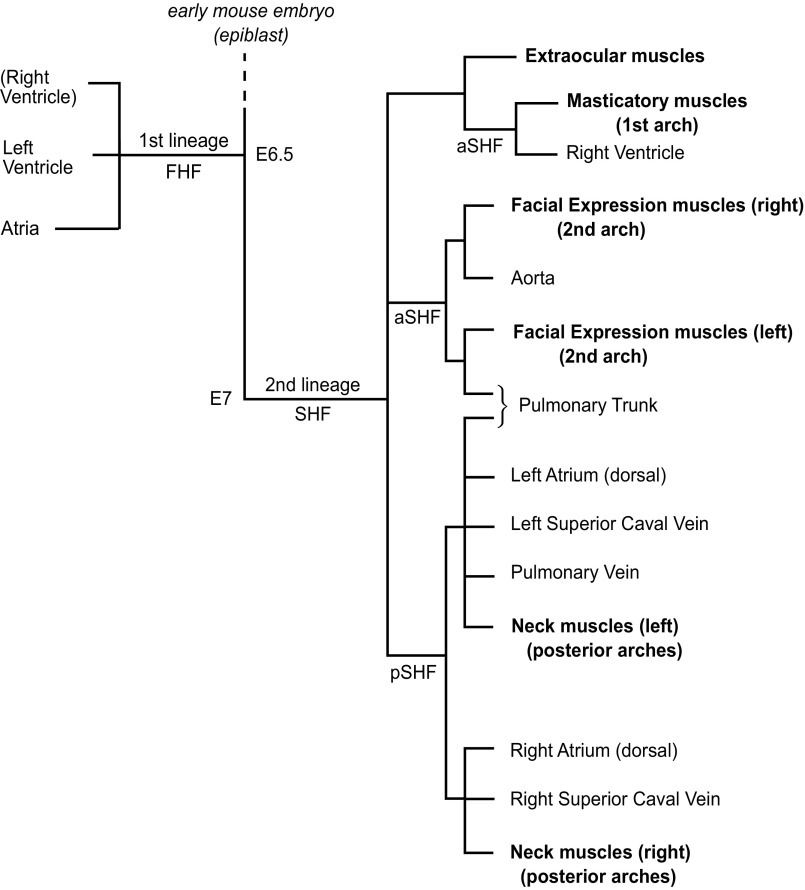
Gene-expression profiles and functional analyses cannot resolve this issue, which can be addressed by cell lineage studies (31). In the avian embryo and, to a limited extent, in cultured mouse embryos, grafting experiments or introduction of dyes or other markers have made it possible to follow the fate of progenitor cells. However, these procedures usually involve labeling groups of cells and have temporal limitations. Genetic analyses in the mouse embryo, adapted to clonal conditions, provide an alternative approach. Retrospective clonal analysis (32) is a powerful method that does not involve any preconceived idea about the location of progenitors. This method depends on the use of a reporter sequence, which contains a duplication that introduces a stop codon, so that the reporter protein is not made. A rare intragenic recombination event resulting in removal of the duplication leads to a functional coding sequence. Thus, a lacZ sequence containing such a duplication (laacZ) will only encode functional β-galactosidase if the duplication is recombined so that it reverts to lacZ. To look at skeletal and cardiac muscle, a laacZ sequence was introduced into an allele of the α-cardiac actin gene, expressed in developing skeletal muscle, as well as in the myocardium. Collections of several thousand embryos were examined for β-galactosidase staining in both types of muscle. Statistical analysis of the frequency of labeling is essential to establish that this labeling results from a single recombination event in a progenitor cell and thus establish clonality. This approach had demonstrated that two cell lineages form the myocardium of the heart (33), with the contribution of the second lineage corresponding to that of the SHF. Analysis of head and neck muscles led to the lineage tree shown in Fig. 3. Somite-derived muscles are not clonally related to myocardium, whereas nonsomitic muscles of the head (10) and neck (16) derive from common progenitors of the second myocardial cell lineage. They have no clonal relationship with muscles of the trunk and limbs. This finding demonstrates that these two skeletal muscle groups not only differ in terms of the regulatory gene network that controls their entry into myogenesis, but also derive from different embryological cell lineages. A first sublineage that forms nonsomitic muscles is constituted by progenitors for the temporalis and masseter muscles derived from the first branchial arches. Extraocular muscles are related to this group, suggesting that paraxial mesoderm as well as prechordal mesoderm contribute to eye muscles. The first branchial arch-derived skeletal muscles show clonality with right ventricular myocardium. Facial-expression muscles, derived from the second branchial arches, are clonally related to the outflow region of the heart, to myocardium at the base of the pulmonary trunk and the aorta. A third sublineage forms the nonsomitic muscles of the neck, notably the trapezius and sternocleidomastoid muscles, and including muscles of the larynx and the nonsomitic component of the splenius muscle (16). This finding is in keeping with observations of Pax3 independence and Tbx1 dependence of the trapezius and sternocleidomastoid muscles (34). The trapezius group of muscles is clonally related to the venous pole of the heart, including the right and left atria, as well as the myocardium at the base of the pulmonary vein and superior caval veins. This sublineage is clonally distinct from the second branchial arch sublineage that contributes to the pulmonary trunk and aorta at the arterial pole of the heart. However, the third sublineage also contributes to the pulmonary trunk, indicating that this myocardium has two origins. This dual origin is of major clinical importance because many heart malformations seen at birth involve this component of the arterial pole (18, 19). The function of Tbx1 at both poles of the heart probably reflects its implication in this sublineage (30). Dye-I labeling of cells in cultured mouse embryos shows that some cells from the posterior part of the SHF, which is the source of myocardial cells at the venous pole of the heart, migrate anteriorly, acquire gene expression patterns typical of the anterior SHF, and contribute to outflow tract myocardium that will form the base of the pulmonary trunk (35).
Fig. 3.
A lineage tree for the myocardium of the heart and for nonsomitic head and neck muscles (in bold), based on retrospective clonal analyses in the mouse embryo. In the case of nonsomitic neck muscles, clonal analysis focused on the trapezius and sternocleidomastoid muscles. The two myocardial lineages can be compared with the two heart fields that constitute sources of equivalent parts of the myocardium. a, anterior; FHF, first heart field; p, posterior.
The three sublineages for nonsomitic skeletal muscles correspond to derivatives of the first, second, and more posterior branchial arches. Their contribution to different regions of the myocardium reflects the progressive posterior movement of the heart in relation to the arches during pharyngeal morphogenesis. The skeletal muscles that derive from the arches show left/right segregation of clones. In the case of second arch derivatives, this segregation correlates with left-hand clones that also colonize the base of the pulmonary trunk, whereas clones on the right-hand side of the face colonize the base of the aorta (10). Similarly for trapezius and sternocleidomastoid neck muscles, left/right labeling correlates with clones in the left part of the venous pole—the left atrium, left superior caval vein, and pulmonary vein, as well as the pulmonary trunk at the venous pole—vs. right-hand labeling in the right atrium and right superior caval vein (16). This left/right segregation takes place at the onset of gastrulation (36). Deducing the timing of events in retrospective clonal analysis (37) depends on how widespread the phenomenon is across sublineages and also on the size of clones, which reflects how early the recombination event took place. Rarely, very large clones label most skeletal muscles and all compartments of the heart, reflecting a recombination event in the very early embryo. In most cases, it is possible to estimate an approximate timing of sublineage segregation based on clone size and therefore an estimated number of progenitor cell divisions. The segregation of somitic and nonsomitic muscles lineages is probably a very early event, as also suggested by fate-mapping experiments (38). In the case of extraocular muscle clones, some showed labeling in head muscles derived from the second as well as the first branchial arches, and in these cases, also had extensive labeling in the heart, including the left ventricle. This finding suggests that recombination took place in a progenitor that predates the segregation of the two myocardial cell lineages during gastrulation (10), perhaps reflecting the prechordal mesoderm contribution. However, other clones extended only to labeling in muscles derived from the first branchial arch, indicating a later common progenitor. The common progenitors that contribute to each of the branchiomeric muscle groups and to the corresponding parts of the heart probably segregate to give rise to the different nonsomitic muscles lineages during or after gastrulation, after the segregation of the second myocardial cell lineage (Fig. 3). The lineage segregation for head or neck muscles from myocardium is a late event in the context of heart development. A late-segregation event is consistent with the shared branchial arch origin of many skeletal and cardiac derivatives and the expression of SHF marker genes in some of these skeletal muscle progenitors.
Sophisticated genetic tracing (31) has the potential for more precise estimates of the time of lineage segregation. By using inducible conditional reporter genes, clonal analysis can be achieved with limiting levels of inducer given over a narrow time window. This approach is exemplified by induction by doxycycline of a Mesp1–rtTA transgene with a responder Tet0–Cre transgene on a genetic background with a conditional Rosa–Confetti reporter. These experiments confirmed the presence of two myocardial cell lineages and showed that the second lineage also contributes to head muscles (39). The timing of doxycycline administration indicated that the first myocardial lineage is established at ∼E6.5 (E6.25–6.75), whereas the second emerges later, at ∼E7 (E6.75–7.25). By using the Confetti reporter, all cell types are visualized, with the demonstration that the first lineage gives rise to monopotent endothelial or cardiac muscle cells, whereas the second lineage generates bipotent cardiac muscle/endothelial, cardiac muscle/smooth muscle, or cardiac muscle/skeletal muscle cell types. Alternative genetic approaches (40) to clonal analysis of cardiac progenitors have used a Mesp1Cre–MADM (mosaic analysis with double markers) method or the conditional Rosa–Confetti reporter with a tamoxifen-inducible Smarcd3–CreERT2 transgene. The transgene is driven by an enhancer of Smard3, encoding a chromatin-remodeling factor expressed early in a subset of Mesp1-positive cardiac progenitor cells. In these experiments, two myocardial cell lineages with the properties described above were identified; however, no labeling of head muscles was reported. The presence of a fluorescent reporter in such inducible Cre genetic approaches also opens the possibility of cell separation by flow cytometry and single-cell transcriptome analyses of different cardiac progenitor types (39), including cardiac/skeletal muscle progenitor cells from the developing embryo.
Evolutionary Considerations
All vertebrates have somites as a source of trunk and limb/wing/fin muscles, with a conserved gene regulatory network underlying myogenesis. There is a great diversity in the deployment of head muscles between vertebrates, according to their feeding requirements and predatory behavior (7); however, the origin and genetic regulation of these muscles is conserved. Many branchiomeric muscles seen in mammals and birds have their homologs in gnathostome (jawed) fish, such as the shark, and cyclostomes (jawless) fish, such as the hagfish or lamprey, also have some of these muscles, together with extraocular muscles and an additional somite-derived group of epibranchial muscles. In the lamprey, homologous genes to Isl1, Nkx2-5, and Tbx1 are expressed in myogenic progenitors of branchiomeric muscles. The link between head and heart is therefore present, with the concept of a cardiopharyngeal field as a mesodermal source of common progenitors (17). In this context, it is intriguing that the emergence of a heart with cardiac chambers (41), with its implications in terms of two myocardial cell lineages, occurred in parallel with head muscle diversity at the base of vertebrate radiation.
Chordates, which are closely related to vertebrates, show interesting analogies with vertebrate muscle formation. The cephalochordate Amphioxus has somites, where a Pax3/7 gene homolog (42) is expressed, and forms myotomal muscles with expression of two MyoD family genes (43). It has branchiomeric head muscles analogous to those of jawless fish (44), which are lost at metamorphosis and then reformed in the adult. However, their formation appears to be independent of myogenic regulatory genes, expressed exclusively in somites (43). Nevertheless, homologs of the same upstream regulatory genes, such as Tbx1/10 (45) or Isl1 (46), are expressed in this pharyngeal region in the embryo. Amphioxus does not have a well-defined heart, although the contractile branchial artery expresses some cardiac markers, such as the homolog of Nkx2-5 (47), and the analogy with cardiopharyngeal mesoderm, which gives rise to heart and head muscles is less evident.
Urochordates, such as the ascidian Ciona, do not form somites and lack the Pax3 gene regulatory network. As in vertebrates, skeletal muscle formation depends on myogenic regulatory genes, represented by a homolog of the MyoD gene family, which is activated at a high level in the few cells in the embryo that are destined to form muscles, exemplified by the tail muscle cell lineage (48). Ciona has a clearly defined beating heart and provides a striking example of the conservation of genetic circuitry in the cardiopharyngeal mesoderm that will form both cardiac and skeletal muscle (17). The heart derives from two Mesp1-positive cells that go on to generate bipotent cells in trunk ventral mesoderm that give rise to both the heart and atrial siphon muscles. These cardiopharyngeal progenitors express homologs of genes such as Nkx2-5 and Tbx1, and functional studies have shown a remarkable degree of conservation with the vertebrate gene regulatory network, reminiscent of cells in the SHF (49). Isl1 is also expressed in these progenitors, although it is maintained in the muscle derivatives, whereas in vertebrates, Islet1 is thought to repress skeletal myogenesis (14). Another apparent difference is the upstream role of Collier/Olf1/EBF in specifying muscle identity in atrial siphon muscles at the expense of heart myocardium. In Drosophila, the homolog of Collier confers identity to specific muscles, as indeed does the Isl1 homolog Tup (50). A myogenic function for Collier may also exist in vertebrates where the homologous genes Ebf2 and Ebf3 have been reported to be potential upstream regulators at sites of muscle formation, including jaw muscles, in Xenopus (51). Another intriguing characteristic of the Ciona cardiopharyngeal gene regulatory network is antagonism between the Nkx2-5 homolog NK4 and Tbx1/10, such that NK4 promotes activation of GATAa (Gata 4/5/6) and heart formation, whereas Tbx1/10 inhibits GATAa and activates Collier, leading to skeletal muscle formation (52), a situation reminiscent of the Pax3:Foxc2 antagonism that controls cell fates in the mouse somite (4). In general, conserved fragments of gene regulatory networks, even in remote species, are instructive, a classic example being the Paired (Pax)/Sine oculis (Six)/Eyes absent (Eya) network first identified for the Drosophila eye (53), which is echoed in the upstream regulation of trunk and limb myogenesis.
Concluding Remarks
Our understanding of the evolution of gene regulatory network circuitry owes much to Eric Davidson’s conceptual insight (54). His and his colleagues’ dissection at a single-cell level of transcriptional regulation in the early sea urchin embryo was a tour de force that led to the precise delineation of gene regulatory networks, such as that governing the specification of the endoderm (55). The gene regulatory networks discussed here for skeletal muscle formation in the mouse embryo are very crude by comparison. They are averaged over populations of cells and lack precise spatiotemporal resolution. As discussed in the context of current studies on myocardial cell lineages, the use of inducible genetic tools and fluorescent markers opens the way to single-cell analysis, but reconstructing lineages and networks at this level in the mouse embryo, or other vertebrates, remains a challenge. In this context, the ascidian Ciona, with a small number of well-defined embryonic progenitor cells, is much more amenable. However, research on vertebrate gene regulatory networks and the nature of cell heterogeneity in progenitor cell populations continues to advance. Eric Davidson’s pioneering work provides a conceptual framework for current and future understanding of how gene regulatory networks function to determine cell fate during development.
Acknowledgments
M.B. thanks all her previous laboratory members and colleagues who have contributed to the work on skeletal myogenesis and cardiogenesis in her laboratory. The Pasteur Institute and CNRS UMR 3738 have provided ongoing support.
Footnotes
The author declares no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Gene Regulatory Networks and Network Models in Development and Evolution,” held April 12–14, 2016, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and video recordings of most presentations are available on the NAS website at www.nasonline.org/Gene_Regulatory_Networks.
This article is a PNAS Direct Submission. E.V.R. is a guest editor invited by the Editorial Board.
References
- 1.Buckingham M, Vincent SD. Distinct and dynamic myogenic populations in the vertebrate embryo. Curr Opin Genet Dev. 2009;19(5):444–453. doi: 10.1016/j.gde.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Buckingham M, Rigby PWJ. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev Cell. 2014;28(3):225–238. doi: 10.1016/j.devcel.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Buckingham M, Relaix F. PAX3 and PAX7 as upstream regulators of myogenesis. Semin Cell Dev Biol. 2015;44:115–125. doi: 10.1016/j.semcdb.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Lagha M, et al. Pax3:Foxc2 reciprocal repression in the somite modulates muscular versus vascular cell fate choice in multipotent progenitors. Dev Cell. 2009;17(6):892–899. doi: 10.1016/j.devcel.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 5.Mayeuf-Louchart A, et al. Notch regulation of myogenic versus endothelial fates of cells that migrate from the somite to the limb. Proc Natl Acad Sci USA. 2014;111(24):8844–8849. doi: 10.1073/pnas.1407606111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daubas P, et al. Fine-tuning the onset of myogenesis by homeobox proteins that interact with the Myf5 limb enhancer. Biol Open. 2015;4(12):1614–1624. doi: 10.1242/bio.014068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noden DM, Francis-West P. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 2006;235(5):1194–1218. doi: 10.1002/dvdy.20697. [DOI] [PubMed] [Google Scholar]
- 8.Gopalakrishnan S, et al. A cranial mesoderm origin for esophagus striated muscles. Dev Cell. 2015;34(6):694–704. doi: 10.1016/j.devcel.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Rios AC, Serralbo O, Salgado D, Marcelle C. Neural crest regulates myogenesis through the transient activation of NOTCH. Nature. 2011;473(7348):532–535. doi: 10.1038/nature09970. [DOI] [PubMed] [Google Scholar]
- 10.Lescroart F, et al. Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo. Development. 2010;137(19):3269–3279. doi: 10.1242/dev.050674. [DOI] [PubMed] [Google Scholar]
- 11.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91(4):1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 12.Comai G, Tajbakhsh S. Molecular and cellular regulation of skeletal myogenesis. Curr Top Dev Biol. 2014;110:1–73. doi: 10.1016/B978-0-12-405943-6.00001-4. [DOI] [PubMed] [Google Scholar]
- 13.Relaix F, et al. Six homeoproteins directly activate Myod expression in the gene regulatory networks that control early myogenesis. PLoS Genet. 2013;9(4):e1003425. doi: 10.1371/journal.pgen.1003425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michailovici I, Eigler T, Tzahor E. Craniofacial muscle development. Curr Top Dev Biol. 2015;115:3–30. doi: 10.1016/bs.ctdb.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Czajkowski MT, Rassek C, Lenhard DC, Bröhl D, Birchmeier C. Divergent and conserved roles of Dll1 signaling in development of craniofacial and trunk muscle. Dev Biol. 2014;395(2):307–316. doi: 10.1016/j.ydbio.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Lescroart F, et al. Clonal analysis reveals a common origin between nonsomite-derived neck muscles and heart myocardium. Proc Natl Acad Sci USA. 2015;112(5):1446–1451. doi: 10.1073/pnas.1424538112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diogo R, et al. A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature. 2015;520(7548):466–473. doi: 10.1038/nature14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly RG. The second heart field. Curr Top Dev Biol. 2012;100:33–65. doi: 10.1016/B978-0-12-387786-4.00002-6. [DOI] [PubMed] [Google Scholar]
- 19.Buckingham M. First and second heart fields. In: Rickert-Sperling S, Kelly RG, Driscoll DJ, editors. Congenital Heart Diseases: The Broken Heart. Springer; Vienna: 2016. pp. 25–40. [Google Scholar]
- 20.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6(11):826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 21.Lescroart F, Mohun T, Meilhac SM, Bennett M, Buckingham M. Lineage tree for the venous pole of the heart: Clonal analysis clarifies controversial genealogy based on genetic tracing. Circ Res. 2012;111(10):1313–1322. doi: 10.1161/CIRCRESAHA.112.271064. [DOI] [PubMed] [Google Scholar]
- 22.Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1(3):435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 23.Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313(5795):1922–1927. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biben C, Hadchouel J, Tajbakhsh S, Buckingham M. Developmental and tissue-specific regulation of the murine cardiac actin gene in vivo depends on distinct skeletal and cardiac muscle-specific enhancer elements in addition to the proximal promoter. Dev Biol. 1996;173(1):200–212. doi: 10.1006/dbio.1996.0017. [DOI] [PubMed] [Google Scholar]
- 25.Sambasivan R, Kuratani S, Tajbakhsh S. An eye on the head: The development and evolution of craniofacial muscles. Development. 2011;138(12):2401–2415. doi: 10.1242/dev.040972. [DOI] [PubMed] [Google Scholar]
- 26.Lyons I, et al. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 1995;9(13):1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- 27.Vincent SD, Buckingham ME. How to make a heart: The origin and regulation of cardiac progenitor cells. Curr Top Dev Biol. 2010;90:1–41. doi: 10.1016/S0070-2153(10)90001-X. [DOI] [PubMed] [Google Scholar]
- 28.Cai C-L, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5(6):877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly RG, Jerome-Majewska LA, Papaioannou VE. The del22q11.2 candidate gene Tbx1 regulates branchiomeric myogenesis. Hum Mol Genet. 2004;13(22):2829–2840. doi: 10.1093/hmg/ddh304. [DOI] [PubMed] [Google Scholar]
- 30.Rana MS, et al. Tbx1 coordinates addition of posterior second heart field progenitor cells to the arterial and venous poles of the heart. Circ Res. 2014;115(9):790–799. doi: 10.1161/CIRCRESAHA.115.305020. [DOI] [PubMed] [Google Scholar]
- 31.Buckingham ME, Meilhac SM. Tracing cells for tracking cell lineage and clonal behavior. Dev Cell. 2011;21(3):394–409. doi: 10.1016/j.devcel.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Bonnerot C, Nicolas JF. Clonal analysis in the intact mouse embryo by intragenic homologous recombination. C R Acad Sci III. 1993;316(10):1207–1217. [PubMed] [Google Scholar]
- 33.Meilhac SM, Esner M, Kelly RG, Nicolas J-F, Buckingham ME. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell. 2004;6(5):685–698. doi: 10.1016/s1534-5807(04)00133-9. [DOI] [PubMed] [Google Scholar]
- 34.Theis S, et al. The occipital lateral plate mesoderm is a novel source for vertebrate neck musculature. Development. 2010;137(17):2961–2971. doi: 10.1242/dev.049726. [DOI] [PubMed] [Google Scholar]
- 35.Domínguez JN, Meilhac SM, Bland YS, Buckingham ME, Brown NA. Asymmetric fate of the posterior part of the second heart field results in unexpected left/right contributions to both poles of the heart. Circ Res. 2012;111(10):1323–1335. doi: 10.1161/CIRCRESAHA.112.271247. [DOI] [PubMed] [Google Scholar]
- 36.Lawson KA, Meneses JJ, Pedersen RA. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development. 1991;113(3):891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- 37.Meilhac SM, Lescroart F, Blanpain C, Buckingham ME. Cardiac cell lineages that form the heart. Cold Spring Harb Perspect Med. 2015;5(2):a026344. doi: 10.1101/cshperspect.a026344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parameswaran M, Tam PP. Regionalisation of cell fate and morphogenetic movement of the mesoderm during mouse gastrulation. Dev Genet. 1995;17(1):16–28. doi: 10.1002/dvg.1020170104. [DOI] [PubMed] [Google Scholar]
- 39.Lescroart F, et al. Early lineage restriction in temporally distinct populations of Mesp1 progenitors during mammalian heart development. Nat Cell Biol. 2014;16(9):829–840. doi: 10.1038/ncb3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Devine WP, Wythe JD, George M, Koshiba-Takeuchi K, Bruneau BG. Early patterning and specification of cardiac progenitors in gastrulating mesoderm. eLife. 2014;3 doi: 10.7554/eLife.03848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moorman AFM, Christoffels VM. Cardiac chamber formation: Development, genes, and evolution. Physiol Rev. 2003;83(4):1223–1267. doi: 10.1152/physrev.00006.2003. [DOI] [PubMed] [Google Scholar]
- 42.Holland LZ, Schubert M, Kozmik Z, Holland ND. AmphiPax3/7, an amphioxus paired box gene: insights into chordate myogenesis, neurogenesis, and the possible evolutionary precursor of definitive vertebrate neural crest. Evol Dev. 1999;1(3):153–165. doi: 10.1046/j.1525-142x.1999.99019.x. [DOI] [PubMed] [Google Scholar]
- 43.Schubert M, Meulemans D, Bronner-Fraser M, Holland LZ, Holland ND. Differential mesodermal expression of two amphioxus MyoD family members (AmphiMRF1 and AmphiMRF2) Gene Expr Patterns. 2003;3(2):199–202. doi: 10.1016/s1567-133x(02)00099-6. [DOI] [PubMed] [Google Scholar]
- 44.Yasui K, Kaji T, Morov AR, Yonemura S. Development of oral and branchial muscles in lancelet larvae of Branchiostoma japonicum. J Morphol. 2014;275(4):465–477. doi: 10.1002/jmor.20228. [DOI] [PubMed] [Google Scholar]
- 45.Mahadevan NR, Horton AC, Gibson-Brown JJ. Developmental expression of the amphioxus Tbx1/ 10 gene illuminates the evolution of vertebrate branchial arches and sclerotome. Dev Genes Evol. 2004;214(11):559–566. doi: 10.1007/s00427-004-0433-1. [DOI] [PubMed] [Google Scholar]
- 46.Jackman WR, Langeland JA, Kimmel CB. Islet reveals segmentation in the Amphioxus hindbrain homolog. Dev Biol. 2000;220(1):16–26. doi: 10.1006/dbio.2000.9630. [DOI] [PubMed] [Google Scholar]
- 47.Holland ND, Venkatesh TV, Holland LZ, Jacobs DK, Bodmer R. AmphiNk2-tin, an amphioxus homeobox gene expressed in myocardial progenitors: insights into evolution of the vertebrate heart. Dev Biol. 2003;255(1):128–137. doi: 10.1016/s0012-1606(02)00050-7. [DOI] [PubMed] [Google Scholar]
- 48.Meedel TH, Chang P, Yasuo H. Muscle development in Ciona intestinalis requires the b-HLH myogenic regulatory factor gene Ci-MRF. Dev Biol. 2007;302(1):333–344. doi: 10.1016/j.ydbio.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stolfi A, et al. Early chordate origins of the vertebrate second heart field. Science. 2010;329(5991):565–568. doi: 10.1126/science.1190181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dubois L, Frendo J-L, Chanut-Delalande H, Crozatier M, Vincent A. Genetic dissection of the transcription factor code controlling serial specification of muscle identities in Drosophila. eLife. 2016;5:e14979. doi: 10.7554/eLife.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Green YS, Vetter ML. EBF proteins participate in transcriptional regulation of Xenopus muscle development. Dev Biol. 2011;358(1):240–250. doi: 10.1016/j.ydbio.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang W, Razy-Krajka F, Siu E, Ketcham A, Christiaen L. NK4 antagonizes Tbx1/10 to promote cardiac versus pharyngeal muscle fate in the ascidian second heart field. PLoS Biol. 2013;11(12):e1001725. doi: 10.1371/journal.pbio.1001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gehring WJ, Ikeo K. Pax 6: Mastering eye morphogenesis and eye evolution. Trends Genet. 1999;15(9):371–377. doi: 10.1016/s0168-9525(99)01776-x. [DOI] [PubMed] [Google Scholar]
- 54.Peter IS, Davidson EH. Evolution of gene regulatory networks controlling body plan development. Cell. 2011;144(6):970–985. doi: 10.1016/j.cell.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peter IS, Davidson EH. A gene regulatory network controlling the embryonic specification of endoderm. Nature. 2011;474(7353):635–639. doi: 10.1038/nature10100. [DOI] [PMC free article] [PubMed] [Google Scholar]