Abstract
The present study aimed to evaluate the inter-individual variability in fermentation of standard fibrous substrates by faecal inocula from ten healthy adult female cats. Substrates were citrus pectin (CP), fructo-oligosaccharides (FOS), guar gum (GG), sugar beet pulp (SBP) and wheat middlings (WM). Each substrate was incubated with faecal inoculum from each cat. Gas production was measured continuously during the 48 h incubation and SCFA and organic matter disappearance (only SBP and WM) after incubation. Out of ten cats, nine produced faeces on the days of inoculum preparation. The substrates contrasted in terms of fermentation parameters measured. The inter-individual variability was in general lower for the more simple and pure substrates (CP, FOS, GG) than for the more complex substrates containing mixtures of fibres (SBP, WM). Furthermore, for total SCFA and gas produced, inter-individual variability was lower than for proportions of butyrate and of branched-chain fatty acids and for the parameters of gas production kinetics. It is concluded that the variability in in vitro fermentation parameters is associated with the complexity of fibrous substrates. The presented data are instrumental for the calculation of number of faecal donors required for precise in vitro characterisation of the fermentability of dietary fibres. In addition, the number of faecal donors should be adjusted to the specific fermentation parameter(s) of interest.
Key words: Cats, Fibre fermentability, In vitro methodology, Gas production, SCFA
Abbreviations: CP, citrus pectin; FOS, fructo-oligosaccharide; GG, guar gum; Rmax, maximum rate of gas production; SBP, molassed sugar beet pulp; Tmax, time at which Rmax occurred; WM, wheat middlings
The properties of dietary fibres may affect cats’ health, digestive processes and faecal characteristics(1). It is therefore important to consider the properties of dietary fibres including their potential fermentability by the intestinal microbiota. To characterise the fermentability of fibres, in vitro methods simulating intestinal fermentation have been developed. In vitro batch culture systems are commonly applied in which the fibrous substrate of interest is incubated with a faecal inoculum from the target animal species. There are, however, methodological aspects that can have an impact on the quality of the results obtained and these are not well described in the literature. For example, the number of faecal donors has been advised to be at least five to control for the effect of inter-individual variation(2). Most studies have used three faecal donors(3). However, some only used one(4). Furthermore, few studies focused on the repeatability and reproducibility of the method, which has been described for our laboratory in a companion article(5). To gain knowledge on the precision of an in vitro method for characterisation of the fermentability of dietary fibres, this study aimed to evaluate the inter-individual variability in fermentation of five standard fibrous substrates by faecal inocula from ten cats.
Experimental methods
Substrates
Selected dietary fibres or fibre sources are used in dry and wet cat foods and contrast in chemical composition and anticipated fermentation characteristics, as shown in previous in vitro studies(6–8). Substrates included citrus pectin (CP; rapidly and highly fermentable, HM Rapid, TIC Gums), fructo-oligosaccharide (FOS; rapidly and highly fermentable; Orafti® IPS, BENEO-Orafti), guar gum (GG; rapidly and highly fermentable, 8/22, TIC Gums), molassed sugar beet pulp (SBP; slowly and highly fermentable; Research Diet Services) and wheat middlings (WM; slowly and moderately fermentable; Research Diet Services).
Animals, housing and care
A total of nine intact and one neutered female European shorthair cats (3 to 5 years old) with a mean body weight of 3·3 (sd 0·6) kg were used. Cats did not receive any antibiotics for at least 6 months prior to faecal collection. The cats were housed in two groups in rooms with an outside area and individually in a metabolic cage during parts of the day (12.00–13.30 and 16.30–09.30 hours). Details of the group rooms and metabolic cages as well the climate and light schedules have been provided by Van Rooijen et al.(9). Litter trays were present in the cage and contained non-absorbent polyethylene litter (Katkor®; Rein Vet Products). On the day of faeces collection the litter and the tray were sterilised with 70 % ethanol. Cats were fed a nutritionally complete (i.e. meeting the FEDIAF standards) commercial dry extruded diet (Perfect Fit In-Home; Mars Petcare) for at least 4 weeks prior to faeces collection. Cats were adapted in 2 weeks to a daily regimen of feeding between 08.30 and 09.30 hours (about 45 % of their daily portion), 12.00 and 13.30 hours (about 10 %) and at 16.30 hours (about 45 %) in their cage and provided food to maintain optimal body weight. In the morning and afternoon, cats went to their group room. Water was available ad libitum during group housing and in the metabolic cages. The health status of the animals was monitored daily and cats were weighed weekly. Animal care and experimental procedures were approved by the Animal Care and Use Committee of Wageningen University, Wageningen, the Netherlands.
Preparation of inoculum and incubation
Within 15 min of defecation, faeces were transferred to sterile 250 ml plastic bottles prefilled with CO2 and 250 ml of CO2 was immediately added to the bottle to ensure anaerobic storage conditions. The bottle with faeces was closed and transported within 5 min to the analytical laboratory. Faeces from cats were not pooled but processed for each cat. All handling procedures and processing of faeces to the inoculum were carried out under a constant stream of CO2. All attached litter particles were manually removed from faeces and faeces were weighed and diluted 1:9 (w/v) in a 39°C anaerobic sterile physiological saline solution (9 g/l NaCl). The diluted mixture was homogenised for 60 s using a hand-blender and filtered through nylon fabric (pore size 40 µm, permeability 30 %; PA 40/30, Nybolt). The filtrate was mixed with a pre-warmed (39°C) N-containing medium(10) in a 5:84 mixture (v/v) and gently flushed for 5 min with CO2. The resulting medium/inoculum mixture was dispensed (89 ml) into pre-warmed and CO2-flushed 250 ml serum bottles (Schott) containing 0·5 g of substrate. Inoculated bottles were then immediately attached to fully automated gas production equipment(11) and gas production was recorded for 48 h. This incubation time is longer than average total tract transit times observed in young adult (36 (sd 14) h) and senior cats (26 (sd 6) h)(12) with a orocaecal transit time of approximately 5 h(13). However, 48 h was estimated to be required for characterising the fermentation kinetics of SBP and WM. After 48 h of incubation, fermentation liquids were sampled for the determination of SCFA (i.e. acetate, propionate, butyrate, iso-butyrate, valerate, iso-valerate) concentrations and for organic matter disappearance of SBP and WM. All incubations were done in triplicate. The study was performed in two runs, with maximally five inocula per run.
Chemical analyses
All substrates were analysed for DM and ash by drying to a constant weight at 103°C and combusting at 550°C, respectively. The SBP and WM were also analysed for crude protein (6·25 × N)(14), for neutral-detergent fibre (NDF)(15,16), and for acid-detergent fibre (ADF) and acid-detergent lignin (ADL)(17). SCFA analyses of fermentation liquids as well as organic matter disappearance were analysed as described by Bosch et al.(18).
Calculations and data analyses
Cumulative gas production (ml) and SCFA production (mmol) were expressed per g organic matter. Monophasic models as described by Groot et al.(19) were fitted to the data for cumulative gas production and the maximum rate of gas production (Rmax in ml/(g organic matter h)) and the time at which it occurred (Tmax in h) were calculated(20). Acetate, propionate and butyrate were expressed as percentage of total SCFA. Similarly, branched-chain proportion was iso-butyrate + iso-valerate on a total SCFA basis. Values of replicates for each substrate–cat combination were averaged and considered as the experimental unit. For each substrate, mean and standard deviation values were calculated and used to compute the CV (sd/mean × 100).
Results and discussion
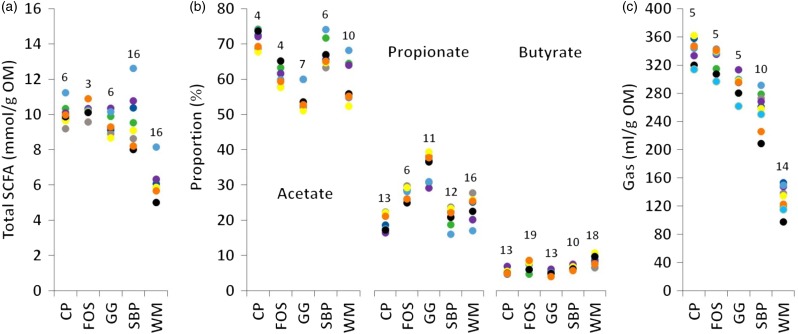
All cats remained healthy throughout the study. Of the ten cats, nine produced faeces on the days of inoculum preparation and for one cat the SCFA analysis failed. DM and ash contents (as is) were CP 90·0 and 1·7 %, FOS 96·5 and 0·0 %, GG 89·4 and 0·7 %, SBP 89·3 and 6·3 % and WM 88·4 and 7·2 %, respectively. Crude protein, NDF, ADF and ADL contents were, respectively, 9·2, 32·2, 16·6 and 1·0 % for SBP and 15·7, 42·3, 14·0 and 3·9 % for WM. The substrates contrasted in terms of fermentation parameters measured, as anticipated (Fig. 1 and Table 1). Furthermore, the ranking of substrates (i.e. relative accuracy(3)) was consistent among cats (based on Spearman's rank correlations; results not shown).
Fig. 1.
Inter-individual variation in in vitro total SCFA production (a), proportions of acetate, propionate and butyrate (b) and gas production (c). Coloured markers represent results from individual cats used as faecal donors (n 8 for SCFA and n 9 for gas production). CV (%) per substrate is indicated above the markers. OM, organic matter; CP, citrus pectin; FOS, fructo-oligosaccharide; GG, guar gum; SBP, molassed sugar beet pulp; WM, wheat middlings.
Table 1.
Mean values and inter-individual variability of in vitro fermentation parameters for fibrous substrates using feline faecal inocula (n 9 donor cats unless otherwise indicated)
| Parameter | Substrate | |||||
|---|---|---|---|---|---|---|
| CP | FOS | GG | SBP | WM | ||
| BCP (% of total SCFA production)* | Mean | 2·3 | 2·3 | 2·9 | 2·8 | 5·7 |
| CV (%) | 10 | 9 | 11 | 22 | 19 | |
| Rmax (ml/(g OM h)) | Mean | 87 | 85 | 51 | – | – |
| CV (%) | 15 | 11 | 32 | – | – | |
| Tmax (h) | Mean | 3·8 | 4·1 | 5·0 | – | – |
| CV (%) | 12 | 17 | 23 | – | – | |
| OMD (% of initial weight) | Mean | – | – | – | 86 | 49 |
| CV (%) | – | – | – | 12 | 12 | |
CP, citrus pectin; FOS, fructo-oligosaccharide; GG, guar gum; SBP, molassed sugar beet pulp; WM, wheat middlings; BCP, branched-chain proportion; Rmax, maximum rate of gas production; OM, organic matter; Tmax, time at which Rmax occurred; OMD, organic matter disappearance.
* n 8.
Total SCFA production from CP, FOS, GG and SBP was similar and greater than the production from WM. Inter-individual CV of total SCFA was larger for SBP and WM than for CP, FOS and GG (Fig. 1(a)). Few other studies focused on inter-individual variation in in vitro fermentation of fibrous substrates using faecal inocula. The CV for in vitro total SCFA production after 24 h of incubation with faecal inocula from healthy human volunteers ranged from 9 % for GG to 15 % for wheat bran (n 6 individuals)(21) and was 11 % for soyabean fibre (n 4)(22). In a large study with eight laboratories and number of faecal inocula from healthy human volunteers ranging from 4 to 7, inter-individual CV of total SCFA production after 24 h of incubation was larger for potato starch being pregelatinised (median 18, range 6–29 %), raw (22, 13–44 %) and retrograded (23, 15–55 %) and for glassy pea starch (22, 18–39 %)(2). The SCFA composition differed between substrates with the greatest acetate and propionate proportions for CP and GG, respectively. The CV of the proportion of acetate was smaller than for propionate and butyrate (Fig. 1(b)), which was also found for soyabean fibre fermented by human faecal inocula(22), i.e. respectively 8, 16 and 45 %. The variation in absolute values was in particular small for butyrate in the present study. Inter-individual CV of gas production was like that for total SCFA: larger for SBP and WM than for CP, FOS and GG (Fig. 1(c)). McBurney & Thompson(21) reported larger CV for gas production ranging from 9 % for kidney beans to 23 % for GG. Incubation with WM resulted in the largest branched-chain proportion (Table 1), which relates to the relatively high protein content, and was also observed in an in vitro study with faecal inocula from dogs(23). The inter-individual variation in branched-chain proportion was in particular large for SBP and WM. Fitting of the model for gas production was not possible for SBP and WM. The Rmax and Tmax were similar for CP and FOS. For GG, Rmax was lower than for CP and FOS and inter-individual variation in Rmax and Tmax was in particular large for GG. Finally, WM had a lower organic matter disappearance than SBP after 48 h of incubation, but both substrates showed similar inter-individual variability.
The observed fibre source-dependent inter-individual variation in various fermentation parameters probably relates to differences in specific microbial taxa observed in cat faeces(24). In healthy humans, variation in microbial taxa was also noted but the functionality of the microbiome (i.e. metabolic pathways) remained fairly stable among individuals(25). Degradation of more complex and mixtures of non-digestible carbohydrates would require a larger array of microbial enzymes than that required for the degradation of relatively simple and pure carbohydrates. Differences in faecal microbial composition and its functional capacities among individual cats may therefore become more pronounced with the more complex fibrous substrates (SBP and WM) relative to the more simple and pure ones (CP, FOS and GG). The variability associated with the complexity of fibrous substrates presented in this study is instrumental for the calculation of the number of faecal donors required for precise in vitro characterisation of the fermentability of dietary fibres. In addition, the number of faecal donors should be adjusted to the specific fermentation parameters of interest.
Acknowledgements
We would like to thank D. Nijkamp-Van de Pol and S. Van Laar-Schuppen for assistance during the execution of the in vitro procedures and M. Breuer for SCFA analyses. This research was funded by Wageningen University.
G. B. contributed to all aspects of this research including research design, data collection, calculations and manuscript drafting. J. W. C., W. F. P. and W. H. H. contributed to research design, data interpretation and manuscript preparation and L. H. and K. M. S. to data collection, calculations, data interpretation and manuscript preparation.
There were no conflicts of interest.
References
- 1.Fahey GC Jr, Flickinger AE, Grieshop CM, et al. (2004) The role of dietary fibre in companion animal nutrition In Dietary Fibre: Bio-Active Carbohydrates for Food and Feed, pp. 295–328 [Van der Kamp JW, Asp N-G, Miller Jones J and Schaafsma G, editors]. Wageningen, The Netherlands: Wageningen Academic Publishers. [Google Scholar]
- 2.Edwards CA, Gibson G, Champ M, et al. (1996) In vitro method for quantification of the fermentation of starch by human faecal bacteria. J Sci Food Agric 71, 209–217. [Google Scholar]
- 3.Coles LT, Moughan PJ & Darragh AJ (2005) In vitro digestion and fermentation methods, including gas production techniques, as applied to nutritive evaluation of foods in the hindgut of humans and other simple-stomach animals. Anim Feed Sci Technol 123–124, 421–444. [Google Scholar]
- 4.Murray J-AMD, McMullin P, Handel I, et al. (2012) The effect of freezing on the fermentative activity of equine faecal inocula for use in an in vitro gas production technique. Anim Feed Sci Technol 178, 175–182. [Google Scholar]
- 5.Bosch G, Heesen L, de Melo Santos K, et al. (2017) Evaluation of an in vitro fibre fermentation method using feline faecal inocula: repeatability and reproducibility. J Nutr Sci 6, doi: 10.1017/jns.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flickinger EA & Fahey GC Jr (2002) Pet food and feed applications of inulin, oligofructose and other oligosaccharides. Br J Nutr 87, S297–S300. [DOI] [PubMed] [Google Scholar]
- 7.Karr-Lilienthal LK, Grieshop CM, Smeets-Peeters MJE, et al. (2002) Selected gelling agents in canned dog food affect nutrient digestibilities and fecal characteristics of ileal cannulated dogs. Arch Anim Nutr 56, 141–153. [DOI] [PubMed] [Google Scholar]
- 8.Barry KA, Wojcicki BJ, Bauer LL, et al. (2011) Adaptation of healthy adult cats to select dietary fibers in vivo affects gas and short-chain fatty acid production from fiber fermentation in vitro. J Anim Sci 89, 3163–3169. [DOI] [PubMed] [Google Scholar]
- 9.Van Rooijen C, Bosch G, Butré CI, et al. (2016) Urinary excretion of dietary Maillard reaction products in healthy adult female cats. J Anim Sci 94, 185–195. [DOI] [PubMed] [Google Scholar]
- 10.Williams BA, Bosch MW, Boer H, et al. (2005) An in vitro batch culture method to assess potential fermentability of feed ingredients for monogastric diets. Anim Feed Sci Technol 123–124, 445–462. [Google Scholar]
- 11.Cone JW, Van Gelder AH, Visscher GJW, et al. (1996) Influence of rumen fluid and substrate concentration on fermentation kinetics measured with a fully automated time related gas production apparatus. Anim Feed Sci Technol 61, 113–128. [Google Scholar]
- 12.Peachey SE, Dawson JM & Harper EJ (2000) Gastrointestinal transit times in young and old cats. Comp Biochem Physiol A 126, 85–90. [DOI] [PubMed] [Google Scholar]
- 13.Papasouliotis K, Sparkes AH, Gruffydd-Jones TJ, et al. (1998) Use of the breath hydrogen test to assess the effect of age on orocecal transit time and carbohydrate assimilation in cats. Am J Vet Res 59, 1299–1302. [PubMed] [Google Scholar]
- 14.International Organization for Standardization (ISO) (2005) Animal Feeding Stuffs – Determination of Nitrogen Content and Calculation of Crude Protein Content – Part 1: Kjeldahl Method (ISO 5983–1). Geneva, Switzerland: International Organization for Standardization. [Google Scholar]
- 15.Van Soest PJ, Robertson JB & Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74, 3583–3597. [DOI] [PubMed] [Google Scholar]
- 16.Goelema JO, Spreeuwenberg MAM, Hof G, et al. (1998) Effect of pressure toasting on the rumen degradability and intestinal digestibility of whole and broken peas, lupins and faba beans and a mixture of these feedstuffs. Anim Feed Sci Technol 76, 35–50. [Google Scholar]
- 17.Van Soest PJ (1973) Collaborative study of acid-detergent fibre and lignin. J Assoc Off Anal Chem 56, 781–784. [Google Scholar]
- 18.Bosch G, Pellikaan WF, Rutten PGP, et al. (2008) Comparative in vitro fermentation activity in the canine distal gastrointestinal tract and fermentation kinetics of fiber sources. J Anim Sci 86, 2979–2989. [DOI] [PubMed] [Google Scholar]
- 19.Groot JCJ, Cone JW, Williams BA, et al. (1996) Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. Anim Feed Sci Technol 64, 77–89. [Google Scholar]
- 20.Bauer E, Williams BA, Voigt C, et al. (2003) Impact of mammalian pretreatment on the fermentability of carbohydrate-rich feedstuffs. J Sci Food Agric 83, 207–214. [Google Scholar]
- 21.McBurney MI & Thompson LU (1989) Effect of human faecal donor on in vitro fermentation variables. Scand J Gastroenterol 24, 359–367. [DOI] [PubMed] [Google Scholar]
- 22.Barry JL, Hoebler C, Macfarlane GT, et al. (1995) Estimation of the fermentability of dietary fibre in vitro: a European interlaboratory study. Br J Nutr 74, 303–322. [DOI] [PubMed] [Google Scholar]
- 23.Bosch G, Wrigglesworth DJ, Cone JW, et al. (2013) Effect of preservation conditions of canine faeces on in vitro fermentation. J Anim Sci 91, 259–267. [DOI] [PubMed] [Google Scholar]
- 24.Ritchie LE, Burke KF, Garcia-Mazcorro JF, et al. (2010) Characterization of fecal microbiota in cats using universal 16S rRNA gene and group-specific primers for Lactobacillus and Bifidobacterium spp. Vet Microbiol 144, 140–146. [DOI] [PubMed] [Google Scholar]
- 25.Huttenhower C, Gevers D, Knight R, et al. (2012) Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]