Abstract
Background:
Little information is available about chemical components of the Cousinia genus. A primary cytotoxicity screening on Cousinia verbascifolia showed moderate cytotoxic activity against OVCAR-3 ovarian and HT-29 colon cancer cells. Therefore, the aim of this study is a phytochemical investigation to identify the compounds responsible for this bioactivity.
Materials and Methods:
Extraction was done through percolation and fractionations by reverse phase column chromatography and normal column chromatography. Using standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay Fr.b8 with moderate cytotoxicity was selected for identification of major components. Fr.b8 was subjected to polyamide column chromatography. More purification was done using a new modified recycle high-performance liquid chromatography (HPLC) with flow splitter.
Results:
Two known compounds: Apigenin (flavone) and caffeic acid (phenolic acid) were obtained from phenolic bioactive fraction for the first time from this plant.
Conclusions:
Apigenin and caffeic acid with known antitumor and matrix metalloproteinase inhibitory effects seem to be the bioactive components responsible for moderate cytotoxicity of phenolic fraction. Recycle HPLC following with flow splitting is a new method useful for isolation of closely eluted compounds in HPLC chromatogram.
Keywords: Apigenin, Asteraceae, caffeic acid, colon cancer, Cousinia verbascifolia, ovary cancer, recycle high-performance liquid chromatography
Introduction
Plant kingdom provides a rich and wonderful source of biologically active compounds. There are about 250,000–500,000 plant species on earth, out of which only about 20% have been submitted to biological and phytochemical screening.[1,2,3] Hence, a huge number of plant species still remain to be studied and described in terms of biological and phytochemical profiles.
Cousinia, one of the largest genera of Asteraceae with 600–700 species, is one of the most diverse genera in central and South-West Asia. About 250 species of the genus are distributed throughout Iran, comprising about 200 endemic species.[4,5,6,7] Based on phytochemical studies on a few Cousinia species chemical constituents including acetylenes, triterpenes, steroids, sesquiterpene lactones, and flavonoids were reported.[8,9,10,11,12,13,14,15,16,17] However, little information is available about the phytochemical and biological properties of the species grow in Iran. In a phytochemical study on the aerial parts of three Iranian species of the genus Cousinia (Cousinia picheriana, Cousinia piptocephala, and Cousinia canescens), all three species yielded guianolide class sesquiterpens including 10 (14), 11 (13)-guiadien, 12, 6-olide, 4 (15), 10 (14)-guiadien, 12, 6-olide, and 4 (15), 10 (14), 11 (13)-guiatrien, 12, 6-olide derivatives mainly differed in functions on C-3, C-4, C-15, and ester located on C-8. In addition, C. canescens contained oxygenated bisabolene derivatives.[9] In another study on cytotoxicity of total ethanol extract of seven Iranian Cousinia species against fibrosarcoma WEHI 164 cancer cells, they showed medium cytotoxicity with inhibitory concentration ranged between 18.4 ± 0.59 and 87.9 ± 0.58 μg/mL. In the same study on fibrosarcoma WEHI 164 cancer cells, most of the Cousinia species had considerable inhibitory effect on matrix metalloproteinase (MMP) activity.[18]
Cousinia verbascifolia Bunge is endemic to Kopetdagh (NE Iran and S Turkmenistan), and could be seen in open areas, scrublands, and stony slopes.[6] It is a monocarpic biennial plant, up to 45 cm high, stems simple or branched from the base, leaves lyrate with spiny-dentate margin, flowers 50–150; corolla pink, rose or purple, 20–35 mm long. The flowering period of the plant is May to July.[6] In a primary study by authors, different fractions of C. verbascifolia showed medium cytotoxicity against OVCAR-3 and HT-29 cells.[20] In this present study, due to the observed inhibitory properties ofsemi-polar fractions of C. verbascifolia's extract against cancer cells, phytochemical investigation on these semi-polar fractions were carried out using a new modified recycle high-performance liquid chromatography (HPLC) technique which was used in the purification process.
Materials and Methods
General
Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance AV 400, using pyridine-d6 as solvent. Electrospray ionization mass spectrometry (ESI-MS) spectra were measured in negative mode on Shimadzu 2010EV LC-MS system (Shimadzu, Japan).
Chromatographic conditions
Column chromatography runs were performed using silica gel, 63–200 μm (Merck), polyamide DC6 (MACHEREY-NAGEL, Duren, Germany), and reverse phase chromatography was done by LiChroprep RP-18, 40–60 μm (Merck). High performance thin layer chromatography was performed on silica gel GF-254 plates (Merck KGaA, Darmstadt, Germany). Plates were developed by natural product reagent (1% methanolic diphenyl-boric acid-ethanolamine) and visualized by ultraviolet (UV) fluorescent colors at 254/366 nm UV lamps. Recycle-high pressure liquid chromatography was done on a Waters HPLC apparatus (Waters Assoc., Milford, MA, USA), at 250 nm using silica gel column (YMC-Pack SIL, 250 mm × 20 mm, YMC Co., Kyoto, Japan).
Plant material
Flowering aerial parts of C. verbascifolia (Asteraceae) were collected from Salehabad, Torbat-e Jam in Razavi Khorasan Province, Iran, in May 2013. It was identified by Dr. Iraj Mehregan. A voucher specimen (No. 2838) was deposited at the Herbarium of the Pharmacognosy Department, School of Pharmacy, Isfahan University of Medical Sciences, Isfahan, I. R. Iran.
Extraction, isolation, and purification
Air dried aerial parts of C. verbascifolia (3.5 kg) were extracted via percolation method using methanol as solvent. Filtration and in vacuum concentration of methanol extract resulted in a green gum which was subjected to reverse phase chromatography for removing fats and chlorophylls using methanol:water (80:20) as solvent. Defatted fraction (41.3 g) was then subjected to a silica gel column chromatography using hexane/acetone as mobile phase with increasing polarity (5→50%) that afforded 11 fractions (Fr.b1-8). These fractions were studied for cytotoxic activity using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide standard method against ovarian and colon cancer cells.[19,20] Detailed description of this study is published by authors elsewhere.[20] The cytotoxicity screening revealed that Fr.b2, Fr.b4, and Fr.b8 were the most cytotoxic fractions.[20] Fr.b2 contained mainly fatty acids and common steroids which were discarded. Inspired from 1H-NMR spectra, Fr.b4 contained sesquiterpene lactones but with inadequate amounts for further analysis that were excluded from the study. Finally, bioactive fraction, Fr.b8, was selected for identification of major components responsible for the observed cytotoxic effects. Fr.b8 was then subjected to polyamide DC6 column (40 cm × 2 cm) using chloroform:methanol (2→20%) with increasing polarity to yield fractions Fr.b8a-d. After thin layer chromatography visualization, Fr.b8d eluted by chloroform:methanol (88:12) with positive reaction to natural product reagent was selected and injected into the recycle HPLC system using YMC-SIL column (25 cm × 2.5 cm), particle size: 10 μm, mobile phase: chloroform:methanol (85:15), injection volume: 2 ml, flow rate: 3 ml/min, chart rate: 0.1 cm/min, and detector wavelength: 250 nm.
In water, 501 HPLC pump a recycle valve and a flow splitter were incorporated in HPLC system between detector and HPLC pump, so that the chromatographic profile could reenter the column after passing through the detector. This process resembles using a longer column and thus provides more theoretical plates leading to increasing the column efficiency and resolution. In the case of observing tailing in the peaks, flow is splitted before reentering the column. Finally, during any cycle in which the desired resolution is achieved, samples are collected. In this process, after three recycles following the flow splitting (80% to pump and 20% to waste), the peaks were sufficiently resolved to compounds A, B, and C [Figure 1]. Compound B was excluded from the study because of insufficient purity, and compounds A and C were subjected to mass and NMR spectroscopy which led to the identification of compounds 1 (30 mg) and 2 (10 mg) [Figure 2].
Figure 1.
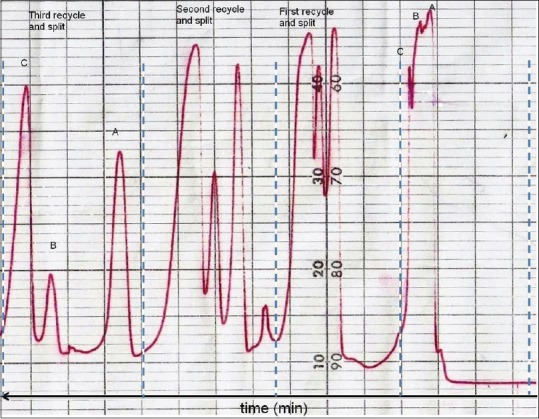
Isolation of apigenin (A) and caffeic acid (C) from Cousinia verbascifolia by implementing recycle high-performance liquid chromatography technique in the purification process. Fraction Fr.b8d was injected into the recycle high-performance liquid chromatography system using YMC-SIL column (10 µm, 25 cm × 2.5 cm) using chloroform:methanol (85:15) at 250 nm. After three recycles, the peaks were sufficiently resolved to compounds A, B, and C
Figure 2.
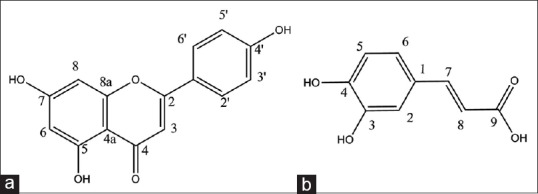
Apigenin (a) and caffeic acid (b) isolated from Cousinia verbascifolia Bunge
Results
Two known compounds were obtained from phenolic bioactive fraction for the first time from this plant. Their physical properties are given bellow:
Compound 1
Pale yellow powder, 1H-NMR in pyridine-d6 (400 MHz) ppm: 7.76 (d, 2H, J = 8.9 Hz), 6.84 (d, 2H, J = 8.8 Hz), 6.50 (s, 1H), 6.37 (d, 1H, J = 2.1 Hz), 6.11 (d, 1H, J = 2.1 Hz); 13C-NMR (100 MHz, pyridine-d6) δC: 163.8 (C2), 104.4 (C3), 183.2 (C4), 106.5 (C4a), 163.2 (C5), 100.5 (C6), 166.4 (C7), 95.3 (C8), 159.0 (C8a), 122.8 (C1’), 129.4 (C2’), 117.3 (C3’),163.3 (C4’), 117.3 (C5’), 122.8 (C6’). ESI negative mass m/z 269 (M–1).
Compound 2
White powder, 1H-NMR in pyridine-d6 (400 MHz) ppm: 9.63 (d, 1H, J = 15.9 Hz, H-7), 9.16 (d, 1H, J = 1.4 Hz, H-2), 8.73 (d, 1H, J = 8.1 Hz, H-5), 8.71 (dd, 1H, J = 1.6 Hz, J = 8.2 Hz, H-6), 8.33 (d, 1H, J = 15.8 Hz, H-8); 13C-NMR (100 MHz, pyridine-d6) δC: 171.7 (C1), 116.7 (C2), 148.7 (C3), 147.9 (C4), 117.8 (C5), 122.7 (C6), 146.2 (C-7), 117.8 (C-8), 170.6 (C-9). ESI negative mass m/z 179 (M–1).
Discussion
Compound 1 was isolated as a pale yellowish solid with positive reaction to FeCl3 and natural product reagents. Molecular formula was determined by NMR data and negative ESI-MS m/z 269 (M–1) as C15H10O5. Eleven degrees of unsaturation and NMR data suggested the presence of one carbonyl carbon, seven olefin bands, and therefore, three rings in the skeleton. The UV spectrum showed absorption maxima at 265 and 340 nm that are characteristic of flavones.[8] The 1H-NMR spectrum displayed meta- coupled doublets at δ 6.11 (1H, d, J = 2 Hz) and 6.37 (1H, d, J = 2 Hz) described to H-6 and H-8. In addition, ortho- coupled proton signals at δ 6.84 (2H, d, J = 8. 5 Hz) and 7.76 (2H, d, J = 8.5 Hz), corresponding to H-5’,3’ and H-6’,2’ as well as δ 6.50 (1H, s) corresponding to H-3 proton, indicated that compound 1 is a flavone derivative. Comparison of the spectral data with those published before allowed us to establish the structure of compound 1 as 4’,5,7-trihydroxyflavon or apigenin.[21]
Compound 2 was yielded as a white solid with positive reaction to FeCl3. The six degrees of unsaturation derived from positive HR-ESI-MS m/z 181.0495 (calcd. for C9H8O4 + H+, 181.0495) and NMR data suggested the presence of one carbonyl carbon, four olefin bands, and therefore, one ring in the skeleton. The 1H-NMR spectra displayed meta- coupled doublets at δ 9.16 (1H, d, J = 1.4 Hz) as well as ortho- coupled proton signals at δH8.73 (1H, d, J = 8.1 Hz) and 8.71 (1H, d, J = 1.6, 8.2 Hz), described to H-2, H-6 and H-8. The above ABX spin coupling system resembled 3,4-disubstituted benzyl derivatives.[22] In addition, two trans-olefin protons at δH9.63 (1H, d, J = 15.9 Hz) and 9.63 (1H, d, J = 15.9 Hz) with relatively large coupling constant J = 15.8 Hz and acidic proton at down field aria at δH10.1 ppm were observed. Comparison of the spectral data with those published before allowed us to establish the structure of compound 2 as 3-(3,4-dihydroxyphenyl)-2-propenoic acid or caffeic acid.[23,24]
Numerous studies have reported anticancer properties of both apigenin and caffeic acid through various mechanisms and pathways.[25,26] In a report by Wang et al., they investigated the cell growth inhibition and cell-cycle arrest caused by apigenin against HT-29 colon cancer cell line. The results showed that apigenin induces a cell cycle arrest at G2/M phase, but this inhibitory effect on cancer cells appeared to be cytostatic, not cytotoxic.[27] Antitumor effects of caffeic acid against colon and hepatocarcinoma cell lines are also reported through MMP-9 inhibitory effects.[28,29,30] There are many evidence supporting those selective MMP inhibitors are effective in prevention and treatment of cancer.[28,29,30,31,32] Caffeic acid and its derivatives selectively inhibited MMP-2 and -9.[29] In a similar study, hepatocarcinoma cells with phorbol ester (PMA)-induced MMP-9 expression were also suppressed followed by caffeic acid derivatives treatment. Their oral administrations significantly reduced the hepatic metastasis.[28,29] Apigenin has also been reported to inhibit MMP-9 secretion.[33] The IC50 of apigenin and caffeic acid against HT-29 colon cancer cell line was reported as 50 and 60 μM, respectively.[27,34] In a previous study, Shahverdi et al. demonstrated cytotoxicity and MMP inhibitory effects of C. verbascifolia.[18] These results together with aforementioned properties of apigenin and caffeic acid may explain the moderate cytotoxicity of C. verbascifolia phenolic fraction.
Conclusion
Two phenolic compounds: Apigenin (flavone) and caffeic acid (phenolic acid) were isolated for the first time from C. verbascifolia. Both of these compounds possess antitumor and MMP inhibitory effects. Thus, the bioactivities observed from C. verbascifolia in the previous studies might be due to the presence of these two phenolic compounds. Moreover, recycle HPLC following with flow splitting is a new method useful for isolation of closely eluted compounds in HPLC chromatogram.
Limitation of the study
The limitation of this study was inadequate amounts of sesquiterpene lactones in fraction Fr.b4 for further purification and elucidation. One another limitation of this study was the sample size due to the low yield of the extract. Although apigenin and caffeic acid seems to be responsible for observed cytotoxic effects but more phytochemical investigation with larger amounts of the plant material is suggested for identification of sesquiterpene lactones as another possible compounds responsible for cytotoxic activities in this plant.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This paper is a part of the thesis of Mehrangiz Haghighi submitted for the fulfillment of the degree of PharmD in Faculty of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Tehran, I.R. Iran.
References
- 1.Dutt R, Garg V, Madan AK. Can plants growing in diverse hostile environments provide a vital source of anticancer drugs. J Cancer Ther. 2014;10:13–37. [Google Scholar]
- 2.Niño J, Narváez DM, Mosquera OM, Correa YM. Antibacterial, antifungal and cytotoxic activities of eight Asteraceae and two Rubiaceae plants from Colombian biodiversity. Braz J Microbiol. 2006;37:566–70. [Google Scholar]
- 3.Pan L, Chai HB, Kinghorn AD. Discovery of new anticancer agents from higher plants. Front Biosci (Schol Ed) 2012;4:142–56. doi: 10.2741/257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attar F, Djavadi SB. A taxonomic revision of Cousinia, sect. Cynaroides (Asteraceae, Cardueae) in the flora of Iran. Iran J Bot. 2012;16:130–84. [Google Scholar]
- 5.Mehregan I. Systematics, Phylogeny and Biogeography of Cousinia (Asteraceae). Ph.D, Thesis, Fachbreich Biologie Der Johannes Gutenberg-Universitat Mainz. 2008 [Google Scholar]
- 6.Mehregan I, Kadereit JW. Taxonomic revision of Cousinia sect. Cynaroideae (Asteraceae, Cardueae) Willdenowia. 2008;38:293–362. [Google Scholar]
- 7.Zare M, Khosravi AR, Joharchi MR. Distribution patterns of the genus Cousinia (Asteraceae) in Iran. Iran J Bot. 2013;19:127–41. [Google Scholar]
- 8.Bohlmann F, Burkhardt T, Zdero C. Naturally Occurring Acetylenes. London: Academic Press; 1973. [Google Scholar]
- 9.Alberto Marco J, Juan FS, Albiach R, Rustayian A, Habibi Z. Bisabolene derivatives and sesquiterpene lactones from Cousinia species. Phytochemistry. 1993;32:395–400. [Google Scholar]
- 10.Rustaiyan A, Niknejad A, Sigari H, Ahmadi A. Guaianolides from Cousinia onopordioides. Fitoterapia. 1981;52:31–2. [Google Scholar]
- 11.Rustaiyan A, Sharif Z, Sadjadi A. Two farnesol derivatives from Cousinia adenostica. Phytochemistry. 1987;26:2635–6. [Google Scholar]
- 12.Turdumambetov K, Rakhimov DA, Malikova MK. Oligo-and polysaccharides from Cousinia umbrosa. Molbank. 2007;43:308–9. [Google Scholar]
- 13.Turdumambetov K, Plekhanova NV, Rakhimov DA, Yagudaev MR. Glucofructans of Cousinia polycephala. Molbank. 1989;25:371–2. [Google Scholar]
- 14.Plekhanova NV, Turdumambetov K, Sudnitsyna IG. Carbohydrates of Cousinia. Molbank. 1983;19:603–4. [Google Scholar]
- 15.Ulubelen A, Tuzlaci E, Mericli A. Triterpenic and steroidal compounds from Cousinia canescens. Fitoterapia. 1986;57:269–70. [Google Scholar]
- 16.lubelen A, Tuzlaci E. Flavonoids and terpenoids from Cousinia eriocephala. Fitoterapia. 1988;59:350. [Google Scholar]
- 17.Azimova S, Glushenkova A. Lipids, Lipophilic Components and Essential Oils from Plant Sources. London: Springer; 2012. pp. 110–3. [Google Scholar]
- 18.Shahverdi AR, Khorramizadeh MR, Attar F, Saadat F, Vahid S, Ghahraman A. Concomitant chemopreventive and antibacterial effects of some Iranian plants from the genus Cousinia (Asteraceae) Braz J Pharmacogn. 2007;17:325–30. [Google Scholar]
- 19.Zarei SM, Ayatollahi AM, Ghanadian M, Kobarfard F, Aghaei M, Choudhary MI, et al. Unusual ingenoids from Euphorbia erythradeni a Bioss. With pro-apoptotic effects. Fitoterapia. 2013;91:87–94. doi: 10.1016/j.fitote.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Sajjadi SE, Ghanadian M, Haghighi M, Mouhebat L. Cytotoxic effect of Cousinia verbascifolia Bunge against OVCAR-3 and HT-29 cancer cells. J Herb Med Pharmacol. 2015;4:15–9. [Google Scholar]
- 21.Markham KR. Techniques of Flavonoid Identification. Vol. 31. London: Academic Press; 1982. [Google Scholar]
- 22.Ghanadian M, Sadraei H, Yousuf S, Asghari G, Choudhary MI, Jahed M. New diterpene polyester and phenolic compounds from Pycnocycla spinosa Decne. Ex Boiss with relaxant effects on KCl-induced contraction in rat ileum. Phytochem Lett. 2014;7:57–61. [Google Scholar]
- 23.Kelley CJ, Harruff RC, Carmack M. Polyphenolic acids of Lithospermum ruderale. II. Carbon-nuclear magnetic resonance of lithospermic and rosmarinic acids. J Org Chem. 1976;41:449–55. [Google Scholar]
- 24.Andary C, Wylde R, Laffite C, Privat G, Winternitz F. Structures of verbascoside and orobanchoside, caffeic acid sugar esters from Orobanche rapum-genistae. Phytochemistry. 1982;21:1123–7. [Google Scholar]
- 25.Lefort ÉC, Blay J. Apigenin and its impact on gastrointestinal cancers. Mol Nutr Food Res. 2013;57:126–44. doi: 10.1002/mnfr.201200424. [DOI] [PubMed] [Google Scholar]
- 26.Rocha LD, Monteiro MC, Teodoro AJ. Anticancer properties of hydroxycinnamic acids. – A review. Cancer Clin Oncol. 2012;1:109–21. [Google Scholar]
- 27.Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ, Birt DF. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinog. 2000;28:102–10. [PubMed] [Google Scholar]
- 28.Rao CV, Desai D, Kaul B, Amin S, Reddy BS. Effect of caffeic acid esters on carcinogen-induced mutagenicity and human colon adenocarcinoma cell growth. Chem Biol Interact. 1992;84:277–90. doi: 10.1016/0009-2797(92)90129-9. [DOI] [PubMed] [Google Scholar]
- 29.Chung TW, Moon SK, Chang YC, Ko JH, Lee YC, Cho G, et al. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: Complete regression of hepatoma growth and metastasis by dual mechanism. FASEB J. 2004;18:1670–81. doi: 10.1096/fj.04-2126com. [DOI] [PubMed] [Google Scholar]
- 30.Chiang EP, Tsai SY, Kuo YH, Pai MH, Chiu HL, Rodriguez RL, et al. Caffeic acid derivatives inhibit the growth of colon cancer: Involvement of the PI3-K/Akt and AMPK signaling pathways. PLoS One. 2014;9:e99631. doi: 10.1371/journal.pone.0099631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li NG, Shi ZH, Tang YP, Duan JA. Selective matrix metalloproteinase inhibitors for cancer. Curr Med Chem Anticancer Agents. 2009;16:3805–27. doi: 10.2174/092986709789178037. [DOI] [PubMed] [Google Scholar]
- 32.Nemunaitis J, Poole C, Primrose J, Rosemurgy A, Malfetano J, Brown P, et al. Combined analysis of studies of the effects of the matrix metalloproteinase inhibitor marimastat on serum tumor markers in advanced cancer: Selection of a biologically active and tolerable dose for longer-term studies. Clin Cancer Res. 1998;4:1101–9. [PubMed] [Google Scholar]
- 33.Lindenmeyer F, Li H, Menashi S, Soria C, Lu H. Apigenin acts on the tumor cell invasion process and regulates protease production. Nutr Cancer. 2001;39:139–47. doi: 10.1207/S15327914nc391_19. [DOI] [PubMed] [Google Scholar]
- 34.Ng SH. Characterization of Colon Cancer Cell Culture Based Screening Assay to Study Effects of Phenolic Acids. Thesis for Ph.D, University of Saskatchewan, Saskatoon, USA. 2011 [Google Scholar]


