Abstract
Background:
Cancer is a term for a large group of different diseases, all involving uncontrolled cell growth. Many of Euphorbiaceae plants have been traditionally used for the treatment of ulcers, tumors, warts, and other diseases. In addition, in the last decade, there are studies showing cytotoxic effects of different species of Euphorbia on tumor cell lines. In this study, we attempted to determine if Euphorbia turcomanica possess any cytotoxic activity.
Materials and Methods:
Solvents extracted the plant powder with various polarities by a maceration method, and qualitative phytochemical analyzes were carried out on them to identify the constituents. On the other hand, the possible cytotoxicity of different extracts on Hela and HT-29 tumor cells was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and 50% reduction in cell survival was considered as a cytotoxic effect. Analyze of variance followed by Student-Newman-Keuls test was used to see the differences among the groups.
Results:
Phytochemical analysis of E. turcomanica showed the presence of flavonoid, alkaloid, anthraquinone and tannin in plant aerial parts. Methanol-water, acetone, dichloromethane, methanol, and heptane extracts of E. turcomanica significantly reduced viability of Hela cells (P < 0.05) with inhibitory concentration 50% (IC50) of 50, 90, 230, 420, and 450 μg/ml, respectively. While methanol-water, dichloromethane, methanol, ethyl acetate, and heptane extracts were cytotoxic with IC50 of 43, 115, 125, 250, and 390 μg/ml, respectively (P < 0.05), on HT-29 cells.
Conclusion:
It can be concluded that E. turcomanica is a good candidate for further study toward cytotoxic agents.
Keywords: 3-(4,5-dimethylthiazol-2-yl)-2; 5-diphenyltetrazolium bromide assay; cytotoxicity; Euphorbia turcomanica; Hela; HT-29; maceration
Introduction
Cancer is a term for a large group of different diseases, all involving uncontrolled cell growth.[1] In 2008, approximately 12.7 million cancers were diagnosed worldwide. Cancer has been a major cause of death where it account for approximately 13% of all deaths in each year.[2] Because of associated side effects and toxicity of synthetic chemotherapeutic drugs, it would be important to discover new biologic agents that are less toxic for normal cells and possess potential antiproliferative effects on cancer cells. Screening active compounds from plants, lead to the discovery of new medicinal drugs that are promising for the treatment of various diseases including cancer. Isolation and identification of some potent antitumor compounds such as colchicine, vincristine, vinblastine, podophyllotoxin, and taxol from plants have encouraged scientists to screen different parts of plant species against cancer cell lines.[3,4,5]
Euphorbiaceae is a large family of flowering plants with 300 genera and around 7500 species. Most are herbs, but some, especially in the tropics, are also shrubs or[6] Euphorbia is a genus of plants belonging to the family Euphorbiaceae consisting of 2008 species.[7] About 70 species of Euphorbia grow in Iran, among them 17 are endemic.[8] These plants are annual or perennial herbs with a caustic, poisonous milky sap (latex). Many of Euphorbiaceous plants have been traditionally used for the treatment of ulcers, tumors, warts, and other diseases.[9]
Recent studies suggested that isolated polycyclic diterpenoids and triterpenoids from the Iranian Euphorbia species have strong cytotoxic activity.[10] Moreover, it was showed that Iranian Euphorbia species inhibit proliferation of tumor cells and possess immunomodulatory effects on lymphocytes.[11]
To our knowledge, anticancer activities of Euphorbia turcomanica extracts have not yet been examined. Therefore, the object of this study was to examine the cytotoxic effect of different E. turcomanica extracts on cervical cancer cell line (Hela) and human colorectal carcinoma (HT-29).
Materials and Methods
Materials and chemicals
RPMI1640, fetal calf serum (FCS), sodium pyruvate, trypsin, L-glutamine were obtained from Gibco Co (USA). Ethyl acetate, heptane, acetone, dichloromethane, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), NaCl, KCl, NaOH, HCl, H2SO4, NaHCO3, and Na2HPO4 were purchased from Merck Co., (Germany). Hela and HT-29 cells were obtained from pasture Institute (Tehran, Iran). All other chemicals were supplied by Sigma Aldrich (USA) unless mentioned otherwise.
Plant collection and authentication
Fresh aerial parts of E. turcomanica were collected from the central part of Iran near Isfahan in June 2011. Mr. Bahram Zehzad authenticated the plant, Department of Biology, University of Shahid Beheshti, and a voucher specimen of the plant (No. 2410) was deposited in the herbarium of School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran.
Extraction and isolation
The aerial parts of the plant were air dried at room temperature and grounded into a fine powder. The herb was extracted by maceration method. The powdered aerial parts were extracted with the different solvent system in order of decreasing polarity. Heptane, ethyl acetate, dichloromethane, acetone, methanol, and methanol-water (70–30) were used as solvents separately. Dried powder (50 g) was soaked in 100 ml of solvent for 24 h under occasional shaking, and the process was repeated three times. The extracts were concentrated to dryness using a rotary evaporator under reduced pressure. The extracts obtained were finally freeze-dried to remove any residual water. The extraction procedures were performed in dim lighting, and all the dried extracts were stored at 4°C until use.
Preparation of the extracts
Dried residues (10 mg) were dissolved in 100 μl of dimethyl sulfoxide (DMSO) and diluted to a final volume of 2 ml with distilled phosphate buffer saline then filtered through 0.22 μ microbiological filters. Dilution was continued to obtain working concentration of 1, 10, 20, 50, 100, and 500 μg/ml.
Preliminary phytochemical analyzes
The presence of flavonoids, tannins, glycosides, alkaloids, saponins, and anthraquinones in the aerial of E. turcomanica were carried out on the specimen using standard procedure to identify the constituents as described earlier.[12,13,14]
Determination of alkaloids
Plant powdered specimen (200 mg) was boiled with water and 10 ml of hydrochloric acid on a water bath. Finally, it was filtered, and its pH was adjusted with ammonia to about 6–7. One milliliter of the filtrate was treated with a few drops of Mayer's reagent (potassium mercuric iodide solution). In addition, 1 ml portion was treated similarly with Wagner's reagent (solution of iodine in potassium iodide). Turbidity or colored precipitation with either of these reagents was taken as evidence for the presence of alkaloids.[13]
Presence of cardiac glycosides
A few drops of the Baljet's reagent (picric acid, ethanol and sodium hydroxide) were added to 2–3 mg of sample. Orange indicated a positive reaction to deep red color.[12]
Tannins testing
One gram of sample was boiled with 20 ml distilled water for 5 min in a water bath and filtered while hot. Then 1 ml of cool filtrate was diluted to 5 ml with distilled water and a few drops (2–3) of 10% ferric chloride were added and observed for the formation of precipitates and any color change. A bluish-black or brownish-green precipitate indicated the presence of tannins.[12]
Test for flavonoids
One gram of powdered sample was boiled with 10 ml of distilled water for 5 min and filtered while hot. A few drops of 20% sodium hydroxide solution were added to 1 ml of the cooled filtrate. A change to yellow color which on the addition of acid changed to colorless solution depicted the presence of flavonoids.[14]
Test for saponins
The extract solution (1 ml) was diluted with distilled water to 20 ml and shaken for 15 min in a graduated cylinder. Development of stable foam suggests the presence of saponins.[14]
Test for combined anthraquinones
Powdered sample (1 g) was boiled with 2 ml of 10% hydrochloric acid for 5 min. The mixture was filtered while hot and the filtrate was allowed to cool. The cooled filtrate was partitioned against the equal volume of chloroform, and the chloroform layer was transferred into a clean, dry test tube using a clean pipette. An equal volume of 10% ammonia solution was added into the chloroform layer, shaken and allowed to separate. The separated aqueous layer was observed for any color change. Rose pink color indicated the presence of anthraquinones.[14]
Maintenance of human cell lines
Cells were grown in RPMI-1640 (supplemented with 10% of FCS, penicillin/streptomycin [50 IU/ml and 50 μg/ml, respectively], sodium pyruvate [1 mM], NaHCO3 and L-glutamine [2 mM]). Completed media was sterilized using 0.22 μm microbiological filters and kept at 4°C prior to use. Cells were maintained and grown in RPMI 1640 up to 15 subcultures.
Cytotoxicity assay
The different extracts of E. turcomanica were tested for in vitro cytotoxicity against Hela and HT-29 human tumor cell lines by a colorimetric assay, using 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide (MTT).[15] This assay is based on the metabolic reduction of soluble MTT by mitochondrial enzyme activity of viable tumor cells, into an insoluble colored formazan product, which can be measured by spectrophotometer after dissolving in DMSO. Overall, 180 μl of cells (5 × 104 cells/ml of media) were seeded in 96 well microplates and incubated for 24 h (37°C, 5% CO2 air humidified). Then the cells were treated with 20 μl of each concentration of different extracts and microplates containing cells and extracts were incubated for another 72 h in the same condition. Doxorubicin was used as a positive control. The first column of each microplate was assumed as a negative control (containing no extracts or doxorubicin). To evaluate cell survival, 20 μl of MTT solution (5 mg/ml in phosphate buffer solution) was added to each well and incubated for three h. Then gently 150 μl of old medium containing MTT was replaced by DMSO and pipetted to dissolve any formed formazan crystals. Absorbance was then determined at 540 nm by ELISA plate reader. Each extract concentration was assayed in eight wells and repeated three times. Standard curves (absorbance against a number of cells) for each cell line were illustrated. Intraday and interday variations were determined. Based on standard curves, percent cell survival was calculated. Percent of cell survival in DMSO (0.5% as a negative control) was assumed 100.
Statistical analysis
SIGMASTAT™ (Jandel Software, San Raphael, CA, USA) was used to perform statistical tests. Analysis of variance followed by Student-Newman-Keuls test was used to see the differences among groups. The significance was assumed at the 5% level.
Results
Preliminary phytochemical studies
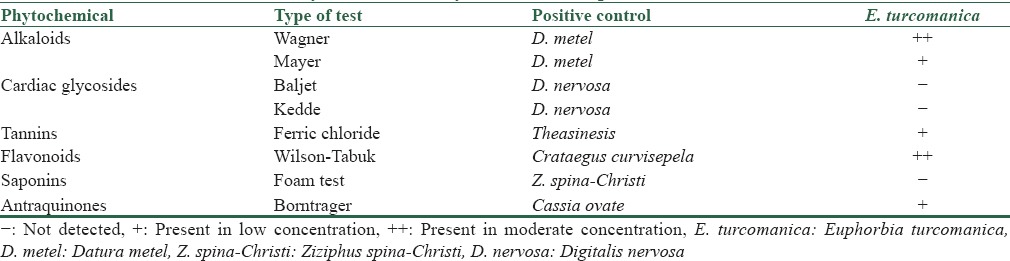
The results of the phytochemical analysis of the aerial parts of E. turcomanica suggested the presence of flavonoid, alkaloid, anthraquinone, and tannin [Table 1]. However, cardiac glycosides and saponin were not detected.
Table 1.
Phytochemical analysis of the aerial parts of E. turcomanica
Cytotoxic effect of Euphorbia turcomanica
A good relationship between absorbance and the number of cells was observed for Hela and HT-29 cell lines, (R2 = 0.975 and 0.985, respectively). Intraday and interday variations for all standard curves were acceptable (%coefficient of variation <15). Doxorubicin (20 μg/ml), a known cytotoxic antibiotic, as a positive control significantly inhibited the proliferation of both cell lines to <25%. Extracts were considered cytotoxic when cell viability decreased to <50%.
To assess acceptable cell lines incubation time with herbal extracts, the growth curve of each cell lines were plotted. According to the measured data, 48 h incubation time was used for MTT cytotoxicity assay.
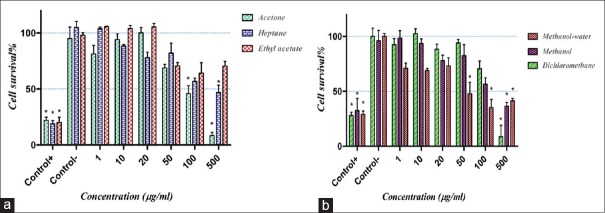
The antiproliferative effects of different extracts of E. turcomanica on Hela and HT-29 cells were assessed by MTT-based cytotoxicity method. Methanol-water, acetone, dichloromethane, methanol, and heptane extracts of E. turcomanica significantly reduced cell viability of Hela cells with inhibitory concentration 50% (IC50) of 50, 90, 230, 420, and 450 μg/ml, respectively [P < 0.05, Figure 1a and b].
Figure 1.
The effects of different extract of Euphorbia turcomanica on Hela cells. Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Survival percent in control group was supposed 100%. (a) Acetone, heptane, and ethyl acetate extracts and (b) methanol-water, methanol and dichloromethane extracts *P < 0.05, n = 9
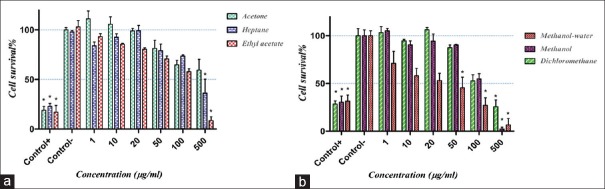
Methanol-water, dichloromethane, methanol, ethyl acetate, and heptane extracts of E. turcomanica were cytotoxic on HT-29 cells with IC50 of 43, 115, 125, 250, and 390 μg/ml, respectively [P < 0.05, Figure 2a and b]. However, ethyl acetate extract of E. turcomanica on Hela cells and acetone extract of this plant on HT-29 cells could not reduce cell viability to <50% up to a concentration of 500 μg/ml.
Figure 2.
The effects of different extract of Euphorbia turcomanica on HT-29 cells. Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Survival percent in control group was supposed 100%. (a) Acetone, heptane, and ethyl acetate extracts and (b) methanol-water, methanol, and dichloromethane extracts. *P < 0.05
Discussion
Cancer is one of the most dangerous, fast progressive with huge mortality rate diseases of the present century even in the developed countries. Roughly 60–75% of currently used anticancer agents are derived from natural sources,[16,17] in which it have been shown that Euphorbia spp. is promising plants antitumor activity.[18,19]
In this regard, the cytotoxic effects of six different extracts of E. turcomanica were evaluated against two tumor cell lines using MTT assay. Methanol-water and acetone extracts of E. turcomanica exhibited statistically significant cytotoxic activity on Hela cells with IC50 values of (50 and 90 μg/ml, respectively). However, acetone extracts of E. turcomanica only significantly inhibited cell growth in Hela cells with no effect on HT-29 cells. Ethyl acetate extracts of E. turcomanica did not show considerable cytotoxicity on Hela cells in concentrations tested. This could be explained by different susceptibility of tumor cells. Javidnia and co-workers showed that Euphorbia hebecarpa extracts were cytotoxic for K562 and U937 cells while they had no effects on KB cells.[20] Ethyl acetate extract was more effective on HT-29 cells than Hela cells. These findings are consistent with those of Sadeghi-Aliabadi et al. who showed that ethyl acetate extracts of E. macroclada were cytotoxic against MDA-MB-468 cells.[21] Various studies have shown that flavonoids possess cytotoxic effects on different cell lines.[22,23] Phytochemical test for flavonoids showed that E. turcomanica contain flavonoids so it can be concluded that at least some of the cytotoxic effects of this plant is due to the presence of flavonoids. As methanol-water and acetone extract of E. turcomanica showed more cytotoxicity and the fact that this solvent could better extract flavonoid glycosides,[24] it can be suggested that these compounds are involved in the cytotoxicity of E. turcomanica.
Recent studies suggested that the main chemical ingredients of Euphorbiaceous plants are diterpeneesters, triterpenoids, alkaloids, acetophenone derivatives, organic acid, and flavonoids.[25,26] It was also showed that Euphorbia plants exhibited a broad range of biological activities such as antianaphylactic, antioxidative, antiproliferative, antiarthrtic, antimicrobial, and cytotoxic activities.[27,28]
From our findings it could be inferred that E. turcomanica constituents extracted by polar solvents possess possible cytotoxic activity against two human tumor cell lines and it needs further investigations for the purification and identification of the active constituents as a new cytotoxic agent.
Financial support and sponsorship
This study was supported by a grant from the Research Council of Isfahan University of Medical Sciences, Isfahan, Iran.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank the School of Pharmacy research department in Isfahan University of Medical Sciences, Isfahan, I.R. Iran, for their co-operation and financial supports.
References
- 1.What is Cancer? National Cancer Institute. [Last cited on 2009 Aug 17]. Available from: http://www.cancer.gov/cancertopics/what-is-cancer .
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Graham JG, Quinn ML, Fabricant DS, Farnsworth NR. Plants used against cancer – An extension of the work of Jonathan Hartwell. J Ethnopharmacol. 2000;73:347–77. doi: 10.1016/s0378-8741(00)00341-x. [DOI] [PubMed] [Google Scholar]
- 4.Mahboobi S, Sellmer A, Beckers T. Development of tubulin inhibitors as antimitotic agents for cancer therapy. In: Attaur R, editor. Studies in Natural Products Chemistry. Philadelphia: Elsevier; 2006. pp. 719–50. [Google Scholar]
- 5.Srivastava V, Negi AS, Kumar JK, Gupta MM, Khanuja SP. Plant-based anticancer molecules: A chemical and biological profile of some important leads. Bioorg Med Chem. 2005;13:5892–908. doi: 10.1016/j.bmc.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 6.Mwine TJ, Van Damme P. Why do Euphorbiaceae tick as medicinal plants?: A review of Euphorbiaceae family and its medicinal features. J Med Plants Res. 2011;5:652–62. [Google Scholar]
- 7.Davis CC, Latvis M, Nickrent DL, Wurdack KJ, Baum DA. Floral gigantism in Rafflesiaceae. Science. 2007;315:1812. doi: 10.1126/science.1135260. [DOI] [PubMed] [Google Scholar]
- 8.Mozaffarian V. A Dictionary of Iranian Plant Names. Tehran: Farhang Moaser Publishers; 1996. [Google Scholar]
- 9.Betancur-Galvis LA, Morales GE, Forero JE, Roldan J. Cytotoxic and antiviral activities of Colombian medicinal plant extracts of the Euphorbia genus. Mem Inst Oswaldo Cruz. 2002;97:541–6. doi: 10.1590/s0074-02762002000400017. [DOI] [PubMed] [Google Scholar]
- 10.Jassbi AR. Chemistry and biological activity of secondary metabolites in Euphorbia from Iran. Phytochemistry. 2006;67:1977–84. doi: 10.1016/j.phytochem.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Amirghofran Z, Malek-hosseini S, Gholmoghaddam H, Kalalinia F. Inhibition of tumor cells growth and stimulation of lymphocytes by Euphorbia species. Immunopharmacol Immunotoxicol. 2011;33:34–42. doi: 10.3109/08923971003699018. [DOI] [PubMed] [Google Scholar]
- 12.Harbone JB. Phytochemical Methods. London: Chapman and Hall; 1973. [Google Scholar]
- 13.Smolenski SJ, Silinis H, Farnsworth NR. Alkaloid screening.V. Lloydia. 1974;37:506–36. [PubMed] [Google Scholar]
- 14.Trease G, Evans W. A Textbook of Pharmacognosy. London: Bailliere Tindall and Company Publishers; 1983. [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 16.Cragg GM, Kingston DG, Newman DJ. Anticancer Agents from Natural Products. New york: CRC Press; 2011. [Google Scholar]
- 17.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–37. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 18.Liang QL, Dai CC, Jiang JH, Tang YP, Duan JA. A new cytotoxic casbane diterpene from Euphorbia pekinensis. Fitoterapia. 2009;80:514–6. doi: 10.1016/j.fitote.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Jafarain A, Asghari G, Ghassami E. Evaluation of cytotoxicity of Moringa oleifera Lam. callus and leaf extracts on Hela cells. Adv Biomed Res. 2014;3:194. doi: 10.4103/2277-9175.140668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Javidnia K, Miri R, Amirghofran Z, Jafari A, Amoozegar Z. Cytotoxicity and antimicrobial assessment of Euphorbia hebecarpa. Iran J Pharm Res. 2004;3:75–82. [Google Scholar]
- 21.Sadeghi-Aliabadi H, Sajjadi SE, Khodamoradi M. Cytotoxicity of Euphorbia macroclada on MDA-MB-468 breast cancer cell line. Iran J Pharm Sci. 2009;5:103–8. [Google Scholar]
- 22.Matsuo M, Sasaki N, Saga K, Kaneko T. Cytotoxicity of flavonoids toward cultured normal human cells. Biol Pharm Bull. 2005;28:253–9. doi: 10.1248/bpb.28.253. [DOI] [PubMed] [Google Scholar]
- 23.Soares VC, Varanda EA, Raddi MS. In vitro basal and metabolism-mediated cytotoxicity of flavonoids. Food Chem Toxicol. 2006;44:835–8. doi: 10.1016/j.fct.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Markham KR. Techniques of Flavonoid Identification. London: Academic Press; 1982. [Google Scholar]
- 25.Ghanadian M, Rahiminejad M, Saeidi H, Ayatollahi A, Shamsabadipour S. Triterpenes from Euphorbia denticulata. Res Pharm Sci. 2012;7:S960. [Google Scholar]
- 26.Fu GM, Qin HL, Yu SS, Yu BY. Yuexiandajisu D, a novel 18-nor-rosane-type dimeric diterpenoid from Euphorbia ebracteolata Hayata. J Asian Nat Prod Res. 2006;8:29–34. doi: 10.1080/10286020500480308. [DOI] [PubMed] [Google Scholar]
- 27.Bani S, Kaul A, Khan B, Gupta VK, Satti NK, Suri KA, et al. Anti-arthritic activity of a biopolymeric fraction from Euphorbia tirucalli. J Ethnopharmacol. 2007;110:92–8. doi: 10.1016/j.jep.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Chaabi M, Freund-Michel V, Frossard N, Randriantsoa A, Andriantsitohaina R, Lobstein A. Anti-proliferative effect of Euphorbia stenoclada in human airway smooth muscle cells in culture. J Ethnopharmacol. 2007;109:134–9. doi: 10.1016/j.jep.2006.07.016. [DOI] [PubMed] [Google Scholar]