Abstract
Background
Bupleuri Radix (Chaihu) represents one of the most successful and widely used herbal medicines in Asia for the treatment of many diseases such as inflammatory disorders and infectious diseases over the past 2000 years. In the Chinese Pharmacopoeia, Chaihu is recorded as the dried roots of Bupleurum chinense DC. and B. scorzonerifolium Willd. (Umbelliferae). However, the widespread demand for the herb has tended to far outstrip the supply. Whether the aerial parts, which account for 70 ~ 85% of the dry weights of Bupleurum species, could be used as an alternative for the root has become an important scientific issue for the sustainable utilization of Bupleurum species. On the other hand, in some areas including the southeast of China as well as in Spain, the aerial parts of Bupleurum species have already been used in the folk medications. Therefore, to clarify whether the root and aerial parts of Bupleurum species are “equivalent” in the types and quantities of chemical constituents which subsequently influence their biological activities and therapeutic effects is of great importance for both the rational and sustainable use of this herb.
Methods
In the present study, the chemical profiles between the root and aerial parts of Bupleurum species from different species and collected from various locations were analyzed and compared by the ultra-high performance liquid chromatography quadrupole/time of flight-mass spectrometry (UHPLC-QTOF-MS).
Results
A total of 56 peaks were identified in the root and/or aerial parts from different batches of Bupleurum species, by comparison of references standards or with those reported in the literature. Principal Component Analysis (PCA) was conducted for displaying the differentiating clustering between these two parts.
Conclusion
The results disclosed the distinct variations between them, which indicated that the aerial parts could not be used as an alternative of root from a chemodiversity perspective. The differentiating markers resulted from the PCA analysis could also be utilized for the differentiation between them. Further validation of their biological differences is anticipated in the future study.
Keywords: Bupleuri Radix, Chemical profiles, Medicinal parts, Metabolomics approach
Background
Bupleuri Radix (Chinese name: Chaihu) represents one of the most popular Traditional Chinese Medicines (TCM) over the past 2000 years. Its TCM indications include the treatment of influenza or common cold with fever, chills and fever from malaria, distending pain in the chest and menstrual disorders [1]. In the Chinese Pharmacopoeia, Chaihu is recorded as the dried roots of Bupleurum chinense DC. and B. scorzonerifolium Willd. (Umbelliferae) [1]. It is often found in clinical prescriptions and proprietary Chinese medicines, such as Xiao-Chaihu-Tang, Xiao-Yao-Wan, Jia-Wei-Xiao-Yao-Wan and Chai-Ling-Tang. In addition to the authentic species of Chaihu, there are more than 20 other species in the genus Bupleurum also habitually utilized as Chaihu in some local areas. Knowing the high demand for Bupleuri Radix and knowing the diversity of species that can be—both rightly and wrongly—used for this herb, the resources of Chaihu are very scare. Today, Chaihu from the species of B. yinchowense Shan et Y. Li has become mainstream in the market. [2] The species of B. yinchowense is abundantly distributed in the Northwest of China and is widely used in folk medicine for relieving fever, soothing liver and improving the symptoms of emotional instability such as depression, anxiety and phobia [3–5]. Additionally, in the Japanese Pharmacopoeia (16th edition), the official botanical origin of Bupleuri Radix (pronounced “saiko” in Japanese) is the roots of B. falcatum L [6]. Actually, B. falcatum is also commonly used in China and Korea [7]. In Japan, B. falcatum L is known for its therapeutic effects in the treatment of chronic hepatitis, auto-immune diseases and diabetes [8–11]. It is also used as an ingredient in herbal tea and traditional fermented beverages [8].
Previous phytochemical studies on approximately 50 Bupleurum species led to the isolation and identification of almost 250 natural compounds from all major phytochemical classes, including mono- and sesquiterpenes (essential oils), triterpenoid glycosides (saikosaponins), sterols, lignans, flavonoid glycosides, coumarins, and polyacetylenes [12–14]. In addition, minor components, including phenylpropanoids, polysaccharides and a few alkaloids, have also been reported [12]. Among them, the saikosaponins (SSs) are acknowledged to be the principal bioactive components, which can be divided into six types on the basis of the aglycones: type I-VI (Fig. 1) [15, 16]. Flavonoids are another class of bioactive secondary metabolites present in all species of the genus Bupleurum [17]. Most flavonoids in the genus are derivatives of the flavonol aglycones kaempferol, isorhamnetin or quercetin [12].
Fig. 1.
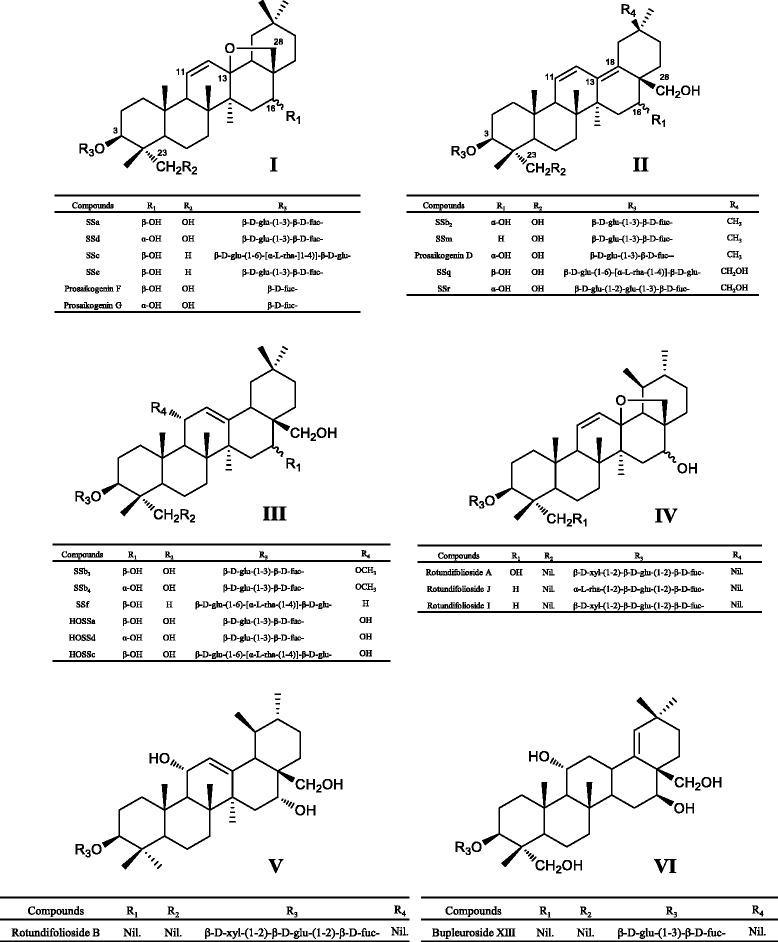
Chemical structures of six types (I-VI) of saikosaponins (SSs) in Bupleurum
Since Chaihu is very rare in nature, the amount of wild samples is not sufficient for commercial exploitation [18]. Although Chaihu have been widely cultivated nowadays, the widespread demand for the herb has still tended to far outstrip the supply [19, 20]. As the aerial part of Bupleurum species accounts for more than half of the whole plant, some areas in the southeast of China use the whole herb for the medication [21]. In Guangdong province of China, the root and aerial parts of Bupleurum species are sold separately for the folk use [22]. Besides, the aerial parts of Bupleurum species are used as a popular topical antiseptic and anti-inflammatory remedy in Spain [23]. Therefore, the short supply and perspective for sustainable utilization of Chaihu has stimulated the interest on comparing the “equivalence” of the root and aerial parts. Whether the root and aerial parts vary in the types and quantities of chemical constituents which subsequently influence their therapeutic effects, would determine whether the aerial parts of Bupleurum species could be used as a suitable substitute for the root. Additionally, whether Chaihu should be prescribed as root, aerial parts or whole plant of Bupleurum species was also needed to be clarified by scientific evidences, so as to prevent or avoid the misuse of this herb. To differentiate the crude materials from which medicinal parts used in proprietary Chinese medicines containing Chaihu is also significant for their quality control. Therefore, a comprehensive analysis of the chemical profiles is highly needed to be conducted for the two different parts.
The objective of the present study is to analyze and compare the chemical profiles between the root and aerial parts of Bupleurum species utilizing the ultra-high performance liquid chromatography quadrupole/time of flight-mass spectrometry (UHPLC-QTOF-MS). The authentic species of B. chinense as well as B. yinchowense and B. falcatum collected from different locations were investigated. The overall results provided comprehensive chemical comparison for the two different parts of Bupleurum species, which was anticipated to advise the possibilities of alternate use of these two parts.
Methods
Plant materials
Eight batches of the whole plant including the root and aerial parts of Bupleurum chinense DC., B. yinchowense and B. falcatum were collected. Details of the sample are shown in Table 1 and Fig. 2. All the herbal samples were authenticated by Prof. Guo Baolin from the Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Peking Union Medical College and deposited in the Bank of China (Hong Kong) Chinese Medicines Centre of Hong Kong Baptist University.
Table 1.
Sample information for the analysis
| No. | Species | Source (geographical location) | Collection time | Deposition numbers |
|---|---|---|---|---|
| BC1 | Bupleurum chinense DC. | Wei Village, Fen Cheng town, Xiang fen County, Shanxi Province, cultivated for three years | 2013–8-7 | SX-0807 |
| BC2 | Bupleurum chinense DC. | Long Xing Village, Kao Lao town, Wan Rong County, Shanxi Province, cultivated for three years | 2013–8-8 | SX-0808 |
| BC3 | Bupleurum chinense DC. | Xiang Quan County, Chen Cang District, Bao Ji City, Shaanxi Province, wild | 2013–9-4 | SX-0917 |
| BC4 | Bupleurum chinense DC. | Xin Min County, Chen Cang District, Bao Ji City, Shaanxi Province, cultivated for three years | 2013–9-4 | SX-0918 |
| BY1 | Bupleurum yinchowense Shan et Y. Li | An Yi Town, Long Xi County, Gansu Province, cultivated for two years | 2013–9-2 | GS-0903 |
| BY2 | Bupleurum yinchowense Shan et Y. Li | Lu Ming Village, Wu Zhu Town, Wei Yuan County, Gansu Province, cultivated for two years | 2013–9-2 | GS-0905 |
| BY3 | Bupleurum yinchowense Shan et Y. Li | Da Hong Yan Village, San Fen Town, Zhang County, Gansu Province, cultivated for two years | 2013–9-3 | GS-0908 |
| BF | Bupleurum falcatum L. | Han Yuan County, Sichuan Province, cultivated for one years | 2013–9-5 | SC-0919 |
Fig. 2.
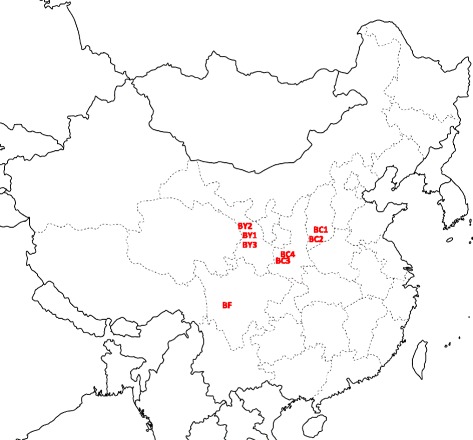
The locations of harvest of Bupleurum plants. (The blank map was obtained from a free Baidu library https://wenku.baidu.com/, and then the locations were marked on the map by the authors)
Chemicals and reagents
Chemical markers of saikosaponins a, c and d were purchased from Chengdu Must Bio-Technology Co., Ltd. (Chengdu, People’s Republic of China). The purities of all saikosaponins were determined to be higher than 98% by HPLC-DAD analysis. The solvents, acetonitrile and methanol, were of HPLC grade from E. Merck (Darmstadt, Germany). Formic acid with a purity of 96% was also of HPLC grade (Tedia, U.S.A.). Water was obtained from a Milli-Q water purification system (Millipore, Bedford, MA, U.S.A.).
Preparation of sample solution
The dried roots and aerial parts of eight batches of samples involving Bupleurum chinense, B. yinchowense and B. falcatum from different growing areas were separated and grinded into homogeneous powders using liquid nitrogen. The dried powder (~0.1 g) was weighed accurately into a 15 mL microcentrifuge tube and was then extracted twice with 10 ml of methanol using an ultrasonicator (1875HTAG, CREST, U.S.A.) for 30 min at room temperature each time. After centrifugation at 3000 rpm for 10 min, the supernatant was transferred into a 25 ml volumetric flask and was adjusted to the volume with methanol. Finally, 1.0 mL extraction was transferred to 1.5 microcentrifuge tube and centrifuged again for 10 min at 12,000 rpm. An aliquot of 90 μl of supernatant was transferred to the glass inserts of 1.5 ml brown HPLC vials (Grace, HK) with plastic bottom springs (400 μl, Grace, UK) and stored at 4 °C pending for analysis.
Stock solutions of saikosaponins a, c and d were prepared individually in methanol. Working solutions were prepared by diluting the stock solutions with methanol to give final concentrations of 36, 10 and 36 μg/ml for saikosaponins a, c and d, respectively.
UHPLC-QTOF-MS analysis
UHPLC–QTOF-MS analysis was performed on an Agilent 6540 ultra-high definition accurate mass quadrupole time-of-flight spec-trometer with UHPLC (UHPLC–QTOF-MS, Agilent Technologies, U.S.A.). A UPLC C18 analytical column (2.1 mm × 100 mm, I.D. 1.7 μm, ACQUITY UPLC®BEH, Waters, U.S.A.) was used for separation, coupled with a C18 pre-column (2.1 mm × 5 mm, I.D. 1.7 μm, Van-GuardTM BEH, Waters, U.S.A.) at room temperature of 20 °C. The mobile phase was a mixture of water (A) and acetonitrile (B), both containing 0.1% formic acid, with an optimized linear gradient elution as follows: 0–5 min, 10–35% B; 5–25 min, 35–55% B; 25–28 min, 55–85% B; 28–30 min, 85–100% B. The injection volume was 4 μL. The flow rate was set at 0.35 mL/min. The mass spectra were acquired in negative mode by scanning from 100 to 1700 in mass to charge ratio (m/z). The MS analysis was performed under the following operation parameters: dry gas temperature 300 °C, dry gas (N2) flow rate 5 L/min, nebulizer pressure 30 psi, Vcap 3000, nozzle voltage 500 V, and fragmentor voltage 200 V.
Data analysis
Data analysis was performed on Agilent MassHunter Workstation software-Qualitative Analysis (version B.04.00, Build 4.0.479.5, Service Pack 3, Agilent Technologies, Inc. 2011). The acquired data in MassHunter Qualitative Analysis software were extracted using the molecular feature extraction (MFE) algorithm and imported into Mass Profier Professional (MPP) V.12.5 for principal component analysis (PCA) to display the difference between the root and aerial parts of eight herbal samples.
Results and discussion
Chemical profiling
The chemical profiles of the root and aerial parts of Bupleurum species were analyzed by UHPLC-QTOF-MS. The representative total ions current (TIC) chromatograms of the different parts from B. chinense, B. yinchowense and B. falcatum are shown in Fig. 3. The major peaks in the TIC chromatograms were identified, with peaks 20, 28 and 43 unambiguously identified as saikosaponins c, a and d (SSc, SSa and SSd) by comparison of their chromatographic retention times, accurate molecular weights and characteristic mass fragment ions with those of the references standards. Other peaks were tentatively identified by comparison of their accurate mass data with those reported in the literature. Detailed information related to the illustration of all 56 peaks was shown in Table 2.
Fig. 3.
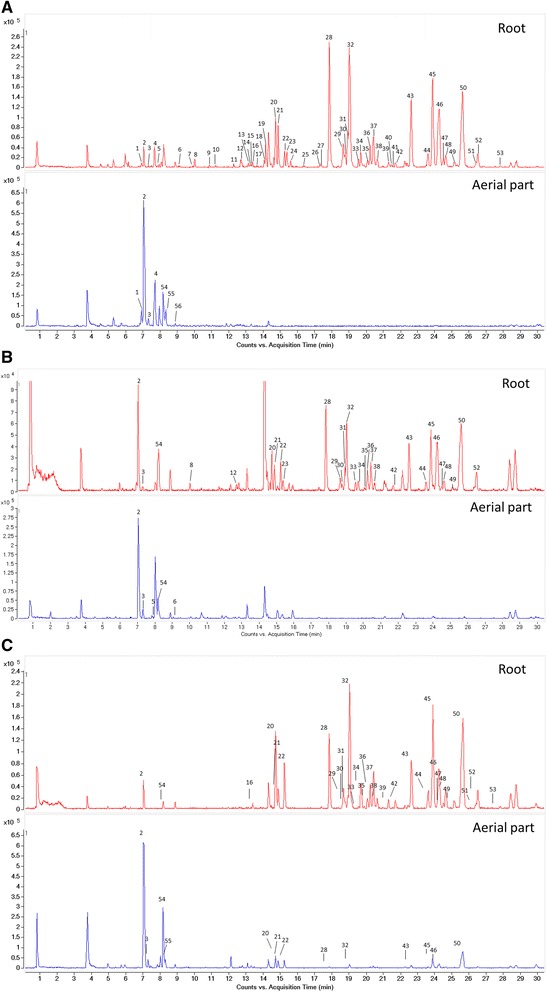
The representative total ions current (TIC) chromatograms of the root and aerial parts from B. chinense DC. a, B. yinchowense Shan et Y. Li b and B. falcatum L. c
Table 2.
Compounds identified from aerial part and root of three Bupleurum species
| Peak No. | Identification | tR (min) | Molecular formula | Molecular ions (m/z) (mass accuracy (ppm)) | Fragments (m/z) | BC | BY | BF | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Root | Aerial | Root | Aerial | Root | Aerial | ||||||
| 1 | Kaempferol-3-O-glucopyranoside-7-O-rhamnopyranoside | 6.90 | C27H30O15 | 593.1537 [M-H]− (4.2) | 431.1086 [M-H-(Glc-H2O)]−, 285.0458 [M-H-(Glc-H2O)-(Rha-H2O)]− | + | + | ||||
| 2 | Rutin | 7.03 | C27H30O16 | 609.1439 [M-H]− (−3.61) | 1219.3206 [2 M–H]−, 300.0334 [M-H-(Glc-H2O)-(Rha-H2O)]− | + | + | + | + | + | + |
| 3 | Isoquercitrin | 7.30 | C21H20O12 | 463.0871 [M-H]− (−2.4) | 300.2900 [M-H-(Glc-H2O)]− | + | + | + | + | + | |
| 4 | Kaempferitrin | 7.67 | C27H30O14 | 577.1589 [M-H]− (4.5) | 431.1079 [M-H-(Rha-H2O)]−, 285.0477 [M-H-2(Rha-H2O)]− |
+ | + | + | |||
| 5 | Quercetin 3′-glucoside-7-acetat | 7.84 | C23H22O13 | 505.0992 [M-H]− (−0.8) | 300.0311 [M-2H-Acetyl-(Glc-H2O)]− | + | + | ||||
| 6 | Quercetin 3,7-diglucoside | 9.05 | C27H30O17 | 625.1433 [M-H]− (3.7) | 463.1016 [M-H-(Glc-H2O)]− | + | + | ||||
| 7 | Quercetin 4′-glucoside | 9.89 | C21H20O12 | 463.0884 [M-H]− (0.4) | 300.283 [M-H-(Glc-H2O)]- | + | |||||
| 8 | Unknown | 10.01 | C42H66O15 | 809.4367 [M-H]− (4.7) 855.4347 [M + HCOO]− (−4.3) |
647.3920 [M-H-(Glc-H2O)]− | + | + | ||||
| 9 | Rotundifolioside I/Rotundioside O | 10.86 | C47H76O16 | 895.5017 [M-H]− (−4.9) | / | + | |||||
| 10 | Rotundioside N | 11.23 | C48H78O18 | 941.5142 [M-H]− (2.87) 987.5146 [M + HCOO]− (−2.43) |
633.4307 [M-H-(Glc-H2O)-(Fuc-H2O)]− | + | |||||
| 11 | Hydroxy-SSa | 12.27 | C42H70O14 | 797.4646 [M-H]− (−5.9) 843.4705 [M + HCOO]− (−5.1) |
635.4320 [M-H-(Glc-H2O)]− | + | |||||
| 12 | Hydroxy-SSd | 12.67 | C42H70O14 | 797.4656 [M-H]− (−4.6)) 843.4711 [M + HCOO]− (−4.4) |
635.4365 [M-H-(Glc-H2O)]− | + | + | ||||
| 13 | Rotundioside W | 13.12 | C48H78O18 | 941.5195 [M-H]− (8.5) 987.5156 [M + HCOO]− (−1.4) |
796.4688 [M-H- (Rha-H2O)]− | + | |||||
| 14 | Acetyl-hydroxy-SSa | 13.22 | C44H72O15 | 839.4793 [M-H]− (−0.6) 885.4824 [M + HCOO]− (−3.28)) |
797.4801 [M-H-Acetyl]−; 635.4276 [M-H-Acetyl-(Glc-H2O)]− |
+ | |||||
| 15 | Acetyl-hydroxy-SSd | 13.29 | C44H72O15 | 839.4777 [M-H]− (−2.5) 885.4822 [M + HCOO]− (−3.5) |
797.4856 [M-H-Acetyl]−; 635.4268 [M-H-Acetyl-(Glc-H2O)]− |
+ | |||||
| 16 | Malonyl-acetyl-hydroxy-SSa/Malonyl-acetyl-hydroxy-SSd | 13.37 | C45H72O17 | 883.4663 [M-H]− (−3.9) | 797.4801 [M-H-Malonyl-Acetyl]−; 635.4276 [M-H-Malonyl-Acetyl- (Glc-H2O)]− |
+ | + | ||||
| 17 | Malonyl-SSn | 13.61 | C51H80O21 | 1027.5194 [M-H]− (6.21) | 985.5383 [M-H-Acetyl]−, 941.5287 [M-H-Acetyl-CO2]− |
+ | |||||
| 18 | Unknown | 14.06 | C51H90O26 | 1117.5640 [M-H]− (−0.7) | 955.5135 [M-H-(Glc-H2O)]− | + | |||||
| 19 | Unknown | 14.15 | C51H90O26 | 1117.5654 [M-H]− (−0.5) | 955.5135 [M-H-(Glc-H2O)]− | + | |||||
| 20 | SSc | 14.73 | C48H78O17 | 925.5168 [M-H]− (0.2) 971.5208 [M + HCOO]− (1.3) |
779.4734 [M-H-(Rha-H2O)]− | + | + | + | + | ||
| 21 | SSf | 14.87 | C48H80O17 | 927.5313 [M-H]− (1.1) 973.5376 [M + HCOO]− (0.2) |
781.4895 [M-H-(Rha-H2O)]− | + | + | + | + | ||
| 22 | Malonyl-Ssc | 15.23 | C51H80O20 | 1011.5162 [M-H]− (−0.8) | 967.5447 [M-H-CO2]−, 779.4838 [M-H-Malonyl-(Rha-H2O)]− | + | + | + | + | ||
| 23 | Malonyl-Ssf | 15.37 | C51H82O20 | 1013.5322 [M-H]− (−0.5) | 969.5611 [M-H-CO2]−, 927.5527 [M-H- Malonyl]−, 781.4795 [M-H-Malonyl-(Rha-H2O)]−, 765.4884 [M-H-Malonyl-(Glc-H2O)]− |
+ | + | ||||
| 24 | Malonyl-acetyl-rotundifolioside B | 15.59 | C52H82O21 | 1041.5205 [M-H]− (−6.8) | 997.5215 [M-H-CO2]−, 835.4661 [M-H-CO2-(Glc-H2O)]− |
+ | |||||
| 25 | SSb3 / SSb4 | 16.34 | C43H72O14 | 811.4847 [M-H]− (−0.3) 857.4985 [M + HCOO]− (0.9) |
649.4344 [M-H-(Glc-H2O)]− | + | |||||
| 26 | SSn | 17.31 | C48H78O18 | 941.5196 [M-H]− (0.8) 987.5127 [M + HCOO]− (0.4) |
779.4722 [M-H-(Glc-H2O)]− | + | |||||
| 27 | Malonyl-SSb3 / SSb4 | 17.38 | C46H74O17 | 897.4823 [M-H]− (3.3) | 853.5090 [M-H-CO2]−, 811.5024 [M-H- Malonyl]−, 649.4453 [M-H-Malonyl-(Glc-H2O)]− |
+ | |||||
| 28 | SSa | 17.83 | C42H68O13 | 779.4558 [M-H]− (−0.4) 825.4629 [M + HCOO]− (−0.2) |
617.4186 [M-H-(Glc-H2O)]− | + | + | + | + | ||
| 29 | Acetyl-SSa | 18.65 | C44H70O14 | 821.4668 [M-H]− (−3.0) 867.4713 [M + HCOO]− (−4.0) |
779.4742 [M-H-Acetyl]−, 617.4236 [M-H-Acetyl-(Glc-H2O)]− |
+ | + | + | |||
| 30 | Acetyl-SSa | 18.77 | C44H70O14 | 821.4660 [M-H]− (−4.0) 867.4718 [M + HCOO]− (−3.4) |
779.4746 [M-H-Acetyl]−, 617.4209 [M-H-Acetyl-(Glc-H2O)]− |
+ | + | + | |||
| 31 | Acetyl-SSa | 18.93 | C44H70O14 | 821.4649 [M-H]− (−5.3) 867.4714 [M + HCOO]− (−3.9) |
779.4752 [M-H-Acetyl]−, 617.4177 [M-H-Acetyl-(Glc-H2O)]− |
+ | + | + | |||
| 32 | Malonyl-SSa | 19.01 | C45H70O16 | 865.4576 [M-H]− (−1.7) | 821.4851 [M-H-CO2]−, 779.4747 [M-H- Malonyl]−, 617.4182 [M-H- Malonyl-(Glc-H2O)]− |
+ | + | + | + | ||
| 33 | Acetyl-SSa | 19.55 | C44H70O14 | 821.4657 [M-H]− (−4.4) 867.4713 [M + HCOO]− (−4.0) |
779.4746 [M-H-Acetyl]−, 617.4188 [M-H-Acetyl-(Glc-H2O)]− |
+ | + | + | |||
| 34 | Dimalonyl-SSa | 19.68 | C52H72O16 | 951.4765 [M-H]− (1.8) | 907.4863 [M-H-CO2]−, 779.4687 [M-H-2 Malonyl]−, 617.4188 [M-H-2 Malonyl-(Glc-H2O)]− |
+ | + | + | |||
| 35 | Dimalonyl-SSa | 20.08 | C52H72O16 | 951.4771 [M-H]− (2.4) | 907.4873 [M-H-CO2]−, 779.4757 [M-H-2 Malonyl]−, 617.4244 [M-H-2 Malonyl-(Glc-H2O)]− |
+ | + | + | |||
| 36 | Dimalonyl-SSa | 20.22 | C52H72O16 | 907.4874 [M-H-CO2]− (2.8) | 779.4717 [M-H-2 Malonyl]−, 617.4192 [M-H-2 Malonyl-(Glc-H2O)]− |
+ | + | + | |||
| 37 | Dimalonyl-SSa | 20.43 | C52H72O16 | 907.4880 [M-H-CO2]− (3.4) | 779.4764 [M-H-2 Malonyl]−, 617.4199 [M-H-2 Malonyl-(Glc-H2O)]− |
+ | + | + | |||
| 38 | Dimalonyl-SSa | 20.64 | C52H72O16 | 907.4865 [M-H-CO2]− (1.8) | 779.4799 [M-H-2 Malonyl]−, 617.4156 [M-H-2 Malonyl-(Glc-H2O)]− | + | + | + | |||
| 39 | Dimalonyl-acetyl-SSa | 21.31 | C54H74O17 | 993.4864 [M-H]− (1.1) | 949.4979 [M-H-CO2]−, 864.5019 [M-2H-Malonyl-Acetyl]−, 821.4803 [M-2Malonyl]−, 761.4609 [M-H-Malonyl-(Fuc-H2O)]− |
+ | + | ||||
| 40 | SSs | 21.44 | C59H74O10 | 941.5269 [M-H]− (6.4) 987.5245 [M + HCOO]− (−1.9) |
780.4802 [M-(Glc-H2O)]−
617.4202 [M-H-2(Glc-H2O)]− |
+ | |||||
| 41 | Dimalonyl-SSa / SSd | 21.53 | C52H72O16 | 907.4865 [M-H-CO2]− (1.8) | 779.4799 [M-H-2 Malonyl]−, 617.4156 [M-H-2 Malonyl-(Glc-H2O)]− |
+ | |||||
| 42 | Malonyl-SSe | 21.68 | C45H70O15 | 849.4689 [M-H]− (5.5) | 805.4903 [M-H-CO2]−, 763.4800 [M-H-Malonyl]−, 643.4376 [M-H-CO-(Glc-H2O)]− 601.4253 [M-H-Malonyl-(Glc-H2O)]− |
+ | + | + | |||
| 43 | SSd | 22.62 | C42H68O13 | 779.4547 [M-H]− (−5.1) 825.4606 [M + HCOO]− (−4.4) |
617.4186 [M-H-(Glc-H2O)]− | + | + | + | + | ||
| 44 | Acetyl-SSd | 23.60 | C44H70O14 | 821.4653 [M-H]− (−4.9) 867.4756 [M + HCOO]− (0.9) |
779.4739 [M-H-Acetyl]−, 617.4196 [M-H-Acetyl-(Glc-H2O)]− |
+ | + | + | |||
| 45 | Malonyl-SSd | 23.85 | C45H70O16 | 865.4571 [M-H]− (−2.3) | 821.4859 [M-H-CO2]−, 779.4716 [M-H- Malonyl]−, 617.4199 [M-H- Malonyl-(Glc-H2O)]− |
+ | + | + | + | ||
| 46 | Acetyl-SSd | 24.22 | C44H70O14 | 821.4648 [M-H]− (−5.5) 867.4722 [M + HCOO]− (−3.0) |
779.4745 [M-H-Acetyl]−, 617.4238 [M-H-Acetyl-(Glc-H2O)]− |
+ | + | + | + | ||
| 47 | Acetyl-SSd | 24.49 | C44H70O14 | 821.4671 [M-H]− (−2.7) 867.4717 [M + HCOO]− (−3.6) |
779.4723 [M-H-Acetyl]−, 617.4218 [M-H-Acetyl-(Glc-H2O)]− |
+ | + | + | |||
| 48 | Dimalonyl-SSd | 24.62 | C52H72O16 | 951.4771 [M-H]− (2.4) | 907.4859 [M-H-CO2]−, 821.4865[M-H- Malonyl-CO2]−, 779.4799 [M-H-2 Malonyl]−, 617.4156 [M-H-2 Malonyl-(Glc-H2O)]− |
+ | + | + | |||
| 49 | Dimalonyl-SSd | 25.14 | C52H72O16 | 907.4863 [M-H-CO2]− (1.5) | 821.4924[M-H- Malonyl-CO2]−, 779.4829 [M-H-2 Malonyl]−, 617.4177 [M-H-2 Malonyl-(Glc-H2O)]− |
+ | + | + | |||
| 50 | Dimalonyl-SSd | 25.60 | C52H72O16 | 907.4873 [M-H-CO2]− (2.6) | 821.4856 [M-H- Malonyl-CO2]−, 779.4761 [M-H-2 Malonyl]−, 617.4191 [M-H-2 Malonyl-(Glc-H2O)]− |
+ | + | + | + | ||
| 51 | Dimalonyl-acetyl-SSd | 26.40 | C54H74O17 | 993.4828 [M-H]− (−2.5) | 949.4978 [M-H-CO2]−, 821.4838 [M-2Malonyl]− |
+ | + | ||||
| 52 | Dimalonyl-acetyl-SSd | 26.52 | C54H74O17 | 993.4896 [M-H]− (4.0) | 949.4976 [M-H-CO2]−, 864.5027 [M-2H-Malonyl-Acetyl]−, 821.4860 [M-2Malonyl]−, 761.4615 [M-H-Malonyl-(Fuc-H2O)]−, |
+ | + | + | |||
| 53 | Malonyl-diacetyl-SSd | 27.81 | C53H73O15 | 949.4971 [M-H]− (1.7) | 863.5013 [M-H-Malonyl]−, 779.4689 [M-H-Malonyl-2Acetyl]− |
+ | + | ||||
| 54 | Isorhamnetin-3-O-rutinoside | 8.20 | C28H32O16 | 623.1651 [M-H]− (5.3) | / | + | + | + | + | + | |
| 55 | Quercetin-3-O-rhamnoside | 8.32 | C21H20O11 | 447.0936 [M-H]− (0.7) | 300.0349 [M-H-(Rha-H2O)]− | + | + | ||||
| 56 | Quercetin 3-(6″-acetylglucoside) | 8.54 | C23H22O13 | 505.0993 [M-H]− (1.0) | 300.0330 [M-2H-Acetyl-(Glc-H2O)]− | + | |||||
In the TIC chromatograms, saikosaponins and flavonoids represents majority of the peaks identified, with flavonoids (peaks 1, 2, 3, 4, 5, 6, 7, 54, 55 and 56) accumulated in the previous 10 min of the eluting time while saikosaponins were eluted at the rest of time. The root and aerial parts of all the three Bupleurum species exhibited varied profiles. It is distinct and consistent for all the roots contain more chromatographic peaks covering both of the saikosaponin and flavonoid part of the TIC chromatograms. The profiles of the roots from the three Bupleurum species were similar especially for the characteristic saikosaponin part (peaks 20–52). In contrast, the aerial parts contain abundantly flavonoids with little or no saikosaponin peaks. The results clearly demonstrated the distinct chemical profiles of the root and aerial parts of Bupleurum species. Yen et al. compared the saikosaponins a, c and d between the root and aerial parts of three Bupleurum species using thin-layer chromatography (TLC) scanning. The results showed that the aerial parts contained low levels of saikosaponins, which were different from that of the root [24]. These were in accordance with those provided in the present study, which thus strengthened the conclusions that the aerial parts could not be used as an alternate of root from a chemodiversity perspective.
Principal component analysis (PCA)
The differences between the root and aerial parts of eight herbal samples were further displayed by the Principal Component Analysis (PCA). The full time-of-flight (TOF) mass spectral data of each sample were first processed by MassHunter Workstation software. Ions were extracted by molecular feature extraction (MFE) algorithm characterized by retention time (RT), intensity in apex of chromatographic peak, and accurate mass, exported as the Compound Exchange Format (.cef) file. These results were then analyzed by Mass Profiler Professional (MPP) software. Entities that present in more than 50% of samples in at least one condition were filtered by frequency before doing Principal Component Analysis (PCA). Finally 258 features were left for further PCA study. The resulting PCA graph also demonstrated the distinct clustering between the root and aerial parts of the investigated samples, which indicating the chemical difference between these two parts (Fig. 4).
Fig. 4.
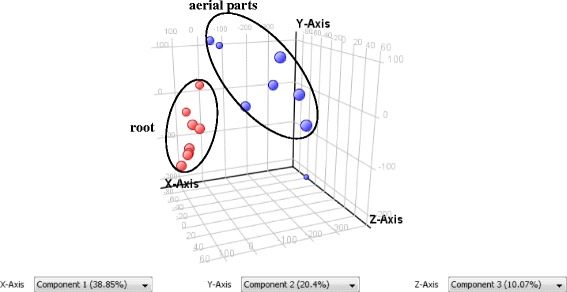
The principal component analysis (PCA) of the root (red) and aerial (blue) parts from eight batches of Bupleurum samples
Potential differentiating markers
In order to find out the potential differentiating markers for distinguishing the different parts of Bupleurum species, significant testing and fold change was investigated to identify statistically differentiative compounds by applying appropriate test and conditions. Nine compounds, out of 258 entities from the above frequency filtration were found to be significantly different among the two parts using one way ANOVA and a level of probability of 0.001 and fold change >2, as listed in Table 3. These differentiating markers with the lowest p-values and highest fold-changes (most significant with greatest abundance differences) posed mostly influential features for the differentiation between the root and aerial parts, which therefore could be used as markers for differentiation.
Table 3.
List of compounds (9 entities) that are distinguished between root and aerial parts at p < 0.001 and fold change (FC) > 3
| Peak No. | Compounds | R.T. (mins) | p-value | Log FC |
|---|---|---|---|---|
| 3 | Isoquercitrin | 7.30 | 2.09 × 10−6 | −15.798277 |
| 23 | Malonyl-Ssf | 15.37 | 7.41 × 10−6 | 15.704748 |
| 28 | Saikosaponin a | 17.83 | 6.74 × 10−6 | 17.776505 |
| 32 | Malonyl-Ssa | 19.01 | 6.61 × 10−6 | 16.194246 |
| 43 | Saikosaponin d | 22.62 | 7.21 × 10−6 | 17.776505 |
| 44 | Acetyl-Ssd | 23.60 | 7.90 × 10−6 | 15.572599 |
| 45 | Malonyl-Ssd | 23.85 | 1.33 × 10−16 | 20.250618 |
| 46 | Acetyl-Ssd | 24.22 | 6.35 × 10−6 | 17.99062 |
| 52 | Dimalonyl-acetyl-Ssd | 26.52 | 6.61 × 10−6 | 16.017168 |
Conclusions
The present study revealed the distinct chemical profiles between the root and aerial parts of Bupleurum species, which indicated that the aerial parts could not be used as an alternative of root from a chemodiversity perspective. Meanwhile, the established UHPLC-QTOF-MS method in the present study as well as the potential differentiating markers could be utilized to profile and distinguish the root and aerial parts of Bupleurum species from different species or locations. Thus, the approach established here will provide a comprehensive analysis of chemical profiles between the root and aerial parts from the commonly used Bupleurum species which will be helpful for testing the crude materials of proprietary Chinese medicines containing Chaihu as well as for establishing guidelines for the appropriate clinical use of Chaihu. Furthermore, this information will be of great significance to the efficient use of botanical resources.
Acknowledgements
The authors sincerely thank Mr. Alan Ho (School of Chinese Medicine, Hong Kong Baptist University) for his technical assistance in the LC-MS experiment.
Funding
This work was partially supported by the Faculty Research Grant of Hong Kong Baptist University (FRG2/15–16/031), the National Natural Science Foundation of China (81603381, 81673691), the Guangdong Natural Science Foundation (2016A030313008) and the Shenzhen Science and Technology Innovation Committee (JCYJ20160518094706544).
Availability of data and materials
The data was included in figures and tables of the manuscript. The supporting data and materials can be obtained upon request via email to the corresponding author.
Authors’ contributions
HBC and BLG provided research inspiration and revised manuscript. LZ and ZTL made the design of the study, took part in all the study and drafted manuscript. ZZZ and JYZ revised the text. TY and YM helped for analyzing data. And all authors contributed to revising the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- MFE
Molecular feature extraction
- PCA
Principal component analysis
- RT
Retention time
- SSa
Saikosaponin a
- SSc
Saikosaponin c
- SSd
Saikosaponin d
- SSs
Saikosaponins
- TCM
Traditional Chinese medicine
- TIC
Total ions current
- TLC
Thin-layer chromatography
- UHPLC-QTOF-MS
Ultra-high performance liquid chromatography quadrupole/time of flight-mass spectrometry
Contributor Information
Lin Zhu, Email: linzhu912@gmail.com.
Zhi-Tao Liang, Email: lzt23@hkbu.edu.hk.
Tao Yi, Email: yitao@hkbu.edu.hk.
Yue Ma, Email: 16440943@life.hkbu.edu.hk.
Zhong-Zhen Zhao, Email: zzzhao@hkbu.edu.hk.
Bao-Lin Guo, Phone: +86 010-5783-3172, Email: guobaolin010@163.com.
Jian-Ye Zhang, Email: jianyez@163.com.
Hu-Biao Chen, Phone: +852-3411-2060, Email: hbchen@hkbu.edu.hk.
References
- 1.The Pharmacopoeia Commission of the PRC. Pharmacopoeia of People’s republic of China. Chemical Industry Press, 2010; Beijing. pp, 263–264.
- 2.Liu XJ, Hu J, Li ZY, Qin XM, Zhang LZ, Guo XQ. Species classification and quality assessment of Chaihu (Radix Bupleuri) based on high-performance liquid chromatographic fingerprint and combined chemometrics methods. Arch Pharm Res. 2011;34(6):961–969. doi: 10.1007/s12272-011-0613-2. [DOI] [PubMed] [Google Scholar]
- 3.Xiao PG. Modern Chinese Medica. Chemical Industry Press, 2002; Beijing.
- 4.Liang ZB, Liu L, Chao Z. Investigation of medicinal Bupleurum resources and current situation of Chaihu production. Lishizhen Medicine and Materia Medica Research. 2012;23(8):2011–2013. [Google Scholar]
- 5.Sun XP, Shi Z, Li TF, Pan RL, Liu XM, Bu LL, et al. Antidepressant-like effects of total saikosaponins of Bupleurum yinchowense in mice. J Med Plants Res. 2012;6(26):4308–16.
- 6.The Society of Japanese Pharmacopoeia. The Japanese Pharmacopoeia, 16th ed., The Ministry of Health, Labor and Welfare, 2011; Tokyo, Japan. 1613–4 (English Version).
- 7.Jang MJ, Kim YS, Bae EY, Oh TS, Choi HJ, Lee JH, et al. Saikosaponin D isolated from Bupleurum falcatum inhibits selectin-mediated cell adhesion. Molecules. 2014;19(12):20340–9. [DOI] [PMC free article] [PubMed]
- 8.Sakurai MH, Matsumoto T, Kiyohara H, Yamada H. B-cell proliferation activity of pectic polysaccharide from a medicinal herb, the roots of Bupleurum falcatum L. and its structural requirement. Immunology. 1999;97(3):540–547. doi: 10.1046/j.1365-2567.1999.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auvinen K, Jalkanen S, Salmi M. Expression and function of endothelial selectins during human development. Immunology. 2014;143(3):406–415. doi: 10.1111/imm.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin CC, Chiu HF, Yen MH, Wu CC, Chen MF. The pharmacological and pathological studies on Taiwan folk medicine (III): the effects of Bupleurum kaoi and cultivated Bupleurum falcatum. Am J Chin Med. 1990;18(3–4):105–112. doi: 10.1142/S0192415X90000149. [DOI] [PubMed] [Google Scholar]
- 11.Kim KS, Lee JY, Lee SY. Comparative transcriptomic analysis of themulti-targeted effects of the herbal extracts against Escherichia coli O157:H7. Biochip Journal. 2012;6(4):379–390. doi: 10.1007/s13206-012-6410-2. [DOI] [Google Scholar]
- 12.Ashour ML, Wink M. Genus Bupleurum: a review of its phytochemistry, pharmacology and modes of action. J Pharm Pharmacol. 2011;63(3):305–321. doi: 10.1111/j.2042-7158.2010.01170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bermejo Benito P, Abad Martínez MJ, Silván Sen AM, Sanz Gómez A, Fernández Matellano L, Sánchez Contreras S, et al. In vivo and in vitro anti-inflammatory activity of saikosaponins. Life Sci. 1998;63(13):1147–56. [DOI] [PubMed]
- 14.Chiang LC, Ng LT, Liu LT, Shieh DE, Lin CC. Cytotoxicity and anti-hepatitis B virus activities of saikosaponins from Bupleurum species. Planta Med. 2003;69(8):705–709. doi: 10.1055/s-2003-42797. [DOI] [PubMed] [Google Scholar]
- 15.Pan SL. Bupleurum Species - Scientific Evaluation and Clinical Application, Taylor and Trancis Group, 2006; Boca Raton FL.
- 16.Tan L, Zhao Y, Tu G, Wang B, Cai S, Zhang R. Saikosaponins from roots of Bupleurum scorzonerifolium. Phytochemistry. 1999;50(1):139–142. doi: 10.1016/S0031-9422(98)00451-8. [DOI] [PubMed] [Google Scholar]
- 17.Zhang T, Zhou J, Wang Q. Flavonoids from aerial part of Bupleurum chinense DC. Biochem Syst Ecol. 2007;35(11):801–804. doi: 10.1016/j.bse.2007.03.023. [DOI] [Google Scholar]
- 18.Wang YQ, Niu YB, Qin XM. The investigation and research wild Bupleurum Chinense DC. resources. Journal of Sangxi Agricultural University (Natural Science Edition), 2007; 27(1): 103–7.
- 19.Qin XM, Wang YQ, Yue JY. Resources analysis of cultivated Bupleurum Chinense DC. Research & Information on Traditional Chinese Medicine. 2005;7(8):30–32. [Google Scholar]
- 20.Yue JY, Qin XM, Wang YQ. The investigation and research on different types of cultivated Bupleurum chinense DC. Zhong Yao Cai. 2005;28(8):650–651. [Google Scholar]
- 21.Feng BL, Wang Q, Zhao XT, Wang MZ, Zhang ZW. The study on the medical parts of Radix Bupleuri. Guangxi Chinese Medicine. 1980;1:59–62. [Google Scholar]
- 22.Gan HS, Chen SW. The quality comparison of root and aerial parts from Bupleurum Chinense DC. China Journal of Chinese Materia Medical. 1982;7(2):7–10. [Google Scholar]
- 23.Prieto JM, Ogunsina MO, Novak A, Joshi A, Kokai J, Rocha Ida C, et al. Comparative study of the in vitro bioactivities of Bupleurum rigidum and B. fruticescens. Nat Prod Commun. 2012;7(6):757–60. [PubMed]
- 24.Yen MH, Lin CC, Chuang CH, Liu SY. Evaluation of root quality of Bupleurum species by TLC scanner and the liver protective effects of "xiao-chai-hu-tang" prepared using three different Bupleurum species. J Ethnopharmacol. 1991;34(2–3):155–165. doi: 10.1016/0378-8741(91)90033-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data was included in figures and tables of the manuscript. The supporting data and materials can be obtained upon request via email to the corresponding author.


