Abstract
Background
Standardized patient-reported outcome (PRO) questionnaires can be utilized to evaluate treatment satisfaction (subjective evaluation of treatment) in patients with type 2 diabetes (T2D). These outcomes are important because they may affect patient adherence and overall study results.
Methods
PROs were evaluated in two randomized 26-week clinical trials in Japanese patients with T2D taking dulaglutide 0.75 mg (dulaglutide) once weekly; comparators were once-daily liraglutide (0.9 mg/day) and once-weekly placebo in one study and once-daily insulin glargine (glargine) in the other study. The Perceptions About Medications-Diabetes 21 Questionnaire - Japanese version (PAM-D21-J) and the Injectable Diabetes Medication Questionnaire - Japanese version (IDMQ-J) were completed by patients in both studies. These measures were both considered exploratory endpoints. All scale scores range from 0 to 100, with higher scores reflecting better outcomes.
Results
Patients reported that dulaglutide was more convenient and flexible than liraglutide (PAM-D21-J Convenience/Flexibility subscale: dulaglutide least-square mean [LSM], 84.58; liraglutide LSM, 78.94; p = .026), and that they were more satisfied with dulaglutide than with liraglutide (IDMQ-J Satisfaction subscale: dulaglutide, 75.24; liraglutide, 69.53; p = .012). Patients also reported that dulaglutide was more convenient and flexible than glargine (PAM-D21-J Convenience/Flexibility subscale: dulaglutide, 87.89; glargine, 79.22; p < .001), and that they were more satisfied with dulaglutide than with glargine (IDMQ-J Satisfaction subscale: dulaglutide, 78.86; glargine, 69.66; p < .001), and felt dulaglutide was more effective than glargine, with fewer symptoms and adverse events (PAM-D21-J Perceived Effectiveness subscale: dulaglutide, 77.61; glargine, 67.22; p < .001; Emotional Effects subscale: dulaglutide, 93.02; glargine, 89.55; p = .017; IDMQ-J Blood Glucose Control subscale: dulaglutide, 76.33; glargine, 67.57; p < .001). In addition, patients responded that dulaglutide was superior to placebo in the PAM-D21-J Convenience/Flexibility, Perceived Effectiveness, and Emotional Effects subscales and all IDMQ-J subscales (Satisfaction, Ease of Use, Lifestyle Impact, Blood Glucose Control).
Conclusions
Overall, after 26 weeks of once-weekly dulaglutide administration in Japanese patients with T2D, PROs were generally positive versus the three comparator treatments (liraglutide, glargine, and placebo), suggesting increased treatment satisfaction through better blood glucose control and convenience/flexibility and reduced negative emotional effects of diabetes.
Trial registration
ClinicalTrials.gov (monotherapy study: NCT01558271, registered March 12, 2012; combination therapy study: NCT01584232, registered April 23, 2012).
Electronic supplementary material
The online version of this article (doi:10.1186/s12955-017-0696-7) contains supplementary material, which is available to authorized users.
Keywords: Type 2 diabetes, Glucagon-like peptide-1, Patient-reported outcomes, Dulaglutide
Background
Diabetes is a chronic metabolic disorder and is a major public health threat, with more than 415 million people globally and 7.2 million people in Japan diagnosed with the disease [1]. In international and Japanese guidelines, treatment of type 2 diabetes (T2D) includes diet, physical exercise, and weight control followed by oral and/or injectable therapies [2, 3].
Due to the progressive nature of the disease, patients require the right treatment at the right time based on their clinical and psychological conditions; to achieve diabetes treatment goals in clinical practice, patients must be fully engaged with their therapy [2, 3]. When choosing a treatment strategy, clinicians must strive for good clinical and physical outcomes for patients while also considering patients’ psychosocial well-being [4]. For example, preliminary evidence suggests that various clinical and psychosocial factors can become barriers to injection therapy [5, 6]. Based on responses of both patients and physicians in a study in Japan and other key research, barriers to the initiation of insulin treatment include fear, pain, and inconvenience associated with injections; concerns about weight gain and cost; and fear that the disease will continue to worsen [5, 7, 8].
In clinical trials, patient-reported outcome (PRO) measures complement clinical measures by providing information beyond traditional efficacy and safety parameters [9]. PRO questionnaires can be used to examine whether drug differences other than in clinical efficacy have an impact on outcomes that may be important to patients. For example, patient satisfaction, the subjective evaluation of treatment (including outcomes and processes), can be assessed with PRO questionnaires. Satisfaction is an important outcome in diabetes treatment because it may affect treatment compliance and adherence [10].
Dulaglutide is a long-acting human glucagon-like peptide-1 (GLP-1) receptor agonist that is administered once weekly via subcutaneous injection. It has been approved for the treatment of T2D in the United States and European Union at doses of 0.75 and 1.5 mg and in Japan at a dose of 0.75 mg [11–13]. PROs were used in two randomized, phase 3 clinical trials of patients treated with once-weekly dulaglutide 0.75 mg (dulaglutide) in Japan to assess treatment satisfaction and psychological perception. One trial (henceforth referred to as “the monotherapy study”) was a 52-week (primary endpoint at 26 weeks) study in which patients received double-blind dulaglutide monotherapy or placebo or open-label once-daily liraglutide 0.9 mg monotherapy; after 26 weeks, patients receiving placebo were switched to dulaglutide (ClinicalTrials.gov: NCT01558271) (Fig. 1) [14]. The other trial (hereafter referred to as “the combination therapy study”) was a 26-week open-label study in which dulaglutide was compared to once-daily insulin glargine (glargine) in patients also treated with sulfonylureas and/or biguanides (ClinicalTrials.gov: NCT01584232) (Fig. 2) [15].
Fig. 1.
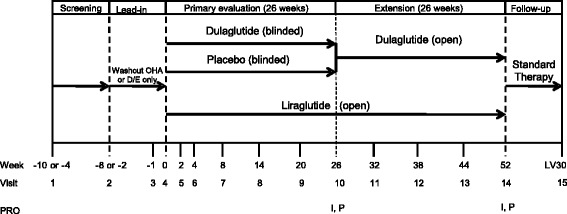
Study Design for the Monotherapy Study. D/E diet and exercise; I Injectable Diabetes Medication Questionnaire - Japanese version; LV30 follow-up visit 30 days after last study visit; OHA oral hypoglycemic agent; P Perceptions About Medications-Diabetes 21 Questionnaire - Japanese version; PRO Patient-Reported Outcomes
Fig. 2.
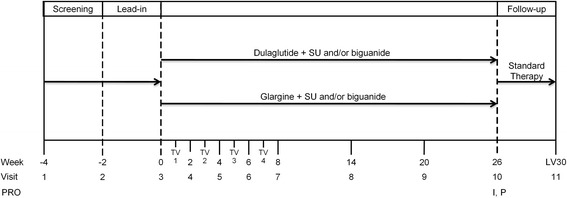
Study Design for the Combination Therapy Study. I Injectable Diabetes Medication Questionnaire - Japanese version; LV30 follow-up visit 30 days after last study visit; P Perceptions About Medications-Diabetes 21 Questionnaire - Japanese version; PRO patient-reported outcomes; SU sulfonylurea; TV telephone visit
This is the first known published report of treatment satisfaction in Asian patients with T2D treated with dulaglutide as of April 2017, although PROs were assessed in some Asian patients in the global AWARD-3 and AWARD-5 studies [16]. The two studies reported here are the only phase 3 studies of dulaglutide in Japan that included PRO measures; the impacts of once-weekly injectable treatment on patients’ satisfaction and perceptions of treatment were of key interest.
Methods
Study design
Dulaglutide was administered in both studies using a prefilled syringe (a device specific to clinical trials) because the device later marketed was not available at the time the clinical studies were conducted.
In the monotherapy study, eligible patients were randomized to treatment in a 4:2:1 ratio (dulaglutide:liraglutide:placebo) [14]. Randomization was stratified by prestudy oral hypoglycemia agent (OHA) status (yes/no), body mass index (BMI; <25 and ≥25 kg/m2), and glycated hemoglobin (HbA1c; ≤8.5 or >8.5%). Patients and investigators were masked to dulaglutide and placebo treatment assignments but were not masked to liraglutide treatment assignment. Liraglutide was administered using a pen injector (marketed product). Placebo was administered using the same prefilled syringe used for dulaglutide administration.
In the combination therapy study, eligible patients were randomized to treatment in a 1:1 ratio (dulaglutide:glargine) [15]. Randomization was stratified by concomitant OHA regimen (sulfonylureas only, biguanides only, or sulfonylurea and biguanide), BMI (<25 and ≥25 kg/m2), and HbA1c (≤8.5 and >8.5%). An open-label design was used, and participants, investigators, and site staff were not masked to treatment assignments. Glargine was administered using a prefilled disposable pen (marketed product).
The primary endpoint of both studies was change from baseline in HbA1c (%) at week 26 [14, 15].
Patients
In the monotherapy study, eligible patients were Japanese males or females with T2D aged 20 years or older with BMIs ≥18.5 and ≤35.0 kg/m2 and HbA1c ≥7.0 and ≤10.0% confirmed at randomization who were OHA-naïve (diet and exercise only) or had discontinued OHA monotherapy [14].
In the combination therapy study, eligible patients were Japanese males or females with T2D aged 20 years or older with a BMI ≥18.5 and <35.0 kg/m2 and HbA1c at screening ≥7.0 and ≤10.0% who were taking stable doses of sulfonylureas and/or biguanides [15].
Patient-reported outcome instruments
PRO measures were considered exploratory endpoints in the studies.
The Perceptions About Medications Diabetes 21 Questionnaire - Japanese version (PAM-D21-J) and the Injectable Diabetes Medication Questionnaire - Japanese version (IDMQ-J) were completed by patients at week 26 in both studies (English-translated versions provided in Additional files 1 and 2 for PAM-D21-J and IDMQ-J, respectively). The PAM-D21-J was developed to assess perceptions about diabetes medications, focusing on frequency, amount, timing, and effectiveness of the drugs as well as on the physical and emotional side effects. It was based on the Perception About Medications-Diabetes Questionnaire (PAM-D) [17]. This scale comprises 21 questions forming four subscales: Convenience/Flexibility (three items), Perceived Effectiveness (three items), Emotional Effects (five items), and Physical Effects (10 items). Item scores for Convenience/Flexibility, Perceived Effectiveness, and Physical Effects were reversed prior to statistical analysis so that for all four subscales a higher score represented a more favorable state. Subscale scores were derived by summing the item scores. The scores for all four subscales were linearly transformed to a 0 to 100 scale using the following formula:
The IDMQ-J was developed to assess treatment satisfaction, ease of use, lifestyle impact, and blood glucose control by injectable diabetes medication, such as GLP-1 receptor agonists, based on the Insulin Delivery System Questionnaire, Japanese version (IDSQ-J) [18]. This scale comprises 11 questions. The scores for items 1 and 2a to 2f range from 1 to 7 and for items 3a to 3c range from 1 to 6, and the score for item 4 ranges from 1 to 5. Satisfaction score (item 1), Ease of Use score (items 2a and 2e), Lifestyle Impact score (items 2c, 2d, and 2f), and Blood Glucose Control score (items 2b, 3a [reverse], and 3b) were derived by summing the corresponding scores and were linearly transformed to a 0 to 100 scale using the formula described previously for the PAM-D21-J. A higher score represented a more favorable state for all four subscales. Because the PAM-D21-J and IDMQ-J have been developed recently, neither instrument has been fully psychometically validated or published elsewhere at this time.
In addition, to assess baseline characteristics of study participants in terms of general health status, the five-response-level EQ-5D-5L questionnaire was administered at baseline in the monotherapy study. The EQ-5D-5L is a widely used, validated generic questionnaire that assesses health-related quality of life [19]. It consists of two parts. The first part assesses five dimensions associated with quality of life (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), which are combined to produce a single index/utility score based on the up-to-date value set of the Japanese population from Ikeda et al. [20]; for this analysis this scoring was done post hoc. For the index score, 1 is the best score (higher scores indicate greater health), a score of 0 represents death, and scores less than 0 represent conditions perceived as worse than death. The second part of the questionnaire consists of a 100-mm visual analog scale (VAS) on which the patient rates his or her perceived health state on that day from 0 mm (worst imaginable health state) to 100 mm (best imaginable health state).
Statistical analysis
Statistical analyses were conducted on the Full Analysis Set (FAS) populations in both studies, defined as all randomized patients who received at least one dose of study medication, with last observation carried forward (LOCF) used to impute missing postbaseline values.
In both studies, the responses to the items of the PAM-D21-J were summarized individually by treatment as counts and percentages, and the PAM-D21-J subscale scores at 26 weeks were described and compared between treatment groups using an analysis of variance (ANOVA) model. The ANOVA included treatment, prestudy therapy, and BMI group at baseline as fixed effects. In both studies, the responses to the items of the IDMQ-J were summarized individually by treatment as counts and percentages. The IDMQ-J subscale scores at 26 weeks were described and compared between treatment groups in each study using the same ANOVA model described above for the PAM-D21-J. Least-square means (LSMs) for treatment differences and p-values for pairwise comparisons between treatments based on the ANOVA model were reported for both instruments. All analyses were prespecified in the study analysis plan.
Results
Baseline characteristics
Patient demographics at baseline for both studies are summarized in Table 1. In both studies, the majority of patients (>70%) were male, and the mean age was approximately 57 years. Mean duration of diabetes was approximately 6 to 7 years in the monotherapy study and approximately 9 years in the combination therapy study. Mean HbA1c at baseline was approximately 8% in both studies.
Table 1.
Patient characteristics at baseline, Full Analysis Set
| Monotherapy Study | Combination Therapy Study | ||||
|---|---|---|---|---|---|
| Dulaglutide 0.75 mg (N = 280) |
Liraglutide 0.9 mg (N = 137) |
Placebo (N = 70) |
Dulaglutide 0.75 mg (N = 181) |
Insulin Glargine (N = 180) |
|
| Females, n (%) | 52 (19%) | 24 (18%) | 15 (21%) | 56 (31%) | 47 (26%) |
| Age (yrs), mean (SD) | 57.2 (9.6) | 57.9 (10.4) | 57.7 (8.3) | 57.5 (10.5) | 56.1 (11.3) |
| Weight (kg), mean (SD) | 71.3 (12.5) | 70.2 (12.5) | 69.3 (11.6) | 70.9 (13.7) | 71.1 (13.8) |
| BMI (kg/m2), mean (SD) | 25.6 (3.6) | 25.5 (3.5) | 25.2 (3.2) | 26.1 (3.6) | 25.9 (3.9) |
| Diabetes duration (yrs), mean (SD) | 6.8 (5.6) | 6.3 (6.0) | 6.3 (5.1) | 8.9 (6.7) | 8.8 (6.1) |
| HbA1c (%), mean (SD) | 8.2 (0.8) | 8.1 (0.9) | 8.2 (0.8) | 8.1 (0.8) | 8.0 (0.9) |
| Receiving OHA at screening, n (%) | 94 (34%) | 48 (35%) | 22 (31%) | 181 (100%) | 180 (100%) |
| OHA-naive, n (%) | 186 (66%) | 89 (65%) | 48 (69%) | 0 (0%) | 0 (0%) |
| Concomitant OHA at baseline, n (%) | |||||
| Sulfonylureas only | NA | NA | NA | 34 (19%) | 33 (18%) |
| Biguanides only | NA | NA | NA | 64 (35%) | 66 (37%) |
| Sulfonylureas and biguanides | NA | NA | NA | 83 (46%) | 81 (45%) |
| EQ-5D-5L Japan Index, mean (SD) | 0.97 (0.06) | 0.97 (0.06) | 0.96 (0.08) | NA | NA |
| EQ-5D VAS, mean (SD) | 82.6 (12.7) | 81.0 (11.4) | 79.3 (14.2) | NA | NA |
BMI Body mass index, HbA1c Glycosylated hemoglobin A1c, NA Not applicable, OHA Oral hypoglycemic agent, SD Standard deviation, VAS Visual analog scale
EQ-5D-5L data have not been often collected or reported in Japanese patients with T2D. EQ-5D-5L Japan Index and VAS scores at baseline by treatment in the monotherapy study are presented in Table 1. Across all three treatment groups, the mean EQ-5D-5L Japan Index score at baseline was approximately 0.97, and the mean EQ-5D VAS was approximately 82.
Key results
At week 26 in the monotherapy study, dulaglutide was superior to placebo for HbA1c change from baseline (the primary objective of the study; p < .001) [14]. Dulaglutide was also noninferior (margin 0.4%), but not superior, to once-daily liraglutide. The LSM (standard error [SE]) changes in HbA1c from baseline to week 26 were −1.43% (0.05) for dulaglutide, −1.33% (0.07) for liraglutide, and 0.14% (0.10) for placebo. The LSM differences were −1.57% (95% confidence interval [CI] [−1.79% to −1.35%]) between dulaglutide and placebo and −0.10% (95% CI [−0.27 to 0.07%]) between dulaglutide and liraglutide. The incidence of hypoglycemia through 26 weeks was 2.1% for dulaglutide, 1.5% for liraglutide, and 1.4% for placebo.
At week 26 in the combination therapy study, dulaglutide was superior to glargine for HbA1c change from baseline (the primary objective of the study; p < .001) [15]. The LSM (SE) changes in HbA1c from baseline to week 26 were −1.44% (0.05) for dulaglutide and −0.90% (0.05) for glargine. The LSM difference between dulaglutide and glargine was −0.54% (95% CI [−0.67% to −0.41%]). The incidence of hypoglycemia through 26 weeks was 26.0% for dulaglutide and 47.8% for glargine.
PRO results at week 26
LSM endpoint scores of the PAM-D21-J and IDMQ-J subscales at week 26 (LOCF) are presented in Fig. 3 for the monotherapy study and in Fig. 4 for the combination therapy study.
Fig. 3.
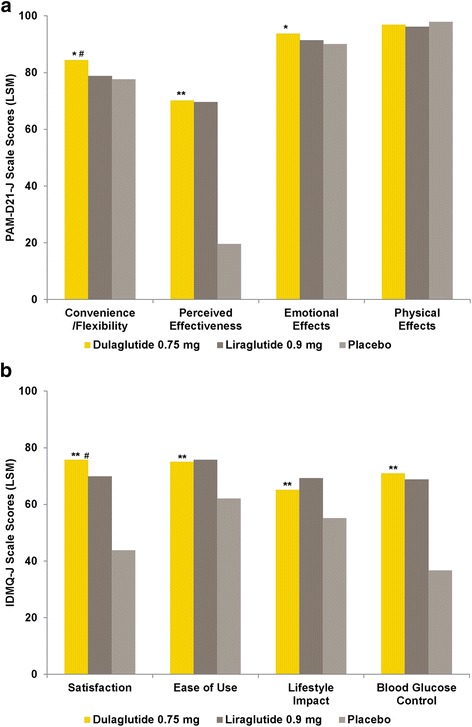
Results of the PAM-D21-J and IDMQ-J at Week 26 in the Monotherapy Study. (a) PAM-D21-J at week 26 (LSM scores by treatment). (b) IDMQ-J at week 26 (LSM scores by treatment). IDMQ-J Injectable Diabetes Medication Questionnaire - Japanese version; LSM least-squares mean; PAM-D21-J Perceptions About Medications-Diabetes 21 Questionnaire - Japanese version. Dulaglutide and placebo administered once weekly; liraglutide administered once daily. For all subscales higher scores represent a more favorable state. *p < .05 vs. placebo, **p < .001 vs. placebo, # p < .05 vs. liraglutide
Fig. 4.
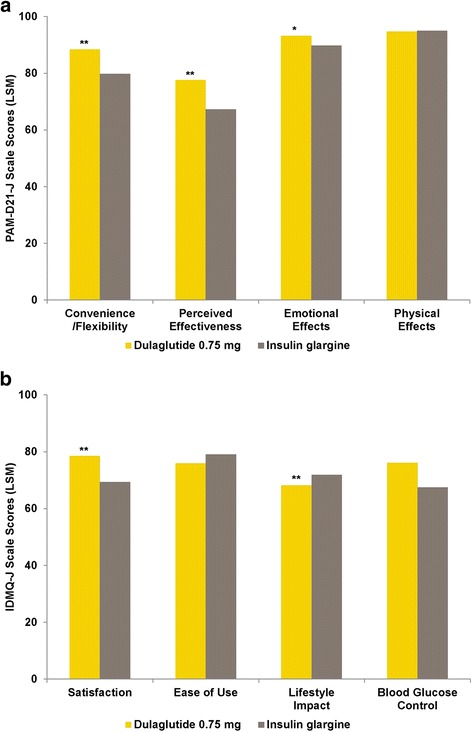
Results of the PAM-D21-J and IDMQ-J at Week 26 in the Combination Therapy Study. (a) PAM-D21-J at week 26 (LSM scores by treatment). (b) IDMQ-J at week 26 (LSM scores by treatment). IDMQ-J Injectable Diabetes Medication Questionnaire - Japanese version; LSM, least-squares mean; PAM-D21-J Perceptions About Medications-Diabetes 21 Questionnaire - Japanese version. Dulaglutide administered once weekly; insulin glargine administered once daily. For all subscales higher scores represent a more favorable state. *p < .05 vs. insulin glargine, **p < .001 vs. insulin glargine
Dulaglutide versus liraglutide (monotherapy study)
Dulaglutide was superior to liraglutide in the PAM-D21-J Convenience/Flexibility subscale (dulaglutide vs. liraglutide LSM, 84.58 vs. 78.94; p = .026). No significant treatment difference was observed in the Perceived Effectiveness (69.50 vs. 69.07; p = .874), Emotional Effects (93.84 vs. 91.48; p = .073), or Physical Effects (96.79 vs. 96.13; p = .336) subscales.
Dulaglutide was superior to liraglutide in the IDMQ-J Satisfaction subscale (dulaglutide vs. liraglutide LSM, 75.24 vs. 69.53; p = .012). No significant treatment difference was observed in the Ease of Use (75.07 vs. 75.80; p = .704), Lifestyle Impact (64.94 vs. 69.15; p = .053), or Blood Glucose Control (70.55 vs. 68.51; p = .300) subscales.
Dulaglutide versus placebo (monotherapy study)
Dulaglutide was superior to placebo in the PAM-D21-J Convenience/Flexibility (dulaglutide vs. placebo LSM, 84.58 vs. 77.85; p = .040), Perceived Effectiveness (69.50 vs. 18.71; p < .001), and Emotional Effects (93.84 vs. 90.13; p = .029) subscales and all IDMQ-J subscales (Satisfaction [75.24 vs. 43.19; p < .001], Ease of Use [75.07 vs. 62.13; p < .001], Lifestyle Impact [64.94 vs. 54.82; p < .001], Blood Glucose Control [70.55 vs. 36.12; p < .001]). No significant treatment difference was observed in the PAM-D21-J Physical Effects subscale (96.79 vs. 97.74; p = .290).
Dulaglutide versus glargine (combination therapy study)
Dulaglutide was superior to glargine in the PAM-D21-J Convenience/Flexibility (dulaglutide LSM, 87.89; glargine LSM, 79.22; p < .001), Perceived Effectiveness (77.61 vs. 67.22; p < .001), and Emotional Effects (93.02 vs. 89.55; p = .017) subscales. No significant treatment difference was observed in the Physical Effects subscale (94.79 vs. 95.02; p = .798).
Dulaglutide was superior to glargine in the IDMQ-J Satisfaction (dulaglutide vs. glargine LSM, 78.86 vs. 69.66; p < .001) and Blood Glucose Control (76.33 vs. 67.57; p < .001) subscales. No significant treatment difference was observed in the Ease of Use (75.77 vs. 78.93; p = .090) or Lifestyle Impact (68.00 vs. 71.58; p = .108) subscales.
Discussion
The two instruments presented here (PAM-D21-J and IDMQ-J) were used as exploratory measures in the studies to evaluate once-weekly injectable treatment with dulaglutide in Japanese patients with T2D.
Overall in two randomized studies of once-weekly dulaglutide in Japan, 26 weeks of treatment with dulaglutide resulted in greater treatment satisfaction and better perception of the treatment compared to liraglutide, glargine, and placebo. The questionnaires evaluated in these studies were chosen based on key attributes of the medications administered in the studies (eg, clinical characteristics of the active ingredients, administration frequency, and injection device).
Dulaglutide versus liraglutide (monotherapy study)
Dulaglutide was superior to liraglutide in the PAM-D21-J Convenvience/Flexibility subscale. Dulaglutide was also superior to liraglutide in the IDMQ-J Satisfaction subscale; the reduced frequency of administration for dulaglutide (once weekly) vs. liraglutide (once daily) might be one of the reasons for this finding [21, 22]. However, there was no significant difference in the IDMQ-J Ease of Use subscale. The PAM-D21-J Convenience/Flexibility subscale and the IDMQ-J Ease of Use subscale assess similar concepts, but it seemed that patients answered differently depending on the exact wording of the questions: the PAM-D21-J Convenience/Flexibility subscale includes a specific question about the frequency of administration, whereas the IDMQ-J Ease of Use subscale includes an abstract question about convenience but does not include any questions about the frequency of administration.
Dulaglutide versus placebo (monotherapy study)
Dulaglutide was superior to placebo in all PAM-D21-J subscales except for the Physical Effects subscale, indicating that patients were more satisfied overall with dulaglutide treatment compared to placebo. The Physical Effects subscale scores in both groups were high, indicating that patients in both groups did not experience negative physical consequences commonly observed with existing diabetes treatments, such as weight gain, abdominal bloating, and nausea.
Despite the fact that dulaglutide and placebo were both administered once weekly with the same device in a double-blind fashion, dulaglutide significantly improved the PAM-D21-J Convenience/Flexibility and IDMQ-J Ease of Use subscales compared to placebo. Although there were no items in these subscales directly related to glycemic control, patients’ satisfaction with their glycemic control during the study may have subconsciously affected their answers to questions about the convenience, flexibility, and ease of use of the study medications [23].
Dulaglutide versus glargine (combination therapy study)
Dulaglutide was superior to glargine in the PAM-D21-J Convenience/Flexibility, Perceived Effectiveness, and Emotional Effects subscales and in the IDMQ-J Satisfaction and Blood Glucose Control subscales. No significant differences were observed for dulaglutide compared to glargine in the IDMQ-J Ease of Use or Lifestyle Impact subscales as were observed in the monotherapy study for dulaglutide compared to liraglutide. This result suggests that the Ease of Use subscale may not be able to accurately measure patients’ perceptions about differences in injection frequency, most likely because the Ease of Use subscale includes an abstract question about convenience but no specific items about injection frequency. The statistically significant differences in the PAM-D21-J Perceived Effectiveness subscale and the IDMQ-J Blood Glucose Control subscale may be explained by the better glycemic control in the dulaglutide group compared to the glargine group. The better clinical outcomes with dulaglutide compared to glargine may also have affected the psychological perception of treatment with dulaglutide, resulting in a higher score in the PAM-D21-J Emotional Effects subscale for dulaglutide compared to glargine, which is consistent with a previous report about the relationship between glycemic control and quality of life [23].
Dulaglutide versus both comparators
Although once-weekly dulaglutide was statistically superior to both once-daily comparators in the IDMQ-J Treatment Satisfaction subscale, it is unknown how much injection frequency affected patients’ assessments of treatment satisfaction for each medication. In addition, the clinical relevance and interpretation of the treatment differences in the scores between dulaglutide and the comparators is unclear, and further research is required to interpret the differences in detail in real-world settings, for instance by measuring treatment adherence anchored to treatment satisfaction.
We expected to observe a greater influence of injection frequency on patient perceptions of convenience/ease of use of the treatments. Although dulaglutide was statistically superior to both active comparators (liraglutide and glargine) in the PAM-D21-J Convenience/Flexibility subscale, the differences in the scores were smaller than expected based on the different injection frequencies (once weekly vs. once daily). As was discussed previously, it appears that the wording of items in the PAM-D21-J Convenience/Flexibility and IDMQ-J Ease of Use subscales substantially affected patient responses, and it seems that these questionnaires may not be specific enough to capture the additional burdens placed on patients with once-daily medication dosing compared to once-weekly dosing.
Mean EQ-5D-5L scores at baseline in this clinical trial setting were numerically slightly higher, but consistent with those observed in previous research in Japan [24].
Results of PRO questionnaires completed by patients treated with dulaglutide 0.75 mg in the global dulaglutide AWARD studies were generally positive for dulaglutide; in particular, it improved treatment satisfaction based on the Diabetes Treatment Satisfaction Questionnaire status version (DTSQs) [16]. In the AWARD-1 study, once weekly dulaglutide 0.75 mg and 1.5 mg both resulted in significantly greater improvement in all DTSQs subscale scores compared to exenatide twice daily at both 26 and 52 weeks [25]. In the AWARD-3 study, no statistically significant differences were observed between the dulaglutide 0.75 mg and 1.5 mg groups and metformin in total treatment satisfaction based on the DTSQs at either 26 or 52 weeks.
Limitations
These instruments were not psychometrically validated and were studied in clinical trial settings; thus, the results obtained in a real-world setting may be different. In addition, the relatively brief duration of the studies in comparison to the long-term treatment required for diabetes should be considered when evaluating these results.
Because these studies were designed to compare dulaglutide to active comparators (liraglutide or glargine) in an open-label fashion (dulaglutide and placebo were blinded in the monotherapy study), the results of the PRO instruments may have been biased. In addition, market formulations were used for the active comparators, whereas a prefilled syringe designed for use in clinical trials was used for dulaglutide and placebo; therefore, because dulaglutide is marketed as a single-dose pen, the interpretation of some of the PRO results, such as findings related to convenience of the treatments, may be challenging. Future research is necessary to evaluate patient perception of the marketed single-dose dulaglutide pen compared to comparators’ devices.
Some of the questions in the questionnaires may not have been applicable to these study medications. For example, “Easy to carry for use away from home” from the IDMQ-J (Lifestyle Impact subscale) is relevant for injectable medications that are administered frequently but may not be relevant for once-weekly medications such as dulaglutide.
Finally, it appeared that the PAM-D21-J and IDMQ-J may not be able to appropriately evaluate patients’ feelings about frequency of administration of antidiabetic medications: for example, dulaglutide significantly improved the PAM-D21-J Convenience/Flexibility subscale and the IDMQ-J Ease of Use subscale compared to placebo even though both medications were administered at the same frequency with the same device. It is believed that perhaps the PRO results were confounded by greater improvements in glycemic control in the dulaglutide group. Future research to develop PRO instruments that will more efficiently elicit and assess patients’ feelings about dosing frequency (eg, once weekly vs. once daily) would be very useful.
Conclusions
Overall, after administration of once-weekly dulaglutide 0.75 mg to Japanese patients with T2D for 26 weeks, patient-reported health outcomes were generally positive versus the three comparator treatments (liraglutide, glargine, and placebo), suggesting increased treatment satisfaction through better blood glucose control and convenience/flexibility and reduced negative emotional effects of diabetes.
Additional files
The Perceptions About Medications-Diabetes 21 Questionnaire - Japanese version (PAM-D21-J). (PDF 61 kb)
Injectable Diabetes Medication Questionnaire - Japanese version (IDMQ-J). (PDF 22 kb)
Ethics review boards (ERBs) which approved the monotherapy study. (PDF 15 kb)
Ethics review boards (ERBs) which approved the combination study. (PDF 14 kb)
Acknowledgements
The authors thank all of the patients and study sites involved in these studies. We also thank Mary Re of inVentiv Health Clinical for writing assistance and Akiko Matsui, PhD, of Eli Lilly Japan K.K. for project management and writing assistance.
Funding
Eli Lilly Japan K.K. funded the research reported here, including the design of the two studies, as well as the collection, analysis, and interpretation of data from the studies and the medical writing for this paper.
Availability of data and materials
Lilly provides access to the individual patient data from studies on approved medicines and indications as defined by the sponsor-specific information on http://clinicalstudydatarequest.com. Researchers need to have an approved research proposal submitted through http://clinicalstudydatarequest.com. Access to the data will be provided in a secure data sharing environment after signing a data sharing agreement.
Authors’ contributions
SS prepared the first draft of the manuscript. TO was responsible for the statistical considerations in the analysis and trial design. MT was responsible for trial design and medical oversight during the trials. All authors participated in reviewing and interpreting the data and providing comments and revisions to the manuscript. All authors approved the final version of the manuscript and take full responsibility for the content.
Competing interests
SS, TO, and MT are employees of Eli Lilly Japan KK, and KSB is an employee of Eli Lilly and Company. TO, MT, and KSB are minor stock shareholders of Eli Lilly and Company.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Both studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Ethics review boards which approved the monotherapy and combination studies are provided in Additional files 3 and 4, respectively. All patients provided written informed consent before receiving treatment in the study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ANOVA
Analysis of variance
- BMI
Body mass index
- CI
Confidence interval
- DTSQs
Diabetes Treatment Satisfaction Questionnaire status version (DTSQs)
- FAS
Full analysis set
- GLP-1
Glucagon-like peptide-1
- HbA1c
Glycated hemoglobin
- IDMQ-J
Injectable Diabetes Medication Questionnaire - Japanese version
- IDSQ-J
Insulin Delivery System Questionnaire - Japanese version
- LOCF
Last observation carried forward
- LSM
Least-square means
- OHA
Oral hypoglycemia agent
- PAM-D
Perception About Medications-Diabetes Questionnaire
- PAM-D21-J
The Perceptions About Medications-Diabetes 21 Questionnaire, Japanese version
- PRO
Patient-reported outcome
- SE
Standard error
- VAS
Visual analog scale
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12955-017-0696-7) contains supplementary material, which is available to authorized users.
Contributor Information
Shuichi Suzuki, Phone: +81-78-242-9732, Email: suzuki_shuichi@lilly.com.
Tomonori Oura, Email: oura_tomonori@lilly.com.
Masakazu Takeuchi, Email: takeuchi_masakazu@yahoo.co.jp.
Kristina S. Boye, Email: boye_kristina_secnik@lilly.com
References
- 1.International Diabetes Federation . IDF diabetes atlas. 7. Brussels: International Diabetes Federation; 2015. [PubMed] [Google Scholar]
- 2.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 3.Japan Diabetes Society (Ed.). Treatment guide for diabetes 2014–2015. http://www.fa.kyorin.co.jp/jds/uploads/Treatment_Guide_for_Diabetes_2014-2015.pdf. Accessed 18 May 2017.
- 4.Bradley C, Gamsu DS. Guidelines for encouraging psychological well-being: report of a Working Group of the World Health Organization Regional Office for Europe and International Diabetes Federation European Region St Vincent Declaration Action Programme for Diabetes. Diabet Med. 1994;11:510–6. doi: 10.1111/j.1464-5491.1994.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 5.Peyrot M, Rubin RR, Lauritzen T, Skovlund SE, Snoek FJ, Matthews DR, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673–9. doi: 10.2337/diacare.28.11.2673. [DOI] [PubMed] [Google Scholar]
- 6.Polonsky WH, Fisher L, Guzman S, Villa-Caballero L, Edelman SV. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care. 2005;28:2543–2545. doi: 10.2337/diacare.28.10.2543. [DOI] [PubMed] [Google Scholar]
- 7.Ishii H, Iwamoto Y, Tajima N. An exploration of barriers to insulin initiation for physicians in Japan: findings from the Diabetes Attitudes, Wishes and Needs (DAWN) JAPAN study. PLoS One. 2012;7:e36361. doi: 10.1371/journal.pone.0036361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng CJ, Lai PS, Lee YK, Azmi SA, Teo CH. Barriers and facilitators to starting insulin in patients with type 2 diabetes: a systematic review. Int J Clin Pract. 2015;69:1050–1070. doi: 10.1111/ijcp.12691. [DOI] [PubMed] [Google Scholar]
- 9.U.S Department of Health and Human Services Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. Accessed 18 May 2017.
- 10.Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39–48. doi: 10.2147/PPA.S24752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trulicity . Prescribing information. Indianapolis: Lilly USA, LLC; 2017. [Google Scholar]
- 12.Trulicity . Summary of product characteristics. Houten: Eli Lilly and Company; 2016. [Google Scholar]
- 13.Trulicity Ateos. [Japan package insert] (in Japanese). Hyogo, Eli Lilly Japan KK; 2016. http://www.info.pmda.go.jp/downfiles/ph/PDF/530471_2499416G1029_1_07.pdf. Accessed 18 May 2017.
- 14.Miyagawa J, Odawara M, Takamura T, Iwamoto N, Takita Y, Imaoka T. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide is non-inferior to once-daily liraglutide and superior to placebo in Japanese patients with type 2 diabetes: a 26-week randomized phase III study. Diabetes Obes Metab. 2015;17:974–983. doi: 10.1111/dom.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Araki E, Inagaki N, Tanizawa Y, Oura T, Takeuchi M, Imaoka T. Efficacy and safety of once-weekly dulaglutide in combination with sulphonylurea and/or biguanide compared with once-daily insulin glargine in Japanese patients with type 2 diabetes: a randomized, open-label, phase III, non-inferiority study. Diabetes Obes Metab. 2015;17:994–1002. doi: 10.1111/dom.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu M, Van Brunt K, Varnado OJ, Boye KS. Patient-reported outcome results in patients with type 2 diabetes treated with once-weekly dulaglutide: data from the AWARD phase III clinical trial programme. Diabetes Obes Metab. 2016;18:419–424. doi: 10.1111/dom.12624. [DOI] [PubMed] [Google Scholar]
- 17.Hayes RP, Meldahl ML. Perceptions about medications-diabetes: further revision and validation. International Society for Quality of life research. 2009. p. A-83. [Google Scholar]
- 18.Ishii H, Tsujii S, Tanaka M, Furuya M, Iburi T, Ueda R, et al. Development of insulin Delivery System questionnaire in Japanese (IDSQ-J) and reproducibility and adequacy assessment (in Japanese) J Japan Diab Soc. 2009;52:209–21. [Google Scholar]
- 19.EuroQol Group EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda S, Shiroiwa T, Igarashi I, Noto S, Fukuda T, Saito S, et al. Developing a Japanese version of the EQ-5D-5L value set (in Japanese; abstract in English) J Natl Inst Public Health. 2015;64:47–55. [Google Scholar]
- 21.Ishii H. Treatment satisfaction among patients with weekly exenatide, measurement with DTSQ (in Japanese) Jpn J Med Pharm Sci. 2014;71:135–43. [Google Scholar]
- 22.Boye KS, Matza LS, Walter KN, Van Brunt K, Palsgrove AC, Tynan A. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ. 2011;12:219–230. doi: 10.1007/s10198-010-0224-8. [DOI] [PubMed] [Google Scholar]
- 23.Ishii H, Anderson JH, Jr, Yamamura A, Takeuchi M, Ikeda I. Improvement of glycemic control and quality-of-life by insulin lispro therapy: assessing benefits by ITR-QOL questionnaires. Diabetes Res Clin Pract. 2008;81:169–78. doi: 10.1016/j.diabres.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Sakamaki H, Ikeda S, Ikegami N, Uchigata Y, Iwamoto Y, Origasa H, et al. Measurement of HRQL using EQ-5D in patients with type 2 diabetes mellitus in Japan. Value Health. 2006;9:47–53. doi: 10.1111/j.1524-4733.2006.00080.x. [DOI] [PubMed] [Google Scholar]
- 25.Reaney M, Yu M, Lakshmanan M, Pechtner V, van Brunt K. Treatment satisfaction in people with type 2 diabetes mellitus treated with once-weekly dulaglutide: data from the AWARD-1 and AWARD-3 clinical trials. Diabetes Obes Metab. 2015;17:896–903. doi: 10.1111/dom.12527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Perceptions About Medications-Diabetes 21 Questionnaire - Japanese version (PAM-D21-J). (PDF 61 kb)
Injectable Diabetes Medication Questionnaire - Japanese version (IDMQ-J). (PDF 22 kb)
Ethics review boards (ERBs) which approved the monotherapy study. (PDF 15 kb)
Ethics review boards (ERBs) which approved the combination study. (PDF 14 kb)
Data Availability Statement
Lilly provides access to the individual patient data from studies on approved medicines and indications as defined by the sponsor-specific information on http://clinicalstudydatarequest.com. Researchers need to have an approved research proposal submitted through http://clinicalstudydatarequest.com. Access to the data will be provided in a secure data sharing environment after signing a data sharing agreement.


