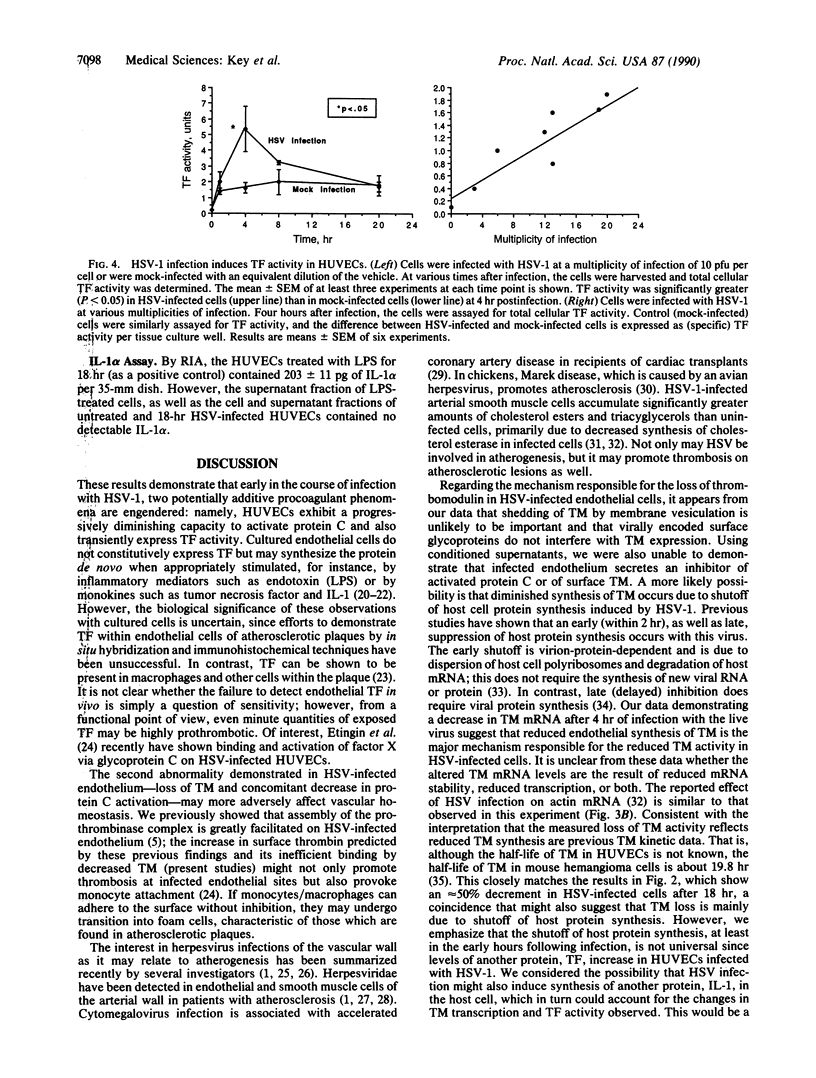
Abstract
Latent infection of vascular cells with herpes-viruses may play a pathogenic role in the development of human atherosclerosis. In a previous study, we found that cultured human umbilical vein endothelial cells (HUVECs) infected with herpes simplex virus 1 (HSV-1) became procoagulant, exemplified both by their enhanced assembly of the prothrombinase complex and by their inability to reduce adhesion of platelets. We now report two further procoagulant consequences of endothelial HSV infection: loss of surface thrombomodulin (TM) activity and induction of synthesis of tissue factor. Within 4 hr of infection of HUVECs, TM activity measured by thrombin-dependent protein C activation declined 21 +/- 3% (P less than 0.05) and by 18 hr, 48 +/- 5% (P less than 0.001). Similar significant TM decrements accompanied infection of bovine aortic endothelial cells. Identical TM loss was induced with HSV-2 infection but not with adenovirus infection. Decreased surface expression of TM antigen (measured by the specific binding of a polyclonal antibody to bovine TM) closely paralleled the loss of TM activity. As examined by Northern blotting, these losses apparently reflected rapid onset (within 4 hr of HSV infection) loss of mRNA for TM. In contrast, HSV infection induced a viral-dose-dependent increase in synthesis of tissue factor protein, adding to the procoagulant state. The results indicate that loss of endothelial protein-synthetic capacity is not a universal effect of HSV infection. We suggest that the procoagulant state induced by reduction in TM activity and amplified tissue factor activity accompanying HSV infection of endothelium could contribute to deposition of thrombi on atherosclerotic plaques and to the "coagulant-necrosis" state that characterizes HSV-infected mucocutaneous lesions.
Full text
PDF

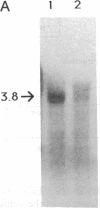
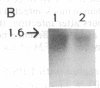
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benditt E. P., Barrett T., McDougall J. K. Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6386–6389. doi: 10.1073/pnas.80.20.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cines D. B., Lyss A. P., Bina M., Corkey R., Kefalides N. A., Friedman H. M. Fc and C3 receptors induced by herpes simplex virus on cultured human endothelial cells. J Clin Invest. 1982 Jan;69(1):123–128. doi: 10.1172/JCI110422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway E. M., Rosenberg R. D. Tumor necrosis factor suppresses transcription of the thrombomodulin gene in endothelial cells. Mol Cell Biol. 1988 Dec;8(12):5588–5592. doi: 10.1128/mcb.8.12.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. J., Pasternak R. C. The potential role of viruses in the pathogenesis of atherosclerosis. Circulation. 1988 May;77(5):964–966. doi: 10.1161/01.cir.77.5.964. [DOI] [PubMed] [Google Scholar]
- Dittman W. A., Kumada T., Sadler J. E., Majerus P. W. The structure and function of mouse thrombomodulin. Phorbol myristate acetate stimulates degradation and synthesis of thrombomodulin without affecting mRNA levels in hemangioma cells. J Biol Chem. 1988 Oct 25;263(30):15815–15822. [PubMed] [Google Scholar]
- Esmon N. L., DeBault L. E., Esmon C. T. Proteolytic formation and properties of gamma-carboxyglutamic acid-domainless protein C. J Biol Chem. 1983 May 10;258(9):5548–5553. [PubMed] [Google Scholar]
- Etingin O. R., Silverstein R. L., Friedman H. M., Hajjar D. P. Viral activation of the coagulation cascade: molecular interactions at the surface of infected endothelial cells. Cell. 1990 May 18;61(4):657–662. doi: 10.1016/0092-8674(90)90477-v. [DOI] [PubMed] [Google Scholar]
- Fabricant C. G., Fabricant J., Litrenta M. M., Minick C. R. Virus-induced atherosclerosis. J Exp Med. 1978 Jul 1;148(1):335–340. doi: 10.1084/jem.148.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick M. L., McMenamin M. M. Early virion-associated suppression of cellular protein synthesis by herpes simplex virus is accompanied by inactivation of mRNA. J Gen Virol. 1984 Jul;65(Pt 7):1225–1228. doi: 10.1099/0022-1317-65-7-1225. [DOI] [PubMed] [Google Scholar]
- Goodman J. L., Stevens J. G. Passage of herpes simplex virus type 1 on chick embryo fibroblasts confers virulence for chick embryos. Virus Res. 1986 Aug;5(2-3):191–200. doi: 10.1016/0168-1702(86)90017-1. [DOI] [PubMed] [Google Scholar]
- Gyorkey F., Melnick J. L., Guinn G. A., Gyorkey P., DeBakey M. E. Herpesviridae in the endothelial and smooth muscle cells of the proximal aorta in arteriosclerotic patients. Exp Mol Pathol. 1984 Jun;40(3):328–339. doi: 10.1016/0014-4800(84)90050-9. [DOI] [PubMed] [Google Scholar]
- Hajjar D. P., Nicholson A. C., Hajjar K. A., Sando G. N., Summers B. D. Decreased messenger RNA translation in herpesvirus-infected arterial cells: effects on cholesteryl ester hydrolase. Proc Natl Acad Sci U S A. 1989 May;86(9):3366–3370. doi: 10.1073/pnas.86.9.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P., Pomerantz K. B., Falcone D. J., Weksler B. B., Grant A. J. Herpes simplex virus infection in human arterial cells. Implications in arteriosclerosis. J Clin Invest. 1987 Nov;80(5):1317–1321. doi: 10.1172/JCI113208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Fiers W., Pober J. S. Membrane interleukin 1 induction on human endothelial cells and dermal fibroblasts. J Immunol. 1987 Oct 1;139(7):2317–2324. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- McDonald K., Rector T. S., Braulin E. A., Kubo S. H., Olivari M. T. Association of coronary artery disease in cardiac transplant recipients with cytomegalovirus infection. Am J Cardiol. 1989 Aug 1;64(5):359–362. doi: 10.1016/0002-9149(89)90535-3. [DOI] [PubMed] [Google Scholar]
- McSorley J., Shapiro L., Brownstein M. H., Hsu K. C. Herpes simplex and varicella-zoster: comparative histopathology of 77 cases. Int J Dermatol. 1974 Mar-Apr;13(2):69–75. doi: 10.1111/j.1365-4362.1974.tb01769.x. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Moore K. L., Andreoli S. P., Esmon N. L., Esmon C. T., Bang N. U. Endotoxin enhances tissue factor and suppresses thrombomodulin expression of human vascular endothelium in vitro. J Clin Invest. 1987 Jan;79(1):124–130. doi: 10.1172/JCI112772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. L., Esmon C. T., Esmon N. L. Tumor necrosis factor leads to the internalization and degradation of thrombomodulin from the surface of bovine aortic endothelial cells in culture. Blood. 1989 Jan;73(1):159–165. [PubMed] [Google Scholar]
- Nawroth P. P., Handley D. A., Esmon C. T., Stern D. M. Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci U S A. 1986 May;83(10):3460–3464. doi: 10.1073/pnas.83.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986 Mar 1;163(3):740–745. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Requirement of protein synthesis for the degradation of host mRNA in Friend erythroleukemia cells infected wtih herpes simplex virus type 1. J Virol. 1978 Sep;27(3):619–627. doi: 10.1128/jvi.27.3.619-627.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrild B., Pedersen B. Effect of tunicamycin on the synthesis of herpes simplex virus type 1 glycoproteins and their expression on the cell surface. J Virol. 1982 Aug;43(2):395–402. doi: 10.1128/jvi.43.2.395-402.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie B. L., Adam E., Melnick J. L. Association of herpesvirus/cytomegalovirus infections with human atherosclerosis. Prog Med Virol. 1988;35:21–42. [PubMed] [Google Scholar]
- Phinney P. R., Fligiel S., Bryson Y. J., Porter D. D. Necrotizing vasculitis in a case of disseminated neonatal herpes simplex infection. Arch Pathol Lab Med. 1982 Feb;106(2):64–67. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Schorer A. E., Rick P. D., Swaim W. R., Moldow C. F. Structural features of endotoxin required for stimulation of endothelial cell tissue factor production; exposure of preformed tissue factor after oxidant-mediated endothelial cell injury. J Lab Clin Med. 1985 Jul;106(1):38–42. [PubMed] [Google Scholar]
- Visser M. R., Jacob H. S., Goodman J. L., McCarthy J. B., Furcht L. T., Vercellotti G. M. Granulocyte-mediated injury to herpes simplex virus-infected human endothelium. Lab Invest. 1989 Feb;60(2):296–304. [PubMed] [Google Scholar]
- Visser M. R., Tracy P. B., Vercellotti G. M., Goodman J. L., White J. G., Jacob H. S. Enhanced thrombin generation and platelet binding on herpes simplex virus-infected endothelium. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8227–8230. doi: 10.1073/pnas.85.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox J. N., Smith K. M., Schwartz S. M., Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiroya H. M., Ghosh L., Yang R., Robertson A. L., Jr Herpesviridae in the coronary arteries and aorta of young trauma victims. Am J Pathol. 1988 Jan;130(1):71–79. [PMC free article] [PubMed] [Google Scholar]