Abstract
Background
Insomnia is comorbid with internalizing and externalizing psychiatric disorders. However, the extent to which the etiologic influences on insomnia and common psychopathology overlap is unclear. There are limited genetically-informed studies of insomnia and internalizing disorders and few studies of overlap exist with externalizing disorders.
Methods
We utilized twin data from the Virginia Adult Twin Studies of Psychiatric and Substance Use Disorders (total n = 7500). Insomnia, internalizing disorders (major depressive disorder [MDD], generalized anxiety disorder [GAD]), and alcohol abuse and dependence (AAD) were assessed at two time points, while antisocial personality disorder (ASPD) was assessed once. Cholesky decompositions were performed in OpenMx and longitudinal measurement models were run on available phenotypes to reduce measurement error.
Results
The latent additive genetic influences on insomnia overlapped significantly (56% for females, 74% for males) with MDD and were shared completely (100%) with GAD. There was significant overlap of latent unique environmental influences, with overlap ranging from 38% to 100% across disorders. In contrast, there was less genetic overlap between insomnia and externalizing disorders, with 18% of insomnia’s heritability shared with AAD and 23% with ASPD. Latent unique environmental overlap between insomnia and both externalizing disorders was negligible.
Conclusions
The evidence for substantial genetic overlap between insomnia and stable aspects of both internalizing disorders suggests that there may be few insomnia-specific genes and investigation into unique environmental factors is important for understanding insomnia development. The modest overlap between insomnia and externalizing disorders indicates that these disorders are genetically related, but largely etiologically distinct.
Keywords: sleep disorders, twin studies, depression, GAD/generalized anxiety disorder, alcoholism/Alcohol Use Disorders, genetics
Introduction
Sleep difficulties have adverse consequences, including both negative physical and mental health outcomes 1, constituting a major public health concern. Approximately 6%–10% of individuals meet diagnostic criteria for insomnia, yet as many as one in three adults report experiencing at least one main nighttime insomnia symptom from DSM-IV criteria 1–3. Sleep disturbances are included within DSM criteria for internalizing disorders such as major depressive disorder (MDD) and generalized anxiety disorder (GAD) 4. Moreover, anxiety and depression have been shown to have reciprocal and causal effects on disrupted sleep 5. Although sleep items are not part of diagnostic criteria for externalizing disorders, such as alcohol abuse or dependence (AAD) or antisocial personality disorder (ASPD) 4, individuals with these externalizing conditions often report disturbed sleep e.g., 6,7–11.
There is a large twin literature examining genetic and environmental contributions to insomnia and related disorders 12, with current estimates indicating that insomnia is moderately heritable in adults (range: 0.25 to 0.57) 13. A recent paper by our group has shown the stability of genetic influences on insomnia symptoms across time and higher heritability for women in a large longitudinal adult sample 14. Additionally, twin studies demonstrate that common internalizing (e.g., MDD, GAD) and externalizing (e.g., AAD, ASPD) disorders also have genetic influences, with moderate to high heritabilities 15. Although the heritability of insomnia and psychopathology has been established, and insomnia is comorbid with both types of disorders e.g., 3,8,16,17, there are a limited number of studies investigating genetic and environmental overlap between these phenotypes.
Prior studies have used multivariate twin modeling to examine shared genetic and environmental contributions between insomnia and internalizing psychopathology. Notably, Gehrman and colleagues 18 demonstrated complete genetic overlap between insomnia and internalizing disorders (i.e., depression and overanxious disorder) in juvenile twins. In a young adult sample, Gregory and colleagues found substantial (but not complete) overlap between genes influencing sleep disturbance and those influencing anxiety and depression symptoms (genetic correlations [rA] were 0.58 and 0.68, respectively) 19. Another study in children found that anxiety was not correlated with sleep problems. However, the genetic correlation between sleep and depression was 0.64 20. The remaining studies had mixed results, finding low or non-significant correlations/overlap between depression or anxiety and sleep 21–24.
Thus, the extant literature base on genetic overlap is small and there is some disagreement over the extent of overlap between insomnia and internalizing disorders. Most studies have been in children, and have not formally examined sex differences. Furthermore, only Gillespie and colleagues 5 used a longitudinal model in adults, and no adult studies to date include externalizing disorders. We aimed to address these gaps in the literature using data from a large adult twin study where the heritability and longitudinal stability of insomnia symptoms has been shown 14. Our sample has several noteworthy features: 1) longitudinal data on insomnia and psychiatric phenotypes; and 2) data on male, female, and opposite-sex twin pairs, allowing examination of sex differences. We examined etiologic overlap between insomnia symptoms and common internalizing (MDD and GAD) and externalizing (AAD and ASPD) psychopathology. We hypothesized that there would be significant genetic overlap between insomnia and both internalizing and externalizing disorders, but that this overlap would be larger in magnitude for internalizing psychopathology. We expected minimal environmental overlap between sleep and all disorders.
Materials and Methods
Sample
Participants came from the Virginia Adult Twin Studies of Psychiatric and Substance Use Disorders (VATSPSUD) study of Caucasians 15, ascertained from the birth certificate-based Virginia Twin Registry. This sample is described in detail elsewhere 15. We utilized data from female-female (FF) and male-male and male-female (MMMF) twin pairs assessed at two separate interview waves (referred to as Time 1 and Time 2). Mean (SD) age at Time 1 was 29.3 (7.7) for FF and 35.1 (9.1) for MMMF. The average number of years between Time 1 and 2 was 5.11 (0.42) for FF, and 1.59 (0.73) for MMMF.
Zygosity was determined by discriminant function analyses using standard twin questions validated against DNA genotyping in 496 pairs 25. The VATSPSUD contains data from approximately 7,500 twins, including both members of 3,084 pairs (503 monozygotic (MZ) FF, 346 dizygotic (DZ) FF, 703 MZ MM, 485 DZ MM, and 1,047 opposite-sex DZ pairs) and 1,325 twins without their cotwin (these numbers do not sum because all possible pairings for triplet and quadruplet sets are included in this total; we excluded triplets and quadruplets).
Measures
Insomnia measurement
Participants completed a shortened version of the Symptom Checklist-90 (SCL-90; 26) at each wave, which uses a past month timeframe. These SCL items include three insomnia symptoms: trouble falling asleep, sleep that is restless or disturbed, and awakening in the early morning (note that these three items are the same as those included in the full SCL). All items were recorded on a 5-point Likert scale (response options ranging from “not at all” to “extremely”) and summed to create a composite score (R=0–12), which was reorganized into 4 categories (as done before 14) for analysis. The sleep items showed sufficient internal reliability across waves (Cronbach’s alpha=0.80). The mean (SD) collapsed insomnia symptom score was 1.16 (1.06) in this sample.
Assessment of psychiatric and alcohol use disorders
Prevalence of psychiatric and AAD were assessed via personal interview administered by trained mental health professionals who were blind to the status of the cotwin, using modifications of the SCID interview and DSM-III-R criteria 27,28. For additional information on measures, see 15. Past year MDD and GAD were assessed at multiple time points (referred to here as Times 1 and 2). A minimum duration of two weeks for MDD ( as per DSM-III-R criteria; 27), and one month for GAD (which is similar to six months; e.g., 29), was required. Since sleep is part of diagnostic criteria for both disorders, we operationalized diagnostic status excluding the sleep items. Thus, individuals had to endorse 5 of the 8 remaining MDD symptoms or 3 of the 5 remaining GAD symptoms, in order to be considered a case. A similar method has been used in other twin sleep overlap papers 18,19. We also utilized data from AAD (Times 1 and 2) and ASPD (only adult antisocial traits; assessed once).
Twin Modeling
In the classic twin model, phenotypic variation is decomposed into additive genetic factors (A) which contribute twice as much to the correlations between MZ twins as they do for DZ twins, common environmental factors (C) which are the shared factors (e.g., parental attitudes, economic disadvantage) that make twins reared together more similar and contribute equally to the correlation between MZ and DZ twins, and individual specific environmental (E) sources, which reflect environmental experiences not shared by twins (e.g., divorce) and therefore contribute to differences between the twins. This component also includes random errors of measurement.
To test the degree to which the covariation between insomnia and psychiatric phenotypes is attributable to common etiological factors, we began by applying a series of bivariate Cholesky models 30. These models specify three latent factors (A1, C1, and E1) influencing both the psychiatric phenotype and insomnia and three factors (A2, C2, and E2) accounting for insomnia-specific influences. Insomnia symptoms were entered after the psychiatric phenotypes based on the temporal order of assessment (past year/lifetime for psychiatric disorders and past 30 days for insomnia).
For all phenotypes except ASPD (assessed once), we utilized two waves of data in a measurement model specifying a stable/shared latent liability to insomnia and internalizing (or externalizing) phenotypes across time. In contrast to a standard bivariate model, a measurement model allows separation of time-specific unique effects/measurement error from “true” individual-specific environmental effects. In these extended Cholesky decompositions, the factor loadings (λ1 and λ2) were constrained to be equal across both time points for identification purposes. Note that for phenotypes with two waves of data, only measurement models are reported in the results1. The bivariate model is reported for ASPD.
For all models (bivariate and longitudinal measurement), we began by testing for quantitative sex differences (i.e., the hypothesis that etiological contributions are equivalent in males and females) by constraining the estimates of A, C, and E to be equal across the sexes. The best-fit model was selected from these quantitative sex effect models, and we then tested for qualitative sex effects (i.e., whether or not the same genetic factors influenced liability to psychiatric phenotypes and insomnia for males and females, quantified by rg-psychiatric phenotype and rg-insomnia). Three models were compared to a saturated model (constraining rg- psychiatric phenotype to 1.0, constraining rg-insomnia to 1.0, and constraining both rg parameters to 1.0). Next, the best-fit full models (ACE) were further tested by examining nested submodels with reduced numbers of parameters (AE; CE). For the longitudinal models, we tested the significance of wave-specific genetic influences on each phenotype in a step-wise fashion (3 models: psychiatric phenotype alone [A3 and A4], insomnia alone [A5 and A6], and both together). Finally, based on results from another study 18, which showed complete genetic overlap between internalizing disorders and insomnia, we tested insomnia-specific genetic effects by setting the A2 parameter to zero. To evaluate model fit, a full information maximum likelihood approach for raw ordinal data was used as implemented in the freely available OpenMx software 31,32. Akaike’s Information Criteria (AIC) was used to evaluate the different model fits. AIC is an information index balancing model-data misfit and model complexity (parsimony) 33.
Results
Internalizing phenotypes
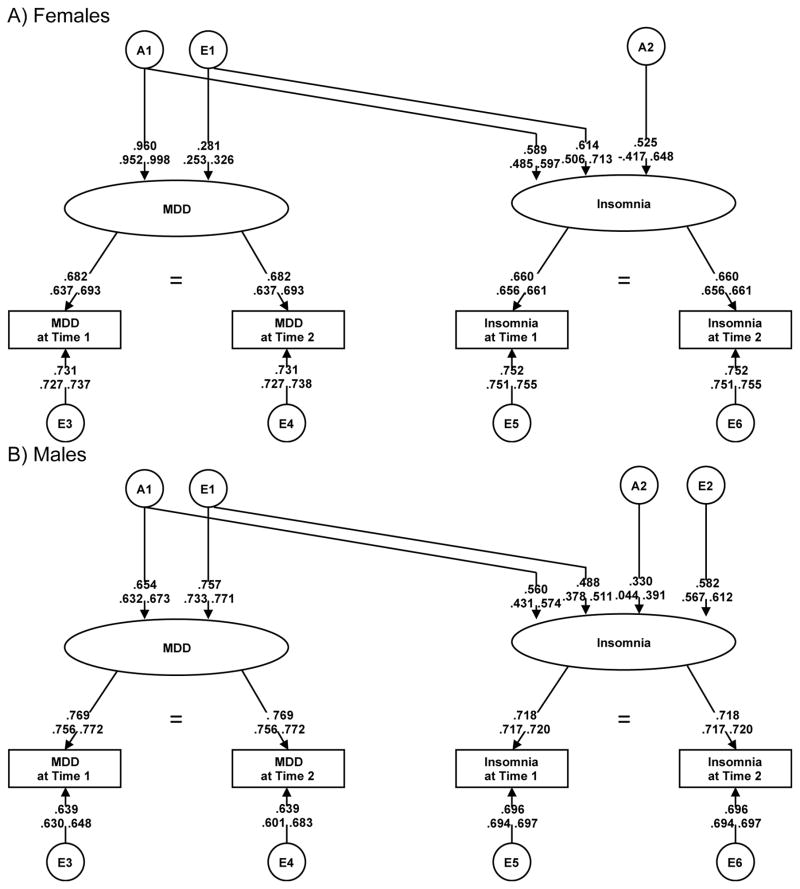
The correlations between insomnia and MDD were moderate (rs ranging from 0.38–0.50 for MZ pairs and 0.36–0.47 for DZ pairs). Similar estimates were found between insomnia and GAD, ranging from 0.36–0.49 (MZ) and 0.30–0.44 (DZ). Table 1 shows the longitudinal measurement models that were fitted for MDD and GAD. For MDD, all initial models were compared to the saturated model (Model I, top half of table). We began by testing for quantitative sex effects (Model II). There was a significant deterioration in model fit, indicating the presence of a quantitative sex effect (i.e., parameter estimates are not equal for males and females, indicating that heritability may be different). Models III–V tested for qualitative sex effects off of Model I. There was no evidence for qualitative sex effects on MDD only (III), insomnia only (IV), or both MDD and insomnia (V), as model fit did not deteriorate when constraining these parameters to 1. Following, we fitted submodels (AE, CE) off of Model V. The C parameter could be dropped without sacrificing model fit (Model VI), but the A parameter could not (Model VII). Next, we constrained the wave-specific genetic effects across phenotypes (Models VIII–X), comparing to the best-fit submodel (Model VI). Finally, we tested the significance of the insomnia-specific genetic parameter (A2; Model XI). A2 could not be dropped, as indicated by significant deterioration in model fit. Given that the E2 estimate was nearly zero in this model, we fit a final model constraining E2 to zero (Model XII), which did not change model fit. Thus, Model XII (Figure 1), an AE model allowing quantitative sex effects, without wave-specific genetic effects, and without a latent insomnia-specific environmental effect, was the best-fit MDD model. The majority of the latent genetic influences on insomnia (56% of insomnia’s 62% heritability for females and 74% of insomnia’s 42% heritability for males) were shared with MDD. Of the 38% total latent unique environmental variance for insomnia in females, 100% overlapped with MDD. For males, 41% of the total 58% of the environmental variance in latent insomnia symptoms was shared with MDD. The genetic correlations between MDD and insomnia were 0.75 [females] and 0.86 [males] in the final model.
Table 1.
Model fitting results for an extended longitudinal bivariate Cholesky decomposition of internalizing disorders and insomnia
| Model | Variables | (Qual MDD or GAD/ Qual Ins) / Quan) | −2LL | AIC | DF | ΔDF | Δ(−2LL) | ΔAIC |
|---|---|---|---|---|---|---|---|---|
| MDD (Prevalence: 9.1% W1; 8.7% W2) | ||||||||
|
| ||||||||
| Sex differences and dropping A/C | ||||||||
| I | ACE | (+ / +) / + | 50242.34 | −14451.66 | 32347 | - | - | - |
| II | ACE | (+ / +) / − | - | - | - | 23 | 43.63 | −2.37 |
| III | ACE | (− / +) / + | - | - | - | 1 | 0.00 | −2.00 |
| IV | ACE | (+ / −) / + | - | - | - | 1 | 0.15 | −1.86 |
| V | ACE | (− / −) / + | - | - | - | 2 | 0.15 | −3.86 |
| VI | AE | (− / −) / + | - | - | - | 16 | 5.95 | −26.06 |
| VII | CE | (− / −) / + | - | - | - | 16 | 46.24 | 14.23 |
| Dropping time-specific effects | ||||||||
| VI | AE | (− / −) / + | 50248.29 | −14477.72 | 32363 | - | - | − |
| VIII | AsMDD = 0 | (− / −) / + | - | - | - | 4 | 1.20 | −6.79 |
| IX | AsIns = 0 | (− / −) / + | - | - | - | 4 | 8.70 | 0.71 |
| X | AsIns/MDD = 0 | (− / −) / + | - | - | - | 8 | 10.10 | −5.90 |
| XI | AsIns/MDD = 0, A22 = 0 | (− / −) / + | - | - | - | 9 | 20.60 | 2.61 |
| XII | AsIns/MDD = 0, E22 = 0* | (− / −) / + | - | - | - | 10 | 10.02 | −7.97 |
|
| ||||||||
| GAD (Prevalence: 5.8% W1; 7.9% W2) | ||||||||
|
| ||||||||
| Sex differences and dropping A/C | ||||||||
| I | ACE | (+ / +) / + | 48637.83 | −16050.18 | 32344 | - | - | - |
| II | ACE | (+ / +) / − | - | - | - | 23 | 29.92 | −16.08 |
| III | ACE | (− / +) / − | - | - | - | 24 | 29.92 | −18.08 |
| IV | ACE | (+ / −) / − | - | - | - | 24 | 31.81 | −16.19 |
| V | ACE | (− / −) / − | - | - | - | 25 | 31.81 | −18.19 |
| VI | AE | (− / −) / − | - | - | - | 32 | 32.83 | −31.17 |
| VII | CE | (− / −) / − | - | - | - | 32 | 62.85 | −1.15 |
| Dropping time-specific genetic effects | ||||||||
| VI | AE | (− / −) / − | 48670.65 | −16081.35 | 32376 | - | - | - |
| VIII | AsGAD = 0 | (− / −) / − | - | - | - | 2 | 3.90 | −0.10 |
| IX | AsIns = 0 | (− / −) / − | - | - | - | 2 | 1.45 | −2.54 |
| X | AsIns/GAD = 0 | (− / −) / − | - | - | - | 4 | 5.36 | −2.64 |
| XI | AsIns/GAD = 0, A22 = 0 | (− / −) / − | - | - | - | 5 | 7.04 | −2.96 |
Abbreviations: Qual=qualitative sex effect; quan=quantitative sex effect; (+) denotes that this sex effect was allowed to vary; (−) denotes that this sex effect was constrained; AsMDD or AsGAD = time-specific genetic effects for MDD or GAD; AsIns = time-specific genetic effects for insomnia; AsIns/MDD or AsIns/GAD = time-specific genetic effects for both MDD or GAD and insomnia; A22 = insomnia-specific genetic effect; E22 = insomnia-specific unique environmental effect.
E22 was fixed to zero in the MDD final model, since the parameter estimate was 0. Bold text indicates the best-fit model.
Figure 1.
Path diagram of extended longitudinal Cholesky decomposition for MDD and insomnia by sex
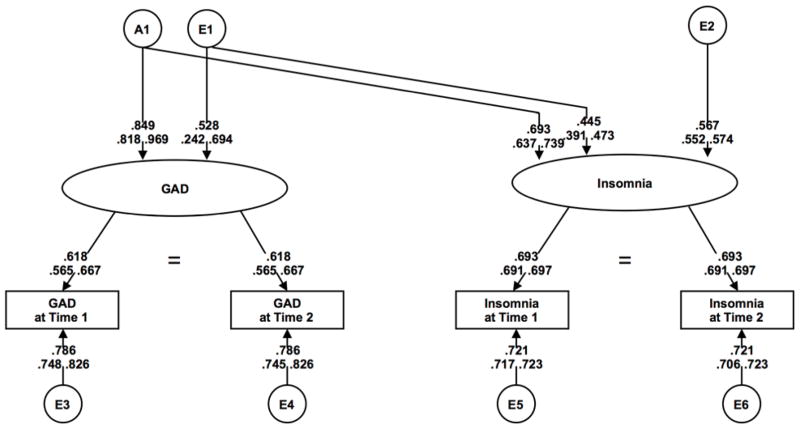
The results of model fitting for GAD are also shown in Table 1 (lower half). We followed the same modeling steps as MDD, beginning with the saturated model (Model I). Model V (no sex effects), was used to fit submodels. We were able to drop C (Model VI), but not A (Model VII). Next, we tested wave-specific genetic effects across phenotypes (Models VIII–X), comparing to Model VI, and found that model fit did not significantly deteriorate in any of these models. In the final GAD model (Model XI), we constrained the insomnia-specific parameter (A2) to 0. Deterioration in model fit was not significant, making the best-fit GAD model an AE model with no sex effects, no wave-specific genetic effects, and no insomnia-specific genetic effects (Model XI; Figure 2). All of the latent genetic influences on insomnia (48%) were shared with GAD. Of the 52% total latent environmental influences on insomnia, 37% overlapped with GAD.
Figure 2.
Path diagram of extended longitudinal Cholesky decomposition for GAD and insomnia
Externalizing phenotypes
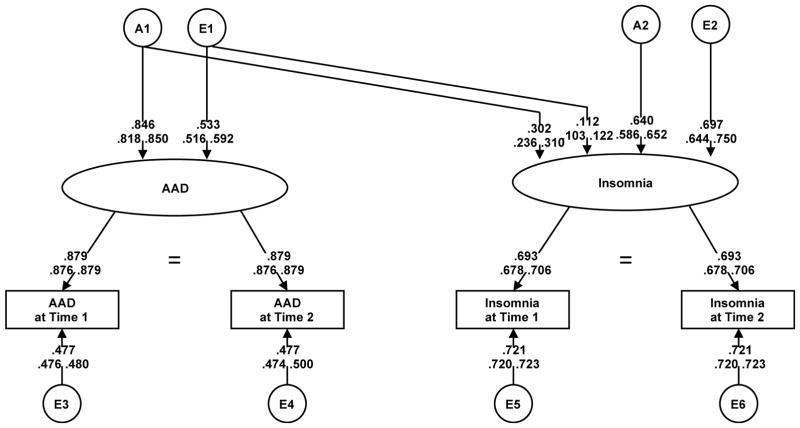
The correlations between insomnia and AAD were lower than for internalizing disorders, with MZ correlations ranging from 0.15–0.23 and DZ correlations ranging from 0.16–0.21. For ASPD, correlations with insomnia were also lower, ranging from 024–0.35 [MZ] and at 0.20–0.23 [DZ]. Table 2 shows longitudinal model results for AAD. Model fitting was carried out similarly to internalizing disorders, using Model I (saturated) for comparison. There was a significant qualitative sex effect on AAD, making Model IV the best-fit model used for submodel testing. There was no deterioration in fit when dropping C (Model V), but A could not be dropped (Model VI). The wave-specific genetic parameters for both AAD and insomnia could also be dropped without significant added misfit (Models VII-IX), but model fit deteriorated when constraining A2 to zero (Model X). The overall best-fit model for AAD was an AE model with a qualitative sex effect on AAD (rg = 0.47) and no wave-specific genetic effects (Model IX; Figure 3). Of the 50% latent heritability estimate of insomnia, 18% overlapped with latent genetic influences on AAD. The genetic correlation between AAD and insomnia was 0.43. There was less environmental overlap, with only 3% of the 50% latent environmental influences on insomnia shared with AAD.
Table 2.
Model fitting results for an extended longitudinal Cholesky decomposition of alcohol abuse or dependence (AAD) and insomnia
| Model | Variables | (Qual AAD / Qual Ins) / Quan) | −2LL | AIC | DF | ΔDF | Δ(−2LL) | ΔAIC |
|---|---|---|---|---|---|---|---|---|
| AAD (Prevalence: 25.7% W1; 28.6% W2) | ||||||||
|
| ||||||||
| Sex differences and dropping A/C | ||||||||
| I | ACE | (+ / +) / + | 57189.38 | 7571.62 | 32380 | - | - | - |
| II | ACE | (+ / +) / − | - | - | - | 23 | 29.00 | −17.00 |
| III | ACE | (− / +) / − | - | - | - | 24 | 44.99 | −3.01 |
| IV | ACE | (+/−) / − | - | - | - | 24 | 29.32 | −18.68 |
| V | AE | (+ / −) / − | - | - | - | 31 | 31.32 | −30.68 |
| VI | CE | (+ / −) / − | - | - | - | 31 | 135.43 | 73.44 |
| Dropping time-specific genetic effects | ||||||||
| V | AE | (+ / −) / − | 57220.70 | 7601.30 | 32411 | - | - | - |
| VII | AsAAD = 0 | (+ / −) / − | - | - | - | 2 | 1.56 | −2.44 |
| VIII | AsIns = 0 | (+ / −) / − | - | - | - | 2 | 2.16 | −1.84 |
| IX | AsIns/AAD = 0 | (+ / −) / − | - | - | - | 4 | 3.72 | −4.28 |
| X | AsIns/AAD = 0, A22 = 0 | (+ / −) / − | - | - | - | 5 | 96.51 | 86.51 |
Abbreviations: Qual=qualitative sex effect; quan=quantitative sex effect; (+) denotes that this sex effect was allowed to vary; (−) denotes that this sex effect was constrained; AsAAD = time-specific genetic effects for AAD; AsIns = time-specific genetic effects for insomnia; AsIns/AAD = time-specific genetic effects for both AAD and insomnia; A22 = insomnia-specific genetic effect. Bold text indicates the best-fit model.
Figure 3.
Path diagram of extended longitudinal Cholesky decomposition for AAD and insomnia
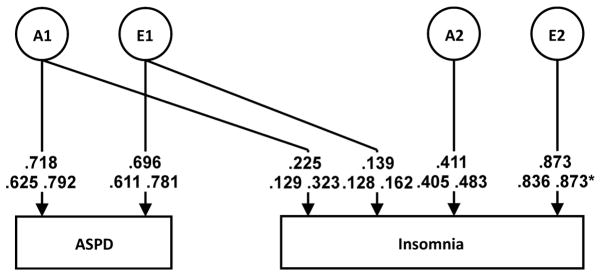
Table 3 shows the bivariate model for ASPD and insomnia. Model fitting steps were similar to prior modeling, testing sex effects (Models II–V) from the saturated model (Model I). No evidence of sex effects were found, so submodels were fit from Model V. The best-fit model was an AE model with no sex effects (Model VI; Figure 4). Twenty-two percent of insomnia’s heritability (23%) was shared with ASPD, whereas only 3% of the environmental influences on insomnia overlapped with those of ASPD. The genetic correlation between ASPD and insomnia was 0.48.
Table 3.
Results of bivariate Cholesky decomposition for antisocial personality disorder and insomnia
| Model | Variables | (Qual ASPD / Qual Ins) / Quan) | −2LL | AIC | DF | ΔDF | Δ( −2LL) | ΔAIC |
|---|---|---|---|---|---|---|---|---|
| ASPD (Prevalence: 9.9%) | ||||||||
|
| ||||||||
| I | ACE | (+ / +) / + | 23389.17 | −4328.83 | 13859 | - | - | - |
| II | ACE | (+ / +) / − | - | - | - | 9 | 2.23 | −15.78 |
| III | ACE | (− / +) / − | - | - | - | 10 | 3.62 | −16.38 |
| IV | ACE | (+/−) / − | - | - | - | 10 | 2.23 | −17.78 |
| V | ACE | (− / −) / − | - | - | - | 11 | 3.86 | −18.14 |
| VI | AE | (− / −) / − | - | - | - | 14 | 9.21 | −18.79 |
| VII | CE | (− / −) / − | - | - | - | 14 | 14.49 | −13.52 |
| VIII | AE, no A22 | (− / −) / − | - | - | - | 15 | 29.53 | −0.48 |
Abbreviations: Qual=qualitative sex effect; quan=quantitative sex effect; (+) denotes that this sex effect was allowed to vary; (−) denotes that this sex effect was constrained; A22 = insomnia-specific genetic effect. Bold text indicates the best-fit model.
Figure 4.
Bivariate Cholesky decomposition, ASPD and Insomnia
*95% confidence interval could not be estimated at a value above the parameter estimate
Discussion
We sought to examine the degree to which the genetic and environmental influences on insomnia are shared with common internalizing and externalizing psychopathology. To our knowledge, this is the first paper utilizing longitudinal data to examine the shared genetic and environmental contributions to the common temporally stable aspects of insomnia and both internalizing and externalizing psychopathology in an adult sample. Overall, there are three main findings: 1) for internalizing disorders, significant genetic overlap was found for the longitudinally stable components, ranging from 56% to 100%; 2) for externalizing disorders, although less genetic overlap was found, the genetic contribution was not completely distinct, suggesting some degree of shared genetic influence; and 3) greater latent environmental overlap was found for internalizing than for externalizing disorders.
Genetic overlap, internalizing disorders
For both sexes, over half (56% for females and 74% for males) of the estimated latent (stable across time) genetic influences on insomnia were shared with MDD. The large genetic correlations between our latent measures of insomnia and MDD (0.75 for females and 0.86 for males) are similar, although on the higher end, to observed estimates in other samples 18,19,21, suggesting that many of the genes influencing insomnia are shared with MDD. In contrast, there was complete overlap between latent genetic influences on insomnia and GAD (no sex effects), indicating that the same genetic architecture may be implicated. Complete genetic overlap of insomnia with overanxious disorder has been shown in youth using a trivariate Cholesky decomposition 18, and we have extended this finding to adults. The longitudinal nature of our study extends existing evidence by examining latent effects and allows us to parse out measurement error, which results in more precise estimates of etiologic influences. Results suggest stability of the phenotypes over time in adults, which aligns with prior work in children 21. The present findings highlight the large genetic covariance between the stable across time components of insomnia and MDD/GAD, indicating that we are likely indexing the genetic risk for internalizing psychopathology when examining insomnia’s heritability.
The molecular genetics literature on insomnia is still in its infancy. Our results suggest that gene-finding efforts for insomnia may benefit from targeted approaches that examine genetic variants associated with risk for MDD and GAD in addition to existing efforts to examine genes relevant to sleep-wake regulation 13. GWAS studies of psychiatric phenotypes such as depression are more developed 34,35, so testing of top hits with insomnia may prove useful. Moreover, it is likely that there are many genes that contribute a small effect 13,36 suggesting the potential benefits of using polygenic risk scores (PRS) to further examine associations.
Genetic overlap, externalizing disorders
There was also evidence for genetic overlap between insomnia and both AAD and ASPD, although the overlap was not as high as for internalizing disorders: Approximately 1/5 of latent genetic influences on insomnia were shared with AAD and 23% of insomnia’s observed heritability was shared with ASPD. To our knowledge, this work represents the first investigation into genetic overlap between insomnia and externalizing disorders, and allows us to reject the notion that the genetic component for insomnia is fully unrelated to the genetic risk for externalizing psychopathology. In a child twin study examining sleep and behavioral problems, no evidence for genetic overlap was found25, while an adult study found genetic overlap between sleep quality and externalizing behaviors (e.g., aggression) 37. The extant literature has examined genetic overlap between many internalizing and externalizing disorders, finding that the genetic influences on both types of disorders are correlated 38 and that much of their comorbidity can be attributed to genetic contributions 39. Given this, along with comorbidity between substance use disorders and insomnia 8 and relationships between sleep disturbance and ASPD 7,11, it is not unexpected that we find some degree of genetic overlap between insomnia and externalizing disorders. These results suggest that insomnia and externalizing disorders are related on a genetic level. Similar to the discussion of internalizing psychopathology, above, research into externalizing disorders may also yield genes relevant to insomnia (via candidate genes, PRS), although likely to a lesser extent given the more modest genetic overlap.
Unique environmental overlap
Significant overlap in latent unique environmental influences on insomnia and internalizing disorders (100% [MDD, females]; 41% [MDD, males]; 38% [GAD]) was found, while latent (AAD) and observed (ASPD) environmental overlap with externalizing disorders was negligible (3%). Thus, insomnia and internalizing disorders may have environmental influences in common, indicating that research into shared enduring environmental influences may be useful for understanding the development of both disorders. However, it should be noted that for all phenotypes analyzed longitudinally, wave-specific unique environmental effects, which also represent measurement error, have a much larger influence on individual time points than latent environmental factors on the across time stable component. This demonstrates the importance of considering acute stressors and environmental events (e.g., construction near one’s residence), which may have a greater influence on current insomnia symptoms. Indeed, prior work has noted evidence of non-shared environmental influences on sleep quality, controlling for genetic and shared environmental effects, that appears to differ between sexes 40.
Limitations
Limitations to be considered include insomnia assessment, which was done subjectively through self-report using a limited number of sleep items, not by formal diagnostic status. However, the items are reflective of DSM-5 insomnia criteria and are similar to phenotypes used in existing literature 41. We have previously published using this phenotype 14. Additionally, data was not available to examine other comorbid sleep disorders such as circadian rhythm disorders. Another important limitation is that MDD and GAD diagnoses were created by excluding sleep items from DSM criteria. Further, while the present study’s longitudinal nature represents a strength, the time intervals between assessments were not equal across all groups. It should also be noted that phenotypes chosen to represent internalizing and externalizing disorders, while common and highly relevant to primary aims, are not all-encompassing. Finally, choice of directionality is a limitation; while study design did not allow for causal determination, we chose to order the psychiatric phenotype first, and this could be reversed.
Conclusions
This study adds to the growing literature examining etiologic overlap between insomnia and common psychiatric disorders by using longitudinal data in a large adult twin sample. Results indicated substantial genetic overlap in stable across time variation between insomnia and internalizing psychopathology and represent the first results for significant, although not complete, genetic overlap between insomnia and externalizing disorders. The significant genetic overlap suggests that individuals with a family history of internalizing, and to a lesser extent externalizing, disorders may also be at higher risk for insomnia (and vice-versa), which can be used by clinicians to help identify individuals at risk. Further, these results suggest that treatment of psychopathology may have positive effects on sleep disturbances, and vice versa. These results can also serve as guidance for future genetic studies of insomnia, which are needed and should capitalize on improved sequencing techniques and utilize better insomnia phenotypes (a pervasive concern in the literature 36). Further, it will be important for future investigations to identify specific environmental contributions to insomnia, the nuances of differential response to current stressors, and the bidirectional nature of sleep concerns and internalizing disorders. Knowledge in these areas will help to better identify and treat the many patients who have, or may be at risk for developing, insomnia.
Acknowledgments
This work was supported in part by NIH grants R01 AA020179, P20 AA107828, R37 AA011408, K02 AA023229, and T32 MH020030. The Mid-Atlantic Twin Registry is supported by NIH grant UL1RR031990. ML is supported by T32 MH020030. Dr. Amstadter is supported by grants R01 AA020179, K02 AA023239, BBRF 20066, R01 MH101518, and P60 MD002256. The authors report no competing interests.
Footnotes
Note that bivariate Cholesky models were run at each time point for all phenotypes and results are available upon request.
Work performed at: Virginia Commonwealth University, Richmond, VA
None of the authors have conflicts of interest with regard to this paper.
References
- 1.Morin CM, Jarrin DC. Epidemiology of Insomnia. Sleep Medicine Clinics. 2013;8(3):281–297. doi: 10.1016/j.jsmc.2022.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 3.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5 Suppl):S7–10. [PMC free article] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 5.Gillespie NA, Gehrman PR, Byrne EM, et al. Modeling the direction of causation between cross-sectional measures of disrupted sleep, anxiety and depression in a sample of male and female Australian twins. J Sleep Res. 2012;21(6):675–83. doi: 10.1111/j.1365-2869.2012.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson EO, Breslau N. Sleep problems and substance use in adolescence. Drug Alcohol Depend. 2001;64(1):1–7. doi: 10.1016/s0376-8716(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 7.Lindberg N, Tani P, Appelberg B, et al. Sleep among Habitually Violent Offenders with Antisocial Personality Disorder. Neuropsychobiology. 2003;47(4):198–205. doi: 10.1159/000071215. [DOI] [PubMed] [Google Scholar]
- 8.Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39(6):411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 9.Prescott CA. Using the Mplus computer program to estimate models for continuous and categorical data from twins. Behav Genet. 2004;34(1):17–40. doi: 10.1023/B:BEGE.0000009474.97649.2f. [DOI] [PubMed] [Google Scholar]
- 10.Kamphuis J, Karsten J, de Weerd A, et al. Sleep disturbances in a clinical forensic psychiatric population. Sleep Med. 2013;14(11):1164–9. doi: 10.1016/j.sleep.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Semiz UB, Algul A, Basoglu C, et al. The relationship between subjective sleep quality and aggression in male subjects with antisocial personality disorder. Turk Psikiyatri Derg. 2008;19(4):373–81. [PubMed] [Google Scholar]
- 12.Barclay NL, Gregory AM. Quantitative genetic research on sleep: a review of normal sleep, sleep disturbances and associated emotional, behavioural, and health-related difficulties. Sleep Med Rev. 2013;17(1):29–40. doi: 10.1016/j.smrv.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Gehrman PR, Byrne E, Gillespie N, et al. Genetics of Insomnia. Sleep Medicine Clinics. 2011;6(2):191–202. [Google Scholar]
- 14.Lind MJ, Aggen SH, Kirkpatrick RM, et al. A Longitudinal Twin Study of Insomnia Symptoms in Adults. Sleep. 2015;38(9):1423–30. doi: 10.5665/sleep.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kendler KS, Prescott CA. Genes, environment, and psychopathology: understanding the causes of pyschiatric and substance use disorders. New York, New York: Guilford Press; 2006. [Google Scholar]
- 16.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 17.McCall WV. A psychiatric perspective on insomnia. J Clin Psychiatry. 2001;62(Suppl 10):27–32. [PubMed] [Google Scholar]
- 18.Gehrman PR, Meltzer LJ, Moore M, et al. Heritability of insomnia symptoms in youth and their relationship to depression and anxiety. Sleep. 2011;34(12):1641–6. doi: 10.5665/sleep.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregory AM, Buysse DJ, Willis TA, et al. Associations between sleep quality and anxiety and depression symptoms in a sample of young adult twins and siblings. J Psychosom Res. 2011;71(4):250–5. doi: 10.1016/j.jpsychores.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Gregory AM, Rijsdijk FV, Dahl RE, et al. Associations between sleep problems, anxiety, and depression in twins at 8 years of age. Pediatrics. 2006;118(3):1124–32. doi: 10.1542/peds.2005-3118. [DOI] [PubMed] [Google Scholar]
- 21.Gregory AM, Rijsdijk FV, Lau JY, et al. The direction of longitudinal associations between sleep problems and depression symptoms: a study of twins aged 8 and 10 years. Sleep. 2009;32(2):189–99. doi: 10.1093/sleep/32.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregory AM, Eley TC, O’Connor TG, et al. Family influences on the association between sleep problems and anxiety in a large sample of pre-school aged twins. Personality and Individual Differences. 2005;39(8):1337–1348. [Google Scholar]
- 23.Van den Oord EJ, Boomsma DI, Verhulst FC. A study of genetic and environmental effects on the co-occurrence of problem behaviors in three-year-old twins. J Abnorm Psychol. 2000;109(3):360–72. doi: 10.1037/0021-843X.109.3.360. [DOI] [PubMed] [Google Scholar]
- 24.Gregory AM, Eley TC, O’Connor TG, et al. Etiologies of associations between childhood sleep and behavioral problems in a large twin sample. J Am Acad Child Adolesc Psychiatry. 2004;43(6):744–51. doi: 10.1097/01.chi/0000122798.47863.a5. [DOI] [PubMed] [Google Scholar]
- 25.Kendler KS, Prescott CA. A population-based twin study of lifetime major depression in men and women. Arch Gen Psychiatry. 1999;56(1):39–44. doi: 10.1001/archpsyc.56.1.39. [DOI] [PubMed] [Google Scholar]
- 26.Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale--preliminary report. Psychopharmacol Bull. 1973;9(1):13–28. [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Fourth Edition. Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- 28.Spitzer RL, Williams J, Gibbon M, et al. Structured Clinical Interview for DSM-IV. Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- 29.Kessler RC, Brandenburg N, Lane M, et al. Rethinking the duration requirement for generalized anxiety disorder: evidence from the National Comorbidity Survey Replication. Psychol Med. 2005;35(7):1073–82. doi: 10.1017/s0033291705004538. [DOI] [PubMed] [Google Scholar]
- 30.Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer; 1992. [Google Scholar]
- 31.Neale MC, Boker SM, Xie G, et al. Mx: statistical modeling. VCU Box 900126, Richmond: VA 23298: Department of Psychiatry; 2002. [Google Scholar]
- 32.Boker S, Neale M, Maes H, et al. OpenMx: An Open Source Extended Structural Equation Modeling Framework. Psychometrika. 2011;76(2):306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52(3):317–332. [Google Scholar]
- 34.Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium. Ripke S, Wray NR, et al. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry. 2013;18(4):497–511. doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Converge consortium. Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature. 2015;523(7562):588–91. doi: 10.1038/nature14659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gehrman PR, Pfeiffenberger C, Byrne E. The Role of Genes in the Insomnia Phenotype. Sleep Med Clin. 2013;8(3):323–331. doi: 10.1016/j.jsmc.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barclay NL, Eley TC, Maughan B, et al. Associations between diurnal preference, sleep quality and externalizing behaviours: a behavioural genetic analysis. Psychol Med. 2011;41(5):1029–40. doi: 10.1017/S0033291710001741. [DOI] [PubMed] [Google Scholar]
- 38.Kendler KS, Aggen SH, Knudsen GP, et al. The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and all axis II disorders. Am J Psychiatry. 2011;168(1):29–39. doi: 10.1176/appi.ajp.2010.10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kendler KS, Prescott CA, Myers J, et al. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60(9):929–37. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 40.Barclay NL, Eley TC, Buysse DJ, et al. Nonshared environmental influences on sleep quality: a study of monozygotic twin differences. Behav Genet. 2012;42(2):234–44. doi: 10.1007/s10519-011-9510-1. [DOI] [PubMed] [Google Scholar]
- 41.Drake CL, Friedman NP, Wright KP, Jr, et al. Sleep reactivity and insomnia: genetic and environmental influences. Sleep. 2011;34(9):1179–88. doi: 10.5665/SLEEP.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]