Abstract
Objective:
New oral anticoagulants (NOACs) are increasingly used both for prevention of stroke in non-valvular atrial fibrillation (NVAF) and the treatment of venous thromboembolism (VTE). In this study, we aimed to evaluate the current patterns of NOACs treatment in Turkey. Moreover, demographic and clinical parameters and bleeding and/or embolic events under NOACs treatment were analyzed.
Methods:
The New Oral Anticoagulants-TURKey (NOAC-TURK) study was designed as a multicenter cross-sectional study. A total of 2,862 patients from 21 different centers of Turkey under the treatment of NOACs for at least three months were included in this study. Demographic, clinical, and laboratory characteristics of study participants with their medications used were obtained through the NOAC-TURK survey database. Additional necessary medical records were obtained from electronic health records of participating centers.
Results:
Of the 2. 862 patients, 1.131 (39.5%) were male and the mean age was 70.3±10.2 years. Hypertension was found as the most frequent comorbidity (81%). The most common indication for NOACs was permanent atrial fibrillation (83.3%). NOACs were mainly preferred because of inadequate therapeutic range or overdose during warfarin usage. The most frequent complication was bleeding (n=217, 7.6%), and major bleeding was observed in 1.1% of the patients. Embolic events were observed in 37 patients (1.3%). Rivaroxaban and dabigatran were both more preferred than apixaban. Almost half of the patients (47.6%) were using lower doses of NOACs, which is definitely much more than expected.
Conclusion:
The NOAC-TURK study showed an important overview of the current NOACs treatment regimens in Turkey. Although embolic and bleeding complications were lower than or similar to previous studies, increased utilization of low-dose NOACs in this study should be considered carefully. According to the results of this study, NOACs treatment should be guided through CHA2DS2-VASc and HASBLED scores to ensure more benefit and less adverse effects in NVAF patients.
Keywords: new oral anticoagulants, atrial fibrillation, embolic complication, bleeding
Introduction
Oral anticoagulants (OACs) are the mainstay therapy used for stroke prevention in non-valvular atrial fibrillation (NVAF) and the treatment of venous thromboembolism (VTE). Atrial fibrillation (AF) is the major indication for OACs use and is one of the leading causes of major cardiovascular events, including mortality and fatal stroke worldwide (1). Conventional preventive strategies of AF, such as proper anticoagulant and rate-limiting therapeutic agents, are crucial to avoid its complications. For over 50 years, vitamin K antagonists such as warfarin, phenprocoumon, and acenocoumarol were the only available oral anticoagulants. Problems like narrow therapeutic window, common food and drug interactions, and the need for repeated blood tests to establish the target international normalized ratio (INR) are the main drawbacks of these drugs during clinical use. These common problems of vitamin K antagonists led to the investigation of more effective and safe anticoagulants. New oral anticoagulants (NOAC) are a result of these studies and have become widely available. Dabigatran (a factor II or thrombin inhibitor), rivaroxaban, and apixaban (factor Xa inhibitors) were approved NOACs in our country. Edoxaban and betrixaban will soon be available worldwide. Dabigatran and rivaroxaban have been approved for non-valvular AF, deep vein thrombosis (DVT), and pulmonary embolism (PE), and apixaban has been approved only for NVAF by the Turkish Ministry of Health. Since their approval, substantial numbers of patients were prescribed NOACs.
The prevalence of AF in Turkey is 1.25% and its incidence is 1.35/1000 person-years according to the Turkish Adult Risk Factor (TARF) study, wherein rheumatic valve disease was regarded as a predisposing factor in only 6.0% of the subjects (2). Reported incidence of VTE along with PE is highly variable because of diagnostic challenges. Estimated annual incidence of VTE ranges from 104 to 183 per 100,000 person-years, and these rates are similar to stroke (1–6). Considering these facts, an increasing number of patients will be prescribed NOACs in our country.
Recently, very important information has been gathered on AF by registries, which include data on large cohorts. These registries were especially important to observe the control of AF and its complications in different patient populations. In the GARFIELD registry, it has been shown that 11.7% of the patients were not using any antithrombotic treatment, whereas 50% were receiving warfarin and 10.8% were using NOACs (7). There are a few recent data sources available in the literature in terms of the efficacy and safety of NOACs, among which only one arose from Turkey (8). However, there is no study that assesses the efficacy and safety of NOACs for all indications, including NVAF, DVT, and PE, in the same study. In this multicenter cross-sectional study, we primarily aimed to assess the current patterns of NOACs treatment to identify therapeutic trends and aspects of the current practice in Turkey. In addition, demographic characteristics, along with bleeding and thromboembolic risk factors of these patients, clinical indications and their conformity to guidelines, adverse effects, and bleeding and embolic complications will be analyzed in this study.
Methods
Study design
The NOAC-TURK study is a national, nonrandomized multicenter cross-sectional study.
Study population
The study was conducted in outpatient cardiology clinics of state, university, private, and training and research hospitals. Included study centers were chosen according to clinical feasibility and also whether or not they represent the Turkish population well. The study centers were initially included in each of the seven geographic regions (Marmara, Aegean, Mediterranean, Central Anatolia, Black Sea, East Anatolia, and Southeast Anatolia), which were composed of 26 different centers in Turkey to provide geographic diversity. However, five centers were excluded from the final data analysis of the study because of non- or low patient (<20 patients/center) recruitment. The study was conducted between August 1, 2015, and January 1, 2016.
In this study, consecutive patients aged ≥18 years with a diagnosis of non-valvular AF, PTE, and/or VTE under the treatment of NOACs for at least three months were included. Patients could be in sinus rhythm or AF at the time of enrollment, but an electrocardiographically confirmed AF episode should have occurred prior to enrollment in NVAF patients. Patients with hypertension; renal failure; coronary artery disease (a history of percutaneous intervention or coronary artery bypass graft surgery); diabetes mellitus; congestive heart failure; and valvular disorders including any degree of mitral regurgitation, aortic stenosis, or aortic regurgitation were included. The exclusion criteria regarding valvular disease were having a mechanical heart valve or any degree of rheumatic mitral stenosis.
The list of participating centers and sub-investigators are presented in Appendix 1.
We planned the present study on behalf of the Young Cardiologists Subgroup of the Turkish Society of Cardiology.
Demographic, clinical, and laboratory characteristics of study participants with their medications used (antiplatelet, anticoagulant, and antiarrhythmic drugs) were obtained through the NOAC-TURK survey database. Additional necessary medical records were obtained from electronic health records of participating centers. The survey included questions about stroke and other embolic adverse events-related risk factors such as coronary heart disease (CHD), hypertension, diabetes mellitus, previous stroke, congestive heart failure (CHF), and vascular disease (prior myocardial infarction, peripheral artery disease). Embolic events were also recorded during the study period. Moreover, hemorrhagic events associated with antiplatelet or anticoagulant drugs were noted. Stroke and thromboembolism risk were assessed using CHA2DS2–VASc (CHF or left ventricular dysfunction, hypertension, age ≥75 or 65–74 years, diabetes, thromboembolism or a history of stroke, vascular disease, and sex) and bleeding risk by HAS-BLED (hypertension, renal or liver failure, stroke history, bleeding history, labile INR, age >65 years, drugs predisposing to bleeding, and alcohol use) score (9, 10). Glomerular filtration rate was calculated using the Modification of Diet in Renal Disease (MDRD) formula (11). Chronic renal failure (CRF) was defined as whether or not GFR was estimated to be <60 mL/min. Major bleeding was defined as a fall in hemoglobin level of at least 2 g/dL or requiring two or more units of whole blood/erythrocyte transfusion or symptomatic bleeding in a critical organ/area, such as intracranial, intraocular, intra-spinal, retroperitoneal, intra-articular, pericardial, and intramuscular bleeding, leading to compartment syndrome or fatal, according to International Society on Thrombosis and Hemostasis criteria (12). Minor bleeding was defined as any bleeding other than major bleeding considered to be related to NOACs use. Mortality data were obtained from electronic health records of participating centers but were not included in the analysis because of the cross-sectional nature of the study.
The study was approved by the local ethics committee (the Ethics Committee of Haydarpaşa Numune Training and Research Hospital; HNEAH-KAEK 2015/KK/60). Written informed consent was obtained from all study patients.
Statistical analysis
Data were analyzed by Statistical Package for Social Sciences (SPSS) version 17.0 for Windows (IBM, Armonk, New York, USA). Whether or not the distributions of continuous variables were normal was determined by the Kolmogorov–Smirnov test. Data were shown as mean±standard deviation or median (min – max) for continuous variables. Number of cases and percentages were used for categorical data. Mean differences between groups were compared by Student’s t-test, whereas the Mann–Whitney U test was applied for comparisons of the not normally distributed data. Categorical variables were analyzed by chi-square or Fisher’s exact test, where applicable. Determining the best predictor(s) that affect(s) each clinical outcome (i.e., bleeding, other embolic events) was performed by using the multiple logistic regression backward LR method. Any variable with a p-value <0.25 in a univariate model was accepted as a candidate for the multiple model along with all variables of known clinical importance (13). Odds ratios and 95% confidence intervals for each independent variable were also calculated. Statistical significance was accepted as p <0.05.
Results
In this cross-sectional study, a total of 2.862 patients from 21 different centers of Turkey under the treatment of NOACs for at least three months were included. Baseline demographic and clinical characteristics are summarized in Table 1. The mean age was 70.3±10.2 years, and 60.5% of the study patients were female. The duration of use of NOACs was approximately 10.8±7.6 months.
Table 1.
Baseline characteristics of study patients
| Variables | All patients (n=2862) |
|---|---|
| Age, years | 70.3±10.2 |
| Male, % | 1131 (39.5) |
| Female, % | 1731 (60.5) |
| Medical history, % | |
| Hypertension | 2320 (81.1) |
| Diabetes mellitus | 568 (19.8) |
| Hyperlipidemia | 1070 (37.4) |
| Chronic heart failure | 765 (26.7) |
| Chronic renal failure | 224 (7.8) |
| GFR, mL/min/1.73 m2 | 78.0±23.1 |
| Cerebrovascular accident | 326 (11.4) |
| Pulmonary embolism | 66 (2.3) |
| Peripheral artery disease | 177 (6.2) |
| Malignancy | 58 (2.0) |
| Smoking | 534 (18.7) |
| Indication for OAC treatment (%) | |
| Permanent AF | 2385 (83.3) |
| Paroxysmal AF | 325 (11.4) |
| Ischemic stroke | 103 (3.6) |
| Deep vein thrombosis | 56 (2.0) |
| Pulmonary embolism | 46 (1.6) |
| Profylaxis for orthopedic surgery | 5 (0.2) |
| Other | 1 (0.0) |
| Baseline risk analysis | |
| CHA2DS2-VASc score | 3.4±1.4 |
| HASBLED score | 1.8±1.0 |
AF - atrial fibrillation; GFR - glomerular filtration rate; OAC - oral anticoagulant
Eighty-one percent of these patients were hypertensive, 19.8% diabetic, 37.4% had dyslipidemia, and only 18.7% were smokers. The history of these patients showed 26.6% CHF, 7.8% CRF, 11.4% cerebrovascular disease, 6.2% PAD, 2.3% PE, and 2.0% malignancy.
The most common indication for NOACs was permanent AF (83.3%), followed by paroxysmal AF (11.4%), ischemic cerebrovascular disease (3.6%), DVT (2.0%), PE (1.6%), and for prophylaxis following orthopedic surgery.
All patients were evaluated for their CHA2DS2-VASc and HASBLED score. The mean value for the CHA2DS2-VASc score was 3.4±1.4, and the HASBLED score was 1.8±1.0.
Novel oral anticoagulant drugs were mainly preferred because of an inadequate therapeutic range or overdose during warfarin usage. The second most common reason was physicians’ preferences.
Medical treatment
All patients were evaluated for their NOAC doses and frequencies (Fig.1). Rivaroxaban and dabigatran were both more preferred than apixaban. A lower dose of dabigatran was more preferred than its recommended dose. Almost half of the patients (47.6%) were using lower doses of NOACs, which is definitely much more than expected. When people using low-dose NOACs were compared with the ones using it in the recommended dose, it was observed that they were usually older, females at a high rate, with high CHA2DS2-VASc and HASBLED scores and low GFR levels (Table 2). This indicates the tendency of the physicians in Turkey to prefer low-dose treatment in high-risk patients.
Figure 1.
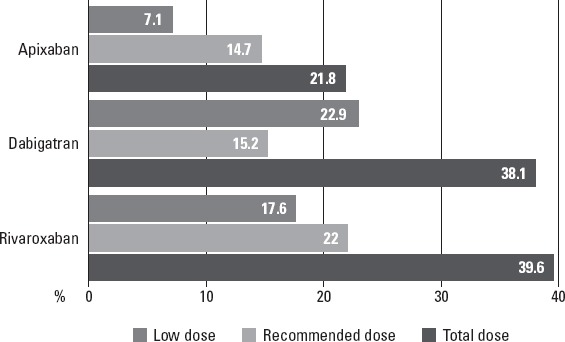
Frequency of the use of each NOAC type and the percentages (prevalence) of the drug use in low and recommended doses among all cases
Table 2.
Clinical characteristics of patients using low dose and recommended dose of NOACs
| Variables | Low dose (n=1361) | Recommended (n=1486) | P |
|---|---|---|---|
| Age, years | 74.6±8.9 | 66.5±9.8 | <0.001† |
| Sex category | 0.048‡ | ||
| Male | 513 (37.7%) | 614 (41.3%) | |
| Female | 848 (62.3%) | 872 (58.7%) | |
| CRF | 162 (11.9%) | 61 (4.1%) | <0.001‡ |
| CHA2DS2-VASc score | 4 (0–9) | 3 (0–9) | <0.001¶ |
| GFR | 72.0 (12.2–212.1) | 81.0 (1.0–233.0) | <0.001¶ |
| HASBLED score | 2 (0–5) | 1.5 (0–5) | <0.001¶ |
Student’s t-test;
Chi-square test;
Mann–Whitney U test. CRF - chronic renal failure; GFR - glomerular filtration rate
Additional antiplatelet drug prescription was detected in 12.9% of the patients. Only 1.8% of this prescription involved clopidogrel, and the others were acetylsalicylic acid in different doses. Since most of these were AF patients, they were also prescribed anti-arrythmic/rate-lowering drugs in addition to NOACs. Two hundred twelve of 2,862 patients (7.4%) were taking antiarrhythmic/rate control drugs. Among these drugs, beta-blockers (45.5%), non-dihydropyridine calcium channel blockers (12.9%) and digoxin (11.3%) were mainly preferred.
Embolic events
Embolic events including transient ischemic attack, stroke, and peripheral embolism were seen in 37 (1.3%) of the patients (Table 3). NOAC treatment was stopped and warfarin treatment was initiated in 12 patients. NOAC doses were increased in 11 patients and another NOAC treatment was initiated following an embolic event in eight patients. There were no significant differences in terms of the number of patient with embolic complication between with and without additional antiplatelet drug treatment [1.2% (n=30) vs. 1.9% (n=7), p=0.317]. In a univariate analysis, the CVA history and smoking were found to be significant predictors in the group that experienced embolic incident, compared with the group that did not experience it (p=0.003 and p=0.002, respectively) (Table 4). As a result of the univariate statistical analyses, all variables identified as p<0.25 were included in the logistic regression model as candidate risk factors. Multiple logistic regression analysis revealed that DVT, CVA, smoking, apixaban treatment, and lower doses of NOACs were the main predictors of embolic events in these patients (Table 5). Embolic events with apixaban were significantly higher in these patients, especially in lower doses, whereas rivaroxaban was associated with significantly lower embolic events (Fig. 2).
Table 3.
Bleeding and embolic complications in patients under NOACs treatment
| Complications | Number of patients (n=2862) |
|---|---|
| Bleeding | 217 (7.6%) |
| Admission count due to bleeding in a year period | 1 (1–5) |
| Bleeding complication in a year period, month | 5 (1–33) |
| Embolism | 37 (1.3%) |
| TIA | 17 (0.6%) |
| Stroke | 16 (0.6%) |
| Peripheral embolism | 4 (0.1%) |
TIA - transient ischemic attack
Table 4.
Demographic and clinical features of groups with and without embolic events
| Some variables and comorbidities | Embolism (–)(n=2825) | Embolism (+) (n=37) | P |
|---|---|---|---|
| Age, years | 70.3±10.2 | 73.3±11.0 | 0.072† |
| Sex category | 0.641‡ | ||
| Male | 1115 (39.5%) | 16 (43.2%) | |
| Female | 1710 (60.5%) | 21 (56.8%) | |
| Diabetes mellitus | 560 (19.8%) | 8 (21.6%) | 0.785‡ |
| Hyperlipidemia | 1056 (37.4%) | 14 (37.8%) | 0.954‡ |
| Hypertension | 2292 (81.1%) | 28 (75.7%) | 0.400‡ |
| Deep venous thrombosis | 87 (3.1%) | 3 (8.1%) | 0.109¶ |
| Coronary artery disease | 757 (26.8%) | 7 (18.9%) | 0.282‡ |
| Chronic heart failure | 751 (26.6%) | 14 (37.8%) | 0.124‡ |
| Chronic renal failure | 222 (7.9%) | 2 (5.4%) | 1.000¶ |
| Malignancy | 57 (2.0%) | 1 (2.7%) | 0.533¶ |
| Peripheral artery disease | 173 (6.1%) | 4 (10.8%) | 0.285¶ |
| Pulmonary embolism | 64 (2.3%) | 2 (5.4%) | 0.209¶ |
| Cerebrovascular accident | 315 (11.2%) | 11 (29.7%) | 0.002¶ |
| Smoking | 520 (18.4%) | 14 (37.8%) | 0.003‡ |
| CHA2DS2-VASC score | 3 (0–9) | 4 (0–7) | 0.199$ |
| GFR, mL/min/1.73 m2 | 76.2 (1–233) | 82 (42.7–118) | 0.158$ |
| HASBLED score | 2 (0–5) | 2 (0–4) | 0.694$ |
| Types of NOACs | |||
| Apixaban | 611 (21.7%) | 14 (37.8%) | 0.019‡ |
| Dabigatran | 1075 (38.3%) | 15 (40.5%) | 0.776‡ |
| Rivaroxaban | 1124 (40.0%) | 8 (21.6%) | 0.023‡ |
| High-dose NOACs | 1474 (52.5%) | 12 (32.4%) | 0.015‡ |
Student’s t-test;
Chi-square test;
Fisher’s exact test;
Mann–Whitney U test. GFR - glomerular filtration rate; NOACs - new oral anticoagulants
Table 5.
Predictors of embolic events in patients under NOACs treatment
| Some risk factors | Odds ratio | 95% Confidence interval | P |
|---|---|---|---|
| DVT | 4.614 | 1.328–16.032 | 0.016 |
| CVA | 2.813 | 1.322–5.982 | 0.007 |
| Smoking | 2.736 | 1.373–5.453 | 0.004 |
| Rivaroxabana | 1.000 | – | – |
| Apixaban | 3.609 | 1.457–8.941 | 0.006 |
| Dabigatran | 1.720 | 0.716–4.135 | 0.225 |
| Low-dose NOACs | 2.913 | 1.385–6.127 | 0.005 |
CVA - cerebrovascular accident; DVT - deep vein thrombosis; NOACs - new oral anticoagulants;
- Reference category
Figure 2.
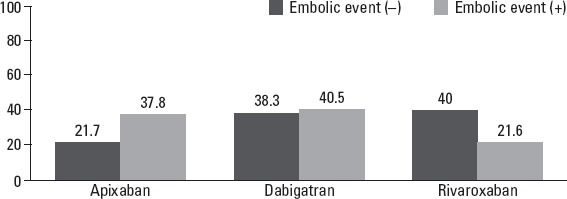
Percentages of NOAC types in the cases that were an embolic incident or not
Bleeding
Bleeding complication was seen in 7.6% (217 patients) of these patients (Table 3). The median number of referrals to the hospital due to bleeding in a one-year period was one, and bleeding complication was observed median five months after NOAC prescription. The most common causes for patients being admitted to the hospital were as follows: nasal bleeding (35.0%), hematuria (25.8%), ecchymosis (17.5%), and conjunctival hemorrhage (17.1%). The upper gastrointestinal bleeding (10.6%) and lower gastrointestinal bleeding (10.1%) rates were similar. Major bleeding was observed in 1.1% of the patients. Intracranial bleeding was observed in two patients (0.9%). Bleeding complications were minor in most of the patients such that 68.2% of these patients were treated in outpatient clinics. 31.8% of these patients were treated hospitalized. Fresh frozen plasma was needed in 16.6% of the patients, prothrombin complex in 1.8%, and erythrocyte suspension in 15.7%. Hemodialysis was used in only one patient (0.5%) for bleeding.
In the univariate analysis, the average age (71.6±9.7 vs. 70.2±10.2) of the group in which bleeding was observed was significantly higher; the DM was lower; and hyperlipidemia, PAD, and smoking were high. The CHA2DS2-VASc score (3.5±1.2 vs. 3.3±1.4; p=0.023) and HASBLED (2.2±1.1 vs. 1.8±0.99) score of the group in which bleeding was observed were significantly higher (Table 6). As a result of the univariate statistical analyses, all variables identified as p<0.25 were included in the logistic regression model as candidate risk factors.
Table 6.
Demographic and clinical features of groups with and without bleeding
| Some risk factors | Bleeding (+) (n=2645) | Bleeding (–) (n=217) | P |
|---|---|---|---|
| Age, years | 70.2±10.2 | 71.7±9.8 | 0.048† |
| Sex category | 0.406‡ | ||
| Male | 1051 (39.7%) | 80 (36.9%) | |
| Female | 1594 (60.3%) | 137 (63.1%) | |
| Diabetes mellitus | 537 (20.3%) | 31 (14.3%) | 0.033‡ |
| Hyperlipidemia | 941 (35.6%) | 129 (59.4%) | <0.001‡ |
| Hypertension | 2140 (80.9%) | 180 (82.9%) | 0.460‡ |
| Deep venous thrombosis | 84 (3.2%) | 6 (2.8%) | 0.739‡ |
| Coronary artery disease | 705 (26.7%) | 59 (27.2%) | 0.864‡ |
| Chronic heart failure | 700 (26.5%) | 65 (30.0%) | 0.264‡ |
| Chronic renal failure | 211 (8.0%) | 13 (6.0%) | 0.295‡ |
| Malignancy | 53 (2.0%) | 5 (2.3%) | 0.800¶ |
| Peripheral artery disease | 127 (4.8%) | 50 (23.0%) | <0.001‡ |
| Pulmonary embolism | 61 (2.3%) | 5 (2.3%) | 0.998‡ |
| Cerebrovascular accident | 296 (11.2%) | 30 (13.8%) | 0.240‡ |
| Smoking | 463 (17.5%) | 71 (32.7%) | <0.001‡ |
| CHA2DS2-VASC score | 3 (0–9) | 4 (1–7) | 0.023$ |
| GFR, mL/min/1.73 m2 | 77 (1–217.3) | 76 (25–233) | 0.542$ |
| HASBLED score | 2 (0–5) | 2 (0–5) | <0.001$ |
| NOAC | |||
| Apixaban | 599 (22.8%) | 26 (12.0%) | <0.001‡ |
| Dabigatran | 1001 (38.0%) | 89 (41.2%) | 0.359‡ |
| Rivaroxaban | 1031 (39.2%) | 101 (46.8%) | 0.029‡ |
| High-dose NOACs | 1364 (51.8%) | 122 (56.5%) | 0.190‡ |
Student’s t-test;
Chi-square test;
Fisher’s exact test;
Mann–Whitney U test. GFR - glomerular filtration rate; NOAC - new oral anticoagulant
Logistic regression analysis revealed that a lack of diabetes mellitus, dyslipidemia, PAD, smoking, higher HASBLED score, dabigatran and rivaroxaban treatment, and higher doses of NOACs were the main predictors for bleeding (Table 7). While bleeding with rivaroxaban was significantly higher especially in high doses in these patients, apixaban was related with significantly lower bleeding rates, especially in higher doses (Fig. 3).
Table 7.
Predictors of bleeding in patients under NOACs treatment
| Odds ratio | 95% Confidence interval | P | |
|---|---|---|---|
| Diabetes mellitus | 0.557 | 0.370–0.839 | 0.005 |
| Hyperlipidemia | 1.873 | 1.376–2.551 | <0.001 |
| PAD | 3.396 | 2.276–5.065 | <0.001 |
| Smoking | 1.781 | 1.282–2.472 | |
| HAS-BLED score | 1.426 | 1.225–1.659 | <0.001 |
| Apixabana | 1.000 | – | – |
| Dabigatran | 2.233 | 1.389–3.590 | <0.001 |
| Rivaroxaban | 2.325 | 1.463–3.697 | <0.001 |
| High-dose NOACs | 1.530 | 1.126–2.078 | 0.006 |
-reference category; NOACs - new oral anticoagulants; PAD - peripheral artery disease
Figure 3.
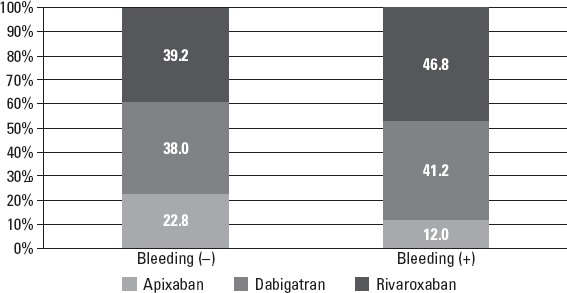
Percentages (prevalence) of NOAC types used in the cases with or without bleeding: cumulative bar graph
Discussion
NOAC-TURK is the first study to report the efficacy and safety of NOACs for all indications, including NVAF, DVT, and PE, in the same study in Turkey. There are many important main findings in this study. First, this study reported that the most common indication for NOACs use in Turkey was permanent AF, which is concordant with large current observational studies (8, 14–16). Another important finding was that nearly half of the study participants were using reduced doses of NOACs, which is discordant with recent phase-III trials and clinical studies (17–19).
In the ARISTOTLE study, reduced doses of apixaban were given to patients with an age of at least 80 years, body weight of no more than 60 kg, or a serum creatinine level of 1.5 mg/dl or more. A 2.5-mg dose of apixaban was administered to only 4.7% of the patients in the ARISTOTLE study; however, 7.1% of the patients in the present study used lower doses of apixaban, which means that almost one-third of all patients were using apixaban (19).
Bleeding is an important life-threatening complication of NOACs use. In the present study, the major bleeding rate was 1.1% and both major and minor bleeding complications were seen in 7.6% of the patients, which is less than the figure in large clinical trials (17–19). Less bleeding complications may be related to the cross-sectional design of the study as well as increased use of lower doses of NOACs. Minor bleeding events occurred more commonly than major events such as intracranial or gastrointestinal sites. Detailed dose and drug relation showed that bleeding with rivaroxaban was significantly higher, especially in recommended doses in patients with AF, and apixaban was related with significantly lower bleeding rates, especially in recommended doses. Diabetes mellitus, dyslipidemia, PAD, smoking, higher HAS-BLED score, dabigatran and rivaroxaban treatment, and higher doses of NOACs were the main predictors for bleeding, which also confirms HAS-BLED score efficiency. Bleeding events occurred in 7.6% of the patients in the NOAC-TURK study, which is similar to the results of the one-year follow up of the EORP-AF study (8.4%) (15). However, the number of bleeding events increased to 11% in the EORP-AF study in the second-year follow up. A lower dosage of NOAC usage could be the reason of facing lower number of bleeding events in our study.
The incidence of embolic events was which is less than previous large clinical trials (17–19). Embolic events with apixaban were significantly higher in these patients, especially in lower doses, whereas rivaroxaban was related with significantly lower embolic events. Logistic regression analysis for predicting embolic complication revealed that apixaban and lower doses of NOACs treatment were two important main predictors. Larsen et al. (20) showed that compared with warfarin, rivaroxaban was related to lower embolic events, and dabigatran and apixaban had similar rates with warfarin. One-year embolic event rates were the highest (4.86%) in the apixaban group in their nationwide cohort study. The embolic event rate was higher in the apixaban group, but it was only 2.24% in our study. The EORP-AF study (15) had higher incidences for embolic events—9.1% for the first-year follow up and 11.6% for the second-year follow up—whereas only 1.3% of our study population had embolic events. We therefore suggested that low embolic complication rate may be due to a lack of follow-up data in our study. Moreover, a heightened effect of apixaban for embolic complications in multiple analyses was due to a high incidence of use of lower doses in all indications.
This study supports the widespread use of NOACs for the prevention of embolic complications in the case of NVAF, DVT, and PE in the daily practice of cardiologists in our country. The RAMSES (8) and the AFTER (14) studies are other large-scale, important cross-sectional observational studies on Turkish populations. Moreover, the NOAC-TURK study, which gives incremental data for the prevention of embolic complications, has some unique features, including both cross-sectional and short-term follow-up data with more wide indications.
The mean age and female dominance were the same as previous observational studies (8, 14–16). Hypertension was the most common etiology for NVAF, which was concordant with previous observational studies (8, 14–16) and randomized controlled trials (17–19). However, the previous incidence of stroke or TIA was lower than both the RAMSES and AFTER studies (8, 14). Permanent AF was the most common indication for prescribing NOACs in our study, which is similar to previous observational studies (8, 14–16). The reason for this majority of AF indication could be because this study was conducted in cardiology clinics. The mean CHA2DS2-VASc score was similar to the RAMSES and the AFTER studies (8, 14).
The disadvantages of warfarin lead to a decreased use of OAC therapy worldwide (8). While a single center study reported a 30.1% incidence of OAC use for AF (21), a tertiary center study presented increased data (67.3%) (22). The RAMSES and the AFTER studies reported incidences for OAC of 72% and 40%, respectively (8, 14). In our study, the rate of previous warfarin use was 48.6% and dabigatran 110 mg was the most commonly prescribed NOAC in patients with NVAF. The main reason for switching warfarin to NOACs may be because of practicability and strong preventive evidence of these new drugs in NVAF patients. Concordant with previous studies, NOACs were preferred over warfarin for embolic complications in patients with NVAF in this study, despite recently emerging in the market of these drugs. Moreover, as with the RAMSES study (8), dabigatran was the most frequently used NOAC in our study, followed by rivaroxaban and apixaban. The highest incidence of dabigatran could be because it was the first NOAC registered by the Ministry of Health in Turkey. However, interestingly, discordant with other previous studies, a 110-mg dose of dabigatran was preferred more frequently than a 150-mg dose for NVAF patients in our population. While a 2.5-mg dose of apixaban was used in only 4.7% of the patients in the ARISTOTLE study (19), 7.1% of the patients in the present study—almost one-third of all patients—used low-dose apixaban. Limited RCT and observational data about efficacy and safety of NOACs may affect physicians’ choice toward a low-dose regimen in the setting of NVAF in our study, which is different from previous studies.
The patients who were on antiplatelet therapy for stable CHD, chronic ischemic heart disease had increased CHA2DS2-VASc and HASBLED scores, which is a big concern in terms of bleeding risk in using NOAC in the setting of AF. Hence, the need for combination therapy should be assessed according to risk–benefit considerations. In our study, the rate of combination therapy was lower than that in previous studies (8, 14–16), which was detected in 12.9% of the patients.
The concomitant use of all rate control/lowering drugs (beta-blockers, non-dihydropridine calcium channel blockers, and digoxin) were higher in the AFTER (14) and RAMSES (8) study populations compared with that in our study’s population (45.5%, 58.7%, 63.3% for beta-blockers; 12.9%, 23.4%, 23.6% for non-dihydropyridine calcium channel blockers; and 11.3%, 27.7%, 20.5% for digoxin usage, respectively, for the NOAC-TURK, AFTER, and RAMSES studies). Moreover, while the concomitant usages of beta-blockers and digoxin are higher in the EORP (15) study, non-dihydropyridine calcium channel blockers and digoxin usages were higher in the NOAC-TURK study.
In the AFTER study (14), the investigators found that only 41.3% of the population had effective INR levels. Furthermore, the mean time in therapeutic range reported 40.5% in RAMSES study (8). We also found that patients are treated with lower doses than clinically indicated. The most common cause was physicians’ neglect in the AFTER study for inadequate anticoagulation; in the NOAC-TURK study, misjudgment of patients’ clinical status could be the reason of treating patients with lower doses.
Study limitations
There are some limitations in our study. Since this study has a cross-sectional design, which is a snapshot of study participants’ characteristics, it could not provide any data in terms of mortality and future embolic and bleeding adverse events. Moreover, the enrollment of patients was limited to only outpatient cardiology clinics. Hence, this study’s results do not extrapolate to all Turkish NOAC-using patients for different indications.
Conclusion
In conclusion, this multicenter cross-sectional study showed an important overview of the current NOACs treatment regimens in Turkey. Although embolic and bleeding complications are lower or similar to various studies, increased utilization of lower doses in this study should be addressed carefully. According to the results of this study, NOACs treatment should be guided through CHA2DS2-VASc and HASBLED scores to ensure more benefit and less adverse effects in NVAF patients.
Collaborators (15)*: Fatma Özpamuk Karadeniz1, Serkan Ünlü2, Ahmet Yanık3, Fatih Mehmet Uçar4, Hakan Duman5, Uğur Canpolat6, Bayram Köroğlu7, Çağrı Yayla8, Kazım Serhan Özcan9, Yalçın Velibey10, Okan Gülel11, Mehmet Emin Kalkan12, Gürkan Karaca13, Mehmet Kadri Akboğa8, Serkan Akdağ14, Mehmet Eren10, Mahmut Şahin11, Seçkin Pehlivanoğlu15
*NOAC-TURK Study Group (by the number of patients enrolled): 1Department of Cardiology, Balıklıgöl State Hospital, Şanlıurfa, 2Department of Cardiology, Faculty of Medicine, Gazi University, Ankara, 3Department of Cardiology, Samsun Training and Research Hospital, Samsun, 4Department of Cardiology, Denizli State Hospital, Denizli, 5Department of Cardiology, Faculty of Medicine, Recep Tayyip Erdoğan University, Rize, 6Department of Cardiology, Faculty of Medicine, Hacettepe University, Ankara, 7Department of Cardiology, Bingöl State Hospital, Bingöl, 8Department of Cardiology, Yüksek İhtisas Training and Research Hospital, Ankara, 9Department of Cardiology, Derince Training and Research Hospital, Kocaeli, 10Department of Cardiology, Dr. Siyami Ersek Thoracic and Cardiovascular Surgery Training and Research Hospital, İstanbul, 11Department of Cardiology, Faculty of Medicine, Ondokuz Mayıs University, Samsun, 12Department of Cardiology, Koşuyolu Training and Research Hospital, Istanbul, 13Department of Cardiology, Osmancık State Hospital, Çorum, 14Department of Cardiology, Faculty of Medicine, Yüzüncü Yıl University, Van, 15Department of Cardiology, Faculty of Medicine, Başkent University, İstanbul
Acknowledgements
We would like to acknowledge Haluk Dülger for developing electronic database and editorial support of this manuscript and we would like to acknowledge Mehmet Erdem Memetoğlu MD, Halil İbrahim Erdoğan for contribution to data collection.
Appendix
Appendix 1.
Participating researchers and centers in the NOAC-TURK study (in order of city name)
| Researcher’s name | Center | Province | Patient number |
|---|---|---|---|
| Çağrı Yayla | Yüksek İhtisas Training and Research Hospital | Ankara | 78 |
| Mehmet Kadri Akboğa | 35 | ||
| Serkan Ünlü | Gazi University Faculty of Medicine | Ankara | 168 |
| Uğur Canpolat | Hacettepe University Faculty of Medicine | Ankara | 100 |
| Bayram Köroğlu | Bingöl State Hospital | Bingöl | 83 |
| Gürkan Karaca | Osmancık State Hospital | Çorum | 47 |
| Fatih Mehmet Uçar | Denizli State Hospital | Denizli | 105 |
| Servet Altay | Edirne State Hospital | Edirne | 256 |
| Lütfü Aşkın | Palandöken State Hospital | Erzurum | 230 |
| Özge Özden Tok | Bakırköy Sadi Konuk Training and Research Hospital | İstanbul | 191 |
| Ümit Yaşar Sinan | İstanbul University, Cardiology Institute | Istanbul | 197 |
| Mehmet Emin Kalkan | Kartal Koşuyolu Training and Research Hospital | İstanbul | 65 |
| Mehmet Erdem Memetoğlu | Dr. Siyami Ersek Thoracic and Cardiovascular Surgery Training and Research Hospital | İstanbul | 10 |
| Yalçın Velibey | 73 | ||
| Kazım Serhan Özcan | Derince Training and Research Hospital | Kocaeli | 77 |
| Halil İbrahim Erdoğan | NEU University, Meram Medical Faculty | Konya | 6 |
| Hüseyin Altuğ Çakmak | Kaçkar State Hospital | Rize | 255 |
| Hakan Duman | Recep Tayyip University, Faculty of Medicine | Rize | 101 |
| Okan Gülel | Ondokuz Mayıs University, Faculty of Medicine | Samsun | 101 |
| Ömer Gedikli | 150 | ||
| Ahmet Yanık | Samsun Training and Research Hospital | Samsun | 131 |
| Feyzullah Beşli | Harran University, Faculty of Medicine | Şanlıurfa | 200 |
| Fatma Özpamuk Karadeniz | Balıklıgöl State Hospital | Şanlıurfa | 172 |
| Serkan Akdağ | Yüzüncü Yıl University, Faculty of Medicine | Van | 31 |
Footnotes
Conflict of interest: None declared.
Funding sources: The study was funded by Turkish Society of Cardiology.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – S.A., S.P., Ö.Y., M.Ş.; Design – S.A., S.P.; Supervision – M.Ş., S.P., M.E.; Fundings – M.Ş., TSC.; Materials – NOACTURK Study Group; Data collection &/or processing – NOACTURK Study Group; Analysis &/or interpretation – S.A., A.Ç., Ö.Y., S.P.; Literature search – Ö.Y., A.Ç.; Writing – S.A., Ö.Y., A.Ç.; Critical review – S.P., Ö.Y.
References
- 1.Pistoia F, Sacco S, Tiseo C, Degan D, Ornello R, Carolei A. The epidemiology of atrial fibrillation and stroke. Cardiol Clin. 2016;34:255–68. doi: 10.1016/j.ccl.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Uyarel H, Onat A, Yüksel H, Can G, Ordu S, Dursunoğlu D. Incidence, prevalence and mortality estimates for chronic atrial fibrillation in Turkish adults. Arch Turk Soc Cardiol. 2008;36:214–22. [PubMed] [Google Scholar]
- 3.Anderson FA, Jr, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933–8. [PubMed] [Google Scholar]
- 4.Huang W, Goldberg RJ, Anderson FA, Kiefe CI, Spencer FA. Secular trends in occurrence of acute venous thromboembolism:the Worcester VTE study (1985–2009) Am J Med. 2014;127:829–39e5. doi: 10.1016/j.amjmed.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study) Lancet. 2004;363:1925–33. doi: 10.1016/S0140-6736(04)16405-2. [DOI] [PubMed] [Google Scholar]
- 6.Koton S, Schneider AL, Rosamond WD, Shahar E, Sang Y, Gottesman RF, et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312:259–68. doi: 10.1001/jama.2014.7692. [DOI] [PubMed] [Google Scholar]
- 7.Bassand JB, Accetta G, Camm AJ, Cools F, Fitzmaurice DA, Fox KAA, et al. Two-year outcomes of patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Eur Heart J. 2016 Jun 29; doi: 10.1093/eurheartj/ehw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Başaran O, Beton O, Doğan V, Tekinalp M, Aykan AC, Kalaycıoğlu E, et al. ReAl-life Multicenter Survey Evaluating Stroke prevention strategies in non-valvular atrial fibrillation (RAMSES study) Anatol J Cardiol. 2016;16:734–41. doi: 10.14744/AnatolJCardiol.2016.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 10.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Schulman S, Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–4. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 13.Hosmer D, Lemeshow S, May S. Model Building Strategies and Methods for Logistic Regression. In: Hosmer D, editor. Applied Logistic Regression. 2nd ed. New Jersey: John Wiley & Sons, Inc. Canaday; 2000. pp. 91–142. [Google Scholar]
- 14.Ertaş F, Kaya H, Yüksel M, Soydinç MS, Alan S, Ülgen MS. Atrial Fibrillation in Turkey: Epidemiologic Registry (AFTER) study design. Anatol J Cardiol. 2013;13:339–43. doi: 10.5152/akd.2013.073. [DOI] [PubMed] [Google Scholar]
- 15.Lip GY, Laroche C, Dan GA, Santini M, Kalarus Z, Rasmussen LH, et al. A prospective survey in European Society of Cardiology member countries of atrial fibrillation management: baseline results of EURObservational Research Programme Atrial Fibrillation (EORP-AF) Pilot General Registry. Europace. 2014;16:308–19. doi: 10.1093/europace/eut373. [DOI] [PubMed] [Google Scholar]
- 16.Proietti M, Laroche C, Opolski G, Maggioni AP, Boriani G, Lip GY, AF Gen Pilot Investigators 'Real-world'atrial fibrillation management in Europe: observations from the 2-year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase. Europace. 2016 May 18; doi: 10.1093/europace/euw112. [DOI] [PubMed] [Google Scholar]
- 17.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. and *the RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 18.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, and the ROCKET AF Steering Committee, for the ROCKET AF Investigators Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 19.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. for the ARISTOTLE Committees and Investigators Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 20.Larsen TB, Skjøth F, Nielsen PB, Kjældgaard JN, Lip GY. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016 Jun 16;:353–3189. doi: 10.1136/bmj.i3189. doi:10.1136/bmj.i3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karacaglar E, Atar I, Yetis B, Corut H, Ersoy B, Yilmaz K, et al. The frequency of embolic risk factors and adequacy of anti-embolic treatment in patients with atrial fibrillation: A single tertiary center experience. Anatol J Cardiol. 2012;12:384–90. doi: 10.5152/akd.2012.123. [DOI] [PubMed] [Google Scholar]
- 22.Ertas F, Duygu H, Acet H, Eren NK, Nazli C, Ergene AO. Oral anticoagulant use in patients with atrial fibrillation. Arch Turk Soc Cardiol. 2009;37:161–7. [PubMed] [Google Scholar]


