Abstract
An injectable local anesthetic producing repeatable on-demand nerve block would be desirable for pain management. Here we present a phototriggerable device to achieve repeatable and adjustable on-demand local anesthesia in superficial or deep tissues, consisting of gold nanorods attached to low temperature sensitive liposomes (LTSL). The particles were loaded with tetrodotoxin and dexmedetomidine. Near infrared light (NIR, 808 nm, continuous wave) could heat GNRs at low fluence (short duration and low irradiance), leading to rapid release of payload. In vivo, 1–2 minutes of irradiation at ≤ 272 mW/cm2 produced repeatable and adjustable on-demand infiltration anesthesia or sciatic nerve blockade with minimal toxicity. The nerve block intensity and duration correlated with the irradiance and duration of the applied light.
Keywords: Phototriggering, Liposome, Gold nanorod, Local anesthesia, Tetrodotoxin
Graphical Abstract
Local treatment of pain can allow analgesia without sedation and other side effects.1 However, local anesthetics are of relatively short duration, require an injection, and – in the case of specific nerve blocks – can require skill and training. Considerable progress has been made in extending the duration of single injections of local anesthetics, by use of synergistic drug combinations and/or sustained release technology.2–6 However, once established, the resulting nerve blocks remain in place until they wear off – they cannot be changed in response to changes in the patient’s condition or needs. It would be desirable to develop on-demand local anesthetics that can be initiated by a single injection then produce repeated adjustable analgesia.
Recently, we reported liposomes containing tetrodotoxin (TTX) and dexmedetomidine (DMED) that could provide adjustable on-demand local anesthesia in vivo, triggered remotely by near infrared (NIR; 808 nm) light.7 We had used TTX, an ultrapotent local anesthetic that blocks site 1 on the outer surface of nerve sodium channels,8–11 because unlike commercially available amino-amide and amino-ester local anesthetics, tissue toxicity from TTX after injection at peripheral nerves can be minimal,12 even when delivered for prolonged periods.5 We had used DMED because it can significantly prolong local anesthesia over that from TTX alone.7, 13
The triggerability of the liposomes in response to NIR light was conferred by gold nanorods conjugated to their surfaces, which would heat when irradiated with NIR light, inducing a phase transition in the lipid bilayers, with consequent release of the encapsulated payload. We7, 14, 15 and others16–18 have explored near infrared (NIR) light for triggerable drug delivery since it can penetrate into soft tissues more efficiently than can other wavelengths.19, 20 However, that light can be significantly attenuated with progressive depth,15, 21 while simply increasing the irradiance can result in tissue injury.15 Consequently, it is important to render the triggerable systems more responsive to the light that reaches it.
We hypothesized that the gold-modified liposome formulation could be made effectively more sensitive to NIR light by making the drug reservoir component more sensitive to heat. To that end we modified the liposomes as has been described for low-temperature-sensitive liposomes (LTSL),22, 23 by incorporating a lipid formulation which forms nanopores upon heating (Please see Methods section for detailed discussion of the formulations and Table S1). A key component of the LTSLs was 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine (MSPC) which is released at mild hyperthermia, forming nanopores through which the payload can escape. PEG2000-DSPE will stabilize the nanopores to achieve rapid release of the payload.22
Here we sought to validate the hypothesis. Thus, Gold nanorods (LTSL-GNRs) were conjugated to the LTSLs to achieve NIR-triggered repeatable on-demand local anesthesia with lower irradiances - which would translate into greater depth of tissue penetration, shorter irradiation, and/or improved safety.
Results and discussion
Synthesis and Characterization of LTSL-GNRs and DMED loaded liposomes (Lip-DMED)
The formulations to be injected consisted of two populations of liposomes to be injected singly or in combination: LTSLs and non-triggerable liposomes. The LTSLs could contain TTX but no GNRs (LTSL-TTX), or GNRs with or without TTX (LTSL-GNR-TTX, LTSL-GNR-0). DMED was encapsulated in separate non-triggerable liposomes (Lip-DMED). TTX and DMED were encapsulated separately because DMED significantly decreased the loading efficiency of TTX.
LTSLs were designed with the following lipid components (with molar ratio in parentheses): DPPC (64%), DPPG (21.5%), MSPC (9.7%), PEG2000-DSPE (3.8%) and biotin-PEG2000-DSPE (1%) for conjugation to the GNRs. The negatively charged lipid DPPG was included to enhance encapsulation of the cationic TTX.
Production of our previous TTX liposomal formulation involved heating during freeze-thaw cycles.5, 7,24 Since that would induce the release of MSPC, here biotinylated LTSL were synthesized by reverse-phase evaporation (see Methods), which can also achieve a high loading of hydrophilic chemicals.25 Biotin-PEG2000-DSPE was anchored in the lipid bilayer to conjugate with streptavidin-modified GNRs, forming LTSL-GNRs. The GNR content in LTSL-GNRs as measured by ICP-MS was 0.023 wt % (see Methods). Rhodamine 6G (R6G) or TTX were loaded in the internal aqueous phase of LTSL-GNRs at a concentration of 0.41 mg/mL and 123 μg/mL, respectively (forming particles abbreviated LTSL-GNR-R6G and LTSL-GNR-TTX; both had total lipid concentration of 35–37 mg/mL). The mean diameters of LTSL were ~0.7 μm, with median zeta potentials of ~−27 mV, irrespective of drug content or GNR conjugation. After storage at 4°C for one week, no leakage of TTX was detected by ELISA from LTSL-GNR-TTX.
Non-triggerable liposomes containing DMED (Lip-DMED) were produced with 1.2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 1.2-distearoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (DSPG), and cholesterol (molar ratio 6:2:3) by modified thin-film hydration as we have previously described (see Methods).5, 24 The liposomes were 2.6 μm in diameter, with −27.4 mV zeta potential. DMED was loaded in the interior aqueous phase of liposomes at a concentration of 284 μg/mL and a lipid concentration of 64 mg/mL. DMED was released from Lip-DMED over two weeks (Figure S1). Liposomes without drug (Lip-0) were prepared by the same procedure but without DMED.
The effect of temperature on the release of payloads was assessed with LTSL-GNR-R6G. R6G had very low fluorescence inside the particles, due to self-quenching,26 but fluoresced once released. LTSL-GNR-R6G were heated at a rate of 1°C/min in a quartz cuvette, and dye release was calculated from the fluorescence intensity (see Methods, Figure S2). Rapid release of R6G was observed at temperatures ranging from 39 °C to 42 °C, indicating that the LTSL-GNRs were sensitive to temperatures above but close to body temperature.
Cryo-electron microscopy image (cryo-EM, Figure 1A) showed GNRs associated with the liposomes. The release profile of TTX from LTSL-GNR-TTX was investigated at different temperatures (Figure 1B). TTX was rapidly released from LTSL-GNR-TTX when the temperature was ≥ 41 °C. Almost 100% of TTX was released within 5 minutes. The release rate of TTX increased in a temperature-dependent manner.
Figure 1.
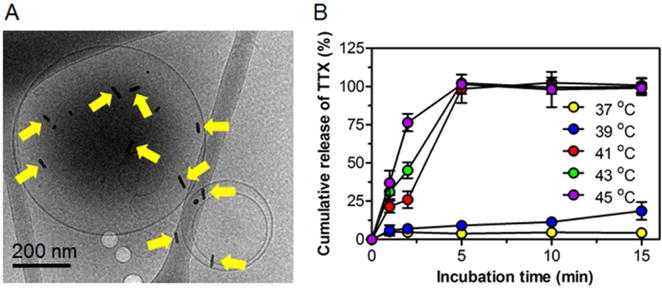
Characterization of low temperature sensitive liposomes conjugated to gold nanorods (LTSL-GNR-TTX). (A) Cryo-EM image displaying gold nanorods on liposomes (yellow arrows). (B) Effect of temperature on cumulative release of TTX from low temperature sensitive liposomes conjugated to gold nanorods (LTSL-GNR-TTX). Data are means ± SD (n = 4).
LTSL-GNR-TTX (18.5 mg/mL lipids) were incubated with C2C12 myotubes or PC12 cells over a four-day period (Figure S3). C2C12 and PC12 cells are commonly used in toxicological assays of muscle and nerve, respectively. The results indicated that LTSL-GNR-TTX were nontoxic to either cell line.
Phototriggered Release of TTX from LTSL-GNR-TTX
In the absence of irradiation, LTSL-GNR-TTX released ca. 19.3% of their TTX payload in 12 hours at 37°C, followed by much slower release (Figure S4). Subsequent studies of TTX release from irradiated LTSL-GNR-TTX were done in particles where the initial rapid release was first removed by dialysis at 37°C for 24 h, to reflect the in vivo reality that injected particles were only irradiated once the initial release would have resolved (see in vivo study below). Release of TTX was measured after repeated irradiation (1 minute for each cycle) at 25, 42 and 75 mW/cm2 (Figure 2). At low irradiances (25 and 42 mW/cm2), each irradiation event induced the release of 2–5% of TTX (Figure 2A). When the irradiance was increased to 75 mW/cm2, the first irradiation triggered the release of 19.2% of TTX, while the fourth irradiation only triggered the release of 7.5% of TTX. After a single 1-minute irradiation at 75 mW/cm2, the size of LTSL-GNR-TTX changed from 0.73 μm to two separate peaks (0.5 μm and 1.38 μm) as measure by DLS (Figure 2B), suggesting that NIR light irradiation could destroy the LTSL; the larger peak could be due to lipid aggregation.
Figure 2.
Effect of continuous irradiation with an 808 nm continuous wave NIR laser on lysolipid-containing thermosensitive liposomes conjugated with gold nanorods (LTSL-GNR-TTX). (A) Repeated release of TTX from repeated 1-minute irradiations at 25, 42 and 75 mW/cm2. Data are means ± SD (n = 4). Asterisks are from comparison of 75 mW/cm2 and 42 mW/cm2 or 75 mW/cm2 and 25 mW/cm2. *p < 0.05, **p < 0.01 and ***p < 0.001, NS indicates p > 0.05. (B) Size distribution of LTSL-GNR-TTX before and after a single irradiation (75 mW/cm2, 1 minute).
Phototriggered On-Demand Local Anesthesia
LTSL (optionally containing TTX) and non-triggerable liposomes (optionally containing DMED) were combined at a volume ratio of 1:1 and injected in a model of infiltration local anesthesia and a model of regional anesthesia (i.e. peripheral nerve block).
Infiltration anesthesia of the hindpaw
One hundred microliters of LTSL-GNR-TTX + Lip-DMED or LTSL-GNR-TTX + Lip-0 were injected subcutaneously into the plantar aspect of the rat left hindpaw under brief isoflurane/oxygen anesthesia; neurobehavioral testing was initiated after the animals woke up. Local anesthesia was assessed by noting the vocal or motor (foot withdrawal) response to mechanical stimulation of the rat footpad with a Touch Test sensory evaluator, and the duration of local anesthesia was calculated (see Methods).7 Injection was followed by initial local anesthesia with a median duration of 4.5 h (4.0–5.8, interquartile range) for LTSL-GNR-TTX + Lip-0 (Figure S5), and 16 h (12–16) h for LTSL-GNR-TTX + Lip-DMED (Figure 3 and Table S2), further confirming that co-delivery of TTX and DMED could prolong local anesthesia. Once daily for three days, starting 24 h after injection and after complete resolution of local anesthesia, both footpads were irradiated once a day for 1 minute. In vitro, LTSL-GNR-TTX could be activated at 25 mW/cm2 (Figure 2), but given that light from an 808 nm NIR laser could be greatly attenuated by rat skin ex vivo.15 the laser irradiance was increased to 272 mW/cm2. Each 1 minute irradiation event (done under isoflurane anesthesia) triggered local anesthesia in the footpad that had been injected with LTSL-GNR-TTX + Lip-DMED (Figure 3) or LTSL-GNR-TTX + Lip-0 (Figure S5 and Table S2) and had no effect of analgesia in the contralateral foot (suggesting a lack of systemically distributed TTX). LTSL-GNR-TTX + Lip-DMED produced longer phototriggered local anesthesia than did LTSL-GNR-TTX + Lip-0 over the first two irradiation events (Figure S5). Modulation of laser intensity (141 and 272 mW/cm2) allowed adjustment of the duration and intensity (%MPE) of phototriggered local anesthesia and the area under the curve (AUC) for those two parameters (Figure 3 and Table S2).
Figure 3.
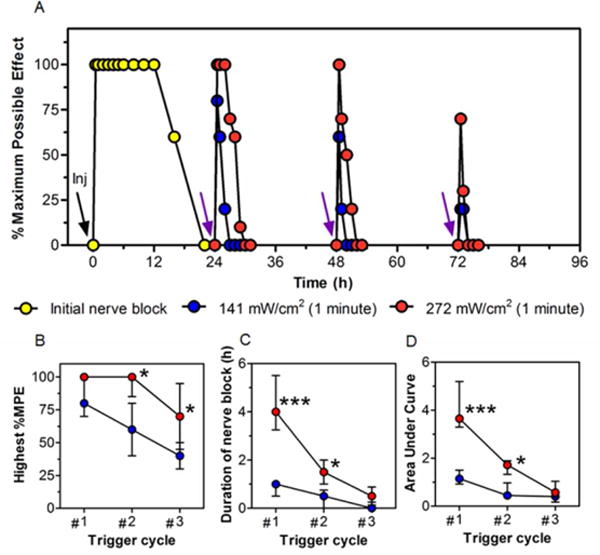
Phototriggered local anesthesia in the rat footpad. (A) Effect on local anesthesia of injection (black arrow labeled “Inj”) of 100 μL of LTSL-GNR-TTX + Lip-DMED and subsequent irradiation (purple arrows, 808 nm continuous wave NIR laser at 141 or 272 mW/cm2, for 1 minute). Local anesthesia is presented as % maximum possible effect; see Methods. Data are medians; Interquartile ranges are in Table S2 (n = 4 per group; for the initial local anesthesia, n = 8 for the 2 groups). (B) The highest %MPE and (C) the duration of local anesthesia after each triggering at different laser irradiances. (D) The AUC of the %MPE-time curves in panel A (see Methods). Data are medians with 25th and 75th percentiles in (B-D). Asterisks are from comparison of 272 mW/cm2 and 141 mW/cm2 *p < 0.05 and ***p < 0.001.
To confirm that triggered local anesthesia was mediated by heating of irradiated GNR, TTX loaded LTSL without gold nanorods were mixed with Lip-DMED (LTSL-TTX + Lip-DMED) and injected (100 μL total volume) into the rat footpad. The initial local anesthesia was of similar duration to that from LTSL-GNR-TTX + Lip-DMED (Figure S6, median duration of 12 h, p = 0.3464). Irradiation (272 mW/cm2 for 1 minute) 24 h after injection did not cause local anesthesia. To further show that localized heating itself was not responsible for local anesthesia, a similar experiment was done with LTSL-GNR-0 + Lip-DMED (no TTX). That combination did not cause local anesthesia, with or without irradiation.
The time course of local anesthesia could be altered by adjusting the timing of irradiation (Figure 4). For example, the duration of continuous local anesthesia could be extended from 14.5 (12.5–15) h to 22.5 (21–23) h by irradiating the footpads of rats injected with 100 μL LTSL-GNR-TTX +Lip-DMED whenever the %MPE dropped below 100%. That prolongation required three irradiation events at 272 mW/cm2 for 1 minute.
Figure 4.
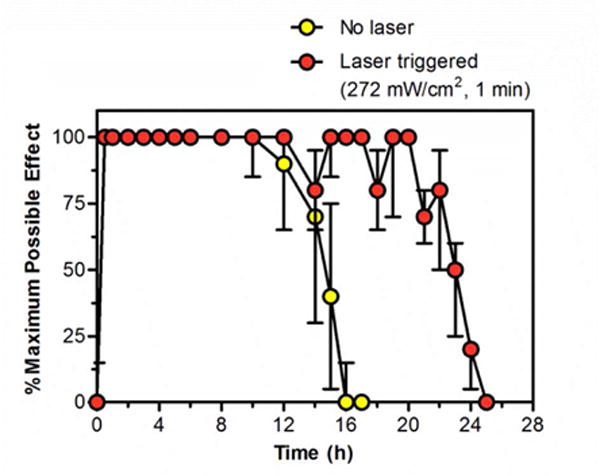
Prolongation of maximal local anesthesia in the rat footpad after injection of 100 μL of LTSL-GNR-TTX + Lip-DMED. Irradiation was with an 808 nm continuous wave NIR laser, 272 mW/cm2, 1 minute, and occurred whenever the %MPE dropped below 100%. Individual irradiation events are not shown since their timing varied from rat to rat. Local anesthesia is presented as % maximum possible effect; see Methods. Data are medians with 25th and 75th percentiles (n = 4).
Sciatic nerve blockade
Three hundred microliters of LTSL-GNR-TTX + Lip-DMED were injected at the sciatic nerve under brief isoflurane/oxygen anesthesia; neurobehavioral testing was initiated after the isoflurane wore off. The initial nerve block had a median duration of 37.4 (35.4–45) h (Figure 5 and Table S3). Once the thermal withdrawal latency in the left leg returned to < 7 s, the injection site was irradiated at 272 mW/cm2 (three cycles in total for each group) for either 1 (Figure 5a) or 2 (Figure 5b) minutes. Each irradiation event (under brief isoflurane/oxygen anesthesia) triggered sciatic nerve block (Figure 5 and Table S3) and had no effect on sensation in the contralateral leg (suggesting a lack of detectable systemic drug distribution). The duration of nerve blockade resulting from irradiation decreased with progressive triggering events, which was consistent with the decreasing triggered flux of TTX occurring with each cycle (Figure 2A). Two minutes of irradiation produced significantly longer block duration than did 1 minute, but only in the first irradiation event (p<0.001, Table S3).The total duration of local anesthesia achieved by this approach was 62 h; 14h of triggered block following 48 h of continuous nerve block.
Figure 5.
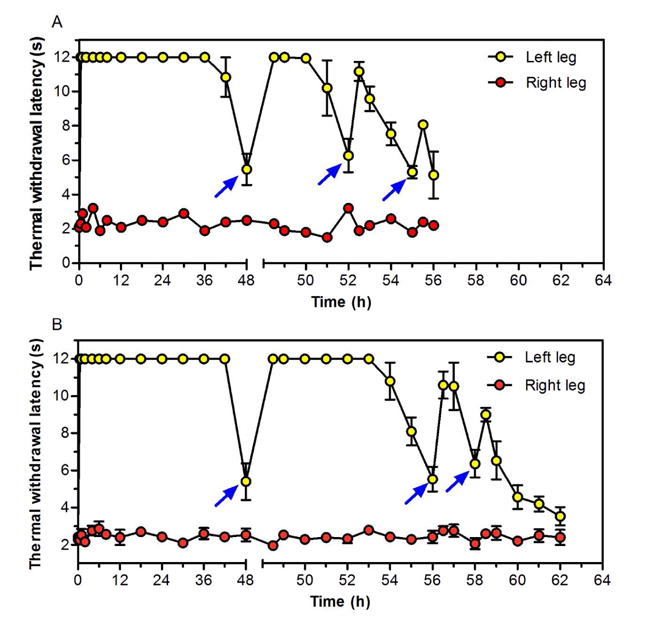
Representative sciatic nerve blocks in single animals treated with LTSL-GNR-TTX + Lip-DMED and subsequent irradiations. Note the discontinuity in the x-axis at 48h, to better demonstrate the triggered events. Arrows indicate irradiation with 808 nm continuous wave NIR laser at 272 mW/cm2 for (A) 1 minute or (B) 2 minutes. Laser irradiation was conducted once the thermal withdrawal latency of the left leg dropped below 7 seconds. Latency in the right (un-injected) leg is also shown.
Tissue reaction
Rats that received intraplantar injection of LTSL-GNR-TTX + Lip-DMED or LTSL-GNR-TTX + Lip-0 (100 μL) with or without 3 cycles of irradiation (272 mW/cm2, 1 minute) were euthanized 8 days after injection. The soft tissues of the footpads were removed and processed for histology. On light microscopy of hematoxylin-eosin stained sections (Figure S7), there was no injury to the skin and underlying tissues but there was inflammation at the injection site, with macrophages and lymphocytes, occurred in all groups, as is commonly seen with injected particles.27, 28 Foamy macrophages were observed, likely reflecting uptake of injected formulations.28, 29
Rats receiving LTSL-GNR-TTX + Lip-DMED at the sciatic nerve with or without 3 cycles of irradiation were euthanized 8 days after injection. The irradiated skin and sciatic nerve with surrounding tissues were dissected and processed into hematoxylin and eosin stained slides (Figure 6A). There was no perceptible damage to the skin, sciatic nerve or surrounding tissues irrespective of irradiation. Inflammation was observed at the injection sites where there was particle residue, with macrophages (many of them foamy) and lymphocytes in both treated groups.
Figure 6.
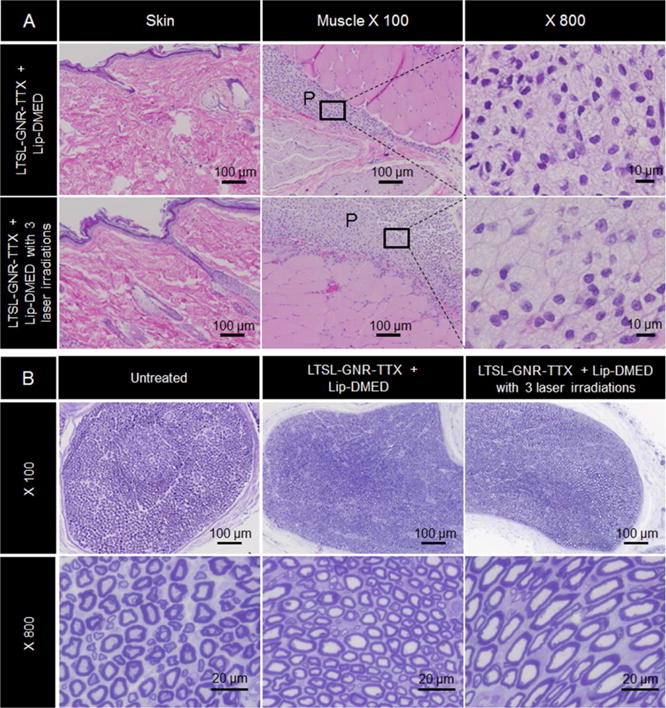
Tissue reaction. (A) Representative light microscopy of hematoxylin/eosin-stained sections of rat skin and muscle near the sciatic nerve 8 days after treatment with LTSL-GNR-TTX + Lip-DMED with or without three cycles of laser irradiation. There was no damage to the skin and underlying tissues (P denotes an area of particle deposition). (B) Toluidine blue stained sections of sciatic nerves harvested from untreated rats and from rats 8 days after treatment with LTSL-GNR-TTX + Lip-DMED with or without three cycles of laser irradiation.
Sciatic nerves were harvested from rats 8 days after injection of LTSL-GNR-TTX + Lip-DMED followed by 3 cycles of irradiation, and toluidine blue-stained sections of them were prepared. No changes in axonal density or myelin structure were observed (Figure 6B). The perineural tissue appeared normal.
Discussion
We have demonstrated an injectable system that can provide on-demand infiltration and regional anesthesia, in superficial and deep tissues respectively. Tissue reaction was benign, with mild inflammation consistent with the injection of particulate material.
This system could be appealing for treating prolonged postoperative pain. The initial nerve block would address the immediate perioperative period, including the duration of a procedure. Near infrared irradiation would subsequently allow on-demand analgesia. The on demand events could be triggered close together, providing continuous pain relief, or spread out. Moreover, the intensity of the analgesia could be adjusted by tuning the intensity and/or duration of irradiation. By providing patient control of pain relief, systems of this type could reduce the role that opioids play in perioperative pain management. This would be important given the frequent side effects of opioids,1 and their potential for addiction30 and diversion.31
The durations of pain relief afforded by the present system are likely too short to make them practical for the management of chronic pain. However, we and others have described reservoir-type devices which could in principle provide very long-term local anesthesia.15 A disadvantage of many such devices is that they often require implantation.
NIR light irradiation is a promising tool for triggering drug release in vivo.21, 32–34 However, high irradiances and/or prolonged irradiation times15 may cause burns. This concern is particularly relevant in devices that can be triggered repeatedly, or that are implanted deep within the body. In the latter case, attenuation of the NIR light by tissues could necessitate high irradiances and/or long durations of irradiation to actuate the device. It was therefore an important goal of this work to reduce the energy required for triggering; this would allow less energy to be used and/or shorter irradiances and/or deeper penetration. The present device was sensitive to 808 nm NIR light, so that low irradiances (≤ 272 mW/cm2) were required over short durations (1–2 minutes) for actuation, and no phototoxicity occurred. By way of comparison, we have found 150 mW/cm2 for 30 min to be safe when triggering an implanted device.15 The short irradiation is important in terms of patient convenience, and the rapidity with which pain relief can be expected.
Another reason that the adjustability of this system is important is that triggered release of TTX from our device would also impair motor function. (This would be true of essentially all local anesthetics.) The ability to modulate the degree of nerve block would allow patients to adjust their regimen in real time in accordance with their needs for movement (e.g. physical therapy).
Tissue reaction was benign, with only mild inflammation, which is characteristic of injected microparticles27. This biocompatibility was in part due to the use of TTX as a local anesthetic. Unlike conventional local anesthetics such as bupivacaine,28, 29, 35 site 1 sodium channel blockers such as TTX cause little or no inflammation,36, 37 myotoxicity,12 or neurotoxicity.38 There was no evidence of systemically distributed TTX, which would have manifested as neurobehavioral deficits in the un-injected extremity.3 This is important since systemic toxicity from TTX can result in respiratory failure. Liposomal encapsulation of site 1 sodium channel blockers can control their systemic distribution very effectively.5, 7,24
We have developed a sensitive externally triggerable device for adjustable on-demand pain relief. The intensity, duration, and timing of local anesthesia could be modulated in both superficial and deep tissues by changing the parameters of irradiation. Tissue reaction (myotoxicity, neurotoxicity, inflammation) was benign.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant GM116920 (to D.S.K).
Footnotes
Supporting information
Methods, the results of release kinetics of DMED from Lip-DMED, Effect of temperature on cumulative release of R6G from LTSL-GNR-R6G, in vitro cytotoxicity assays, release kinetics of TTX from LTSL-GNR-TTX, effect of laser irradiation on local anesthesia of the footpad of injection of LTSL-GNR-TTX + Lip-0 and LTSL-GNR-TTX + Lip-DMED, effect of laser irradiation on local anesthesia of the footpad after injection of LTSL-TTX + Lip-DMED or LTSL-GNR-0 + Lip-DMED, histology studies, the comparison of liposomal formulations, and the list of the duration of local anesthesia in the rat footpad and sciatic nerve.
Author contributions
C.Z. and D.S.K. designed research; C.Z., W.W., C.S., B.W., A.R. and B.P.T. performed research; C.Z., W.W. and D.S.K analyzed data; and C.Z., W.W., B.P.T. and D.S.K. wrote the paper.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Pain physician. 2008;11(2 Suppl):S105–20. [PubMed] [Google Scholar]
- 2.Adams HJ, Blair MR, Jr, Takman BH. Anesth Analg. 1976;55(4):568–73. [PubMed] [Google Scholar]
- 3.Kohane DS, Yieh J, Lu NT, Langer R, Strichartz GR, Berde CB. Anesthesiology. 1998;89(1):119–31. doi: 10.1097/00000542-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 4.McAlvin JB, Kohane DS. Prolonged Duration Local Anesthesia. In: Domb JA, Khan W, editors. Focal Controlled Drug Delivery. Springer US; Boston, MA: 2014. pp. 653–677. [Google Scholar]
- 5.Epstein-Barash H, Shichor I, Kwon AH, Hall S, Lawlor MW, Langer R, Kohane DS. Proc Natl Acad Sci U S A. 2009;106(17):7125–30. doi: 10.1073/pnas.0900598106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lobo K, Donado C, Cornelissen L, Kim J, Ortiz R, Peake RW, Kellogg M, Alexander ME, Zurakowski D, Kurgansky KE, Peyton J, Bilge A, Boretsky K, McCann ME, Berde CB, Cravero J. Anesthesiology. 2015;123(4):873–85. doi: 10.1097/ALN.0000000000000831. [DOI] [PubMed] [Google Scholar]
- 7.Zhan C, Wang W, McAlvin JB, Guo S, Timko BP, Santamaria C, Kohane DS. Nano Lett. 2016;16(1):177–81. doi: 10.1021/acs.nanolett.5b03440. [DOI] [PubMed] [Google Scholar]
- 8.Catterall WA. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- 9.Terlau H, Heinemann SH, Stuhmer W, Pusch M, Conti F, Imoto K, Numa S. FEBS Lett. 1991;293(1–2):93–6. doi: 10.1016/0014-5793(91)81159-6. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz JR, Ulbricht W, Wagner HH. The Journal of physiology. 1973;233(1):167–94. doi: 10.1113/jphysiol.1973.sp010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang GK. The Journal of general physiology. 1990;96(5):1105–27. doi: 10.1085/jgp.96.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padera RF, Tse JY, Bellas E, Kohane DS. Muscle Nerve. 2006;34(6):747–53. doi: 10.1002/mus.20618. [DOI] [PubMed] [Google Scholar]
- 13.McAlvin JB, Zhan C, Dohlman JC, Kolovou PE, Salvador-Culla B, Kohane DS. Invest Ophthalmol Vis Sci. 2015;56(6):3820–6. doi: 10.1167/iovs.15-16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rwei AY, Lee JJ, Zhan C, Liu Q, Ok MT, Shankarappa SA, Langer R, Kohane DS. Proc Natl Acad Sci U S A. 2015;112(51):15719–24. doi: 10.1073/pnas.1518791112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timko BP, Arruebo M, Shankarappa SA, McAlvin JB, Okonkwo OS, Mizrahi B, Stefanescu CF, Gomez L, Zhu J, Zhu A, Santamaria J, Langer R, Kohane DS. Proc Natl Acad Sci U S A. 2014;111(4):1349–54. doi: 10.1073/pnas.1322651111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu G, Mikhailovsky A, Khant HA, Fu C, Chiu W, Zasadzinski JA. J Am Chem Soc. 2008;130(26):8175–7. doi: 10.1021/ja802656d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keurentjes JT, Kemmere MF, Bruinewoud H, Vertommen MA, Rovers SA, Hoogenboom R, Stemkens LF, Peters FL, Tielen NJ, van Asseldonk DT, Gabriel AF, Joosten EA, Marcus MA. Angew Chem Int Ed Engl. 2009;48(52):9867–70. doi: 10.1002/anie.200904172. [DOI] [PubMed] [Google Scholar]
- 18.Rwei AY, Wang W, Kohane DS. Nano Today. 2015;10(4):451–467. doi: 10.1016/j.nantod.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simpson CR, Kohl M, Essenpreis M, Cope M. Phys Med Biol. 1998;43(9):2465–78. doi: 10.1088/0031-9155/43/9/003. [DOI] [PubMed] [Google Scholar]
- 20.Weissleder R. Nat Biotechnol. 2001;19(4):316–7. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 21.Timko BP, Kohane DS. Expert opinion on drug delivery. 2014;11(11):1681–5. doi: 10.1517/17425247.2014.930435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Needham D, Park JY, Wright AM, Tong J. Faraday Discuss. 2013;161:515–34. doi: 10.1039/c2fd20111a. discussion 563–89. [DOI] [PubMed] [Google Scholar]
- 23.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. Cancer Res. 2000;60(5):1197–201. [PubMed] [Google Scholar]
- 24.Shankarappa SA, Tsui JH, Kim KN, Reznor G, Dohlman JC, Langer R, Kohane DS. Proc Natl Acad Sci U S A. 2012;109(43):17555–60. doi: 10.1073/pnas.1214634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szoka F, Jr, Papahadjopoulos D. Proc Natl Acad Sci U S A. 1978;75(9):4194–8. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magde D, Wong R, Seybold PG. Photochemistry and photobiology. 2002;75(4):327–34. doi: 10.1562/0031-8655(2002)075<0327:fqyatr>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Anderson JM. Eur J Pharm Biopharm. 1994;40(1):1–8. [Google Scholar]
- 28.Kohane DS, Lipp M, Kinney RC, Anthony DC, Louis DN, Lotan N, Langer R. Journal of biomedical materials research. 2002;59(3):450–9. doi: 10.1002/jbm.1261. [DOI] [PubMed] [Google Scholar]
- 29.McAlvin JB, Padera RF, Shankarappa SA, Reznor G, Kwon AH, Chiang HH, Yang J, Kohane DS. Biomaterials. 2014;35(15):4557–64. doi: 10.1016/j.biomaterials.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juurlink DN, Dhalla IA. Journal of medical toxicology: official journal of the American College of Medical Toxicology. 2012;8(4):393–9. doi: 10.1007/s13181-012-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, Green JL. N Engl J Med. 2015;372(3):241–8. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal A, Mackey MA, El-Sayed MA, Bellamkonda RV. ACS Nano. 2011;5(6):4919–26. doi: 10.1021/nn201010q. [DOI] [PubMed] [Google Scholar]
- 33.Dai Y, Xiao H, Liu J, Yuan Q, Ma P, Yang D, Li C, Cheng Z, Hou Z, Yang P, Lin J. J Am Chem Soc. 2013;135(50):18920–9. doi: 10.1021/ja410028q. [DOI] [PubMed] [Google Scholar]
- 34.You J, Shao R, Wei X, Gupta S, Li C. Small. 2010;6(9):1022–31. doi: 10.1002/smll.201000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padera R, Bellas E, Tse JY, Hao D, Kohane DS. Anesthesiology. 2008;108(5):921–8. doi: 10.1097/ALN.0b013e31816c8a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simons EJ, Bellas E, Lawlor MW, Kohane DS. Molecular pharmaceutics. 2009;6(1):265–73. doi: 10.1021/mp800167a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohane DS, Langer R. Chemical Science. 2010;1(4):441–446. [Google Scholar]
- 38.Sakura S, Bollen AW, Ciriales R, Drasner K. Anesth Analg. 1995;81(2):338–46. doi: 10.1097/00000539-199508000-00023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


