Abstract
Objective:
The goal of the present study was to investigate the effects of 5-lipoxygenase (5-LOX) inhibition, alone and with cyclooxygenase (COX) inhibitors, on inflammatory parameters and apoptosis in ischemia/reperfusion (I/R)-induced myocardial damage in rats. For this purpose, zileuton, a selective and potent inhibitor of 5-LOX, resulting in suppression leukotriene production, was used.
Methods:
Male Wistar rats (200-250 g; n=12 per group) were used in the study. I/R was performed by occluding the left coronary artery for 30 minutes and 2 hours of reperfusion of the heart. Experimental groups were I/R group, sham I/R group, zileuton (5 mg/kg orally, twice daily)+I/R group, zileuton+indomethacin (5 mg/kg intraperitoneally)+I/R group, zileuton+ketorolac (10 mg/kg subcutaneously)+I/R group, and zileuton+nimesulide (5 mg/kg subcutaneously)+I/R group. Following I/R, blood samples were collected to measure tumor necrosis factor alpha (TNF-α), and left ventricles were excised for evaluation of microscopic damage; malondialdehyde (MDA), glutathione, nuclear factor (NF)-κB assays; and evaluation of apoptosis.
Results:
Left ventricle MDA in I/R group was higher compared to sham group; however, it did not show significant change with zileuton. Although tissue injury in I/R group was less severe in all treatment groups, it was not statistically significant. NF-κB H-score and apoptotic index, which were higher in I/R group compared to sham I/R, were decreased with application of zileuton (H-score: p<0.01; apoptotic index: p<0.001). Zileuton had no significant effect on increased serum TNF-α levels in I/R group.
Conclusion:
5-LOX inhibition in rat myocardial infarction model attenuated increased left ventricle NF-κB expression and apoptosis and these actions were not modulated by COX inhibitors. (Anatol J Cardiol 2017; 17: 269-75)
Keywords: cyclooxygenase, ischemia/reperfusion, myocardial infarction, rat, zileuton, 5-lypoxygenase
Introduction
Oxidative stress and tissue inflammation are the main pathophysiological processes involved in ischemia/reperfusion (I/R) injury of the myocardium and contribute to increased cardiovascular morbidity and mortality. Acute myocardial infarction is due to death of myocardial cells caused by thrombotic occlusion of the coronary artery. Therapeutic coronary artery recanalization by thrombolytic therapy or percutaneous coronary intervention is used in clinical medicine to minimize infarct size. However, reoxygenation of ischemic heart may lead to irreversible loss of myocardial function via signaling molecules such as reactive oxygen species (ROS), which are able to damage several proteins, lipids, and deoxyribonucleic acid (1).
Leukotrienes (LT) are metabolites of arachidonic acid (AA) formed from the 5-lipoxygenase (5-LOX) pathway. They exhibit a number of biological effects such as contraction of smooth muscles, especially bronchoconstriction, increased vascular permeability, and migration of leukocytes to areas of inflammation. 5-LOX acts with 5-lipoxygenase-activating protein (FLAP) to form leukotriene A4 (LTA4), which is converted to either LTB4 or the cysteinyl LTs, LTC4, LTD4, and LTE4. LTs induce a variety of responses including chemotaxis of leukocytes, smooth muscle contraction, and increase in vascular permeability. They play a pivotal role in pathophysiology of various clinical conditions including asthma and psoriasis, as well as conditions associated with I/R of the skin, brain, and kidney (2). LTB4 is known to increase after experimental myocardial infarction in the rat, and also in patients with cardiac ischemia (3,4). Moreover, antileukotriene drugs have demonstrated cardioprotective effects in rat, rabbit, and dog models. The 5-LOX inhibitor nafazatrom was shown to reduce myocardial infarct size in dogs and LTB4 antagonism protected the myocardium in a rabbit model of myocardial infarction (5, 6). In contrast, LT antagonists LY 255283 and FLP-55712 were found to be ineffective in cardiac ischemia in canines and rats, respectively (7, 8).
Cyclooxygenase (COX) enzymes are also involved in metabolism of AA. COX-1 is the constitutively expressed form found in most cells, and COX-2 is the inducible form rapidly induced by cytokines, growth factors, hormones, and oncogenes (9). Previous studies demonstrated induction of COX-2 in the myocardium of patients with ischemic heart disease and congestive heart failure (10). Saito et al. (11) reported that selective inhibition of COX-2 in rat model of myocardial infarction improved myocardial dysfunction.
It has been proposed that inhibition of COX pathway could enhance LT generation via increasing substrate for 5-LOX (12). That is, COX inhibition might lead to shunt of AA metabolism toward 5-LOX pathway. Hudson et al. (13) demonstrated enhanced synthesis of LTB4 in patients with rheumatoid arthritis under treatment with non-steroidal anti-inflammatory drugs for more than 3 months. Findings of an in vitro study conducted by Kwak et al. (14) demonstrated that 5-LOX inhibition by zileuton induced COX-2 expression and protected cardiomyocytes from H2O2-induced death and suggested that COX-2 is involved in protection by zileuton. This finding is of special interest as it describes a shunt from 5-LOX pathway to COX. Moreover, data of in vitro studies on rat peritoneal macrophages and human whole blood, and in vivo rat carregeenan-induced pleurisy model revealed that 5-LOX inhibition by zileuton inhibited prostaglandin production by interfering at the level of AA release (15). In light of these observations, it has been suggested that dual inhibition of 5-LOX and COX pathways might exert synergistic anti-inflammatory effects in various inflammatory settings. Thus, in the present study, we aimed to evaluate the effect of 5-LOX inhibition on extent of inflammatory reaction in I/R-induced myocardial damage and modulation of this effect by COX inhibition.
Methods
Animals
Male Wistar rats (200–250 g), receiving a standard diet and water ad libitum, were used for this study. The study protocol was approved by the Marmara University Animal Care and Use Committee.
Rats were randomized into 6 groups (n=12 per group): sham I/R group, I/R group, zileuton+I/R group, zileuton+indomethacin+I/R group, zileuton+ketorolac+I/R group, and zileuton+nimesulide+I/R group. 5-LOX inhibitor zileuton (5 mg/kg, orally twice daily) was given alone or with non-selective COX inhibitor indomethacin (5 mg/kg, intraperitoneally), selective COX-1 inhibitor ketorolac (10 mg/kg, orally) or selective COX-2 inhibitor nimesulide (10 mg/kg, subcutaneously). COX inhibitors were given 15 minutes before zileuton administration. All drugs were given for 3 days prior to I/R or sham I/R procedure. Dose of zileuton used in this study (5 mg/kg, twice daily) was previously demonstrated to be the effective dose to inhibit 5-LOX (16). Rats in sham I/R group received the vehicle of zileuton orally. Zileuton was dissolved in dimethyl sulfoxide (DMSO) and further dilutions were made using saline to achieve a final DMSO concentration of 1%.
Induction of myocardial infarction
As has been previously described, model was constructed by occluding the left coronary artery for 30 minutes followed by 2 hours of reperfusion, which closely simulates acute myocardial I/R injury (17, 18). In brief, rats were anesthetized intraperitoneally with 1 g/kg urethane. The heart was exposed through left thoracic incision and a 6–0 silk suture slip knot was placed around the left anterior descending coronary artery. After 30 minutes of ischemia, ligature was loosed and reperfusion was performed for 2 hours. During the course of the procedure, rats were ventilated (60–70 stroke/min) through tracheotomy using small animal ventilator (Ugo Basile Srl, Varese, Italy) and standard limb lead II of electrocardiogram (ECG) was continuously monitored using computerized data acquisition system (MP150 Data Acquisition System, Biopac Systems, Inc., CA, USA). Ischemia of myocardial tissue was confirmed by ST segment elevation in ECG recording and presence of cyanosis of ischemic area in the left ventricle. Reperfusion was confirmed by ST segment reversal and removal of left ventricular cyanosis (19). For sham I/R group, all operative procedures were performed identically, except for coronary artery ligation. At conclusion of reperfusion period, rats were euthanized via blood withdrawal though cardiac puncture. Blood samples were used to determine level of tumor necrosis factor alpha (TNF-α). Hearts were excised for biochemical and histological examinations. Six hearts in each group were used for determination of malondialdehyde (MDA) and glutathione content, and 6 hearts were used for histopathological examination, immunostaining, and evaluation of apoptosis.
Biochemical analysis and histological examination
Lipid peroxidation was estimated by measuring formation of MDA. Content of MDA and glutathione in left ventricle was detected using spectrophotometric methods, as described previously (20, 21).
Tissue samples were fixed for histological evaluation. Paraffin-embedded sections (5 µm thick) were deparaffinized with xylene and rehydrated with graded alcohol prior to hematoxylin and eosin staining. Sections were examined with microscope (BX51, Olympus, Tokyo, Japan). Five to 9 sections were randomly chosen from the left ventricle of each animal. From each section, 5 areas were randomly selected for histopathological examination. Histopathological score was determined by experienced histologist (F.E.) who was unaware of the treatment groups.
Semi-quantitative analysis of infarct size, hemorrhage, and leukocyte infiltration was scored as: none=0, weak=1, moderate=2, strong=3, and very strong=4. Score of 0 was defined as absence of infarct; score of 1 indicated infarct size of 1% to 25% of the area; score of 2 was defined as infarct size of 26% to 50%; score of 3 was used for infarct size of 51% to 75%; and score of 4 reflected infarct size >76% of the area examined. Leukocyte infiltration was scored as none/absent=0, weak/occasional (1–10 cells)=1, moderate/focal (10–50 cells)=2, and strong/focal (>50 cells) and/or diffuse=3 (22).
NF-kB immunostaining
Cardiac tissue samples were immersed in 10% neutral formal-dehyde for fixation and embedded in paraffin blocks. Sections (5 µm) were placed in 0.1% sodium citrate and 0.1% Triton X-100 for 4 minutes at 4°C. To recover antigens, citrate buffer (0.01 M; pH=6.0) was applied for 45 seconds in a microwave oven. Endogenous peroxidase activity was halted by incubating slides in 0.3% hydrogen peroxide solution for 30 minutes. Slides were blocked for nonspecific binding with Vectastain Universal Quick kit (RTU Vectastain; Vector Laboratories, Burlingame, CA, USA). Active NF-κB was probed using anti-p65 antibody (3987S; Cell Signaling Technology, Danvers, MA, USA) at 1:100 dilution. Finally, chromogen diaminobenzidine tetrahydrochloride (TA 125 TD; ThermoFisher Scientific, Leicestershire, UK) was used, which produces a brownish precipitate. Slides were counterstained for 10 minutes with Mayer’s hematoxylin. Tissue specimens were evaluated by an observer blinded to study groups (Ü.U.) using Leica DM6000B microscope (Leica Microsystems, Wetzlar, Germany). Immunohistochemical labeling was scored considering both intensity and distribution of specific staining. Intensity of staining was reported based on H-score method, using 0 for negative staining, 1+ for weak staining, 2+ for moderate staining, and 3+ for strong staining. From these semiquantitative estimates of immunostaining, H-score was derived according to modification of previously reported method (23). Formula provided below produces an H-score in the range of 0–300, where 300 equals 100% of NF-κB positive cells stained strongly (i.e., 3+). H-score = (% of cells stained at intensity category 1x1) + (% of cells stained at intensity category 2x2) + (% of cells stained at intensity category 3x3).
Immunohistochemistry processing and counting for apoptosis
Cardiac tissue samples were immersed in 10% neutral formaldehyde in 0.1 M phosphate buffered solution (pH=7.4). Paraffin-embedded sections of 5 µm thickness were stained using a modified terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) technique (24). Each section was fractionated optically using Stereo Investigator version 7.5 image analysis software (MBF Bioscience, Williston, VT, USA). In each frame, a counting area was designated without bias and apoptotic index was calculated as apoptotic cells/total cells.
Measurement of serum TNF-α level
After centrifugation of blood samples at 3,000 rpm/min for 15 minutes, supernatant was collected and stored at -80oC. Serum content of inflammatory cytokine TNF-α was measured using rat TNF-α immunoassay enzyme-linked immunosorbent assay kit (Assaypro LLC, St. Charles, MO, USA) according to manufacturer’s instructions.
Statistical analysis
Microscopic score data were analyzed using Mann-Whitney U test and expressed as median and interquartile range (IQR). Other data were analyzed using one-way analysis of variance followed by Tukey’s multiple comparison test and expressed as mean±SD. Values of p <0.05 were regarded as significant. Calculations were carried out using InStat statistical analysis package (GraphPad Software, San Diego, CA, USA).
Results
Biochemical analysis
MDA content of the left ventricle in I/R group was found to be markedly increased in comparison to sham I/R group (66.59±16.77 nmoL/g and 18.80±9.08 nmoL/g, respectively; p<0.001). Treatment of I/R group with zileuton did not cause significant change in this parameter (p=0.123); however, in zileuton+nimesulide-treated I/R group, MDA level was significantly lower compared to zileuton-treated I/R group (59.24±22.12 nmoL/g and 77.68±10.92 nmoL/g, respectively; p<0.05) (Fig. 1).
Figure 1.
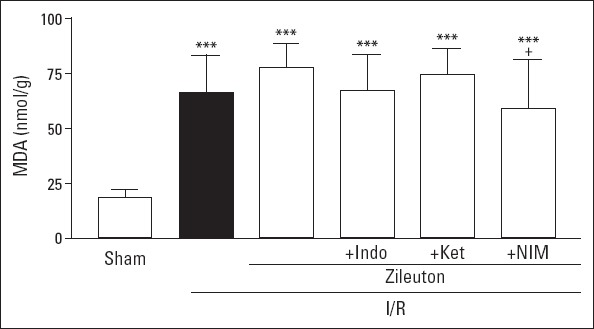
Left ventricle malondialdehyde (MDA) levels in experimental groups. Values are expressed as mean±SD (n=6). Indo - indomethacin; Ket - ketorolac; NIM - nimesulide. ***P<0.001 vs sham I/R group; +P<0.05 vs. I/R group
Glutatione content of the left ventricle in I/R group showed tendency to increase when compared to sham I/R group; however, this effect did not reach statistically significant level (0.27±0.10 µmol/g and 0.36±0.21 µmol/g, respectively; p=0.278). Except for zileuton+nimesulide-treated I/R group, in other treatment groups, tissue glutathione content values were not statistically different from that of I/R group (Fig. 2).
Figure 2.
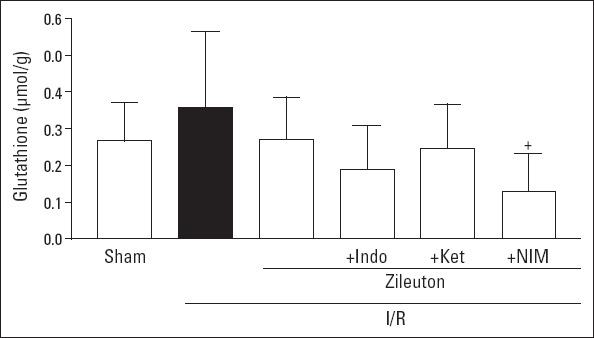
Left ventricle glutathione contents in experimental groups. Values are expressed as mean±SD (n=6). Indo - indomethacin; Ket - ketorolac; NIM - nimesulide. +P<0.05 vs. I/R group
Histological examination
Histological examination of left ventricle samples revealed focal hemorrhage, inflammatory cell infiltration, and disorganized muscle fiber architecture in I/R group. In zileuton-treated I/R group, microscopic score was lower (4.50; 4.00–7.00) in comparison to I/R group (8.00; 7.50–9.00); however, this did not reach statistically significant level (Table 1).
Table 1.
Left ventricle microscopic score values in experimental groups
| Experimental groups | Microscopic score |
|---|---|
| Sham I/R | 0.01 (0.05–0.10) |
| I/R | 8.00 (7.50–9.00)*** |
| Treatment groups | |
| Zileuton+I/R | 4.50 (4.00–7.00)** |
| Zileuton+indomethacin+I/R | 6.00 (8.00–4.50)** |
| Zileuton+ketorolac+I/R | 6.50 (4.50–7.50)** |
| Zileuton+nimesulide+I/R | 5.00 (3.00–7.00)** |
Values are expressed as median and interquartile range (n=6). I/R - ischemia/reperfusion.
P<0.01 and
P<0.001, vs. sham I/R group
NF-kB immunostaining
H-score as an indicator of NF-κB expression in heart tissue was found to be markedly higher in I/R group when compared to sham I/R group (21.67±3.83 and 1.25±0.72; p<0.001). In zileuton-treated I/R group, this value decreased to 15.50±1.70 (p<0.01). Effect of zileuton to decrease NF-κB expression in the left ventricle did not change significantly in the presence of COX inhibitors (Table 2). As demonstrated in Figure 3, cardiac muscle cells in I/R group had higher NF-κB staining intensity in comparison to sham I/R group. I/R group treated with zileuton revealed significantly lower level of NF-κB staining when compared to I/R group. Zileuton+COX inhibitor-treated I/R groups were comparable with zileuton-treated I/R group in terms of tissue NF-κB expression levels.
Table 2.
Left ventricle H-score values showing NF-κB immunostaining in experimental groups
| Experimental groups | H-score |
|---|---|
| Sham I/R | 1.25±0.72 |
| I/R | 21.67±3.83*** |
| Treatment groups | |
| Zileuton+I/R | 15.50±1.70***++ |
| Zileuton+indomethacin+I/R | 12.83±2.07***+++ |
| Zileuton+ketorolac+I/R | 15.65±2.84***+ |
| Zileuton+nimesulide+I/R | 15.16±3.84***++ |
Values are expressed as mean±SD (n=6). I/R - ischemia/reperfusion.
P<0.001 vs. sham I/R group;
P<0.05,
P<0.01,
P<0.001 vs. I/R group
Figure 3.
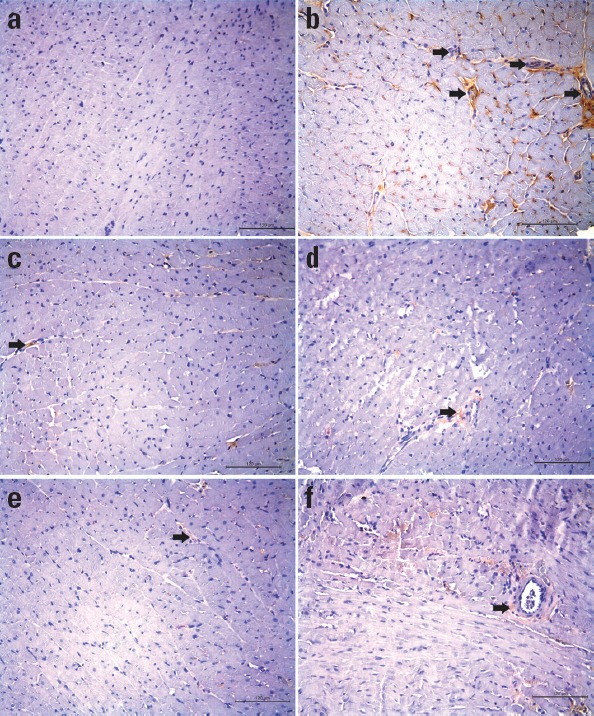
NF-κB immunohistochemical staining images of the representative heart sections from experimental groups (Sham I/R group, a; I/R group, b; zileuton+I/R group, c; zileuton+indomethacin+I/R group, d; zileuton+ketorolac+I/R group, e; and zileuton+nimesulide+I/R group, f). Black arrow indicates NF-κB positive cardiomyocytes. Original magnification 20 x. Scale bar represents 120 µm
Evaluation of apoptosis
Apoptotic index value of the left ventricle in I/R group was found to be higher in comparison to that of sham I/R group (0.23±0.02 and 0.05±0.01, respectively; p<0.001). Zileuton treatment given to I/R rats decreased apoptotic index significantly (0.11±0.01; p<0.001). Treatment with COX inhibitors did not seem to change the effect of zileuton on this parameter (Fig. 4).
Figure 4.
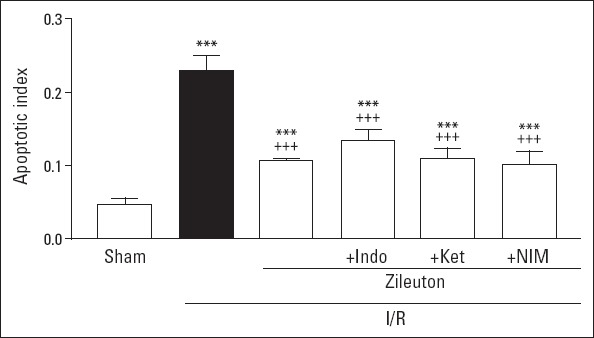
Left ventricle apoptotic index values in experimental groups. Values are expressed as mean±SD (n=6). Indo - indomethacin; Ket - ketorolac; NIM - nimesulide. ***P<0.001 vs. sham I/R group; +++P<0.001 vs. I/R group
Serum TNF-α levels
Myocardial I/R increased serum TNF-α levels, as demonstrated in Table 3. Serum TNF-α levels in sham I/R and I/R groups were 56.76±27.51 ng/mL and 461.00±283.10 ng/mL, respectively (p<0.05). High serum TNF-α level due to I/R did not seem to change with prior treatment with zileuton alone or along with COX inhibitors.
Table 3.
Serum TNF-α levels in experimental groups
| Experimental groups | TNF-α (ng/mL) |
|---|---|
| Sham I/R | 56.76±27.51 |
| I/R | 461.00±283.10* |
| Treatment groups | |
| Zileuton+I/R | 486.10±384.30* |
| Zileuton+indomethacin+I/R | 323.20±140.50** |
| Zileuton+ketorolac+I/R | 475.90±179.07*** |
| Zileuton+nimesulide+I/R | 470.70±239.80** |
Values are expressed as mean±SD (n=6). I/R - ischemia/reperfusion; TNF-α - tumor necrosis factor-α.
P<0.05,
P<0.01,
P<0.001 vs. sham I/R group
Discussion
In the present study, I/R was induced in rats by occluding the left descending coronary artery for 30 minutes and subsequently subjecting the heart to 2 hours of reperfusion. Effect of 5-LOX inhibition on myocardial I/R injury was investigated using selective 5-LOX inhibitor zileuton, which is currently used in therapy of patients suffering from asthma.
Occlusion of coronary arteries induces myocardial necrosis and restoration of blood flow during reperfusion can provoke further myocardial damage. In the present model, I/R group revealed marked focal hemorrhage, inflammatory cell infiltration, and disorganized muscle fiber architecture in the left ventricle. It is possible that this effect was partially mediated by postischemic, infiltrating inflammatory cells, in particular neutrophils, which induce tissue injury and necrosis through production and release of reactive oxygen metabolites and cytotoxic proteins.
LTs are known to be produced after cardiac injury (3,4). LTs are released by activated neutrophils following oxygenation of AA by the enzyme 5-LOX. Thus, 5-LOX mediates generation of ROS. ROS, because of their high chemical reactivity, lead to tissue injury, mainly due to enhanced lipid peroxidation. Lipid peroxides are metabolized via beta-oxidation and lead to formation of MDA and 4-hydroxynonenal (4-HNE). Thus, increased MDA in tissue, an end product of lipid peroxidation, reflects oxidative stress-induced damage. In our study, morphological disturbances observed in the myocardium of I/R group were accompanied by markedly increased MDA levels in the left ventricle. NF-kB, which is activated by ROS, is a key transcription factor for inducible expression of inflammatory cytokines (25). Indeed, in the present study, I/R group revealed increased NF-kB expression in the left ventricle and increased TNF-α levels in the blood. Additionally, immunostaining results demonstrated increased apoptosis in cardiac myocytes of I/R group. It has been demonstrated that cardiomyocytes undergo apoptosis as a consequence of oxidative stress (26). Hydrogen peroxide induces apoptosis in cultured cardiomyocytes by increasing expression of tumor suppressor transcription factor p53 and Bad, as well as release of cytochrome c (27).
The role of 5-LOX in the pathophysiology of I/R injury has been investigated in various experimental models. In mouse renal I/R model, Patel et al. (28) demonstrated that degree of renal dysfunction and tissue injury was preserved along with reduced intercellular adhesion molecule-1 expression and neutrophil infiltration in kidneys in 5-LOX knockout mice and by treatment with 5-LOX inhibitor zileuton. These findings were in accordance with those of a previous study demonstrating that zileuton reduced CD18 adhesion receptor expression on systemic neutrophils and attenuated I/R injury in guinea pig skin flaps (29). In rat brain damage induced by cerebral ischemia, zileuton was found to be effective in reducing brain damage via decreasing MDA content, and inhibition of NF-kB and inducible nitric oxide synthase (iNOS) expression (30). In contrast, findings of a study conducted by Kitagawa et al. (31) revealed that 5-LOX knockout mice were not protected against permanent and transient cerebral ischemia. Similarly, in 5-LOX-deficient mice that underwent 30 minutes of coronary artery ligation and 24 hours of reperfusion, ischemic tissue and inflammation response were not protected against cardiac I/R injury (32). Thus, in light of these observations, the authors suggest that the effect of LOX on I/R-induced injury might be organ specific. In our study, 5-LOX inhibition by zileuton was investigated in rat myocardial I/R model established by occluding the left coronary artery for 30 minutes followed by 2 hours of reperfusion of the myocardium. Morphological examination revealed slight protection as result of zileuton treatment in I/R group in comparison to untreated group when extent of hemorrhage, inflammatory cell infiltration, and muscle fiber architecture were taken into account. On the other hand, we observed marked suppression of NF-kB expression in the myocardium in this group. This finding is in agreement with findings of a study reported by Tu et al. (30) showing inhibition of expression of NF-kB in rats with permanent cerebral ischemia through zileuton treatment. It is known that NF-kB expression might be regulated by TNF-α, which attracts leukocytes to inflammatory sites, enhancing ROS generation. Although zileuton treatment was not effective in attenuating increased serum TNF-α levels in rats that underwent I/R in our study, this does not exclude the possibility that it might modulate TNF-α expression in the heart.
A recent in vitro study compared the direct free radical scavenging properties of the most widely used LOX inhibitors in rat cortex homogenates. Their results indicated that at concentrations higher than 5 µM, zileuton may protect against lipid peroxidation, and at high concentrations (50 µM) it significantly prevents carbonyl group formation, which is an indicator of protein oxidation (33). Although it is difficult to translate these findings into in vivo conditions, in our experimental model, high tissue lipid peroxidation (as assessed by MDA assay) in I/R group did not seem to change with zileuton treatment at a daily dose of 10 mg (40 mM)/kg. Lack of efficiency of zileuton on this parameter might be overcome by changing the dose of the drug and/or duration of treatment.
The other finding of the present study is suppression of apoptosis in myocardium by zileuton in I/R group. According to data of a previous in vitro study conducted by Kwak et al. (14), zileuton has a protective effect on cardiomyocytes via PI3K/Akt signaling pathway, which plays a crucial role in cell proliferation, differentiation, inflammation, and apoptosis. This finding was also supported by 2 in vivo studies. In mouse experimental spinal cord injury model, both 5-LOX inhibitor zileuton and LT receptor antagonist montelukast improved motor recovery and reduced tissue injury, cell infiltration at the injured site, and apoptotic cell death (as assessed by annexin V, TUNEL and Fas ligand staining) (34). More recently, Shi et al. (35) demonstrated that zileuton reduced the extent of brain damage and neuronal apoptosis in rats following middle cerebral artery occlusion via inhibition of caspase-1 and regulation of caspase-3. Although we are unable to explain the underlying pathway(s), our findings implying increased apoptosis in myocardial I/R and its suppression by zileuton are in accordance with previous observations.
In the heart, AA is converted by LOX to LTs and hydroxyeicosatetraenoic acids, and by COX to a variety of prostaglandins and thromboxanes. Cyclooxygenase-1 is present in most cells and is responsible for constitutive prostaglandin formation and COX-2 expression is induced in cardiomyocytes in response to stress, including ischemia (36). COX-1- or COX-2-knockout mice were suggested to be susceptible to cardiac I/R-induced injury (37). Recent investigations suggest that inhibition of 1 or both COX enzymes may lead to a shunt of AA metabolism toward 5-LOX pathway. On the other hand, data of Peters-Golden et al. (38) did not support the arachidonate shunt hypothesis, as they found that COX and 5-LOX utilize distinct arachidonate pools. Recently, a concomitant decrease of prostaglandin production has been observed in the presence of 5-LOX inhibitor zileuton (39, 40). In rat esophageal adenocarcinoma model, zileuton was found to be effective to suppress both increased LTB4 and prostaglandin E2 (PGE2) levels in esophageal tissues (39). In Syrian Golden hamsters with ductal pancreatic adenocarcinoma, treatment with zileuton decreased intrametastatic PGE2 concentration (40). In accordance with these observations, zileuton also inhibited prostaglandin production in mouse peritoneal macrophages and J774 macrophages, as well as in carrageenan-induced pleurisy model in rats (15). Therefore, it appears that anti-inflammatory actions of zileuton are not just restricted to inhibition of LT formation, but also involve inhibition of prostaglandins. In our study, we used non-selective and COX-1 and -2 selective inhibitors to examine possible role of COX inhibition in effects of zileuton on I/R-induced myocardial injury. However, according to our findings, COX inhibitors do not seem to modulate beneficial effects of zileuton on NF-κB expression and apoptosis. As we did not measure levels of COX and 5-LOX metabolites in this setting, it is not clear whether inhibiting 1 of the 2 pathways would shift the arachidonate metabolism toward the other. This issue needs to be re-evaluted in additional experimental models.
Study limitations
This study investigated the effect of 5-LOX inhibition on I/R-induced myocardial injury using zileuton. The antiapoptotic effect of 5-LOX inhibition in our experimental model is an interesting finding. The underlying mechanisms of this effect need further clarification. Additionally, effects of zileuton beyond 5-LOX inhibition should also be taken into consideration in experiments aiming to examine myocardial oxidant/antioxidant balance and extent of tissue damage following I/R.
Conclusion
To conclude, treatment of rats with 5-LOX inhibitor zileuton attenuated I/R-stimulated NF-κB expression and apoptosis in the left ventricle, and effects of zileuton were not modulated when I/R rats were administered COX enzyme inhibitors along with zileuton. This study, for the first time, explored the effect of zileuton on a rat model of myocardial infarction. These findings may provide the base for further studies to clarify the effectiveness of 5-LOX inhibition in I/R-related pathologies in both experimental and clinical settings.
Acknowledgements
The authors thank Marmara University Scientific Research Project Commission for the financial support of this study (SAG-C-YLP-150513-0152).
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – L.A., İ.A., Ü.U.; Design – L.A., İ.A., Ü.U.; Supervision – F.E., İ.A., Ü.U.; Funding – Ü.U., A.C., A.V.Ö., F.E.; Materials – Ü.U., F.E., A.V.Ö., A.C.; Data collection &/or processing – L.A., A.C., A.V.Ö.; Analysis and/or interpretation – L.A., İ.A., Ü.U.; Literature review – L.A., İ.A.; Writing – İ.A.; Critical review – İ.A.
References
- 1.Bagatini MD, Martins CC, Battisti V, Gasparetto D, da Rosa CS, Spanevello RM, et al. Oxidative stress versus antioxidant defenses in patients with acute myocardial infarction. Heart Vessels. 2011;26:55–63. doi: 10.1007/s00380-010-0029-9. [DOI] [PubMed] [Google Scholar]
- 2.Henderson WR. The role of leukotrienes in inflammation. Ann Intern Med. 1994;121:684–97. doi: 10.7326/0003-4819-121-9-199411010-00010. [DOI] [PubMed] [Google Scholar]
- 3.Sasaki K, Ueno A, Katori M, Kikawada R. Detection of leukotriene B4 in cardiac tissue and its role in infarct extension through leucocyte migration. Cardiovasc Res. 1988;22:142–8. doi: 10.1093/cvr/22.2.142. [DOI] [PubMed] [Google Scholar]
- 4.Carry M, Korley V, Willerson JT, Weigelt L, Ford-Hutchinson AW, Tagari P. Increased urinary leukotriene excretion in patients with cardiac ischemia. In vivo evidence for 5-lipoxygenase activation. Circulation. 1992;85:230–6. doi: 10.1161/01.cir.85.1.230. [DOI] [PubMed] [Google Scholar]
- 5.Hock CE, Beck LD, Papa LA. Peptide leukotriene receptor antagonism in myocardial ischaemia and reperfusion. Cardiovasc Res. 1992;26:1206–11. doi: 10.1093/cvr/26.12.1206. [DOI] [PubMed] [Google Scholar]
- 6.Fiedler VB, Mardin M. Effects of nafazatrom and indomethacin on experimental myocardial ischemia in the anesthetized dog. J Cardiovasc Pharmacol. 1985;7:983–9. doi: 10.1097/00005344-198509000-00027. [DOI] [PubMed] [Google Scholar]
- 7.Hahn RA, MacDonald BR, Simpson PJ, Potts BD, Parli CJ. Antagonism of leukotriene B4 receptors does not limit canine myocardial infarct size. J Pharmacol Exp Ther. 1990;253:58–66. [PubMed] [Google Scholar]
- 8.Shekher A, Singh M. Role of eicosanoid inhibition of ischemia reperfusion injury: intact and isolated rat heart studies. Methods Find Exp Clin Pharmacol. 1997;19:223–9. [PubMed] [Google Scholar]
- 9.Wu KK. Inducible cyclooxygenase and nitric synthase. Adv Pharmacol. 1995;33:179–207. doi: 10.1016/s1054-3589(08)60669-9. [DOI] [PubMed] [Google Scholar]
- 10.Wong SCY, Fukuchi M, Melnyk P, Rodger A, Giaid A. Induction of cyclooxygenase-2 and activation of nuclear factor-κB in myocardium of patients with congestive heart failure. Circulation. 1998;98:100–3. doi: 10.1161/01.cir.98.2.100. [DOI] [PubMed] [Google Scholar]
- 11.Saito T, Rodger IW, Hu F, Shennib H, Giaid A. Inhibition of cyclooxygenase-2 improves cardiac function in myocardial infarction. Biochem Biophys Res Commun. 2000;273:772–5. doi: 10.1006/bbrc.2000.3010. [DOI] [PubMed] [Google Scholar]
- 12.Hamad AM, Stcliffe AM, Knox AJ. Aspirin-induced asthma: clinical aspects, pathogenesis and management. Drugs. 2004;64:2417–32. doi: 10.2165/00003495-200464210-00004. [DOI] [PubMed] [Google Scholar]
- 13.Hudson N, Balsitis M, Everitt S, Hawkey CJ. Enhanced gastric mucosal leukotriene B4 synthesis in patients taking non-steroidal anti-inflammatory drugs. Gut. 1993;34:742–7. doi: 10.1136/gut.34.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwak HJ, Park KM, Choi HE, Lim HJ, Park JH, Park HY. The cardioprotective effects of zileuton, a 5-lipoxygenase inhibitor, are mediated by COX-2 via activation of PKCdelta. Cell Signal. 2010;22:80–7. doi: 10.1016/j.cellsig.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Rossi A, Pergola C, Koeberle A, Hoffmann M, Dehm F, Bramanti P, et al. The 5-lipoxygenase inhibitor, zileuton, suppresses prostaglandin biosynthesis by inhibition of arachidonic acid release in macrophages. Br J Pharmacol. 2010;161:555–70. doi: 10.1111/j.1476-5381.2010.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter GW, Young PR, Albert DH, Bouska J, Dyer R, Bell RL, et al. 5-lipoxygenase inhibitory activity of Zileuton. J Pharmacol Exp Ther. 1991;256:929–37. [PubMed] [Google Scholar]
- 17.Wu Y, Lu X, Xiang FL, Lui EM, Feng Q. North American ginseng protects the heart from ischemia and reperfusion injury via upregulation of endothelial nitric oxide synthase. Pharmacol Res. 2011;264:195–202. doi: 10.1016/j.phrs.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Elsherif L, Wang X, Grachoff M, Wolska BM, Geenen DL, O'Bryan JP. Cardiac-specific expression of the tetracycline transactivator confers increased heart function and survival following ischemia reperfusion injury. PLoS ONE. 2012;7:e30129. doi: 10.1371/journal.pone.0030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed LA, Salem HA, Attia AS, Agha AM. Pharmacological preconditioning with nicorandil and pioglitazone attenuates myocardial ischemia/reperfusion injury in rats. Eur J Pharmacol. 2011;663:51–8. doi: 10.1016/j.ejphar.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 20.Aykaç G, Uysal M, Yalçın AS, Koçak-Toker N, Sivas A, Öz H. The effect of chronic ethanol ingestion on hepatic lipid peroxide, glutathione peroxidase and glutathione transferase in rat. Toxicology. 1985;46:71–6. doi: 10.1016/0300-483x(85)90008-3. [DOI] [PubMed] [Google Scholar]
- 21.Casini A, Ferrali M, Pompella AS, Maellaro E, Comporti M. Lipid peroxidation and cellular damage in extrahepatic tissues of bromobenzene intoxicated mice. Am J Pathol. 1986;123:520–31. [PMC free article] [PubMed] [Google Scholar]
- 22.Güven BA, Ercan E, Asgun HF, İçkin M, Ercan F, Yavuz O, et al. Experimental acute myocardial infarction in rats: HIF-1α, caspase-3, erythropoietin and erythropoietin receptor expression and the cardioprotective effects of two different erythropoietin doses. Acta Histochem. 2013;115:658–68. doi: 10.1016/j.acthis.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 23.McCarty KS, Jr Miller LS, Cox EB, Konrath J, McCarty KS., Sr Estrogen receptor analyses: correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;709:716–21. [PubMed] [Google Scholar]
- 24.Karakoyun B, Uslu U, Ercan F, Aydın MS, Yüksel M, Öğünç AV, et al. The effect of phosphodiesterase-5 inhibition by sildenafil citrate on inflammation and apoptosis in rat experimental colitis. Life Sci. 2011;89:402–7. doi: 10.1016/j.lfs.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Bonizzi G, Piette J, Schoonbroodt S, Greimers R, Havard L, Merville MP, et al. Reactive oxygen intermediate-dependent NF-kappaB activation by interleukin-1beta requires 5-lipoxygenase or NADPH oxidase activity. Mol Cell Biol. 1999;19:1950–60. doi: 10.1128/mcb.19.3.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erusalimsky JD. Vascular endothelial senescence: From mechanisms to pathophysiology. J Appl Physiol 1985. 2009;106:32–32. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Harsdorf R, Li PF, Dietz R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation. 1999;99:2934–41. doi: 10.1161/01.cir.99.22.2934. [DOI] [PubMed] [Google Scholar]
- 28.Patel NS, Cuzzocrea S, Chatterjee PK, Di Paola R, Sautebin L, Britti D, et al. Reduction of renal ischemia reperfusion injury in 5-lipoxygenase knockout mice and by the 5-lipoxygenase inhibitor zileuton. Mol Pharmacol. 2004;66:220–7. doi: 10.1124/mol.66.2.220. [DOI] [PubMed] [Google Scholar]
- 29.Dolan R, Hartshorn K, Andry C, McAvoy D. Systemic neutrophil intrinsic 5-lipoxygenase activity and CD18 receptor expression linked to reperfusion injury. Laryngoscope. 1998;108:1386–9. doi: 10.1097/00005537-199809000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Tu XK, Yang WZ, Shi SS, Chen CM, Wang CH. 5-lipoxygenase inhibitor zileuton attenuates ischemic brain damage: involvement of matrix metalloproteinase 9. Neurol Res. 2009;31:848–52. doi: 10.1179/174313209X403913. [DOI] [PubMed] [Google Scholar]
- 31.Kitagawa K, Matsumoto M, Hori M. Cerebral ischemia in 5-lipoxygenase knockout mice. Brain Res. 2004;1004:198–202. doi: 10.1016/j.brainres.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Adamek A, Jung S, Dienesch C, Laser M, Ertl G, Bauersachs J, et al. Role of 5-lipoxygenase in myocardial ischemia-reperfusion injury in mice. Eur J Pharmacol. 2007;571:51–4. doi: 10.1016/j.ejphar.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 33.Czapski GA, Czubowicz K, Strosznajder RP. Evaluation of the antioxidative properties of lipoxygenase inhibitors. Pharmacol Rep. 2012;64:1179–88. doi: 10.1016/s1734-1140(12)70914-3. [DOI] [PubMed] [Google Scholar]
- 34.Genovese T, Rossi A, Mazzon E, Di Paola R, Muià C, Caminiti R, et al. Effects of zileuton and montelukast in mouse experimental spinal cord injury. Br J Pharmacol. 2008;153:568–82. doi: 10.1038/sj.bjp.0707577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi SS, Yang WZ, Tu XK, Wang CH, Chen CM, Chen Y. 5-Lipoxygenase inhibitor zileuton inhibits neuronal apoptosis following focal cerebral ischemia. Inflammation. 2013;36:1209–17. doi: 10.1007/s10753-013-9657-4. [DOI] [PubMed] [Google Scholar]
- 36.Smith WL, Garavito RM, De Witt DL. Prostaglandin endoperoxide H synthases (cyclo-oxygenases)-1 and -2. J Biol Chem. 1996;271:33157–60. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 37.Abbate A, Santini D, Biondi-Zoccai GG, Scarpa S, Vasaturo F, Liuzzo G, et al. Cyclo-oxygenase-2 (COX-2) expression at the site of recent myocardial infarction: friend or foe?Heart. 2004;90:440–3. doi: 10.1136/hrt.2003.010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters-Golden M, Brock TG. Intracellular compartmentalization of leukotriene biosynthesis. Am J Respir Crit Care Med. 2000;161:S36–S40. doi: 10.1164/ajrccm.161.supplement_1.ltta-8. [DOI] [PubMed] [Google Scholar]
- 39.Chen X, Wang S, Wu N, Sood S, Wang P, Jin Z, et al. Overexpression of 5-lipoxygenase in rat and human esophageal adenocarcinoma and inhibitory effects of zileuton and celecoxib on carcinogenesis. Cancer Res. 2004;10:6703–9. doi: 10.1158/1078-0432.CCR-04-0838. [DOI] [PubMed] [Google Scholar]
- 40.Gregor JI, Kilian M, Heukamp I, Kiewert C, Kristiansen G, Schimke I, et al. Effects of selective COX-2 and 5-LOX inhibition on prostaglandin and leukotriene synthesis in ductal pancreatic cancer in Syrian hamster. Prostaglandins Leukot Essent Fatty Acids. 2005;73:89–97. doi: 10.1016/j.plefa.2005.04.016. [DOI] [PubMed] [Google Scholar]


