Abstract
Objective:
The aim of this study was to evaluate the effectiveness of computed tomography (CT) attenuation values in the characterization of pericardial effusion.
Methods:
This study consisted of 96 patients with pericardial effusion who underwent pericardiocentesis. For further diagnostic evaluation of pericardial effusion, all the patients were assessed by thorax CT. CT attenuation values were measured from at least 5 different areas of pericardial fluid by specifying the largest region of interest. The average of these measurements was computed and considered as the CT attenuation value of the patient. The patients were classified into two groups: patients with transudative pericardial effusion and those with exudative pericardial effusion.
Results:
CT attenuation values were significantly higher in patients with exudative pericardial effusion than in those with transudative pericardial effusion [14.85±10.7 Hounsfield unit (HU) vs. 1.13±4.3 HU, p<0.001]. CT attenuation values had a close correlation with the pericardial fluid albumin (r=0.829), protein (r=0.752), and LDH (r=0.708) levels; WBC count (r=0.564); protein ratio (r=0.739); and LDH ratio (r=0.689) as well as the albumin gradient (r=–0.725). A cut-off value of 4.7 HU had 80% sensitivity and 87.7% specificity for the identification of exudative pericardial effusion. In addition, a cut-off value of 6.5 HU had 71.4% sensitivity and 72.3% specificity for the prediction of cardiac tamponade.
Conclusion:
In patients with pericardial effusion, CT attenuation values seem to be correlated with the characterization parameters of the fluid and may distinguish exudative pericardial effusion from transudative pericardial effusion. This parameter was also found to be a predictor of cardiac tamponade. CT attenuation values can be a useful tool in the clinical evaluation of patients with pericardial effusion.
Keywords: computed tomography, attenuation, Hounsfield unit, pericardial effusion
Introduction
Pericardial effusion is a common clinical finding in cardiology practice with a wide clinical spectrum from asymptomatic mild effusion to cardiac tamponade. In most cases, the etiology can be derived from clinical data, but in some cases, the diagnosis can not be accomplished despite invasive procedures such as pericardiocentesis (P/S) (1–4). Beyond a life-saving strategy, P/S has severe complications, including cardiac perforations, serious arrhythmias, arterial bleeding, pneumothorax, and infections (5, 6). Besides, some patients have unfavorable locations that are not amenable for P/S. A noninvasive method to characterize pericardial effusion would be advantageous for avoiding the potential complications associated with P/S and may be used to tailor the therapy. In addition, such a method may be particularly beneficial in the identification of the nature of the pericardial fluid in patients who are not candidates for diagnostic P/S.
Echocardiography is not only the primary diagnostic approach for the detection of pericardial effusion but also provides information of the hemodynamic significance of the pericardial effusion (7, 8). However, some cases with poor sonographic windows necessitate additional imaging modalities such as computed tomography (CT) or cardiac magnetic resonance imaging (8, 9). CT has a superior diagnostic role, particularly in patients with false-positive findings due to adjacent pathological conditions such as pleural effusion, atelectasis, masses, and mediastinal lesions (8–12).
As an additional benefit, CT can be used to evaluate the nature of pericardial effusion. Features including pericardial thickening, calcification, loculation, and enlarged mediastinal lymph nodes can be assessed and can contribute to the differential diagnosis of pericardial effusion etiology (11, 13, 14). CT attenuation values may provide additional data about the characteristics of the pericardial effusion. Exudative pericardial effusion consists of high levels of protein and lactate dehydrogenase (LDH) and increased inflammatory activity; thus, they may show greater attenuation values on CT (8, 15, 16). Previous studies have demonstrated the utility of CT attenuation in the discrimination of pleural fluid (16–18).
Based on these considerations, we aimed to evaluate the feasibility of CT attenuation values in the characterization of pericardial effusion.
Methods
Study design and patient selection
Consistent with the Declaration of Helsinki, the study protocol was approved by the Ethics Committee. The study consisted of 253 consecutive patients who were diagnosed with pericardial effusion between January 2012 and June 2015. Informed consent was obtained from all the participants. The study was designed to assess patients who underwent P/S and CT examination within a week after or before the P/S procedure. After the evaluation of these inclusion criteria, a total of 96 patients were enrolled in the study. P/S was performed because of the emergence of cardiac tamponade in 67 patients and with the diagnostic purpose in 29 patients with large pericardial effusion. Cardiac tamponade was defined as hemodynamically significant cardiac compression (including elevated central venous pressure, pulse paradoxes, tachycardia, and hypotension). Large pericardial effusion was defined as at least 20 mm echo-free space surrounding the entire heart on 2-dimentional transthoracic echocardiography at end diastole.
Pericardiocentesis procedure
All the patients underwent echocardiography-guided subxiphoid P/S. Care was taken to perform a procedure via atraumatic puncture. The puncture site was determined as 1 mm left to the post-xiphoid angle. After being anesthetized locally with lidocaine (1%–2%), an 18 G puncture needle was introduced from the right side of the xiphoid and then advanced subcostally, routed to the left shoulder with the application of continuous suction to a syringe. When pericardial fluid was noticed in the syringe, a floppy guidewire was inserted. After widening the skin with a 6F dilatator, a pigtail catheter was sent over the wire. Localization of the catheter was ascertained by echocardiography. Then, the catheter placed at the appropriate site and maintained until the amount of fluid drainage was lower than 25 mL/day.
Analysis of pericardial fluid and classification of pericardial effusion
Samples of the fluid were submitted for cytological examination, microbiological culture, and biochemical tests for glucose, protein, LDH, and adenosine deaminase levels, acid-fast bacilli staining, aerobic and anaerobic bacterial cultures, fluid complete blood count, and polymerase chain reaction analyses. Fluid analysis was based on Light’s criteria. For the diagnosis of exudate, one or more of the following criteria should be met: (a) fluid total protein/serum total protein ratio >0.5, (b) fluid lactic dehydrogenase (LDH)/serum LDH ratio >0.6, or fluid LDH > two-thirds of the upper limits of the normal serum LDH value (19).
Each patient underwent a complete clinical evaluation, which included a complete medical history-taking physical examination; electrocardiography; chest radiography; echocardiography; complete blood count; wide serum biochemical profile; high-sensitive CRP; thyroid function tests; and tests for rheumatoid factor, antibodies against DNA, antinuclear antibodies, and tumor markers (CA 125, CA 19–9, alpha-fetoprotein, and carcinoembryonic antigen). Malignancy and rheumatological diseases were investigated in all the patients.
Pericardial effusion secondary to acute pericarditis was diagnosed on the basis of at least one of the following symptoms or signs: typical chest pain, pericardial friction rub, and typical electrocardiographic changes. In addition, the presence of inflammatory signs and recent history of respiratory tract infection suggested the diagnosis of acute pericarditis possibly of a viral origin. If the cause of the effusion was not apparent after thorough evaluation, the diagnosis of idiopathic pericardial effusion was made. In patients with idiopathic pericardial effusion, no further diagnostic tests were performed, as stated in the current guidelines of European Society of Cardiology (20).
CT protocol
CT was performed using a 64-multidetector CT scanner (Siemens, Somatom Emotion, Erlangen, Germany) for all the patients. All the patients were evaluated using the same CT scanner. Thorax CT scanning was performed at 5 mm slice thickness, 1.5 pitch, 110 kV, 70–90 mAs, and 160–200 ms temporal resolution. The intravenous contrast agent was not administered to patients with renal dysfunction or known allergy to the agent; contrast-enhanced CT was performed in 58 patients. Among these patients, 44 patients had pre- and post-contrast CT images. The patients underwent standard thorax scans after a standard contrast media injection protocol [100 mL of iopamidol-300 (Ultravist, Bayer Schering Pharma, Berlin, Germany)] at an injection rate of 2–2.5 mL/s. Axial images were transferred to a standard, commercially available workstation (Advantage Windows 3.1; GE Medical Systems). This workstation was used to measure Hounsfield unit (HU) at five different locations. In total, 84 patients underwent CT before P/S. The patients who underwent CT after P/S had enough residual PE to analyze the mean HU of the fluid.
Analysis of images and measurement of attenuation values
The pericardial fluid CT attenuation values (HU) were measured for all the patients using the imaging data. Because pericardial effusion composition may be affected by gravitational factors, HU was measured in up to five different, circular regions of interest (ROIs) within the effusion, including anterior, left anterolateral, left posterior, right anterolateral, and right posterior in the section in which the largest effusion was observed. To obtain precise values, we evaluated largest possible (at least 2 cm2 area) circular or elliptical ROIs. Based on previous reports, we measured the mean HU of each ROI (21). We calculated the average of the five measurements to minimize the misinterpretation. In case ROI could not be enough for evaluaton in the pre-determined five regions, the average HU was determined from as many as five adequate regions. The image interpreters took care to not include areas in proximity to the epicardial fat tissue, paracardiac fat tissue, or thickened pericardial areas (Fig. 1). To examine the effect of contrast media on CT attenuation values, the pre- and post-contrast HU were determined in patients who had both pre- and post-contrast images (Fig. 2). CT features such as presence and pattern of pericardial thickening (irregular or smooth), distribution of pericardial effusion (circumferential or loculated), and enlargement of mediastinal lymph nodes (greater than 10 mm in the shortest dimension) were evaluated for the discrimination of exudates and transudates. The thickness of the pericardium was measured with electronic calipers after magnification of a region of interest at the most thickened area, as visualized from the imaging data.
Figure 1.
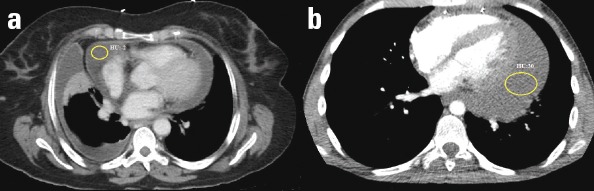
(a) Contrast-enhanced axial CT scan of the heart at the level of the aortic valve in a 62-year-old female patient with loculated pericardial effusion. The mean attenuation value of the effusion (circle) was –2 HU. Pericardial fluid was demonstrated as a transudate after pericardiocentesis. (b) Contrast-enhanced axial CT scan of the heart at the level of 4-chamber view in a 19-year-old male with loculated pericardial effusion. The mean attenuation value of the effusion (circle) was 30 HU. The pericardial fluid was shown as an exudate after pericardiocentesis
Figure 2.
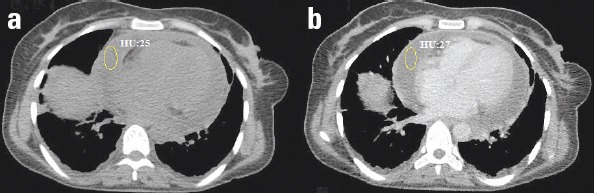
(a) Pre- and (b) post-contrast-enhanced axial CT scan of the heart at the level of 4-chamber view in a 40-year-old female patient with circumferential pericardial effusion
Image analysis was separately conducted by one experienced cardiologist (M.C.) and one experienced radiologist (M.O.) who were blinded to all clinical information and the nature of pericardial effusion as well as the other interpreter’s assessment. In addition, the measurements were analyzed again after 2 days by the same interpreters to evaluate intraobserver agreement.
Statistical analysis
Continuous variables were reported as the mean±standard deviation (SD), and categorical variables were expressed as the number of patients and percentages. HU measurements were compared for both intraobserver and interobserver agreement using intraclass correlation analysis. Kolmogorov–Smirnov tests were used to assess the normality of data distribution. Pearson’s chi-square test was used to compare categorical variables between groups. Independent sample T-test was used to compare continuous variables between groups. Paired sample T-test was used to analyze the difference in pre- and post-contrast images of same patients. Correlation analysis was performed with Pearson’s and Spearman’s correlation coefficients. Receiver operating curve (ROC) analysis was performed to investigate the efficacy of CT attenuation values in discriminating the exudate and transudate pericardial effusion and predicting cardiac tamponade. The sensitivity, specificity, p value, and area under the curve (AUC) were calculated for the attenuation values. The cut-off values were determined to predict the differentiation of exudative and transudate pericardial effusion. Statistical significance was defined as p<0.05. Data were analyzed using SPSS 20.0 software.
Results
Ninety-six patients were categorized into two groups according to Light’s criteria: patients with exudative pericardial effusion (n=66 patients) and those with transudative pericardial effusion (n=30 patients). In total, 16 patients were diagnosed with acute pericardial effusion (<1 week), 48 patients with subacute pericardial effusion (1 week–3 months), and 32 patients with chronic pericardial effusion (>3 months).
Baseline characteristic and CT findings of the groups are represented in Table 1. CT attenuation values were significantly higher in patients with exudative pericardial effusion than in those with transudative pericardial effusion (14.85±10.7 HU vs. 1.13±4.3 HU, p<0.001) (Table 1, Fig. 3). In a detailed analysis of subgroups according to the clinical evaluation and pathology results of pericardial fluid, CT attenuation values were higher in patients with haemopericardium, purulent pericardial effusion, and malignant pericardial effusion (Fig. 4). Loculation was more prevalent in patients with exudative pericardial effusion than in those with transudative pericardial effusion (24.2% vs. 13.3%), but this difference did not reach statistical significance (p=0.222). Pathological mediastinal lymph node enlargement was found to be more prevalent in patients with exudative pericardial effusion than in those with transudative effusion (54.5% vs. 26.7%, p=0.011).
Table 1.
Baseline characteristics and CT findings of groups according to types of pericardial effusion
| Variables | Transudative PE (n=30 patients) | Exudative PE (n=66 patients) | P |
|---|---|---|---|
| Gender, male % | 17 (56.7%) | 31 (47.0%) | 0.378 |
| Age, years | 62.1±12.6 | 57.0±15.5 | 0.110 |
| CT attenuation, HU | 1.13±4.3 | 14.85±10.7 | <0.001 |
| Pericardial irregularity | 6 (20.0%) | 17 (25.8%) | 0.540 |
| Pericardial thickness, mm | 2.23±0.2 | 2.11±1.3 | 0.647 |
| Loculation | 4 (13.3%) | 16 (24.2%) | 0.222 |
| Maximal pericardial effusion thickness | 23.4±13.8 | 20.1±13.1 | 0.268 |
| Mediastinal lymph node (size >1 cm) | 8 (26.7%) | 36 (54.5%) | 0.011 |
Data are means±SD or n (%). CT - computed tomography; HU - Hounsfield unit; PE - pericardial effusion
Figure 3.
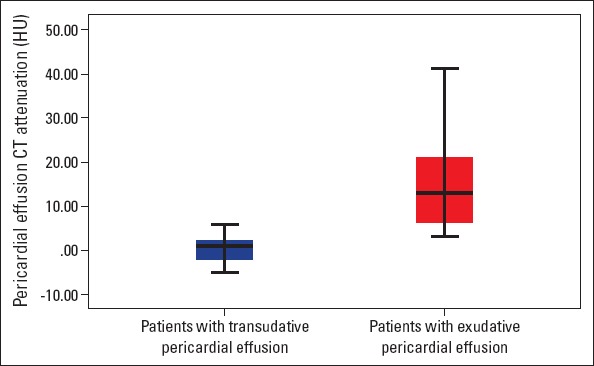
Box plots showing the attenuation values for the transudate and exudate groups. The boxes stretch from the 25th to 75th percentile. The horizontal line across each box is the mean. The vertical lines with whiskers extending below and above the boxes indicate the minimum and maximum values, respectively
HU - hounsfield unit.
Figure 4.
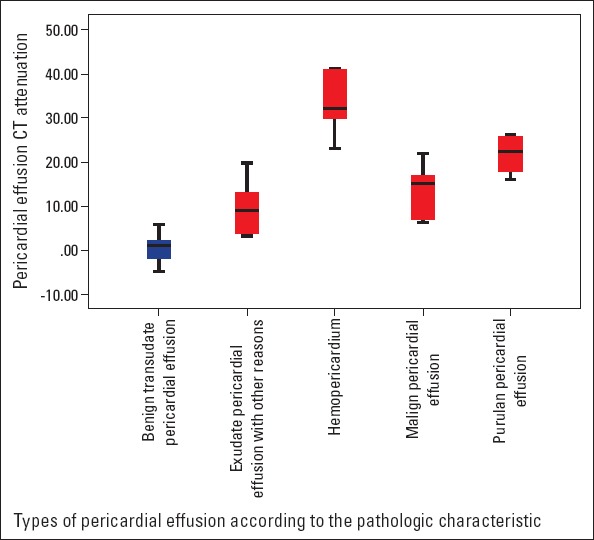
Box plots showing the attenuation values for the types of pericardial effusion according to pathological characteristics. The boxes stretch from the 25th to 75th percentile. The horizontal line across each box is the mean. The vertical lines with whiskers extending below and above the boxes indicate the minimum and maximum values, respectively
HU - hounsfield unit.
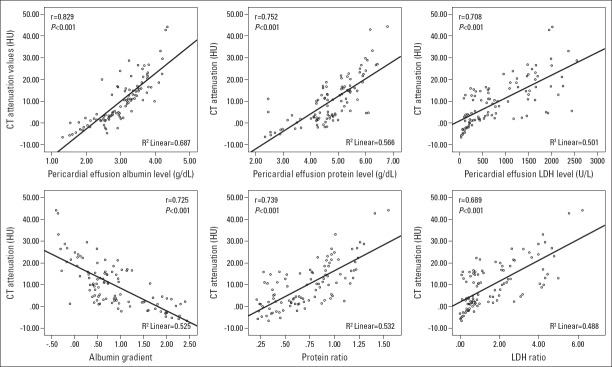
CT attenuation values had a close positive correlation with the pericardial fluid albumin (r=0.829, p<0.001), protein (r=0.752, p<0.001), and LDH (r=0.708, p<0.001) levels; WBC count (r=0.564, p=0.007); protein ratio (r=0.739, p<0.001); and LDH ratio (r=0.689, p<0.001) and a negative correlation with the albumin gradient (r=–0.725, p<0.001) (Fig. 5).
Figure 5.
Correlation between CT attenuation values and pericardial fluid albumin level, protein level, LDH–albumin gradient, protein ratio, and LDH ratio. Each dot represents one patient; the straight line represents the best fit line obtained by linear regression analysis
CT - computed tomography; HU - hounsfield unit; LDH - lactate dehydrogenase.
The diagnostic performance of CT attenuation values in the determination of exudative and transudate pericardial effusion was assessed by ROC analysis. A cut-off value of 4.7 HU for CT attenuation had 80% sensitivity and 87.7% specificity for the identification of exudative pericardial effusion (AUC, 0.935; 95% CI, 0.887–0.988). In addition, a cut-off value of 6.5 HU for CT attenuation had 71.4% sensitivity and 72.3% specificity for the prediction of cardiac tamponade (AUC, 0.729; 95% CI, 0.612–0.845).
The effect of contrast on CT attenuation values was analyzed in 44 patients. The pre- and post-contrast CT attenuation values were not significantly different (8.7±9.6 vs. 8.9±10.4, p=0.841).
In total, 84 patients underwent CT before P/S. The mean CT attenuation values of patients in whom P/S preceded CT did not statistically differ from those of others (10.47 HU vs. 10.81 HU p=0.895).
The intra- and interobserver correlations for the determination of CT attenuation values were evaluated and were found to be in good agreement [intraclass correlation coefficient, 0.967 (95% confidence interval, CI, 0.919–0.987) and 0.931 (95% CI, 0.902–0.963), respectively].
Discussion
To the best of our knowledge, this is the first study investigating the utility of CT attenuation values in the characterization of pericardial effusion. In our study, the CT attenuation value was determined to be a useful tool for the discrimination of exudative and transudative pericardial effusion. CT attenuation values had significant correlations with effusion protein, albumin, and LDH levels; WBC counts; and related ratios as primary determinants of exudative pericardial effusion. CT attenuation values deemed to reflect the higher density and increased inflammatory activity. Besides the effectiveness of CT attenuation values in the characterization of pericardial effusion, the results of our study demonstrated that this parameter may be used to predict the prognosis of patients with pericardial effusion.
Pericardial effusion is a relatively common pathology in clinical cardiology practice. In most cases (some data report 60% of all cases), the etiology of pericardial effusion can be diagnosed with initial clinical evaluation (1). Sometimes, the diagnosis and management can be challenging. Echocardiography, as the most available and reliable imagining modality for detecting the presence of pericardial effusion, enables assessment of the severity, distribution, and hemodynamic importance of pericardial effusion. However, in the context of the diagnostic approach, it seems underpowered (7). CT and MR offer valuable data in patients with pericardial effusion. CT is less operator dependent and enables evaluation of the mediastinum and lungs, and related abnormalities, including pericardial calcification and thickness (8, 9). CT also shows more precise spatial distribution of pericardial effusion, particularly in circumstances where epicardial fat, pericardial hematoma, and clots make diagnosis insufficient (8, 9). CT can provide valuable information about the hemodynamic significance of pericardial effusion. Superior and inferior vena cava enlargement, periportal lymphedema, contrast material reflux to the IVC and azygos vein, enlargement of the hepatic and renal veins, flattening of the anterior surface of the right ventricle, coronary sinus compression, and bowing of the interventricular septum may indicate cardiac tamponade, particularly in suspected cases (8).
CT attenuation is an overlooked radio-diagnostic parameter, particularly in cardiovascular diseases. There are scarce data about the visceral effusion and CT attenuation relationship. In the first study on this issue, Nandalur et al. (17) investigated the clinical use of CT attenuation values to characterize pleural fluid. Consistent with our results, they reported that exudative pleural effusion had significantly higher CT attenuation values than transudative effusion, and CT attenuation values had a positive correlation with the pleural total protein and pleural/serum protein ratio. However, they concluded that the accuracy of this parameter was moderate because of the overlap in attenuation values between transudates and exudates (17). In the prompting study about the feasibility of this parameter in pleural effusion, Çullu et al. (18) demonstrated CT attenuation as a useful diagnostic tool in differentiating exudates from transudates. A considerable overlap also existed in their study, but they recommended that evaluation of attenuation values with clinical findings could handle this problem (18). In contrast, we did not find any considerable overlap in CT attenuation values of exudate and transudate pericardial effusion. We postulated that the different biochemical properties of pericardial and pleural fluid lead to this difference. Ben-Horin et al. (22) reported that pericardial fluid had significantly higher LDH and protein levels and ratios than pleural fluid. Despite the use of Light’s criteria in both fluids, the different compositions of these fluids should be assessed further. The effectiveness of Hounsfield density measurement in the evaluation of pericardial effusion composition was studied by Rifkin et al. (21). They investigated the association of CT attenuation values with pericardial effusion hematocrit and total protein levels and found a significant correlation between these parameters. Contrary to our results, they concluded that in a linear combination, the correlation between total protein levels and CT attenuation was not statistically significant (21). We proposed that the differences in the study population may lead to this discrepancy. Almost half of their patient population consisted of post-cardiotomy patients who underwent P/S because hemopericardium was suspected. In our study, the percentage of patients with hemopericardium was only 6.3%. The elevated HCT level in patients with hemopericardium may cause this difference.
In addition, in our study, we investigated the importance of other CT findings in pericardial effusion characterization. Pericardial irregularity, thickness, and loculation and maximal pericardial effusion thickness were not different in patients with exudative and transudative pericardial effusion. In addition to CT attenuation values, pathological mediastinal lymph node enlargement differed in exudative and transudative pericardial effusion. In the study of Sun et al. (14), the enlargement of mediastinal lymph nodes was more prevalent in malignant effusion from benign cases.
Clinical implications
The use of CT attenuation values to characterize pericardial effusion can be particularly beneficial in patients with pericardial effusion who are not candidated for P/S because of the localization of fluid or any contraindications. In addition, this measurement may be used in the assessment of patients with pericardial effusion, in whom the effusion is not sufficient to perform P/S.
This measurement may decrease the necessity of diagnostic P/S and may at least give clinicians an idea about pericardial effusion before P/S.
Study limitations
The sample size was relatively small, and the results of this study should be confirmed in larger prospective studies. Long-term prognostic data such as those of mortality and recurrence of pericardial effusion were not evaluated.
Conclusion
In conclusion, CT attenuation values seem to enable the characterization of pericardial effusion, particularly the discrimination of exudative and transudative pericardial effusion. CT attenuation values demonstrated significant correlations with major determinants of pericardial effusion. This parameter can facilitate the clinical evaluation of patients with pericardial effusion.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – M.S.Ç., E.H.Ö.Ç., A.T.; Design – M.S.Ç., S.A., E.H.Ö.Ç., A.T.; Supervision – M.S.Ç., S.T., E.H.Ö.Ç., A.T.; Fundings – All authors; Materials – M.S.Ç., M.Ö., E.H.Ö.Ç.; Data collection &/or processing – M.S.Ç., M.Ö., E.H.Ö.Ç., D.A., S.A.; Analysis &/or interpretation – M.S.Ç., M.Ö., E.H.Ö.Ç., D.A., S.T.; Literature search – M.S.Ç., M.Ö., E.H.Ö.Ç., D.A., S.T.; Writing – M.S.Ç., M.Ö., E.H.Ö.Ç., D.A., S.T.; Critical review – M.S.Ç., M.Ö., E.H.Ö.Ç., D.A., S.T.
References
- 1.Sagristà-Sauleda J, Mercé J, Permanyer-Miralda G, Soler-Soler J. Clinical clues to the causes of large pericardial effusions. Am J Med. 2000;109:95–101. doi: 10.1016/s0002-9343(00)00459-9. [DOI] [PubMed] [Google Scholar]
- 2.Corey GR, Campbell PT, Van Trigt P, Kenney RT, O'Connor CM, Sheikh KH, et al. Etiology of large pericardial effusions. Am J Med. 1993;95:209–13. doi: 10.1016/0002-9343(93)90262-n. [DOI] [PubMed] [Google Scholar]
- 3.Levy PY, Corey R, Berger P, Habib G, Bonnet JL, Levy S, et al. Etiologic diagnosis of 204 pericardial effusions. Medicine (Baltimore) 2003;82:385–91. doi: 10.1097/01.md.0000101574.54295.73. [DOI] [PubMed] [Google Scholar]
- 4.Permanyer-Miralda G, Sagristá-Sauleda J, Soler-Soler J. Primary acute pericardial disease: a prospective series of 231 consecutive patients. Am J Cardiol. 1985;56:623–30. doi: 10.1016/0002-9149(85)91023-9. [DOI] [PubMed] [Google Scholar]
- 5.Tsang TS, Enriquez-Sarano M, Freeman WK, Barnes ME, Sinak LJ, Gersh BJ, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002;77:429–36. doi: 10.4065/77.5.429. [DOI] [PubMed] [Google Scholar]
- 6.Tsang TS, Freeman WK, Sinak LJ, Seward JB. Echocardiographically guided pericardiocentesis: evolution and state-of-the-art technique. Mayo Clin Proc. 1998;73:647–52. doi: 10.1016/S0025-6196(11)64888-X. [DOI] [PubMed] [Google Scholar]
- 7.D'Cruz I, Rehman AU, Hancock HL. Quantitative Echocardiographic Assessment in Pericardial Disease. Echocardiography. 1997;14:207–14. doi: 10.1111/j.1540-8175.1997.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 8.Restrepo CS, Lemos DF, Lemos JA, Velasquez E, Diethelm L, Ovella TA, et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics. 2007;27:1595–610. doi: 10.1148/rg.276065002. [DOI] [PubMed] [Google Scholar]
- 9.Rajiah P, Kanne JP. Computed tomography of the pericardium and pericardial disease. J Cardiovasc Comput Tomogr. 2010;4:3–18. doi: 10.1016/j.jcct.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Rienmüller R, Gröll R, Lipton MJ. CT and MR imaging of pericardial disease. Radiol Clin North Am. 2004;42:587–601. doi: 10.1016/j.rcl.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Silverman PM, Harell GS, Korobkin M. Computed tomography of the abnormal pericardium. AJR Am J Roentgenol. 1983;140:1125–9. doi: 10.2214/ajr.140.6.1125. [DOI] [PubMed] [Google Scholar]
- 12.Olson MC, Posniak HV, McDonald V, Wisniewski R, Moncada R. Computed tomography and magnetic resonance imaging of the pericardium. Radiographics. 1989;9:633–49. doi: 10.1148/radiographics.9.4.2756190. [DOI] [PubMed] [Google Scholar]
- 13.Axel L. Assessment of pericardial disease by magnetic resonance and computed tomography. J Magn Reson Imaging. 2004;19:816–26. doi: 10.1002/jmri.20076. [DOI] [PubMed] [Google Scholar]
- 14.Sun JS, Park KJ, Kang DK. CT findings in patients with pericardial effusion: differentiation of malignant and benign disease. AJR Am J Roentgenol. 2010;194:W489–94. doi: 10.2214/AJR.09.2599. [DOI] [PubMed] [Google Scholar]
- 15.Kopcinovic LM, Culej J. Pleural peritoneal and pericardial effusions - a biochemical approach. Biochem Med (Zagreb) 2014;24:123–37. doi: 10.11613/BM.2014.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abramowitz Y, Simanovsky N, Goldstein MS, Hiller N. Pleural effusion: characterization with CT attenuation values and CT appearance. AJR Am J Roentgenol. 2009;192:618–23. doi: 10.2214/AJR.08.1286. [DOI] [PubMed] [Google Scholar]
- 17.Nandalur KR, Hardie AH, Bollampally SR, Parmar JP, Hagspiel KD. Accuracy of computed tomography attenuation values in the characterization of pleural fluid: an ROC study. Acad Radiol. 2005;12:987–91. doi: 10.1016/j.acra.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Çullu N, Kalemci S, Karaka şÖ, Eser İ, Yalçın F, Boyacı FN, et al. Efficacy of CT in diagnosis of transudates and exudates in patients with pleural effusion. Diagn Interv Radiol. 2014;20:116–20. doi: 10.5152/dir.2013.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spodick DH. Spodick The Pericardium: A Comprehensive Textbook. 1st ed. New York: CRC Press; 1996. [Google Scholar]
- 20.Maisch B, Seferović PM, Ristić AD, Erbel R, Rienmüller R, Adler Y, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary;The Task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004;25:587–610. doi: 10.1016/j.ehj.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Rifkin RD, Mernoff DB. Noninvasive evaluation of pericardial effusion composition by computed tomography. Am Heart J. 2005;149:1120–7. doi: 10.1016/j.ahj.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Horin S, Bank I, Shinfeld A, Kachel E, Guetta V, Livneh A. Diagnostic value of the biochemical composition of pericardial effusions in patients undergoing pericardiocentesis. Am J Cardiol. 2007;99:1294–7. doi: 10.1016/j.amjcard.2006.12.048. [DOI] [PubMed] [Google Scholar]