Abstract
Lipids serve essential functions in cells as signaling molecules, membrane components, and sources of energy. Defects in lipid metabolism are implicated in a number of pandemic human diseases, including diabetes, obesity, and hypercholesterolemia. Many aspects of how fatty acids and cholesterol are absorbed and processed by intestinal cells remain unclear and present a hurdle to developing approaches for disease prevention and treatment. Numerous studies have shown that the zebrafish is an excellent model for vertebrate lipid metabolism. In this chapter, we review commercially available fluorescent lipids that can be deployed in live zebrafish to better understand lipid signaling and metabolism. In this chapter, we present criteria one should consider when selecting specific fluorescent lipids for the study of digestive physiology or lipid metabolism in larval zebrafish.
Introduction
A significant body of work links alterations in lipid metabolism (induced through genetic mutation and/or lifestyle) with cardiovascular disease, diabetes mellitus, and obesity (Bastien, Poirier, Lemieux, & Despres, 2014; Bauer, Briss, Goodman, & Bowman, 2014; Rankinen, Sarzynski, Ghosh, & Bouchard, 2015). A better understanding of the genetic and environmental circumstances that perturb lipid uptake, trafficking, and storage in an organism is required to fully assess the impact of metabolic regulators and hormones on lipid-associated diseases. Our lab and others have made great strides in developing techniques to enable the study of lipid metabolism in vivo using zebrafish larvae (Carten, Bradford, & Farber, 2011; Clifton et al., 2010; Farber et al., 2001; Hama et al., 2009; Ho, Lorent, Pack, & Farber, 2006; Ho et al., 2004; Miyares et al., 2013; Semova et al., 2012).
The Need for Whole Animal Studies of Lipid Metabolism
In vitro studies have laid much of the groundwork for our biochemical understanding of lipid metabolism; however, a number of caveats arise when attempting to study lipid metabolism in vitro. Such studies are often carried out in transformed cultured cells, such as liver HepG2, intestinal Caco2, and adipocyte 3LT3 cells. Such cell lines are comprised of a single cell type, which cannot duplicate the cellular heterogeneity of an entire organ, such as the intestine, that is composed of stem, enteroendocrine, immune, and goblet cells. These multiple cell types are known to influence each other through paracrine signaling that can have global effects on lipid uptake and processing. Furthermore, bile, intestinal mucus, and the gut microbiota are all known to greatly influence dietary lipid processing and absorption in the intestine (Backhed et al., 2004; Field, Dong, Beis, & Stainier, 2003; Kruit, Groen, van Berkel, & Kuipers, 2006; Martin et al., 2008; Moschetta et al., 2005; Pack et al., 1996; Semova et al., 2012; Titus & Ahearn, 1992; Turnbaugh, Backhed, Fulton, & Gordon, 2008) and are absent in cultured cell models. For these reasons, employing whole animal in vivo strategies, in addition to cultured cell work, is vital to understanding how metabolic dysfunction arises and manifests itself in an organism.
While the zebrafish has been established as a powerful model for the study of early development, thanks largely to its accessibility, fast development, and optically clear embryos, few researchers have exploited this model organism to visualize lipid uptake and processing. In 2013, James Rothman, Randy Schekman, and Thomas Südhof received the Nobel Prize in Physiology or Medicine for their work in yeast and cultured cells to elucidate the cellular machinery that regulates intracellular vesicle traffic. Schekman stated, “Many fascinating questions remain at the molecular mechanistic level about how vesicles form and how they are directed to their target and fuse with a target membrane” (Schekman & Sudhof, 2014). Experiments using fluorescent lipids in larval zebrafish build on the pioneering studies of Rothman and Schekman to better understand critical vesicular trafficking events that move lipid and/or lipoprotein cargos in a live vertebrate. Many aspects regarding how digestive organs regulate the flow of lipids through specialized cells such as the liver hepatocyte and the intestinal enterocyte remain to be discovered, and these insights are expected to impact a host of prevalent worldwide diseases.
The ability to perform forward genetic studies in zebrafish by mutagenizing the entire genome and screening for particular phenotypes has made this vertebrate model widely used (Driever et al., 1996; Haffter et al., 1996). Mutagenesis methods commonly utilized in the zebrafish include soaking founder fish in mutagenic chemicals, such as ethylnitrosourea (ENU) to generate point mutations (Driever et al., 1996; Haffter et al., 1996), and retrovirus- or transposon-mediated gene insertions (Chen & Farese, 2002; Ivics et al., 2004). We perform ongoing screens based on ENU and gene-break transposon mutagenesis methods to search for mutations that perturb lipid processing. To find and characterize mutants, we immerse zebrafish larvae in fluorescent lipid reporters that are ingested and allow lipid processing events to be visualized in vivo (Farber et al., 2001).
Both in the wild and in the laboratory, zebrafish consume a lipid-rich diet (≥ 10% by weight) high in triacylglycerol (TAG), phospholipids, and sterols (Enzler, Smith, Lin, & Olcott, 1974; Spence, Gerlach, Lawrence, & Smith, 2008). Prior to absorption by the intestine, these lipids must be processed and solubilized by the digestive enzymes and bile that make up the intraluminal intestinal milieu. As in humans, bile is produced by hepatocytes and secreted into an extensive network of intrahepatic ducts, which drains into the gall bladder. In response to hormonal stimulation triggered by food consumption, bile is released into the intestinal lumen to emulsify dietary fat and facilitate its absorption by intestinal enterocytes. After dietary fat is emulsified, TAG and phospholipids must be broken down by luminal lipases to release free fatty acid (FA) or mono- and diacylglycerols, which can then enter the absorptive cells (enterocytes) that line the gut (Thomson, Keelan, Cheng, & Clandinin, 1993). Zebrafish enterocytes are highly similar to mammalian enterocytes (Buhman et al., 2002), with the characteristic microvilli and basal nuclei. After food consumption, zebrafish accumulate cytoplasmic lipid droplets in their enterocytes (unpublished). Fats are burned via oxidative pathways in the mitochondria or peroxisomes or packaged into chylomicrons, which are secreted from the basolateral surface of enterocytes into lymphatic or blood vessels (Field, 2001; Levy et al., 2007). While it remains to be seen how closely the zebrafish system will model human intestinal lipoprotein metabolism, it is likely that many of the mechanisms of lipoprotein production are conserved. We can readily follow and describe these processes through the application of fluorescent lipids to live zebrafish larvae.
In this chapter, we present criteria one should consider when selecting specific fluorescent lipids for the study of digestive physiology or lipid metabolism in larval zebrafish. Determining the appropriate lipid analog or reporter to use for a given experiment will ultimately provide a better understanding of subcellular processes being examined. It is our standpoint that live-imaging studies and fluorescence-based screens are well suited for the larval zebrafish and will greatly broaden our understanding of digestive physiology in the years to come.
1. Forward Genetic Screening With Fluorescent Lipids
1.1 PED6
Using lipids that alter their spectral properties when they are in specific cellular structures or are metabolized by specific enzymes can provide valuable information about where a lipid-related event occurs in the cell. One such fluorescent reporter we have used in our genetic screens is the phosphoethanolamine analog PED6, [N-((6-(2,4-dinitro-phenyl)amino)hexanoyl)-1-palmitoyl-2-BODIPY-FL-pentanoyl-sn-glycerol-3-phosphoethanolamine]. This reporter exhibits altered spectral characteristics upon processing by lipid-modifying enzymes (Farber et al., 2001; Hama et al., 2009; Hendrickson, Hendrickson, Johnson, & Farber, 1999) (Fig. 1A). Following ingestion by larvae, PED6 is cleaved by phospholipase A2 (PLA2), resulting in the release of the fluorescent BODIPY-labeled acyl chain (Farber, Olson, Clark, & Halpern, 1999) (Fig. 1B and C). When zebrafish larvae (5 dpf) are immersed in media containing PED6, bright green fluorescence is observed in the intestine, gall bladder, and liver (Fig. 1C). Because this fluorescent reporter provides a rapid readout of both lipid metabolism and digestive organ morphology in living larvae, it was used to perform the first physiological genetic screen in the zebrafish (Farber et al., 2001). This screen identified Vps51 (fat-free) as both a mediator of intestinal lipid processing and Golgi morphology (Ho et al., 2006). Vps51 is a component of the Golgi-associated retrograde protein (GARP) complex (Frohlich et al., 2015) and, as suggested by the fat-free phenotype that includes increased number of lipid droplets in liver and intestine (Liu, Lee, Tsai, & Ho, 2010), has recently been implicated in regulating cellular lipid homeostasis.
Figure 1. Fluorescent lipids visualize digestive organ uptake and transport in larval zebrafish.
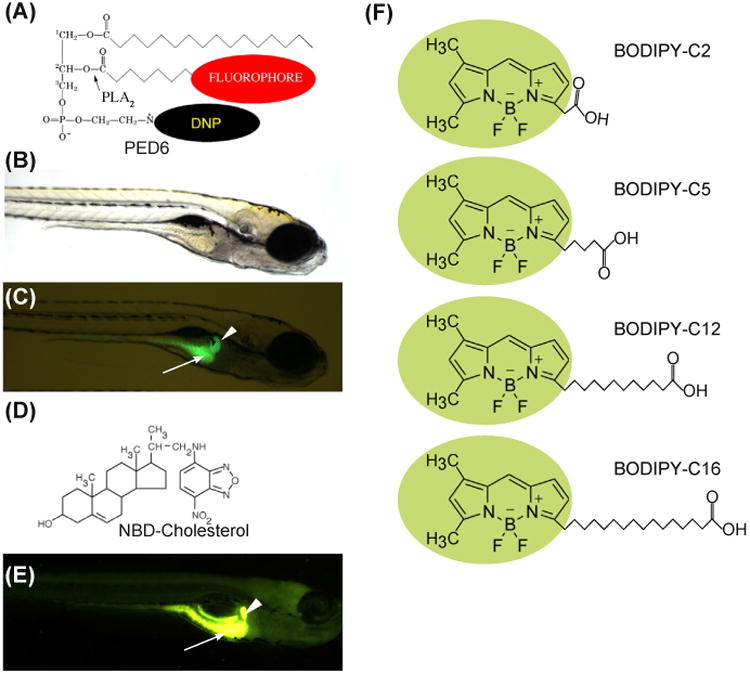
(A) The chemical structures of PED6. The BODIPY-labeled acyl chain of PED6 is normally quenched by the dinitrophenyl group at the sn-3 position. Upon PLA2 cleavage at the sn-2 position, the BODIPY-labeled acyl chain is unquenched and can fluoresce. Bright field (B) and fluorescent (C) images of 5-dpf larva following soaking in PED6 for 6 h. PED6 labeling reveals lipid processing in the gall bladder (arrowhead) and intestine (arrow). (D) The chemical structure of NBD-cholesterol. The NBD-cholesterol analog contains an NBD fluorophore where the alkyl tail at the terminal end of cholesterol would normally reside. (E) Soaking zebrafish larvae (5 dpf) in NBD-cholesterol (3 mg/mL, solubilized with fish bile)for 2 h visualizes cholesterol uptake in the gall bladder (arrowhead) and intestine (arrow). (F) The chemical structures of BODIPY fatty acids. (See color plate)
1.2 NBD- and BODIPY-Cholesterol
Continuously fluorescent lipids are equally powerful in studies performed in larval zebrafish due to the ability to follow their uptake, transport, and storage over time across multiple organs in one organism. We have utilized the sterol analog 22-NBD-cholesterol (22-[N-(7-nitronbenz-2-oxa-1,3-diazol-4-yl) amino]-23,24-bisnor-5-cholen-3-ol) (Fig. 1D) to visualize cholesterol absorption in live larvae and to screen for mutants (Fig. 1E). This reagent is different from PED6 in that it continuously fluoresces and it is more difficult to solubilize and administer via feeding. Thus, it may be more efficient to use in studies involving its direct injection into the yolk or yolk syncytial layer.
NBD-cholesterol was initially created to visualize cholesterol partitioning into membranes. While NBD-cholesterol, such as cholesterol, can be esterified by Acyl CoA:cholesterol acyltransferase (ACAT) to produce cholesterol ester (Lada et al., 2004), it was found to preferentially enter into liquid-disordered lipid domains (cholesterol enters liquid-ordered domains) (Li, Mintzer, & Bittman, 2006). This lipid packing difference makes this probe less useful as a model for all aspects of cholesterol processing. To address this limitation, a BODIPY-tagged cholesterol analog was synthesized with a modified fluorophore linker (Li & Bittman, 2007). Studies on its membrane packing found that BODIPY-cholesterol partitioned into the cholesterol-rich liquid-ordered membrane domain (Ariola, Li, Cornejo, Bittman, & Heikal, 2009) and interacted with membranes in ways similar to native sterols, making it a powerful new tool for imaging sterol trafficking in live cells (Marks, Bittman, & Pagano, 2008).
Studies done in the zebrafish have found that embryos microinjected with BODIPY-cholesterol exhibited fluorescence predominately in the yolk of developing zebrafish larvae (Holtta-Vuori et al., 2008). Ongoing work in the Farber lab involves following the path of BODIPY-cholesterol in larval intestinal enterocytes after a high-fat meal and comparing its localization to that of lipid droplets (revealed by BODIPY-labeled FA). Recent data suggest that sterol and FA initially segregate into nonoverlapping compartments (Farber, unpublished). Despite the physiologically normal lipid packing of BODIPY-cholesterol, it is an extremely poor ACAT substrate (Holtta-Vuori et al., 2008). Since this is a major pathway for dietary cholesterol processing, the lack of esterification potentially limits the degree to which this fluorophore models native cholesterol. It is our expectation that minor adjustments to BODIPY cholesterol's structure may enhance its ability to be esterified.
We have developed a number of novel feeding assays that enable us to observe fluorescently labeled dietary lipids incorporate into fat deposits within cells and track their subsequent movements between cells and digestive tissues in live zebrafish (Carten et al., 2011). Feeding fluorescent lipids together with a high-lipid meal produced striking images of lipid droplets in specific subcellular compartments and has enhanced our understanding of dietary lipid metabolism (Avraham-Davidi et al., 2012; Carten et al., 2011; Clifton et al., 2010; Delous et al., 2012; Otis & Farber, 2013; Sadler, Rawls, & Farber, 2013; Semova et al., 2012). The zebrafish is well suited to genetic manipulation because it is a genetic model organism wherein we can mutate/modify existing genes with genome engineering techniques (Bedell et al., 2012; Hwang et al., 2015; Shah, Davey, Whitebirch, Miller, & Moens, 2015) and/or introduce fluorescent proteins that can serve as transcriptional reporters (Gut et al., 2013). Similarly, we can use Tol2-mediated transgenesis (Kawakami et al., 2004) to introduce any gene of interest encoding a fluorescent fusion protein or metabolic sensor. Taken together, this approach and the unique advantages of larval zebrafish enable the visualization of specific tissues and organs in vivo in conjunction with the biochemical processing of lipid precursors within single cells and throughout the living animal.
2. Visualizing Lipid Metabolism Using BODIPY Fatty Acid Analogs
The wide variety of fluorescent lipid analogs commercially available allows one to fully exploit the optical clarity of zebrafish larvae to study lipid metabolism. One type of analog widely used in cultured cells to visualize lipid dynamics is the BODIPY-conjugated FA. These analogs consist of an acyl chain of variable length attached to the BODIPY (4,4-difluoro-4-bora-3a, 4a-diaza-S-indacene) fluorescent moiety (Fig. 1F). First synthesized by Treibs and Kreuzer in 1968 (Treibs & Kreuzer, 1969), the BODIPY fluorophore possesses a number of advantageous qualities including high photostability, strong and narrow wavelength emission in the visible spectrum, and an overall uncharged state (Monsma et al., 1989; Pagano, Martin, Kang, & Haugland, 1991).
To administer BODIPY FA analogs to live zebrafish larvae, we developed a novel feeding assay that generates liposomes to create an emulsion of relatively hydrophobic FA analogs in embryo media (Carten et al., 2011). Following a short liposome feed, digestive organ structure and metabolic function can be assessed, as the fluorescent FAs accumulate readily throughout numerous larval organs and tissues. With this assay, we have observed that different chain length FAs (short, medium, and long) accumulate in distinct patterns throughout digestive organs and tissues, with each chain length suited to visualize particular larval organs and cellular structures. During processing of a meal delivering BODIPY-conjugated lipid, the specific transport pathway from the intestine to the liver depends on the properties of the labeled lipid. Regardless of the transport details, hepatobiliary function can be readily assayed by observing the accumulation of fluorescence in the gall bladder using a low-power (10× objective) fluorescent stereo microscope. While this approach has been covered in prior editions of this volume (Anderson, Carten, & Farber, 2011), this chapter will focus on organ morphology and subcellular lipid trafficking that can be observed with confocal microscopy (40–63× objective) of live zebrafish larvae.
2.1 BODIPY (Excitation/Emission Maxima ∼503/512 nm)
The unconjugated BODIPY fluorophore has been primarily used to label cytoplasmic lipid droplets (LD) (Brasaemle et al., 2000; Gocze & Freeman, 1994). Administering a high-fat meal to zebrafish larvae leads to the production of copious LDs in both the intestine and liver that can be readily labeled by free BODIPY (data not shown). However, the signal-to-background noise ratio is low, and the image quality is inferior compared to data from studies based on medium- and long-chain BODIPY-conjugated FAs (described in the following section). This is most likely due to its lack of incorporation into complex lipids (Carten et al., 2011).
2.2 BODIPY C2
When comparing the chain length of a BODIPY-conjugated FA to an unlabeled FA, we estimate an additional two to four carbons account for the addition of the BODIPY fluorophore. We consider BODIPY C2 to act as a short-chain fatty acid (SCFA; a fatty acid of less than six carbon atoms) (Fig. 1F). Larvae labeled with this reagent reveal fluorescence primarily in the hepatic and pancreatic ducts (Fig. 2A), suggesting BODIPY C2 is particularly suited to illuminate the ductal networks of digestive organs. The speed of hepatic labeling (<2 h) suggests that this reagent can readily diffuse to the liver and does not require lipoproteins to transit from the intestine. One explanation is that this fluorophore engages the cellular xenobiotic efflux system, enabling its rapid appearance in ductal networks. This efflux pathway is active in the renal proximal tubule, intestine, liver, and blood–brain barrier (Thiebaut et al., 1987). To address this possibility, inhibitors of xenobiotic efflux transporter P-glycoprotein could be exploited to test for suppression of BODIPY C2's labeling pattern.
Figure 2. Dietary BODIPY fatty acid can be used to label digestive organs and simultaneously interrogate organ physiology.

(A) BODIPY-FL C2 does not participate appreciably in lipid metabolism. Fluorescence appears primarily in the intestinal lumen of larvae (not shown). In the liver (left panel), BODIPY-FL C2 accumulates in the hepatic ducts (arrow) and diffusely in hepatocytes, whose nuclei are discernable (empty arrowhead). The ductal network of the exocrine pancreas (right panel) is also labeled (arrow). The gall bladder (filled arrowhead) is indicated. Scale bars = 10 μm. (B) BODIPY-FL C5 reveals significant subcellular details within larval digestive organs (6 dpf). In enterocytes of the anterior intestine, BODIPY-FL C5 appears in lipid droplets as well as in small endosomal-like compartments (not shown) and illuminates the intrahepatobiliary ductal network (left panel). In the right liver lobe, a single long duct (arrow), numerous interconnecting ducts and terminal ductules (arrowhead) are illuminated. Subcellular details of larval hepatocytes are also revealed (right panel). Lipid droplets (filled arrowhead), hepatocyte nuclei (empty arrowhead), and intrahepatic ducts (arrow) are indicated. Scale bars = 10 μm. (C) BODIPY-FL C12 is absorbed and transported in the digestive organs of zebrafish larvae. The analog appears in the intestinal lumen as well as in lipid droplets in intestinal enterocytes (not shown) and readily accumulates in the liver, visualizing large lipid droplets (filled arrowhead), hepatic ducts (arrow), and hepatocyte nuclei (empty arrowhead) (left panel). BODIPY-FL C12 accumulates in fluorescent foci (arrowhead) in the exocrine pancreas (Right panel). Scale bars = 10 μm. (D) BODIPY-FL C16 accumulates in lipid droplets in the digestive organs of live larval zebrafish. Enterocytes of the anterior intestinal bulb readily absorb BODIPY-FL C16 following an egg yolk and analog feed (not shown) and accumulates within large hepatic lipid droplets (filled arrowhead) and faintly in hepatic ducts (arrow) (left panel). Hepatocyte nuclei (empty arrowhead) are also visible. BODIPY-FL C16 accumulates throughout the exocrine pancreas, forming small fluorescent foci (arrowhead) (right panel). Beneath the exocrine pancreas, an intestinal blood vessel (arrow) running the length of the intestine fluoresces, indicating analog entry into the vascular system. Scale bars = 10 μm. In all images, anterior is to the right to enhance liver viewing.
2.3 BODIPY C5
Using BODIPY C5, we attain the most extensive cellular labeling. As a medium-chain fatty acid (MCFA; 6–12 carbon atoms), it can be transported directly to the liver and/or incorporated into more complex lipids and travel via the lipoprotein pathway. We found that LDs throughout a wide range of cell types were labeled, as well as ductal and arterial networks, thereby revealing the subcellular structures of multiple cell types (Fig. 2B). The ability of BODIPY C5 to label both cell membranes and TAG-rich lipid droplets is consistent with our biochemical analyses that revealed its incorporation into both phospholipids and TAG (Carten et al., 2011). The hepatic fluorescence accumulation patterns (in the cytoplasm and in lipid droplets) suggest that BODIPY C5 utilizes both chylomicron-mediated and basic secretion (chylomicron-independent) mechanisms of transport. In summary, BODIPY C5 is ideal for studies that seek widespread labeling of cellular structures and digestive organ morphology.
2.4 BODIPY C12/C16
BODIPY C12 and BODIPY C16 act as long-chain fatty acids (LCFAs; 13–21 carbon atoms) and, as such, can only be transported from the intestine via lipoproteins due to their hydrophobicity. In contrast to the shorter chain BODIPY FAs, both these fluorescent analogs appear in larval hepatic ductal networks after longer labeling times. Both fluorescent lipids readily accumulate in cytoplasmic lipid droplets (LD) in enterocytes and hepatocytes (Fig. 2C and D). Where we observe a difference between these LCFA BODIPY lipids is their ability to label cell membranes. BODIPY C12, like BODIPY C5, is incorporated into membrane phospholipids in a similar pattern to radiolabeled H3 palmitate (C16:0) (Miyares, de Rezende, & Farber, 2014), whereas BODIPY C16 is only incorporated into TAG (Carten et al., 2011).
In summary, the key factors driving the choice of BODIPY-conjugated FA for subsequent studies are their differential ability to label ductal networks, LDs, and cell membranes.
Summary
In this chapter, we have summarized the labeling patterns of BODIPY-conjugated FAs. The optical transparency of zebrafish larvae can be fully exploited by using transgenic lines, fluorescent reporters, and lipid analogs to visualize metabolic events. Furthermore, the high genetic conservation across lipid signaling and metabolic pathways allows pharmacological regents to be utilized to study the importance of lipids during early development. The high fecundity, small size, and genetic tractability of these organisms make them ideal for high-throughput screening efforts to identify genes involved in lipid metabolism and thus identify potential therapeutic targets for human diseases.
Acknowledgments
This work was supported in part by the National Institute of Diabetes and Digestive and Kidney (NIDDK) F31DK091129 to J.D.C. and RO1DK093399 to S.A.F., National Institute of General Medicine (GM) RO1GM63904 to the Zebrafish Functional Genomics Consortium (Stephen Ekker and S.A.F.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH). Additional support for this work was provided by the Carnegie Institution for Science endowment and the G. Harold and Leila Y. Mathers Charitable Foundation to the laboratory of S.A.F.
List of Abbreviations
- ACAT
Acyl CoA:cholesterol acyltransferase
- FA
Fatty acid
- LCFA
Long-chain fatty acid
- LD
Lipid droplet
- MCFA
Medium-chain fatty acid
- SCFA
Short-chain fatty acid
- TAG
Triacylglycerol
References
- Anderson JL, Carten JD, Farber SA. Zebrafish lipid metabolism: from mediating early patterning to the metabolism of dietary fat and cholesterol. Methods in Cell Biology. 2011;101:111–141. doi: 10.1016/B978-0-12-387036-0.00005-0. http://dx.doi.org/10.1016/B978-0-12-387036-0.00005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariola FS, Li Z, Cornejo C, Bittman R, Heikal AA. Membrane fluidity and lipid order in ternary giant unilamellar vesicles using a new bodipy-cholesterol derivative. Biophysical Journal. 2009;96(7):2696–2708. doi: 10.1016/j.bpj.2008.12.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham-Davidi I, Ely Y, Pham VN, Castranova D, Grunspan M, Malkinson G, et al. Yaniv K. ApoB-containing lipoproteins regulate angiogenesis by modulating expression of VEGF receptor 1. Nature Medicine. 2012 doi: 10.1038/nm.2759. http://dx.doi.org/10.1038/nm.2759. [DOI] [PMC free article] [PubMed]
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien M, Poirier P, Lemieux I, Despres JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Progress in Cardiovascular Diseases. 2014;56(4):369–381. doi: 10.1016/j.pcad.2013.10.016. http://dx.doi.org/10.1016/j.pcad.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Bauer UE, Briss PA, Goodman RA, Bowman BA. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384(9937):45–52. doi: 10.1016/S0140-6736(14)60648-6. http://dx.doi.org/10.1016/S0140-6736(14)60648-6. [DOI] [PubMed] [Google Scholar]
- Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG, 2nd, Ekker SC. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491(7422):114–118. doi: 10.1038/nature11537. http://dx.doi.org/10.1038/nature11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasaemle DL, Rubin B, Harten IA, Gruia-Gray J, Kimmel AR, Londos C. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. The Journal of Biological Chemistry. 2000;275(49):38486–38493. doi: 10.1074/jbc.M007322200. http://dx.doi.org/10.1074/jbc.M007322200. [DOI] [PubMed] [Google Scholar]
- Buhman KK, Smith SJ, Stone SJ, Repa JJ, Wong JS, Knapp FF, Jr, et al. Farese RV., Jr DGAT1 is not essential for intestinal triacyl-glycerol absorption or chylomicron synthesis. Journal of Biological Chemistry. 2002;277:25474–25479. doi: 10.1074/jbc.M202013200. [DOI] [PubMed] [Google Scholar]
- Carten JD, Bradford MK, Farber SA. Visualizing digestive organ morphology and function using differential fatty acid metabolism in live zebrafish. Developmental Biology. 2011 doi: 10.1016/j.ydbio.2011.09.010. http://dx.doi.org/10.1016/j.ydbio.2011.09.010. [DOI] [PMC free article] [PubMed]
- Chen HC, Farese RV., Jr Fatty acids, triglycerides, and glucose metabolism: recent insights from knockout mice. Current Opinion in Clinical Nutrition and Metabolic Care. 2002;5(4):359–363. doi: 10.1097/00075197-200207000-00002. [DOI] [PubMed] [Google Scholar]
- Clifton JD, Lucumi E, Myers MC, Napper A, Hama K, Farber SA, et al. Pack M. Identification of novel inhibitors of dietary lipid absorption using zebrafish. PLoS One. 2010;5(8) doi: 10.1371/journal.pone.0012386. http://dx.doi.org/10.1371/journal.pone.0012386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delous M, Yin C, Shin D, Ninov N, Debrito Carten J, Pan L, et al. Stainier DY. Sox9b is a key regulator of pancreaticobiliary ductal system development. PLoS Genetics. 2012;8(6):e1002754. doi: 10.1371/journal.pgen.1002754. http://dx.doi.org/10.1371/journal.pgen.1002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever W, Solnica-Krezel L, Schier AF, Neuhauss SC, Malicki J, Stemple DL, et al. Boggs C. A genetic screen for mutations affecting embryogenesis in zebrafish. Development. 1996;123:37–46. doi: 10.1242/dev.123.1.37. [DOI] [PubMed] [Google Scholar]
- Enzler L, Smith V, Lin JS, Olcott HS. The lipids of Mono Lake, California, brine shrimp (Artemia salina) Journal of Agricultural and Food Chemistry. 1974;22(2):330–331. doi: 10.1021/jf60192a017. [DOI] [PubMed] [Google Scholar]
- Farber SA, Olson ES, Clark JD, Halpern ME. Characterization of Ca2+-dependent phospholipase A2 activity during zebrafish embryogenesis. The Journal of Biological Chemistry. 1999;274(27):19338–19346. doi: 10.1074/jbc.274.27.19338. [DOI] [PubMed] [Google Scholar]
- Farber SA, Pack M, Ho SY, Johnson ID, Wagner DS, Dosch R, et al. Halpern ME. Genetic analysis of digestive physiology using fluorescent phospholipid reporters. Science. 2001;292(5520):1385–1388. doi: 10.1126/science.1060418. [DOI] [PubMed] [Google Scholar]
- Field FJ. Regulation of intestinal cholesterol metabolism. In: Mansbach CM, Tso P, Kuksis A, editors. Intestinal Lipid Metabolism. New York: Kluwer Academic; 2001. pp. 235–255. [Google Scholar]
- Field HA, Dong PD, Beis D, Stainier DY. Formation of the digestive system in zebrafish. II. Pancreas morphogenesis. Developmental Biology. 2003;261(1):197–208. doi: 10.1016/s0012-1606(03)00308-7. [DOI] [PubMed] [Google Scholar]
- Frohlich F, Petit C, Kory N, Christiano R, Hannibal-Bach HK, Graham M, et al. Walther TC. The GARP complex is required for cellular sphingolipid homeostasis. eLife. 2015:4. doi: 10.7554/eLife.08712. http://dx.doi.org/10.7554/eLife.08712. [DOI] [PMC free article] [PubMed]
- Gocze PM, Freeman DA. Factors underlying the variability of lipid droplet fluorescence in MA-10 Leydig tumor cells. Cytometry. 1994;17(2):151–158. doi: 10.1002/cyto.990170207. http://dx.doi.org/10.1002/cyto.990170207. [DOI] [PubMed] [Google Scholar]
- Gut P, Baeza-Raja B, Andersson O, Hasenkamp L, Hsiao J, Hesselson D, et al. Stainier DY. Whole-organism screening for gluconeogenesis identifies activators of fasting metabolism. Nature Chemical Biology. 2013;9(2):97–104. doi: 10.1038/nchembio.1136. http://dx.doi.org/10.1038/nchembio.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA, et al. Nusslein-Volhard C. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development. 1996;123:1–36. doi: 10.1242/dev.123.1.1. [DOI] [PubMed] [Google Scholar]
- Hama K, Provost E, Baranowski TC, Rubinstein AL, Anderson JL, Leach SD, Farber SA. In vivo imaging of zebrafish digestive organ function using multiple quenched fluorescent reporters. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2009;296(2):G445–G453. doi: 10.1152/ajpgi.90513.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson HS, Hendrickson EK, Johnson ID, Farber SA. Intramolecularly quenched BODIPY-labeled phospholipid analogs in phospholipase A(2) and platelet-activating factor acetylhydrolase assays and in vivo fluorescence imaging. Analytical Biochemistry. 1999;276(1):27–35. doi: 10.1006/abio.1999.4280. [DOI] [PubMed] [Google Scholar]
- Ho SY, Thorpe JL, Deng Y, Santana E, DeRose RA, Farber SA. Lipid metabolism in zebrafish. Methods in Cell Biology. 2004;76:87–108. doi: 10.1016/s0091-679x(04)76006-9. [DOI] [PubMed] [Google Scholar]
- Ho SY, Lorent K, Pack M, Farber SA. Zebrafish fat-free is required for intestinal lipid absorption and Golgi apparatus structure. Cell Metabolism. 2006;3(4):289–300. doi: 10.1016/j.cmet.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtta-Vuori M, Uronen RL, Repakova J, Salonen E, Vattulainen I, Panula P, et al. Ikonen E. BODIPY-cholesterol: a new tool to visualize sterol trafficking in living cells and organisms. Traffic. 2008;9(11):1839–1849. doi: 10.1111/j.1600-0854.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- Hwang WY, Fu Y, Reyon D, Gonzales AP, Joung JK, Yeh JR. Targeted mutagenesis in zebrafish using CRISPR RNA-Guided Nucleases. Methods in Molecular Biology. 2015;1311:317–334. doi: 10.1007/978-1-4939-2687-9_21. http://dx.doi.org/10.1007/978-1-4939-2687-9_21. [DOI] [PubMed] [Google Scholar]
- Ivics Z, Kaufman CD, Zayed H, Miskey C, Walisko O, Izsvak Z. The Sleeping Beauty transposable element: evolution, regulation and genetic applications. Current Issues in Molecular Biology. 2004;6(1):43–55. [PubMed] [Google Scholar]
- Kawakami K, Takeda H, Kawakami N, Kobayashi M, Matsuda N, Mishina M. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Developmental Cell. 2004;7(1):133–144. doi: 10.1016/j.devcel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Kruit JK, Groen AK, van Berkel TJ, Kuipers F. Emerging roles of the intestine in control of cholesterol metabolism. World Journal of Gastroenterology. 2006;12(40):6429–6439. doi: 10.3748/wjg.v12.i40.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lada AT, Davis M, Kent C, Chapman J, Tomoda H, Omura S, Rudel LL. Identification of ACAT1- and ACAT2-specific inhibitors using a novel, cell-based fluorescence assay: individual ACAT uniqueness. Journal of Lipid Research. 2004;45(2):378–386. doi: 10.1194/jlr.D300037-JLR200. http://dx.doi.org/10.1194/jlr.D300037-JLR200. [DOI] [PubMed] [Google Scholar]
- Levy E, Spahis S, Sinnett D, Peretti N, Maupas-Schwalm F, Delvin E, et al. Lavoie MA. Intestinal cholesterol transport proteins: an update and beyond. Current Opinion in Lipidology. 2007;18(3):310–318. doi: 10.1097/MOL.0b013e32813fa2e2. [DOI] [PubMed] [Google Scholar]
- Li Z, Bittman R. Synthesis and spectral properties of cholesterol- and FTY720-containing boron dipyrromethene dyes. The Journal of Organic Chemistry. 2007;72(22):8376–8382. doi: 10.1021/jo701475q. http://dx.doi.org/10.1021/jo701475q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Mintzer E, Bittman R. First synthesis of free cholesterol-BODIPY conjugates. The Journal of Organic Chemistry. 2006;71(4):1718–1721. doi: 10.1021/jo052029x. [DOI] [PubMed] [Google Scholar]
- Liu HY, Lee N, Tsai TY, Ho SY. Zebrafish fat-free, a novel Arf effector, regulates phospholipase D to mediate lipid and glucose metabolism. Biochimica et Biophysica Acta. 2010;1801(12):1330–1340. doi: 10.1016/j.bbalip.2010.08.012. http://dx.doi.org/10.1016/j.bbalip.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Marks DL, Bittman R, Pagano RE. Use of Bodipy-labeled sphingolipid and cholesterol analogs to examine membrane microdomains in cells. Histochemistry and Cell Biology. 2008;130(5):819–832. doi: 10.1007/s00418-008-0509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FP, Wang Y, Sprenger N, Yap IK, Lundstedt T, Lek P, et al. Nicholson JK. Probiotic modulation of symbiotic gut microbial-host metabolic interactions in a humanized microbiome mouse model. Molecular Systems Biology. 2008;4:157. doi: 10.1038/msb4100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyares RL, Stein C, Renisch B, Anderson JL, Hammerschmidt M, Farber SA. Long-chain Acyl-CoA synthetase 4A regulates Smad activity and dorsoventral patterning in the zebrafish embryo. Developmental Cell. 2013;27(6):635–647. doi: 10.1016/j.devcel.2013.11.011. http://dx.doi.org/10.1016/j.devcel.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyares RL, de Rezende VB, Farber SA. Zebrafish yolk lipid processing: a tractable tool for the study of vertebrate lipid transport and metabolism. Disease Models & Mechanisms. 2014;7(7):915–927. doi: 10.1242/dmm.015800. http://dx.doi.org/10.1242/dmm.015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma FJ, Jr, Barton AC, Kang HC, Brassard DL, Haugland RP, Sibley DR. Characterization of novel fluorescent ligands with high affinity for D1 and D2 dopaminergic receptors. Journal of Neurochemistry. 1989;52(5):1641–1644. doi: 10.1111/j.1471-4159.1989.tb09220.x. [DOI] [PubMed] [Google Scholar]
- Moschetta A, Xu F, Hagey LR, van Berge-Henegouwen GP, van Erpecum KJ, Brouwers JF, et al. Hofmann AF. A phylogenetic survey of biliary lipids in vertebrates. Journal of Lipid Research. 2005;46(10):2221–2232. doi: 10.1194/jlr.M500178-JLR200. [DOI] [PubMed] [Google Scholar]
- Otis JP, Farber SA. Imaging vertebrate digestive function and lipid metabolism. Drug Discovery Today Disease Models. 2013;10(1) doi: 10.1016/j.ddmod.2012.02.008. http://dx.doi.org/10.1016/j.ddmod.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack M, Solnica-Krezel L, Malicki J, Neuhauss SC, Schier AF, Stemple DL, et al. Fishman MC. Mutations affecting development of zebrafish digestive organs. Development. 1996;123:321–328. doi: 10.1242/dev.123.1.321. [DOI] [PubMed] [Google Scholar]
- Pagano RE, Martin OC, Kang HC, Haugland RP. A novel fluorescent ceramide analogue for studying membrane traffic in animal cells: accumulation at the Golgi apparatus results in altered spectral properties of the sphingolipid precursor. The Journal of Cell Biology. 1991;113(6):1267–1279. doi: 10.1083/jcb.113.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankinen T, Sarzynski MA, Ghosh S, Bouchard C. Are there genetic paths common to obesity, cardiovascular disease outcomes, and cardiovascular risk factors? Circulation Research. 2015;116(5):909–922. doi: 10.1161/CIRCRESAHA.116.302888. http://dx.doi.org/10.1161/CIRCRESAHA.116.302888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler KC, Rawls JF, Farber SA. Getting the inside tract: new frontiers in zebrafish digestive system biology. Zebrafish. 2013;10(2):129–131. doi: 10.1089/zeb.2013.1500. http://dx.doi.org/10.1089/zeb.2013.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R, Sudhof T. An interview with Randy Schekman and Thomas Sudhof. Trends in Cell Biology. 2014;24(1):6–8. doi: 10.1016/j.tcb.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Semova I, Carten JD, Stombaugh J, Mackey LC, Knight R, Farber SA, Rawls JF. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host & Microbe. 2012;12(3):277–288. doi: 10.1016/j.chom.2012.08.003. http://dx.doi.org/10.1016/j.chom.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AN, Davey CF, Whitebirch AC, Miller AC, Moens CB. Rapid reverse genetic screening using CRISPR in zebrafish. Nature Methods. 2015;12(6):535–540. doi: 10.1038/nmeth.3360. http://dx.doi.org/10.1038/nmeth.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence R, Gerlach G, Lawrence C, Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biological Reviews. 2008;83(1):13–34. doi: 10.1111/j.1469-185X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus E, Ahearn GA. Vertebrate gastrointestinal fermentation: transport mechanisms for volatile fatty acids. American Journal of Physiology. 1992;262(4 Pt 2):R547–R553. doi: 10.1152/ajpregu.1992.262.4.R547. [DOI] [PubMed] [Google Scholar]
- Thomson AB, Keelan M, Cheng T, Clandinin MT. Delayed effects of early nutrition with cholesterol plus saturated or polyunsaturated fatty acids on intestinal morphology and transport function in the rat. Biochimica et Biophysica Acta. 1993;1170(1):80–91. doi: 10.1016/0005-2760(93)90178-c. [DOI] [PubMed] [Google Scholar]
- Treibs A, Kreuzer F. Difluorboryl-Komplexe von Di- und Tripyrrylmethenen. Justus Liebigs Annalen der Chemie. 1969;721:116–120. [Google Scholar]
- Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host & Microbe. 2008;3(4):213–223. doi: 10.1016/j.chom.2008.02.015. http://dx.doi.org/10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


