SUMMARY
Objective
The mouse 6 Hz model of psychomotor seizures is a well-established and commonly used pre-clinical model for antiseizure drug (ASD) discovery. Despite its widespread use both in the identification and differentiation of novel ASDs in mice, a corresponding assay in rats has not been developed. We established a method for 6 Hz seizure induction in rats, with similar seizure behaviors as those observed in mice including head nod, jaw clonus, and forelimb clonus.
Methods
A convulsive current that elicits these seizure behaviors in 97% of rats (CC97) was determined using a Probit analysis. Numerous prototype ASDs were evaluated in this model using stimulus intensities of 1.5× and 2× the CC97, which is comparable to the approach used in the mouse 6 Hz seizure model (e.g., 32 and 44 mA stimulus intensities). The ASDs evaluated include carbamazepine, clobazam, clonazepam, eslicarbazepine, ethosuximide, ezogabine, gabapentin, lacosamide, lamotrigine, levetiracetam, phenobarbital, phenytoin, rufinamide, tiagabine, topiramate, and sodium valproate. Median effective dose (ED50) and median toxic (motor impairment) dose (TD50) values were obtained for each compound.
Results
Compounds that were effective at the 1.5×CC97 stimulus intensity at PI values > 1 included clobazam, ethosuximide, ezogabine, levetiracetam, phenobarbital, and sodium valproate. Compounds that were effective at the 2×CC97 stimulus intensity at PI values > 1 included ezogabine, phenobarbital, and sodium valproate.
Significance
In a similar manner to use of the mouse 6 Hz model, development of a rat 6 Hz test will aid in the differentiation of antiseizure drugs, as well as in study design and dose selection for chronic rat models of pharmacoresistant epilepsy. The limited number of established ASDs with demonstrable efficacy at the higher stimulus intensity suggests that, like the mouse 6 Hz 44 mA model, the rat 6 Hz seizure model may be a useful screening tool for pharmacoresistant seizures.
Keywords: psychomotor seizures, antiseizure drugs, animal models, pharmacoresistant epilepsy
INTRODUCTION
The mouse 6 Hz model of psychomotor seizures was originally developed in the 1950s 1; 2 and has been used to characterize established antiseizure drugs (ASDs) as well as novel compounds for their potential utility in the treatment of epilepsy 3–12. Efficacy in the mouse 6 Hz model has been observed for compounds comprising several distinct mechanisms of action, including sodium channel blockers, benzodiazepines and barbiturates, the SV2A ligands, δ2δ ligands, potassium channel modifiers, and others, with varying degrees of efficacy 3; 5; 6; 13. This model has also been used to identify and screen novel compounds and distinguish lead analogs 4; 8; 14. Moreover, in some cases where compounds may effectively block seizures at lower stimulus intensities it has been observed that this efficacy is lost at the 44 mA stimulus intensity. For example, levetiracetam has a median effective dose (ED50) value that is at least 50 times higher at the 44 mA stimulus intensity versus the 32 mA stimulus intensity, which has led to the designation of 44 mA as a more discriminatory pharmacoresistant screening model when compared to 32 mA 3. Thus, the 44 mA stimulus intensity distinguishes between compounds active at this stimulus intensity versus those that lose efficacy between 32 mA and 44 mA. Moreover, the 44 mA stimulus intensity is currently used in the screening and identification of novel compounds submitted to the Epilepsy Therapy Screening Program (ETSP) of the National Institute of Neurological Diseases and Stroke (http://www.ninds.nih.gov/research/asp/index.htm). Therefore, this seizure model in mice has been a useful tool in the identification and characterization of novel compounds with potential efficacy against pharmacoresistant seizures. Despite the successful application of this model in mice to a variety of ASDs, a comparable model in rats has previously not been developed.
Maximal electroshock (MES) and subcutaneous pentylenetetrazol (s.c. PTZ) seizure models have been long-standing models of pre-clinical identification and differentiation of ASDs15–17. Testing methods in mice and rats are similar for both MES and s.c. PTZ-induced seizures, with changes in either the current applied (for MES testing) or the dose of PTZ used. Conversely, despite the widespread use of the mouse 6 Hz model, the methodology for mouse 6 Hz seizures has not been translated to rats and consequently this is a critical missing component to pre-clinical antiseizure profiles for established ASDs and novel investigational compounds. In addition, the development of a rat 6 Hz seizure model will aid in study design and dose selection in other rat models such as the lamotrigine-resistant kindled rat18; 19 or the post-kainic acid spontaneously epileptic rat, which may be a more accurate representation of human temporal lobe epilepsy20–22. As these tests are generally more costly, evaluation of 6 Hz-induced seizures in rats helps to address an unmet need for development of new models of pharmacoresistant epilepsy that are suitable for screening23.
In the present studies, we sought to develop a model of 6 Hz seizures in rats that produced behaviors following stimulation that are similar to those observed in mice and would allow for similar quantification. Pharmacologic efficacy in the rat 6 Hz seizure model could then be compared to mouse 6 Hz seizure profiles for selected compounds. Further, as the rat MES model has been used routinely in the identification and differentiation of ASDs, efficacy in the rat 6 Hz model was also compared to the rat MES test. In order to fully characterize the rat 6 Hz seizure model, a seizure threshold was first determined and this was then followed by suprathreshold stimulation following administration of one of several ASDs at varying doses. Pharmacologic characterization of this preclinical seizure model will allow for integration of this model into a standard screening paradigm for characterization of novel antiseizure therapies.
METHODS
Compound Preparation
All compounds were prepared in 0.5% methylcellulose (Sigma; St. Louis, MO, USA) suspensions, with the exception of sodium valproate, which was prepared in saline (0.9% NaCl). Carbamazepine, clobazam, clonazepam, ethosuximide, ezogabine, phenobarbital, phenytoin, and sodium valproate were obtained from Sigma (St Louis, MO, USA). Eslicarbazepine, gabapentin, levetiracetam, rufinamide, tiagabine, and topiramate were obtained from TCI America (Portland, OR, USA). Lacosamide was obtained from Axon Medchem (Groningen, Netherlands). Lamotrigine was obtained from AK Scientific (Union City, CA, USA).
Animals and Compound Administration
Adolescent and adult male Sprague-Dawley rats (100–120 g and 200–220g, respectively) and adult male CF-1 mice (20–30 g) were obtained from Charles River (Raleigh, NC, USA and Kingston, NY, USA for rats and mice, respectively). Animals were allowed free access to food and water, except during testing periods. After delivery, animals were allowed sufficient time to acclimate to housing conditions prior to testing (~1 week). All mice were housed in plastic cages in rooms with controlled humidity, ventilation, and lighting (12 hours on – 12 hours off). The animals were housed and fed in a manner consistent with the recommendations in the “Guide for Care and Use of Laboratory Animals” (National Research Council). Housing, handling, and testing was performed in accordance with Public Health Service policy guidelines and a protocol approved by the Institutional Animal Care and Use Committee of the University of Utah. Animal experiments were conducted in a manner consistent with ARRIVE guidelines (https://www.nc3rs.org.uk/arrive-guidelines). Test compounds were administered using an optimal fluid volume to body fluid ratio. Test compounds were administered to mice in a volume of 0.04 ml/10 g body weight in rats and 0.01 ml/g body weight in mice. All compounds were administered by intraperitoneal (IP) injection 0.25 h (carbamazepine, clonazepam, ethosuximide, lacosamide, tiagabine), 0.5 h (clobazam, eslicarbazepine, ezogabine, phenytoin, rufinamide, sodium valproate), 1.0 h (lamotrigine, levetiracetam), or 2.0 h (gabapentin, phenobarbital, topiramate) prior to testing. For each compound evaluated, a time-of-peak effect (TPE) was determined in the MES test (rats and mice) or in the 6 Hz test (mice) (data not shown) prior to dose-response studies described herein. All animals were used for testing only once, with the exception of rats that were implanted with surface electroencephalogram (EEG) electrodes (250–350 g at testing) see description below), which received up to 3 separate simulations (3–7 days apart).
6 Hz Psychomotor Seizure Test
Behavioral evaluation of 6 Hz-induced seizures in rats
Focal seizures were induced in mice and rats via corneal stimulation (6 Hz, 0.2 msec rectangular pulse, 3 sec duration)3. Prior to stimulation, drops of 0.5% tetracaine were applied to each eye. For both mouse and rat studies, animals were observed for signs of behavioral seizure activity including head nodding, jaw clonus, forelimb clonus, and twitching of the vibrissae. Animals not displaying these behaviors within a 1-minute observation period were considered protected. Mice were tested at either 32 mA or 44 mA stimulus intensities, which represent stimulus intensities 1.5× and 2x, respectively, the convulsive current for inducing seizures in 97% of mice (CC97)3. For dose-response studies in rats, a Grass S48 stimulator was used with similar settings to those used for mice. Specifically, for the induction of 6 Hz seizures in mice, the stimulus isolation unit resistance setting on the Grass S48 stimulator is R = 250 ohm. In addition, the corneal electrode used for mice includes an in-series resistor (R = 3000 ohm). Conversely, for seizure induction in rats, the internal Grass S48 resistance is set to 25 ohm and the corneal electrode does not include an internal resistor. As this stimulation is under constant voltage, current can only be estimated based on the known resistance and voltage settings and Ohm’s law (voltage = current × resistance). However, using only this calculation for both rats and mice does not accurately reflect resistance within the circuit and thereby the actual current experienced during seizures. Therefore, we sought to determine actual currents and resistance experienced by rats and mice during 6 Hz stimulation according to the methods described below.
Determination of electroconvulsive currents experienced by rodents when stimulated by constant-voltage mode stimulators
In order to determine currents experienced by rodents experimentally, a stimulation wand was constructed with a 100 Ohm resistor (Rm) in series with the positive output lead of a Model 4100 Isolated High Power Stimulator (A-M systems, Carlsborg, WA, USA). The voltage across this resistor was measured using pClamp 10 interfaced to a Digidata 1440A data acquisition board (Molecular Devices, Sunnyvale, CA, U.S.A.) at a sampling rate of 250 kHz and low-pass filtered at 10 kHz. The average amplitude of 18 square voltage deflections (6 Hz stimulation lasting 3 seconds) was converted to a corresponding current (mA) according to Ohm’s Law:
The effective resistance of the rat or mouse (Rrodent) was calculated according to a voltage divider equation:
This equation was rearranged to solve for Rrodent:
The true voltage experienced by the rodent (Vrodent), which deviated from Vo due to a fractional voltage drop across the 100 Ohm series resistor (Rm), was calculated according to the following voltage divider equation:
These adjusted voltages were used for current vs. voltage relationship graphs as well as all CC50 and CC97 probit calculations when the data used to obtain these values was acquired with the modified stimulation wand containing the 100 Ohm series resistor
EEG recordings of 6 Hz seizures
In order to determine the electrographic characteristics of 6 Hz-induced seizures in rats, we implanted 12 adult rats with surface EEG electrodes. Male rats (180–200g) were anesthetized with 2–5% isofluorane and implanted with two EEG (surface) electrodes. A midline incision (0.5–1 cm) was made through the skin on the head after which the skin and other tissue were bluntly dissected away from the skull. Two holes were drilled on one side of the skull using a hand drill. Wires were placed inside the holes and held in place using dental acrylic. EEG and video recordings were captured prior to (1–5 min), during (1–3 min), and following (3–5 min) stimulation24. Spectrograms were calculated using the chronux toolbox24. The computed spectral power was summed between 20 and 70 Hz for two minutes following stimulation, and normalized to the summed 20–70Hz spectral power one minute prior seizure.
Maximal Electroshock Seizure Testing in Rats
The MES test consists of electrical stimulation of the cornea using 60 Hz of alternating current (150 mA), with a stimulus duration of 0.2 sec, using a custom-build MES stimulator. This stimulation produces tonic hindlimb extension in rats, and animals that did not display this behavior following ASD administration were considered protected. On the day prior to treatment and testing in the MES model, all rats received MES stimulation and rats that did not display tonic hindlimb extension were excluded from further testing. Previous experience suggests that approximately 10% of rats stimulated in the MES model do not display tonic hindlimb extension under baseline conditions. Therefore, on the day prior to treatment and testing in the MES model, all rats received MES stimulation and rats that did not display tonic hindlimb extension were excluded from further testing.
Minimal Motor Impairment (MMI) Assays in Rats and Mice
Rats were considered to be impaired if they displayed two or more of the following behaviors: abnormal gait, body posture, tremors, hyperactivity, lack of exploratory behavior, somnolence, stupor, catalepsy, loss of placing response and changes in muscle tone. Motor impairment in mice was quantified using a rotarod assay wherein mice were placed on a rotating knurled steel rod (6 rpm). Since mice can maintain equilibrium for long periods of time on the 6 rpm rotarod test, they are considered impaired if they fell off the rotarod three times during the one-minute observation period performed immediatelly prior to stimulation (rotarod failure).
Statistical Analysis
Data are presented as means + standard error. Median convulsive current (CC50) and CC97 values were calculated using a Probit analysis25. A median toxic dose (TD50) and 95% confidence interval (CI) was determined for each compound evaluated in either the MMI test in rats or rotarod test in mice using Probit analysis25. Median effective dose (ED50) was determined for each compound evaluated in the 6 Hz and MES assays using a Probit analysis. Protective index (PI) was calculated as TD50/ED50 for each compound in the mouse 6 Hz (32 and 44 mA, 1.5×CC97 and 2×CC97, respectively), rat 6 Hz (1.5×CC97 and 2×CC97), and rat MES tests.
RESULTS
Evaluation of 6 Hz Seizures in Rats and Mice
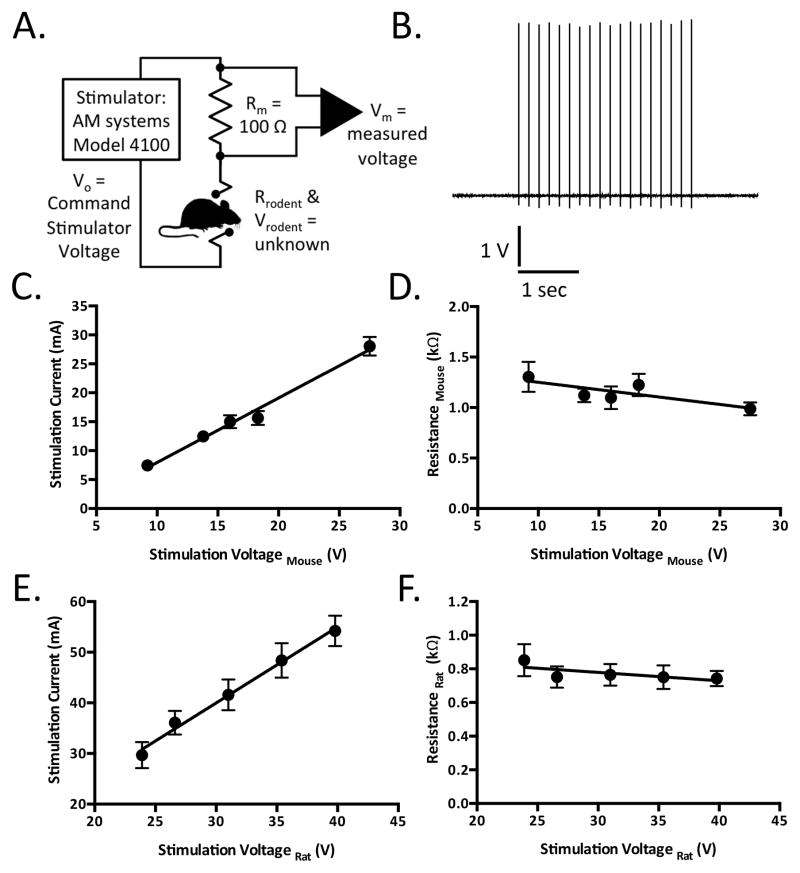
Seizures arising from 6 Hz stimulation in rats are similar to those observed for mice3, characterized by a minimal clonic phase followed by stereotyped automatistic behaviors including stun, forelimb clonus, twitching of the vibrissae, and Straub-tail. In rats, seizure behaviors similarly consist of head nodding, jaw clonus, forelimb clonus with occasional bilateral forelimb clonus, rearing, and loss of the righting reflex. In order to determine a CC97 for rats receiving 6 Hz stimulation, separate groups of rats were subjected to varying voltages using the Grass stimulator under constant voltage stimulation (25 V – 100 V), as shown in Table S1. There was a stimulus-dependent increase in the observation of seizures, with no seizures observed at the lowest stimulus intensity (25 V), and all rats experiencing seizures at stimulus intensities of 50 V and 100 V. The CC97 (95% CI) was determined to be 40 V(35.5 – 70.7) (see also Table 1). As shown in Figure 1, electroconvulsive currents generated using constant voltage mode obey Ohm’s law and can therefore be used to determine rodent resistances. Figure 1A includes a circuit diagram of the experimental design for determination of current and resistance. Figure 1B is a representative trace of a 6 Hz stimulation waveform (250 kHz sampling rate), measured across a 100 Ohm resistor in series with the rat receiving stimulation. The stimulation current (Figure 1C) and resistance (Figure 1D) were determined during stimulation at five different voltages in CF-1 mice. The mean electrical resistance in mice was found to be 1.15 + 0.05 kΩ (N=29). Similarly, stimulation current (Figure 1E) and resistance (Figure 1F) were determined during stimulation at five different voltages in Sprague-Dawley rats. The mean electrical resistance in rat was found to be 0.77 + 0.03 kΩ (N=30). Table 1 includes a summary of seizure threshold (CC50 and CC97) values as determined using Grass (constant voltage) and AM Systems (constant current and constant voltage) stimulators.
Table 1.
Summary of Seizure Threshold (CC50 and CC97) Determination using Grass (Constant Voltage) and AM Systems (Constant Current and Constant Voltage).
| Constant Voltage (V) Setting | Derived Calculated Current (mA) | Constant Current Setting Measured Current (mA) | ||||
|---|---|---|---|---|---|---|
| Stimulator | Grass S48 | AM Systems | Grass S48 | AM Systems | Grass S48 | AM Systems |
| Mouse CF-1 | ||||||
| CC50 | -- | 17.2 | 19.4a | 17.7 | n.a. | 14.7 |
| 95% CI | -- | 13.9 – 20.2 | 18.4 – 20.0 | 14.3 – 20.8 | n.a. | 11.6 – 17.2 |
| CC97 | -- | 22.8 | 22a | 23.5 | n.a. | 25.8 |
| 95% CI | -- | 19.6 – 99.6 | -- | 20.2 – 102.5 | n.a. | 20.7 – 44.1 |
| Rat Sprague-Dawley | ||||||
| CC50 | 31.9 | 26.4 | 42.2 | 35.0 | n.a. | 32.3 |
| 95% CI | 29.0 – 35.9 | 19.1 – 29.7 | 38.4 – 47.5 | 25.3 – 39.3 | n.a. | 26.9 – 35.6 |
| CC97 | 40.0 | 40.1 | 53.0 | 60.9 | n.a. | 45.3 |
| 95% CI | 35.5 – 70.7 | 33.7 – 99.7 | 47.0 – 93.6 | (51 – 142) | n.a. | 39.4 – 76.3 |
n.a. – not applicable; Grass S48 stimulator does not allow for constant current stimulation
(--) – not available
Barton et al. 20013
N=6 per group; rats (100–120g), mice (20–30g)
Figure 1.
Electroconvulsive currents experienced by rodents being stimulated in constant-voltage mode obey Ohm’s-law and can be used to determine rodent resistances. A. Circuit diagram illustrating experimental design. B. Representative trace of a 6 Hz stimulation waveform, sampled at 250 kHz, as measured across a 100 Ohm resistor in series with a male Sprague Dawley rat (100–120g, N=6–8 per group). Scale bar represents 1 V and 1 second. C–D. Stimulation currents (C) and mouse resistances (D) as a function of the stimulation voltage experienced by CF-1 mice (20–30 g). Each point represents the mean ± SEM (N=6 per group). Solid lines represent linear regression curves fit to the data. E–F. Stimulation currents (E) and mouse resistances (F) as a function of the stimulation voltage experienced by Sprague Dawley rats. Each point represents the mean ± SEM (N=6 per group). Solid lines represent linear regression curves fit to the data.
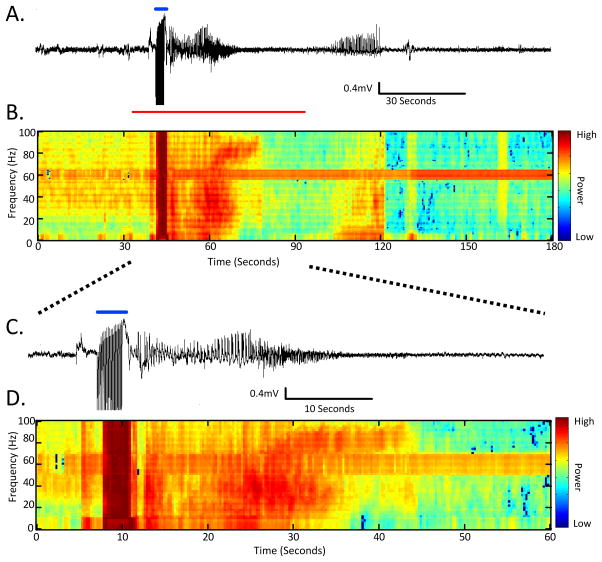
In order to better understand both the behavioral and non-behavioral electrographic components of seizures that arise from 6 Hz stimulation in rats, a group of animals implanted with cortical EEG electrodes were evaluated before, during, and after acute 6 Hz stimulation. The recordings shown in Figure 2, as well as Figure S1 – video recording of behavioral seizure activity with concomitant EEG recording, show electrographic seizure activity that persists for up to 2 min following 6 Hz stimulation. The majority of high-frequency epileptiform discharges occur immediately following 6 Hz stimulation (0–30 sec). Behavioral activity was always correlated with concurrent aberrant electrographic activity, however, electrographic discharges could persisted beyond cessation of behavioral seizure activity (see Figure 2, Figure S1).
Figure 2.
Example of electrographic activity from a convulsive seizure induced using 60V stimulation (1.5CC97, Grass S48 stimulator, constant voltage stimulation). A. 3 minutes of EEG activity surrounding a single stimulation. The time of stimulation is marked by a blue line. B. The time-frequency power normalized across frequency bands of the EEG from part (A) demonstrates the changing high-powered frequency content during epileptiform discharges inherent to a 6 Hz-induced convulsive seizure (arbitrary units). C. An enlargement of the EEG activity from the part in (A) highlighted by a red line. D. The time-frequency power normalized across frequency bands of the EEG from part (C) (arbitrary units). The behavioral aspects of this example seizure are demonstrated in the video as Figure S1. (N=6 per group, 250–350g at testing).
Following 6 Hz stimulation, rats were observed for signs of behavioral seizures (described above) and those not displaying any of these behaviors following ASD administration were considered “protected”. Several established ASDs were evaluated in the MMI, 6 Hz, and MES tests in rats in order to compare efficacy in both the MES and 6 Hz tests to motor toxicity and establish PI values (TD50/ED50) for each compound in these respective models (see Table 2). In the 6 Hz assay, compounds were screened at two stimulus intensities, 1.5× and 2× the CC97 stimulus intensity (60 V and 80 V, Grass stimulator, constant voltage). Each compound was evaluated at the previously determined TPE (e.g. previous testing in either 6 Hz or MES models was used to determine pre-treatment times), and 6 Hz testing generally occurred at the same TPE as that used in MMI and MES tests. Several doses for each compound were evaluated and PI values were determined for 6 Hz (1.5×CC97 and 2×CC97) and MES tests.
Table 2.
Anticonvulsant Profile of Prototype Compounds in Rats Following IP Administration
| Compound | TD50 or ED50 (mg/kg) and PI | |||
|---|---|---|---|---|
| MMI | 6 Hz 1.5×CC97 | 6 Hz 2×CC97 | MES | |
| Carbamazepine | 36.7 (21.7–45.1) | >40 PI<0.9 |
>40 PI<0.9 |
4.1 (2.7–5.8) PI 9.0 |
| Clobazam | 15.7 (8.0–24.3) | 12.4 (5.0–21.4) PI 1.3 |
22.1 (15.0–36.1) PI 0.7 |
36.3 (29.4–46.3) PI 0.4 |
| Clonazepam | 0.5 (0.3–1.9) | 0.7 (0.4–1.2) PI 0.7 |
>1.2 PI<0.4 |
0.7 (0.4–1.3) PI 0.7 |
| Eslicarbazepine | >100 | >200 PI n.c. |
>200 PI n.c. |
8.1 (3.9–12.4) PI>12.3 |
| Ethosuximide | 189 (140–228) | 172 (75–301) PI 1.1 |
242 (150–427) PI 0.8 |
>200 PI<0.9 |
| Ezogabine | 42.1 (37.7–48.1) | 9.1 (3.9–14.5) PI 4.6 |
18.9 (12.9–28.4) PI 2.2 |
5.1 (3.3–7.7) PI 8.3 |
| Gabapentin | 91 (63–130) | >300 PI<0.3 |
>300 PI<0.3 |
19.0 (8.8–43.8) PI 4.8 |
| Lacosamide | 13.0 (7.9–19.9) | 15.6 (6.3–30.0) PI 0.8 |
>50 PI<0.3 |
2.4 (1.8–3.1) PI 5.4 |
| Lamotrigine | 22.2 (15.5–29.9) | >60 PI<0.4 |
>60 PI<0.4 |
2.6 (2.1–3.5) PI 8.5 |
| Levetiracetam | >100 | 13.8 (3.2–25.7) PI>7.2 |
>800 PI n.c. |
>100 PI n.c. |
| Phenobarbital | 41.2 (37.0–46.6) | 27.3 (11.8–75.2) PI 1.5 |
41.2 (21.4–76.2) PI 1.0 |
2.6 (1.7–4.0) PI<15.8 |
| Phenytoin | 15.2 (11.9–19.2) | 30.7a PI 0.5 |
>100 PI<0.2 |
2.4 (1.4–3.7) PI 6.3 |
| Rufinamide | >350 | 42.7 (30.2–59.5) PI 8.2 |
n.c.b | 7.0 (3.1–10.4) PI>50 |
| Tiagabine | 8.0 (6.6–9.4) | 12.5a PI 0.6 |
19.9a PI 0.4 |
n.p. |
| Topiramate | 32.7 (20.2–48.5) | >300 PI n.c. |
>300 PI n.c. |
3.1 (1.3–5.7) PI 10.5 |
| Sodium Valproate | 351 (324–373) | 238 (167–309) PI 1.5 |
291 (243–347) PI 1.2 |
117 (74–160) PI 3.0 |
Minimal motor impairment (MMI); not calculated (n.c.); not performed (n.p.); Protective Index (PI; TD50/ED50)
95% confidence interval exceeded the dose range tested
Partial efficacy (50–65% efficacy) observed at doses of 80–200 mg/kg; reduced activity observed at a dose of 350 mg/kg
N=6–8 per group, 100–120g
Evaluation of ASDs Acting on Sodium Channels
As described above, seizures induced in rats using the 6 Hz stimulation paradigm are similar to those observed in mice. We sought to directly compare antiseizure efficacy for prototype ASDs between mouse and rat 6 Hz seizure models at 1.5× and 2×CC97 stimulation intensities. As shown in Table 3, compounds that act on sodium channels were generally not effective in the rat 6 Hz model, with the exception of rufinamide (Table 3). In the mouse 6 Hz model using the 32 mA (1.5×CC97) stimulus intensity, all sodium channel-acting compounds that were evaluated blocked seizures at ED50 concentrations and exhibited PI values of 1.0 or greater (Table 3). Of these, all but lamotrigine (PI = 0.7) remained effective, with PI values of 1.0 or greater in the mouse 6 Hz 44 mA test (Table 3). By contrast, in the rat 6 Hz model, with the exception of rufinamide, no compounds within this mechanism of action class effectively blocked seizures at the 1.5×CC97 and 2×CC97 stimulus intensities at PI values of 1.0 or greater. Rufinamide was efficacious at the 1.5×CC97 stimulus intensity but was only partially effective at the 2×CC97 stimulus intensity (see Table 3).
Table 3.
Comparison of Mouse and Rat 6 Hz Pharmacology for ASDs Acting on Na+ Channels
| Compound | ED50 and PI | ||||
|---|---|---|---|---|---|
| Mechanism of Action | Mouse Stimulus Intensity | Rat Stimulus Intensity | |||
| 1.5×CC97 (32mA) | 2×CC97 (44mA) | 1.5×CC97 | 2×CC97 | ||
| Carbamazepine | Blocks fast inactivation | 23.7 (18.3–26.3) PI 1.9 | 38.2 (25.8–69.0) PI 1.2 | >40 PI<0.9 |
>40 PI<0.9 |
| Eslicarbazepine | Blocks fast inactivation | 60.7 (46.1–72.4) PI 1.6 |
77.6 (61.3–92.4) PI 1.3 |
>200 PI n.c. |
>200 PI n.c. |
| Lacosamide | Enhance slow inactivation | 10.0 (7.7–12.8) PI 2.7 |
12.9 (9.8–18.2) PI 2.1 |
15.6 (6.3–30.0) PI 0.8 |
>50 PI<0.3 |
| Lamotrigine | Blocks fast inactivation | 19.5 (12.9–30.8) PI 1.5 |
43.4 (33.0–52.2) PI 0.7 |
>60 PI<0.4 |
>60 PI<0.4 |
| Phenytoin | Blocks fast inactivation | 37.5 (30.1–52.7) PI 1.3 |
44.6 (38.2–48.9) PI 1.1 |
30.7a PI 0.5 |
>100 PI<0.2 |
| Rufinamide | Enhance slow inactivation | 23.1 (16.8–34.0) PI >2.0 |
32.9 (23.2–39.5) PI >1.4 |
42.7 (30.2–59.5) PI 8.2 |
n.c.b |
not calculated (n.c.); Protective Index (PI; TD50/ED50)
95% CI exceeded the dose range tested
Partial efficacy (50–65% efficacy) observed at doses of 80–200 mg/kg; reduced activity observed at a dose of 350 mg/kg
N=6–8 per group, 100–120g
Evaluation of ASDs Acting on GABA Receptors or GABA Uptake
As shown in Table 4, the benzodiazepines clobazam and clonazepam, as well as the barbiturate phenobarbital and the GABA transporter blocker tiagabine were compared for antiseizure efficacy in the mouse and rat 6 Hz models. In the mouse 6 Hz model, all compounds evaluated in this group were active at both 32 mA and 44 mA stimulus intensities at PI values greater than 1.0. In the rat 6 Hz model, only clobazam and phenobarbital were active at PI values greater than 1.0 for both stimulus intensities, and only phenobarbital was efficacious in the 2× CC97 stimulus intensity.
Table 4.
Comparison of Mouse and Rat 6 Hz Pharmacology for ASDs Acting on GABA Receptors or Uptake
| Compound | ED50 and PI | ||||
|---|---|---|---|---|---|
| Mechanism of Action | Mouse Stimulus Intensity | Rat Stimulus Intensity | |||
| 1.5×CC97 (32mA) | 2×CC97 (44mA) | 1.5×CC97 | 2×CC97 | ||
| Clobazam | GABAA receptor allosteric modulation | 4.1 (2.6–4.7) PI 11.1 |
4.5 (3.1–6.6) PI 10.1 |
12.4 (5.0–21.4) PI 1.3 |
22.1 (15.0–36.1) PI 0.7 |
| Clonazepam | GABAA receptor allosteric modulation | 0.04 (0.03–0.06) PI 60 |
0.17 (0.14–0.19) PI 14.1 |
0.7 (0.4–1.2) PI 0.7 |
>1.2 PI<0.4 |
| Phenobarbital | GABAA receptor allosteric modulation | 14.8 (8.9–23.9) PI 4.7a |
35.3 (29.0–41.30 PI 1.9 |
27.3 (11.8–75.2) PI 1.5 |
41.2 (21.4–76.2) PI 1.0 |
| Tiagabine | GABA uptake inhibition | 0.7 (0.2–1.2) PI 2.0 |
1.0 (0.7–1.4) 2.0PI 1.3 |
12.5b PI 0.6 |
19.9b PI 0.4 |
Protective Index (PI; TD50/ED50)
Barton et al., 20013
95% confidence interval exceeded the dose range tested
N=6–8 per group, 100–120g
Evaluation of ASDs with Unique or Mixed Mechanisms of Action
As shown in Table 5, several compounds were active in the rat 6 Hz 1.5×CC97 stimulus intensity test, with ED50 values equal to or greater than TD50 values. By contrast, potency was lost between 1.5× and 2×CC97 stimulus intensities for several compounds in this group and only ezogabine, gabapentin, and sodium valproate were efficacious with PI values of 1.0 or greater. Of note, levetiracetam was potently active in the 1.5×CC97 whereas potency was substantially reduced in the 2×CC97 stimulus intensity. This observation was similar to that observed in the mouse 6 Hz model, where levetiracetam is highly efficacious using the 32 mA (1.5×CC97) stimulus intensity but not using the 44 mA (2×CC97) stimulus intensity3.
Table 5.
Comparison of Mouse and Rat 6 Hz Pharmacology for ASDs with Unique or Mixed Mechanisms of Action
| Compound | Mechanism of Action | ED50 and PI | |||
|---|---|---|---|---|---|
| Mouse Stimulus Intensity | Rat Stimulus Intensity | ||||
| 1.5×CC97 (32mA) | 2×CC97 (44mA) | 1.5×CC97 | 2×CC97 | ||
| Ethosuximide | T-type Ca2+ channels | 167 (114–223) PI 2.0a |
271 (234–309) PI 1.3 |
172 (75–301) PI 1.1 |
242 (150–427) PI 0.8 |
| Ezogabine | K+ channels | 42.5 (32.1–53.6) PI 0.4 |
32.5 (20.7–46.3) PI 0.5 |
9.1 (3.9–14.5) PI 4.6 |
18.9 (12.9–28.4) PI 2.2 |
| Gabapentin | α2δ subunit of voltage-gated Ca2+ channels | >350 PI n.c. |
>500 PI n.c. |
>300 PI n.c. |
>300 PI n.c. |
| Levetiracetam | SV2A modulation | 33.1 (9.9–81.2) PI>15.1 |
>1000 PI n.c. |
13.8 (3.2–25.7) PI>7.2 |
>800 PI n.c. |
| Topiramate | Mixed | >300 PI<1.3a |
>300 PI<1.3 |
>300 PI n.c. |
>300 PI n.c. |
| Sodium Valproate | Mixed | 126 (94.5–152) PI 3.2 |
239 (193–266) PI 1.7 |
238 (167–309) PI 1.5 |
291 (243–347) PI 1.2 |
Not calculated (n.c.); Protective Index (PI; TD50/ED50)
Barton et al., 20013
N=6–8 per group, 100–120g (rats), 20–30g (mice)
Additional testing using sodium valproate was also performed on rats with surface EEG electrodes. In a manner consistent with testing in non-implanted rats, sodium valproate dose-dependently reduced seizure activity at both 1.5×CC97 and 2×CC97 stimulus intensities (see Table S2). Interestingly EEG recordings from these animals do not suggest any change in normalized spectral power, despite protection from behavioral seizure activity (See Table S2).
Evaluation of ASDs in the Rat 6 Hz Model in Larger Rats
In order to determine whether age of animals contributed to ASD efficacy in the rat 6 Hz model, we evaluated carbamazepine, sodium valproate, and levetiracetam using both 60 V and 80 V (1.5× and 2× CC97 values, respectively; Grass stimulator, constant voltage) stimulus intensities, in both adolescent (100–120 g, 5–6 weeks old) or adult rats (200–220 g, 7–8 weeks old). There were no notable shifts in potency of the ASDs when tested in both ages of rats (see Table S3).
DISCUSSION
The mouse 6 Hz model of psychomotor seizures has become an important pre-clinical screening tool for evaluation of novel ASDs with diverse mechanisms of action; however, the translation of pharmacologic efficacy in this model to other species has been limited due to lack of a comparable animal model in rats. By contrast, the mouse and rat MES models have been useful screening models for identification and quantification of ASDs, and compounds that are active in the mouse MES model are generally active in rat MES. Therefore, a 6 Hz model in rats that utilizes comparable electrical stimulation and seizure behavior parameters is an important missing component to the current approach for identification and differentiation of novel ASDs that may be efficacious in a pharmacoresistant model. We observed that EEG recordings of seizures induced by 6 Hz stimulation were similar in all animals evaluated and that there were no notable differences in spectral power between two stimulus intensities (1.0× and 1.5× CC97). Interestingly, electrographic seizure activity persisted longer than behavioral seizure activity (normally lasting up to 30 s). The precise mechanism for latent electrographic seizure activity is unknown. Further, sodium valproate was without significant effect in reducing normalized spectral EEG power, despite reducing seizures in a dose-dependent manner at both stimulus intensities. Therefore, additional study is warranted using EEG recordings in rats following 6 Hz-induced seizures to explore the potential effects that various ASDs might have on electrographic seizure activity in this model. The electrographic seizures observations, however, are similar to video-EEG recordings in mice, which include both motor and non-motor seizures in addition to electrographic seizure activity in the frontal cortex and hippocampus26. It is noteworthy that only surface electrodes were used in the studies described herein. Future studies using depth electrodes can further elucidate the duration of electrographic seizure activity following 6 Hz stimulation.
Although the mouse 6 Hz test has been used for several years and by numerous groups to evaluate preclinical ASD pharmacology, differences in efficacy have been noted for some ASDs when evaluated by different research groups27. This can perhaps be attributed to changes in testing environments, mouse strain, and ASD manufacturers, as well as the stimulator systems used. In the studies described herein, however, we conclude that there are no substantial differences between stimulators even when different stimulation paradigms (i.e. constant current or constant voltage) are being used. For several years, the Anticonvulsant Drug Development Program has evaluated novel and prototype ASD compounds using the Grass S48 stimulator, which uses constant voltage stimulation. Previously, the stimulus intensity current was derived (using known voltages and an estimated resistance of the animal). However, in studies described herein for both mice and rats we utilize both constant current and constant voltage stimulation parameters and demonstrate that there are no substantial differences in current between Grass (constant voltage only) and AM Systems (constant current and constant voltage options). Further, we demonstrate that the calculated current is comparable to the measured current output at CC50 and CC97 intensities. Therefore, despite differences in either equipment used or stimulation mode (i.e., constant current or constant voltage), we show that seizure thresholds are similar.
It is noteworthy that some compounds with efficacy in the mouse 6 Hz model are not as potently active in the rat 6 Hz model. For example, carbamazepine and other sodium channel blockers are active at minimally toxic doses in the mouse model but not in the rat model. Clobazam and clonazepam are also potently active in the mouse model and show reduced efficacy or are not active in the rat model. By contrast, ezogabine is highly efficacious in the rat model, whereas in the mouse model, efficacy was limited. It is not currently known whether these discrepancies can be attributed to differences in pharmacokinetics between the two species or whether 6 Hz stimulation in rats produces a different pattern of neuronal or brain region-specific activation.
Several established ASDs were active in the mouse 44 mA (2×CC97) assay with ED50 values at or below the calculated TD50 values for motor impairment (carbamazepine, clobazam, clonazepam, eslicarbazepine, ethosuximide, lacosamide, phenobarbital, phenytoin, rufinamide, tiagabine, sodium valproate). The mouse 6 Hz model (44 mA stimulus intensity) has previously been described as a model of pharmacoresistance3 and is routinely used as a screening and quantification tool for novel ASDs. Similarly, in the rat 6 Hz model, few compounds were able to effectively reduce seizures using the 2×CC97 stimulus intensity in rats at ED50 values at or below the TD50 for motor impairment (e.g., ezogabine, phenobarbital, sodium valproate). Therefore, the rat 6 Hz model, using the 2×CC97 stimulus intensity, can be used as a screening tool to identify novel ASDs with potential activity against pharmacoresistant seizures.
In the rat MES model, several compounds comprising diverse mechanisms of action were effective (carbamazepine, eslicarbazepine, ethosuximide, ezogabine, gabapentin, lacosamide, lamotrigine, phenytoin, rufinamide, topiramate, sodium valproate). The rat 6 Hz model also demonstrated efficacy for several compounds at the 1.5×CC97 stimulus intensity (clobazam, ethosuximide, ezogabine, levetiracetam, phenobarbital, rufinamide, sodium valproate), whereas few compounds were effective at both 1.5× and 2×CC97 stimulus intensities (ezogabine, phenobarbital, sodium valproate). In addition, compounds demonstrating efficacy in the rat 6 Hz model include a broad range of mechanisms of action (e.g., sodium channel blockers, modulation of GABA, calcium, potassium, and SV2A, as well as other mechanisms). Therefore, the rat 6 Hz model is a useful tool for the identification and differentiation of novel ASDs.
A majority of sodium channel blockers were inactive in the rat 6 Hz model, although the sodium channel blocker rufinamide was potently active at the 1.5×CC97 stimulus intensity in rats. By contrast, performance of these compounds against MES seizures demonstrates the utility of the MES model in identifying compounds acting on sodium channels, though this model has not been effective in identifying some mechanistically novel ASDs such as levetiracetam. Although use of the MES model may bias drug discovery toward compounds with mechanisms of action that are well-suited to block MES-induced seizures (e.g., sodium channel blockers)17, it is also noteworthy that compounds with mechanisms of action that extend beyond sodium channel blockade (e.g., felbamate, topiramate, ezogabine, sodium valproate) are also active in the MES model, which suggests that further use of this model may help to identify compounds with unique mechanisms of action.
In comparison to mouse 6 Hz testing, compounds acting on sodium channels were generally less efficacious in the rat 6 Hz model than in the mouse 6 Hz model. For example, carbamazepine, lacosamide, and phenytoin were all effective in both 32 mA and 44 mA stimulus intensities in mice (albeit minimally toxic doses were required to block 44 mA seizures), but were ineffective at both stimulus intensities tested in rats. It is noteworthy that phenytoin was active against mouse 6 Hz seizures, with PI values of 1.1 and 1.3 for 32 mA and 44 mA, respectively, which is in contrast with previously reported data in this model showing lower potency for this compound 3. Further, although sodium channel blockers show lower potency in 6 Hz models, it is noteworthy that lacosamide and rufinamide, both of which act to enhance slow inactivation of sodium channels, were more efficacious in the mouse 6 Hz model. It is possible that 6 Hz seizures may be more sensitive to this mechanism of action, particularly in mice.
Some of the values for mouse 6 Hz efficacy differ from those reported previously. For example, we observed greater potency for phenytoin and lamotrigine (for both 32 mA and 44 mA stimulus intensities), than those previously reported3. The ED50 value we report (6 Hz 32 mA, ED50 37.5 mg/kg) is comparable to that reported by Leclercq and Kaminski (ED50 31 mg/kg)28. Similarly, carbamazepine shows approximately two-fold greater potency in the 32 mA mouse test than previously observed3. While it is possible that these differences may result from changes in supplier/formulation of phenytoin, lamotrigine, and carbamazepine, further studies would be needed to compare between different supplies of each compound to verify this potential discrepancy. In these studies, the mouse strain was the same (CF-1 mice), which is important since it has been shown that differences in the both seizure threshold and ASD potency have been reported in different mouse strains27; 28. Further, anecdotal findings suggest that even the same strain from the same supplier but shipped from two separate locations can produce differences in performance in the 6 Hz assay. In addition, it is noteworthy that 6 Hz efficacy was evaluated at the same time points used for MES testing and it is possible that different time points used may have contributed to the discrepancies between these studies and others in the mouse 6 Hz model.
The benzodiazepines, phenobarbital, and tiagabine were either ineffective (PI < 1) or demonstrated low/moderate efficacy (maximum PI 1.5) in the rat 6 Hz model at both stimulation intensities. In the rat MES test, the benzodiazepines and phenobarbital were ineffective. Interestingly, these compounds were generally more efficacious in the mouse 6 Hz model. It is likely that the mouse 6 Hz model would suggest a greater degree of efficacy for compounds acting on GABA receptors or transport; activity in this group of compounds in the rat 6 Hz model was limited to only phenobarbital.
Levetiracetam has been previously identified to have a notable shift in potency in mouse 6 Hz seizures between 32 mA and 44 mA stimulus intensities 3. A substantial shift in potency was also noted here for the two stimulus intensities used in the rat model. In the rat 6 Hz model, levetiracetam was potently active at the 1.5×CC97 stimulus intensity but this efficacy diminished at the higher stimulus intensity. In this way, both mouse and rat 6 Hz seizures demonstrate similar abilities to identify and distinguish the SV2A acting compound levetiracetam, though further testing is required to verify whether this effect is consistent for other SV2A ligands such as brivaracetam.
While we have evaluated a number of ASD compounds in both mice and rats for the purposes of comparing antiseizure pharmacology between species using 6 Hz-evoked seizures, future studies should endeavor to include collection of brain and plasma samples in tandem with collection of efficacy data. Thereby, we may be able to better assess whether discrepancies between efficacy in mouse and rat are due to differences in pharmacokinetics between the two species. Further, the inclusion of pharmacokinetic analysis concomitant with verification of antiseizure efficacy can be useful for dose guidance in clinical studies27. Therefore, future testing of novel ASD compounds in both the rat and mouse 6 Hz seizure models should include plasma and brain sampling for pharmacokinetic analysis whenever possible.
Since the mouse 6 Hz model was introduced and utilized as a screening tool, ASD compounds have been evaluated primarily using either 32 mA or 44 mA, representing the derived calculated currents corresponding to 1.5× and 2×CC97 values in CF-1 mice, respectively3 (see also Table 1). Similarly we report CC97 values in mice, obtained using either constant current or constant voltage stimulation, that are similar to those described previously. It is noteworthy however, that the nomenclature used to describe 6 Hz testing in mice (e.g. 32 mA, 44 mA) may not accurately reflect the true current that elicits seizures in mice. These studies suggest that the more accurate description would be in reference to either the 1.5× or the 2×CC97 values obtained experimentally. This approach may improve reproducibility between laboratories using the 6 Hz model.
Although 6 Hz-induced seizures in rats are generated in a similar manner to those used for the mouse 6 Hz model, and while both evoked seizure models can demonstrate similar behavioral endpoints, it is noteworthy that efficacy comparisons between species may be influenced by specific factors. For example, motor impairment measurements are different in mice (rotarod) and rats (MMI). The MMI assay in rats may be a more sensitive measure for motor impairment than the rotarod test in mice. It was further noted that pharmacologic profiles for several compounds were different between these models. Ezogabine was active in the rat 6 Hz model but less efficacious in the mouse 6 Hz model. Conversely carbamazepine, clonazepam, eslicarbazepine, lacosamide, phenytoin, and tiagabine were efficacious in the mouse 6 Hz model but less active or inactive in the rat 6 Hz model. It is noteworthy that the PI values observed for these compounds suggest that efficacy was noted at minimally toxic doses. Therefore, compounds with greater PI values (e.g. 4 or higher) may be more desirable to differentiate them from established ASDs. In addition, compounds with efficacy in the mouse 6 Hz model that are less effective in the rat 6 Hz model suggest that the rat 6 Hz model is less sensitive to compounds acting on sodium channels, GABAA receptors, or GABA uptake in comparison to the mouse 6 Hz model. This also suggests that the rat 6 Hz model can be helpful in detecting compounds with novel mechanisms of action and potential activity against pharmacoresistant seizures. As nearly 30% of patients with epilepsy are refractory to existing ASDs, it is critical that drug discovery efforts use novel models that are also refractory to existing ASDs 15. The newly described rat 6 Hz refractory seizure model can be used for differentiation of ASDs and, in comparison to evaluation in other testing paradigms such as the lamotrigine-resistant kindled rat or post-status epilepticus spontaneous seizures, the rat 6 Hz model is inexpensive and rapid.
Supplementary Material
Behavioral activity associated with the 6 Hz seizure shown in Figure 2. 6 Hz stimulation was generated using 60V (1.5 CC97; Grass S48 stimulator, constant voltage stimulation). Note that the motor seizure behavioral component (head nodding, forelimb clonus) finishes at ~30 seconds (~20 seconds following stimulation), but is followed by a stunned behavior (reduced movement) that persists for the duration of the recording. Epileptiform activity (e.g. high frequency and high amplitude EEG activity) occurs both in the presence and the absence of behavioral (motor seizure) activity.
KEY POINTS.
Widespread use of the mouse 6 Hz (44 mA) model as a pre-clinical screening tool has aided in the development of novel seizure drugs, but no comparative model has been developed in rats
The approach to developing a rat 6 Hz model has utilized similar methodology as is currently used for mouse 6 Hz testing, namely the use of 1.5× and 2× the CC97 stimulus intensities as a means of differentiating antiseizure drugs
The rat 6 Hz model produces similar seizure behaviors to those observed in the mouse 6 Hz model
Few compounds are effective (i.e. reduce seizures at doses below the median toxic dose) at the higher stimulus intensity
Development of the rat 6 Hz test will aid in the differentiation of antiseizure drugs as well as in study design and dose selection for chronic models of pharmacoresistant epilepsy
Acknowledgments
The authors would like to thank Stan Draper, Kristina Johnson, and Carlos Rueda for their technical contributions to this manuscript. The authors would also like to thank the Epilepsy Therapy Screening Program at the National Institutes of Neurological Diseases and Stroke for their review and comments on this manuscript. This project has been funded in whole or in part with Federal funds from the National Institute of Neurological Disorders and Stroke, Epilepsy Therapy Screening Program, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN271201100029C.
Footnotes
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Literature Cited
- 1.Toman JE. Neuropharmacologic considerations in psychic seizures. Neurology. 1951;1:444–460. doi: 10.1212/wnl.1.11-12.444. [DOI] [PubMed] [Google Scholar]
- 2.Brown WC, Schiffman DO, Swinyard EA, et al. Comparative assay of an antiepileptic drugs by psychomotor seizure test and minimal electroshock threshold test. J Pharmacol Exp Ther. 1953;107:273–283. [PubMed] [Google Scholar]
- 3.Barton ME, Klein BD, Wolf HH, et al. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–227. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
- 4.Barton ME, Peters SC, Shannon HE. Comparison of the effect of glutamate receptor modulators in the 6 Hz and maximal electroshock seizure models. Epilepsy Res. 2003;56:17–26. doi: 10.1016/j.eplepsyres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Bialer M, Johannessen SI, Levy RH, et al. Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI) Epilepsy Res. 2013;103:2–30. doi: 10.1016/j.eplepsyres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Bialer M, Johannessen SI, Levy RH, et al. Progress report on new antiepileptic drugs: A summary of the Twelfth Eilat Conference (EILAT XII) Epilepsy Res. 2015;111:85–141. doi: 10.1016/j.eplepsyres.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Wojda E, Wlaz A, Patsalos PN, et al. Isobolographic characterization of interactions of levetiracetam with the various antiepileptic drugs in the mouse 6 Hz psychomotor seizure model. Epilepsy Res. 2009;86:163–174. doi: 10.1016/j.eplepsyres.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Bulaj G, Green BR, Lee HK, et al. Design, synthesis, and characterization of high-affinity, systemically-active galanin analogues with potent anticonvulsant activities. J Med Chem. 2008;51:8038–8047. doi: 10.1021/jm801088x. [DOI] [PubMed] [Google Scholar]
- 9.White HS, Scholl EA, Klein BD, et al. Developing novel antiepileptic drugs: characterization of NAX 5055, a systemically-active galanin analog, in epilepsy models. Neurotherapeutics. 2009;6:372–380. doi: 10.1016/j.nurt.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bialer M, Johannessen SI, Kupferberg HJ, et al. Progress report on new antiepileptic drugs: a summary of the Eigth Eilat Conference (EILAT VIII) Epilepsy Res. 2007;73:1–52. doi: 10.1016/j.eplepsyres.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Bialer M, Johannessen SI, Levy RH, et al. Progress report on new antiepileptic drugs: a summary of the Ninth Eilat Conference (EILAT IX) Epilepsy Res. 2009;83:1–43. doi: 10.1016/j.eplepsyres.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Bialer M, Johannessen SI, Levy RH, et al. Progress report on new antiepileptic drugs: a summary of the Tenth Eilat Conference (EILAT X) Epilepsy Res. 2010;92:89–124. doi: 10.1016/j.eplepsyres.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Large CH, Sokal DM, Nehlig A, et al. The spectrum of anticonvulsant efficacy of retigabine (ezogabine) in animal models: implications for clinical use. Epilepsia. 2012;53:425–436. doi: 10.1111/j.1528-1167.2011.03364.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaminski K, Wiklik B, Obniska J. Synthesis and anticonvulsant activity of new -phenyl-2-(4-phenylpiperazin-1-yl)acetamide derivatives. Med Chem Res. 2015;24:3047–3061. doi: 10.1007/s00044-015-1360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilcox KS, Dixon-Salazar T, Sills GJ, et al. Issues related to development of new antiseizure treatments. Epilepsia. 2013;54(Suppl 4):24–34. doi: 10.1111/epi.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov. 2010;9:68–82. doi: 10.1038/nrd2997. [DOI] [PubMed] [Google Scholar]
- 17.Loscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20:359–368. doi: 10.1016/j.seizure.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Srivastava AK, Alex AB, Wilcox KS, et al. Rapid loss of efficacy to the antiseizure drugs lamotrigine and carbamazepine: a novel experimental model of pharmacoresistant epilepsy. Epilepsia. 2013;54:1186–1194. doi: 10.1111/epi.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srivastava AK, White HS. Carbamazepine, but not valproate, displays pharmacoresistance in lamotrigine-resistant amygdala kindled rats. Epilepsy Res. 2013;104:26–34. doi: 10.1016/j.eplepsyres.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorter JA, van Vliet EA, Lopes da Silva FH. Which insights have we gained from the kindling and post-status epilepticus models? J Neurosci Methods. 2016;260:96–108. doi: 10.1016/j.jneumeth.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Reddy DS, Kuruba R. Experimental models of status epilepticus and neuronal injury for evaluation of therapeutic interventions. Int J Mol Sci. 2013;14:18284–18318. doi: 10.3390/ijms140918284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams PA, White AM, Clark S, et al. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci. 2009;29:2103–2112. doi: 10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loscher W. The Search for New Screening Models of Pharmacoresistant Epilepsy: Is Induction of Acute Seizures in Epileptic Rodents a Suitable Approach? Neurochem Res. 2016 doi: 10.1007/s11064-016-2025-7. [DOI] [PubMed] [Google Scholar]
- 24.Mitra P, Bokil H. Observed Brain Dynamics. Oxford University Press; Oxford, UK: 2007. [Google Scholar]
- 25.Finney DJ. Probit Analysis. 3. Cambridge University Press; 1971. [Google Scholar]
- 26.Giordano C, Vinet J, Curia G, et al. Repeated 6-Hz Corneal Stimulation Progressively Increases FosB/DeltaFosB Levels in the Lateral Amygdala and Induces Seizure Generalization to the Hippocampus. PLoS One. 2015;10:e0141221. doi: 10.1371/journal.pone.0141221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loscher W. Fit for purpose application of currently existing animal models in the discovery of novel epilepsy therapies. Epilepsy Res. 2016;126:157–184. doi: 10.1016/j.eplepsyres.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Leclercq K, Kaminski RM. Genetic background of mice strongly influences treatment resistance in the 6 Hz seizure model. Epilepsia. 2015;56:310–318. doi: 10.1111/epi.12893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Behavioral activity associated with the 6 Hz seizure shown in Figure 2. 6 Hz stimulation was generated using 60V (1.5 CC97; Grass S48 stimulator, constant voltage stimulation). Note that the motor seizure behavioral component (head nodding, forelimb clonus) finishes at ~30 seconds (~20 seconds following stimulation), but is followed by a stunned behavior (reduced movement) that persists for the duration of the recording. Epileptiform activity (e.g. high frequency and high amplitude EEG activity) occurs both in the presence and the absence of behavioral (motor seizure) activity.