Abstract
Peripheral nerve injuries (PNIs) can be most disabling, resulting in the loss of sensitivity, motor function and autonomic control in the involved anatomical segment. Although injured peripheral nerves are capable of regeneration, sub-optimal recovery of function is seen even with the best reconstruction. Distal axonal degeneration is an unavoidable consequence of PNI. There are currently few strategies aimed to maintain the distal pathway and/or target fidelity during regeneration across the zone of injury. The current state of the art approaches have been focussed on the site of nerve injury and not on their distal muscular targets or representative proximal cell bodies or central cortical regions. This is a comprehensive literature review of the neurochemistry of peripheral nerve regeneration and a state of the art analysis of experimental compounds (inorganic and organic agents) with demonstrated neurotherapeutic efficacy in improving cell body and neuron survival, reducing scar formation and maximising overall nerve regeneration.
KEY WORDS: Cell body, nerve regeneration, nerve repair, neuron survival, peripheral nerve injury
INTRODUCTION
Peripheral nerve injuries (PNIs) are estimated to occur in 2.8% of multiple trauma patients, or 5% if root and plexus injuries are included. PNI can result from motor vehicle accidents, sharp lacerations, penetrating trauma, stretching or crushing trauma and fractures, and gunshot wounds. In any case, treatment costs, morbidity and loss of function that impact quality of life are significant.[1] Novel treatments to restore and augment nerve regeneration and accelerate functional recovery would be greatly beneficial in the management and rehabilitation of PNIs. Identifying strategies that maximise nerve regeneration and enable motor/sensory recovery after injury can help patients to return to functional or employable status while improving performance, morale, mobility, agility and capability.
OVERARCHING RESEARCH CHALLENGES IN NERVE REGENERATION
The management principles of nerve injuries have not changed in the past 50 years [Figure 1]. Despite substantially increased understanding of neuropathophysiology and experimental evidence of promising neurotherapies, obtaining satisfactory clinical outcomes are challenging in cases of avulsion, crush or blast injuries of peripheral nerves.[2]
Figure 1.
Rat sciatic nerve transection (a) and primary repair with epineural sutures (b) is seen
Although active rehabilitation following nerve injury may minimise the risk of disuse atrophy in muscle following nerve transection or trauma, denervation atrophy of muscle targets is a major hurdle to nerve regeneration strategies. Even the best neuroregeneration, outcomes may be compromised due to the lack of a viable neuromuscular junction (NMJ) or muscle target leading to poor functional results.
Any approach to peripheral nerve regeneration must thus be comprehensive and multifaceted, simultaneously facilitating natural host reparative processes and reintegration mechanisms, both proximal and distal to the zone of injury to overcome obstacles to axonal regeneration and successful reinnervation of the target organs. The primary research challenges involve:
The cell body (withdrawal of target-derived neurotrophic support; alterations in gene/protein expression and antidromic action potentials affecting key neuronal survival pathways in the dorsal root ganglia and cell bodies causing up-regulation in several genes associated with apoptosis; loss of neuroprotection and regeneration in distal segments)
Zone of injury and distal segment (molecular and cellular changes secondary to Wallerian degeneration [WD]; denervation of Schwann cells [SCs], and down-regulation of structural proteins such as protein zero, myelin basic protein and myelin-associated glycoprotein; slow, insufficient or misdirected axonal outgrowth and damaging intracellular cascades across and distal to site of injury)
Target organs (misdirection towards wrong targets, ‘pruning’ of growth cones that do not reach the correct target or lose the support of their endoneurial tubes chronically denervated SCs in axon cone, down-regulation of growth factors that support axonal progression, exhaustion of trophic factors in denervated target organs leading to NMJ loss and muscle fibre atrophy).
PATHOPHYSIOLOGY OF NERVE DEGENERATION AND REGENERATION
The classifications of Seddon and Maggi et al. are based on the anatomical and pathophysiological changes in the nerve following injury that dictate the necessity of surgical repair and impact prognosis of recovery.[3,4] However, regardless of the type of PNI, neuronal survival is key to overall outcomes.
Neuronal response to injury
Nerve injury results in both retrograde (cell body and dorsal root ganglia proximal to the site of injury) and anterograde (neuronal elements in the distal segment) effects and causes apoptotic cell death. The extent of neuronal death depends on the developmental age (adult neurons are less likely to die) and level and type of injury (dorsal root avulsion is more destructive than the sharp transection of distal axonal segment) with sensory neurons more affected than motor neurons (10%–30% compared to 0%–10% as seen in sciatic nerve injury models).[5,6]
Like most cells, neuronal survival depends on an ideal milieu of growth factors, hormones, cytokines and extracellular matrix (ECM) factors. Deprivation of extracellular signals (especially the target-derived neurotrophic support) triggers apoptotic cascades leading to neuronal death.[7] Programmed neuronal death following injury involves the formation of reactive oxygen species and reactive nitrogen species (ROS/RNS), mitochondrial damage, activation of the Bcl family of proteins and release of apoptosis-inducing factors into the cytosol. Nerve avulsion in rats results in both apoptosis and necrosis,[1,8] possibly due to the effect of RNS such as peroxynitrite (ONOO−).[9] Depending on the degree of PNI, oxidative stress and dose-dependent activation of ROS or RNS[10] can significantly influence the mechanism and extent of neuronal death.
After axotomy, injury signals generate a change in gene expression, switching the neuron from its signal transmission to regenerative status. This involves a change in molecular synthesis from neurotransmitters to cytoskeleton and growth associated proteins (GAPs).[11,12] The action potentials generated by disruption of the neurilemma and the influx of sodium and calcium are amongst the first to determine this change.[13] Negative signals related to loss of end-organ derived neurotrophic factors (the same loss that triggers apoptosis), paradoxically contribute to the survival of the neuron and regenerative switch.[14] Different signalling molecules involving the pro-apoptotic Bcl family (BAD, Hrk, Bid, Bik, Bak, Bax), as well as a pro-survival (Bcl-XL, Bcl-2, Bcl-W, Mcl-1) pathways are up-regulated after injury.[7] For example, a decrease in nerve growth factor (NGF) transport from the periphery to the neuronal cell body is an important ‘negative’ injury signal that induces axotomy related changes in gene expression and transcription factors.[14] Nerve injury causes structural alterations in axoplasmic proteins (such as transcription STAT3), which contribute to the regenerative switch after retrograde neuronal transport.[15]
Changes in the axonal stump
Nerve injury impairs axonal transport, acutely increases intracellular calcium, and activates axonal proteases (calpains) that lead to distal axonal breakdown. This process, called WD[16] helps the scavenging of myelin and axon growth inhibitors (such as myelin-associated glycoprotein) at the site of injury and promotes a pro-regenerative environment in the distal axonal stump. Degeneration involves also a short segment of the proximal stump, extending retrograde to the first node of Ranvier.[17] The SCs change from a myelinating to a growth promoting and cytokine-secreting phenotype. The dedifferentiation is induced by the loss of axonal contact and molecules secreted by the injured axon (neuregulin, calcitonin gene-related peptide).[18] The dedifferentiated SCs support nerve regeneration through two mechanisms. First, SCs initiate myelin clearance and up-regulate cytokines and chemokines for recruiting macrophages that complete removal of axonal and myelin debris. Calpain[19] and toll-like receptors (TLRs) play an important role in promoting cytokine secretion. TLRs bind to microbial ligands, as well as endogenous molecules (heat shock proteins [Hsps], ECM components) that signal injury and mediate the action of SCs during the inflammatory response.[20,21]
Second, dedifferentiated SCs multiply and organise within the basal laminar scaffold (that spared by WD in the endoneurial tubes), to form the bands of Büngner, guiding the axonal sprouts and ensuring the neurotrophic and neurotropic support.[22]
The growth cone
In the proximal stump, the retrograde morphological changes that reflect nerve regeneration consist of the transformation of the stable axonal segment into a highly active growth cone. The growth cone guides the regenerating neurites to their target end organs according to instructive neural cues made up by gradients of neurotrophic and neurotropic factors.[23]
The anatomy of the growth cone is divided into three main domains. The peripheral domain (P), highly motile, has a veil-like structure – lamellipodia with actin arranged in a meshwork with finger like extensions-filopodia, containing bundles of actin. The transition zone (T) is set between the peripheral and central domain, rich in myosin that contracts the actin network. The central domain contains microtubules (MTs). As the growth cone advances and the nascent axon elongates, MT becomes incorporated in the newly synthesised cytoskeleton and stabilised by the microtubule-associated proteins (MAPs). GAP-43 is a MAP, its expression being associated with high activity of the growth cone.[24]
Neurotrophic factors
Polypeptides
Numerous polypeptides, produced by non-neuronal cells after injury, ensure the survival, growth and guidance of regenerating nerves. Amongst the most studied of these factors are NGF, brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), neuregulins, pleiotrophin, insulin-like growth factors-1/2 ([IGF1, IGF2], interleukin-1 [IL-1], IL-6), transforming growth factor-beta 1 (TGF-b1), fibroblast growth factor (FGF), leukaemia inhibitory factor (LIF), vascular endothelial growth factor (VEGF) and sonic hedgehog/shh.[14,25]
Lipid rafts
Lipid rafts are membrane microdomains enriched in cholesterol and sphingolipids that help growth cone migration and orientation. They are associated with receptors for neurotrophic factors, which on receptor ligation, trigger receptor translocation to the lipid rafts. The asymmetric association and membrane polarisation of lipid rafts offers directional guidance to the growth cones.[26]
The extracellular matrix
The ECM, including the basal lamina, plays a very complex role in nerve regeneration. Immediately after injury, laminin, fibronectin, integrins and their ligands on the cell surface undergo upregulation. Apart from acting as a substratum for the migrating SCs, the ECM also possesses chemoattractant and mitogen properties. The ECM levels modulate integrin expression by influencing their rate of removal and recycling. Cell integrins bind to ECM proteins and transmit signals to actin filaments generating mechanical forces.[27,28]
NEUROINFLAMMATION – BLESSING AND BANE TO NERVE REGENERATION
Inflammation is an important phenomenon in nerve degeneration and regeneration. The timing and degree of inflammation may improve or impede the regeneration process. Cytokines released by macrophages and fibroblasts have a mitotic effect on SCs that facilitate remyelination. Proliferation of fibroblasts in the epineurium, perineurium or endoneurium can result in scar that includes inhibitory proteoglycans (such as chondroitin sulphate) or semaphorins.[29,30] The scar forms mechanical barrier to the regenerating axons, causes shrinkage of the endoneurial sheaths that contain the axons and contributes to the development of multiple branched axonal terminals to form a neuroma.[31]
The key for optimal nerve regeneration is to maintain or maximise the pro-regenerative capacity of the de-axonised distal nerve by ensuring the cell body and neuronal survival, to support recipient axonal regeneration (gap crossing and orientation of neuritis) to distal sensory/motor targets (NMJ and target muscle trophicity) as well as prevent neuroma (scar) formation. In addition to overall regeneration, the kinetics of regeneration is critical for functional outcomes as the distal targets need to be reinnervated before denervation atrophy. Various inorganic and organic compounds have been studied thus for to provide optimal nerve regeneration following PNI.
PROMISING INORGANIC AND ORGANIC COMPOUNDS WITH NEUROTHERAPEUTIC EFFICACY
Despite decades of experimental research on the role and relevance or effectiveness and efficacy of various small molecules, neurotransmitters, biologics and other pharmacologic agents in peripheral nerve regeneration, there are no approved pharmacotherapies for clinical application after nerve injury or repair. An exhaustive review of the literature provides us an understanding of potentially promising agents (inorganic and organic compounds) that could facilitate natural host reparative processes (e.g., SC migration and organisation), promote expeditious and targeted axonal outgrowth, and provide trophic support to maintain the efficacy of the distal pathway beyond the graft/lesion site in a manner consistent with functional reinnervation and recovery.
Antioxidants
Oxidative stress is considered to be one of the main causes of neural damage after injury. Previous studies have shown that PNI induces the production of ROS and nitric oxide in axotomised neurons. Cells and tissues are equipped with a variety of enzymatic and non-enzymatic antioxidants to remove excess ROS and protect against oxidative injury. These substances involved in scavenging free radicals are used experimentally to promote nerve regeneration. Glutathione and ascorbic acid (Vitamin C) are the most well-known and frequently studied anti-oxidants used for enhancing nerve regeneration.[32,33]
Melatonin
Melatonin is an indoleamine synthesised from tryptophan and pass freely through lipid-rich membranes and to cross blood-brain, blood-testis and maternofetal barriers.[34] Melatonin's main superpower is cell protection from ROS and RNS. At the mitochondrial level, it can reduce the ROS/RNS formation by decreasing electron leakage from the respiratory chain; it also reduces the release of mitochondrial pro-apoptotic factors by regulating the mitochondrial permeability transition pores.[35] In terms of nerve injury and regeneration, melatonin has neuroprotective effect on injured neuron, anti-inflammatory effects at the injury site, scar-reducing effect on coaptation site, proliferative effects on SCs and stimulating effects on axonal regeneration.[36,37,38,39,40] All these studies show that melatonin is an important molecule in nerve regeneration and exerts its effects more than one mechanism.
N-acetylcysteine
N-acetylcysteine (NAC) is a neuroprotective agent and a cysteine donor in the synthesis of glutathione, the free-radical scavenger. It stabilises the mitochondrial membrane by up-regulating antiapoptotic family of proteins Bcl-2 and down-regulating Bax and caspase 3, increasing survival of the primary sensory afferent neuron population.[41] Its neuroprotective effect on motor neurons also was evidenced by Zhang et al. on ventral root avulsion and ventral rhizotomy injury model in rats.[42]
Acetyl-L-carnitine
Acetyl-L-carnitine is a peptide with anti-oxidant and anti-apoptotic activity.[43] In the central nervous system (CNS), it has been shown to prevent cell damage by ROS and cell apoptosis by preventing failure of mitochondrial oxidative metabolism.[44] In the peripheral nervous system, it was also shown to reduce neuron death and to eliminate sensory neuron loss after peripheral nerve transection, most probably involving the same extracellular signal-regulated kinase (ERK) pathway.[45] Wilson et al. proved acetyl-L-carnitine's efficiency in improving sensory and motor neuron regeneration.[46]
FK506 (tacrolimus), cyclosporine A and non-immunosuppressant analogues
FK506 is an immunosuppressant agent, used to help nerve allografts in-take. It also possesses neuroprotective and neurotrophic properties. Its actions are receptor dependent. These receptors called immunophilins or FK506 binding proteins with different effects: (1) Immunosuppressant (2) neuroregenerative.[47]
The immunosuppressant role is exerterted by calcineurin inhibition;[48] however, the neuroprotective effect is independent of the calcineurin pathway. It is due to FK506 anti-oxidative/antinitrosative and anti-apoptotic features mediated by thioredoxin and glutathione redox systems and anti-apoptotic mediators (Bcl-2).[49] By reducing inflammation, FK506 reduces also scar formation, promoting nerve regeneration, increasing the density of myelinated axons, with larger diameters and thicker myelin sheath.[50] FK506 enhances nerve regeneration by binding to Hsp-90. By binding to Hsp-90, FK506 causes dissociation of p23 and activation of transcription, with increased ERK ½ phosphorylation and GAP-43 synthesis.[51] The use of FK506 in peripheral nerve repair and regeneration is restricted by its immunosuppressant effect. Inhibiting inflammation may cause a slowing down in myelin debris removal and in neurite outgrowth.[52] Efforts have been made in finding FK506 analogues deprived of immunosuppressive undesirable effect.[47,53,54] Cyclosporine A, an immunophilin ligand to cyclophilin, sharing similar immunosuppressant properties as FK506, through the same calcineurin inhibiting mechanism, fails to enhance nerve regeneration.[55]
Antineoplastic agents
Chemotherapy drugs have also been tested hoping to reduce scar formation by inhibiting fibroblast activation and collagen formation. Local administration of doxorubicin (DXR) at the site of neurolisis of a sciatic nerve in rats and extraneurial injury by scraping the surface of biceps femori muscles was the model used. Light and electron microscopic evaluation revealed a lower scar tissue formation index and scar density scores in the DXR than in the control group.[56] The same injury model was successfully used to test mitomycin C for its antiscarring properties.[57] Geldanamycin is an antibiotic and anti-neoplasic agent, analogue to FK506 but without its immunosuppressant effect. It was shown to accelerate nerve regeneration compared to FK506, in vivo, in rats.[58,59]
Rolipram and chondroitinase ABC
Rolipram is an inhibitor of cyclic adenosin monophosphate (AMPc) phosphodiesterase Type IV receptor, preventing the levels of AMPc to decline after neuronal injury. Among other, rolipram has a powerful anti-inflammatory action.[60] Udina et al. evidenced rolipram's utility in the accelerating nerve regeneration of both motor and sensory components of a transected and directly repaired common peroneal nerve in rats. The results were compared to a group receiving chondroitinase ABC (ChABC) instead of rolipram. Proteoglycans like chondroitin sulphate proteoglycan (CSPG) inhibit neurites’ elongation. ChABC creates a permissive distal stump environment by degrading CSPG. The results were similar. No cumulative effect was noted in the group receiving both rolipram and ChABC.[61]
Corticosteroids
Steroid medication, due to its property to limit inflammation and consequent scar formation was investigated to evaluate any beneficial effect on nerve regeneration and repair. Karlidag et al. conducted a comparative study, on a facial nerve transection and direct coaptation injury model in rabbits and compared methylprednisolone (MP) and NAC. Compared to the control group, the best regeneration was achieved in the NAC group. The highest degree of degeneration was observed in the MP group.[62] The results come in contradiction with the findings of an earlier study, that of Becker et al., who showed that scarring was less, and better results were obtained after local application of corticoids.[63] In another comparative study MP was without effect in the section injury model and it reduced axonal and myelin degeneration alongside oedema in the compression group.[64] Seth et al. compared the effects of local versus systemic administration of dexamethasone on a model of facial nerve axotomy and direct coaptation. Both the treatment groups failed to achieve statistical significance over the control group.[65]
Calcium channel blockers
Calcium channel blockers are thought to have antiscarring effects. By blocking movement of Golgi-derived vesicles, an intracellular accumulation of procollagen proteins occur. This triggers a negative feedback on procollagen gene transcription. Based on these findings, Xue et al. showed that Type I collagen was less in the group receiving verapamil; and transmission electron microscopy showing less collagen fibres and structurally uniform in the treatment group.[66]
Hyaluronic acid
Hyaluronic acid (HA) is a mucopolysaccharide, a basic component of the ECM. It favours cell proliferation and migration, it reduces the scar formation by inhibiting lymphocyte migration, proliferation and chemotaxis, granulocyte phagocytosis and degranulation, macrophages motility.[67]
Several experimental studies confirm that HA reduces perineurial nerve tissue adhesion formation, without impairment of the wound healing process [Figure 2].[68,69] The evaluation of scar formation and adhesion was done macroscopically (Petersen's grading scheme) and histologically (scar tissue formation index). The axon counts, axon diameter and mean fibre diameter were all better in the HA treatment group.
Figure 2.
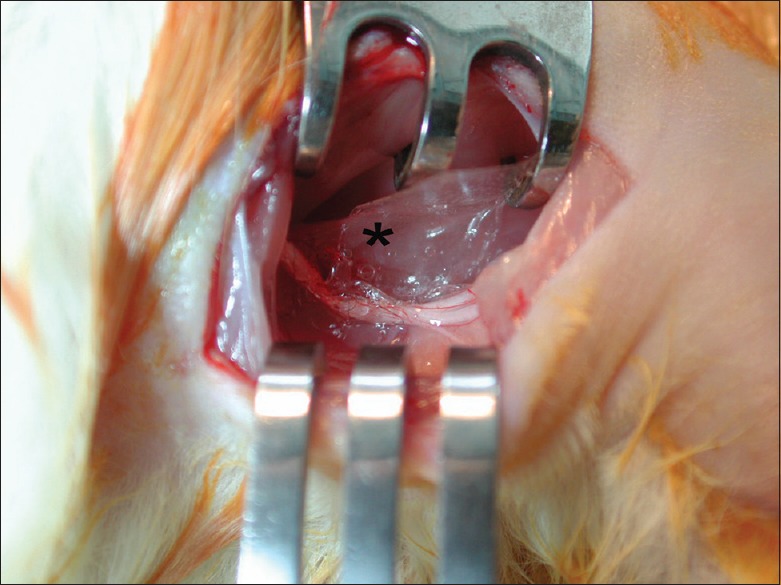
An easy and effective method of local drug delivery at the nerve repair site is the film sheath application. Following nerve repair, hyaluronic acid film sheath (*) is applied at the transection and repair site
A comparative study between FK506 and HA was conducted. Electrophysiological studies and functional evaluation (toe-spreading index) revealed similar results for FK506 and HA.[70] Zor et al. investigated the effect of HA (film sheath at the site of neurorrhaphy), VEGF (VEGF gene in plasmid injection) and combined therapy, on axonal regeneration. The group subject to combined therapy had well myelinated, compact fibres aligned with SCs.[71] HA-carboxymethyl cellulose was proved by Agenor et al. to prevent aberrant outgrowth of regenerating axons, thus avoiding painful neuroma formation.[72]
Citicoline
Together with choline and cytidine, its metabolites, citicoline represents a substrate for phosphatidylcholine, a primary component of neuron membrane. Citicoline is a membrane stabilizer, reducing membrane breakdown and toxic products secondary to ischaemia.[73] Functional, electrophysiological and histological evaluation showed that citicoline improved functional recovery and neuronal regeneration and reduced scar formation, following primary repair.[74,75]
Chitooligosaccharides
Low molecular weight chitooligosaccharides has antioxidative, anti-inflammatory, antiproliferative and antibacterial properties,[76] and also proven to have neuroprotective effects and to enhance nerve regeneration.[77,78]
Fasudil
Fasudil is an inhibitor of the Rho kinase. Rho is a major signalling pathway in the CNS. It blocks neuron regeneration by blocking neurites’ outgrowth. In PNI models, it causes collapse of the growth cones by hindering the actin cytoskeleton dynamics.[79,80] Rho kinase inhibition by fasudil was shown to be associated with an increase in neurite outgrowth in vitro and in vivo, in both sensory and motor neurons.[81,82] Ibuprofen, due to its anti-inflammatory and Rho inhibitory actions, has a positive effect on nerve regeneration functionally and electrophysiologically.[83]
Arachidonic acid derivatives
WD is in part mediated by neuroglia as well as infiltrating inflammatory cells and regulated by inflammatory mediators. Part of this neuroimmune signalling is mediated by the innate immune system, including arachidonic acid derivatives such as prostaglandins and leukotrienes. The enzymes responsible for their production, cyclooxygenases and lipooxygenases, also participate in nerve degeneration and regeneration.[84,85,86,87] The findings point towards a new avenue in the pharmacological treatment of nerve injury, based on a pathophysiologically relevant paradigm.
Gels
Aprotinin (trasylol), was tested by Görgülü et al. to see the antiscarring effects on PNI: external neurolysis, abrasive injury, direct coaptation. Aprotinin was effective in reducing epineural scar formation in all three types of injury.[88] ADCON-T/N, a glycosaminoglycan polymer gel was tested by several authors. Compared to control gel, it reduced scar formation in all three types of nerve injury described above, without impeding neuronal regeneration.[89,90,91] Other bioabsorbable adhesion barrier gel solutions such as hyaloglide-gel and auto-cross-linked polysaccharide proved to be just as efficient as ADCON-T/N.[92]
Growth factors and cytokines
Over the years, the possibility of enhancing the regenerative potential of lesioned neurons by exogenous application of growth-promoting molecules has been addressed in a number of studies. These molecules include neurotrophins (NGF, BDNF), GDNF family members (GDNF, neurturin, persephin, artemin), FGF-1, FGF-2, IGF-I, IGF-II, neuregulins (glial growth factor), neuropeptides (galanin, vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide), and neuropoietic cytokines belonging to the gp130 family (ciliary neurotrophic factor, LIF, cardiotrophin-1, oncostatin M, IL-6, IL-11) in the lesioned peripheral nervous system.[93,94,95]
Growth factors elicit a response via receptor tyrosine kinases at the plasma membrane of neurons. The main signalling cascades required for axon growth are the Ras/ERK and the phosphatidylinositol-3 kinase/Akt pathways.[96] Another important growth factor, VEGF, increases neovacsularisation at the nerve repair site and improves nerve regeneration.[71]
Although there are very encouraging results, usage of growth factors and cytokines in peripheral nerve regeneration has two major drawbacks. First, it is possible that growth factors may cause neuronal apoptosis. Increased FGF levels, for example, may lead to neuron death.[97] Second, these are peptide structures and have a short half-life.
CELLULAR AND TISSUE ENGINEERED THERAPIES
Although the primary aim of this manuscript is the chemical substances that are used for enhancing nerve regeneration, cellular and tissue engineered therapies should be briefly mentioned.
Nerve tissue engineering refers to the fabrication of biocompatible constructs with or without cellular components that enhance nerve regeneration. Axonal elongation has a close relation with ECM and SC. Thus, tissue engineered scaffolds or nerve conduits can be used for SC migration with minimising significant fibrosis.[98] A new technology, 3D printing technology, has been combined with micro-computed tomography to both demonstrate and replicate the internal structure of acellular nerve allografts.[99]
Cellular therapies in nerve regeneration may be performed with stem cells or SCs. Stem cells can differentiate into glial fibrillary acidic protein-positive SCs and can support myelination and the repair process.[100] Stem cells may also differentiate into fibroblast-like cells capable of producing neurotrophic factors in addition to ECM proteins.
CLINICAL APPLICATIONS AND FUTURE PERSPECTIVES
Despite the progress in surgical techniques, today it is clear that peripheral nerve repair is a matter of multidisciplinary and integrated treatment rather than surgical repair. This multidisciplinary approach includes not only surgical repair but also tissue engineering, cellular therapies, nerve regeneration enhancing strategies.[101] Translational and clinical studies are needed to transfer these basic information to standard clinical practice. It is known that each substance has different sites of action. Thus, new experimental and clinical studies which aim to investigate the effects of combined therapy of these substances, are required the further increase the functional outcome.[102] Multicentered studies will also help to determine the ideal substance or substance combination, which will be accepted as standard of care in peripheral nerve regeneration.
New animal studies showed reported that motor and sensory nerves have different denervation and regeneration features. Moreover, the time passed from initial nerve injury is an important factor and different cellular or chemical substances may act differently in acute or chronic injuries.[103] Thus, the regeneration and time relation should be also needed to be evaluated.
There are still basic research, which is dedicated to the identification of new molecules that might promote nerve regeneration, for example, inflammatory cytokines, growth factors and Lipotropic factors, as well as their controlled local release.[104] The list of chemicals used to reduce inflammation and scarring is still opened. Substances such as halofuginon (a Type I collagen synthesis inhibitor) proven to reduce fibrosis and adhesion in abdominal, pelvic and tendon surgery, are still to be tested on PNI and regeneration.[105,106]
Decorin another antifibrotic and anti-inflammatory agent which traps TGF-β in the ECM before it can bind to its receptors on the cell surface.[107] It has been shown to promote axon growth across adult rat spinal cord injuries.[108] These in vitro studies on neurons belonging to the CNS could be taken one step further, by testing decorin's antiscarring effects in vivo and on PNI models also.
CONCLUSIONS
Due to the varied changes in nerves following different types of PNI, the key is to maintain or maximise the pro-regenerative capacity of the de-axonised distal nerve, to support recipient axonal regeneration to distal sensory/motor targets and to achieve functional neuro-integration and neuro-rehabilitation.
Treatment paradigms that have been tested and proven in experimental models have included selective neurotrophic agents (drugs/biologics/growth factors) or cellular neurotherapies (SC/mesenchymal stem cell/adipose-derived stem cell), when delivered in a targeted fashion act through multiple, non-redundant cellular/molecular mechanisms or pathways and have a global, complementary impact on the cellular, scaffold, signalling, inflammation, vascularisation process critical for nerve regeneration.
We have summarised promising inorganic and organic compounds that may have clinical, translational relevance in nerve regeneration. These agents may have multifaceted effects on neuroprotection (pharmacological prevention of some of the damaging intracellular cascades that lead to secondary tissue loss), axonal regeneration (increase of growth factors, neutralisation of inhibitory factors, reduction in scar formation), and help maintain distal neuronal pathways or targets.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Goulart CO, Martinez AM. Tubular conduits, cell-based therapy and exercise to improve peripheral nerve regeneration. Neural Regen Res. 2015;10:565–7. doi: 10.4103/1673-5374.155424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zochodne DW. The challenges and beauty of peripheral nerve regrowth. J Peripher Nerv Syst. 2012;17:1–18. doi: 10.1111/j.1529-8027.2012.00378.x. [DOI] [PubMed] [Google Scholar]
- 3.Seddon HJ. A classification of nerve injuries. Br Med J. 1942;2:237–9. doi: 10.1136/bmj.2.4260.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maggi SP, Lowe JB, 3rd, Mackinnon SE. Pathophysiology of nerve injury. Clin Plast Surg. 2003;30:109–26. doi: 10.1016/s0094-1298(02)00101-3. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Houenou LJ, Wu W, Lei M, Prevette DM, Oppenheim RW. Characterization of spinal motoneuron degeneration following different types of peripheral nerve injury in neonatal and adult mice. J Comp Neurol. 1998;396:158–68. [PubMed] [Google Scholar]
- 6.Hart AM, Terenghi G, Wiberg M. Neuronal death after peripheral nerve injury and experimental strategies for neuroprotection. Neurol Res. 2008;30:999–1011. doi: 10.1179/174313208X362479. [DOI] [PubMed] [Google Scholar]
- 7.Nuñez G, del Peso L. Linking extracellular survival signals and the apoptotic machinery. Curr Opin Neurobiol. 1998;8:613–8. doi: 10.1016/s0959-4388(98)80089-5. [DOI] [PubMed] [Google Scholar]
- 8.Petit PX, Susin SA, Zamzami N, Mignotte B, Kroemer G. Mitochondria and programmed cell death: Back to the future. FEBS Lett. 1996;396:7–13. doi: 10.1016/0014-5793(96)00988-x. [DOI] [PubMed] [Google Scholar]
- 9.Korkmaz A, Reiter RJ, Topal T, Manchester LC, Oter S, Tan DX. Melatonin: An established antioxidant worthy of use in clinical trials. Mol Med. 2009;15:43–50. doi: 10.2119/molmed.2008.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saito Y, Nishio K, Ogawa Y, Kimata J, Kinumi T, Yoshida Y, et al. Turning point in apoptosis/necrosis induced by hydrogen peroxide. Free Radic Res. 2006;40:619–30. doi: 10.1080/10715760600632552. [DOI] [PubMed] [Google Scholar]
- 11.Navarro X. Chapter 27: Neural plasticity after nerve injury and regeneration. Int Rev Neurobiol. 2009;87:483–505. doi: 10.1016/S0074-7742(09)87027-X. [DOI] [PubMed] [Google Scholar]
- 12.Abe N, Cavalli V. Nerve injury signaling. Curr Opin Neurobiol. 2008;18:276–83. doi: 10.1016/j.conb.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandolesi G, Madeddu F, Bozzi Y, Maffei L, Ratto GM. Acute physiological response of mammalian central neurons to axotomy: Ionic regulation and electrical activity. FASEB J. 2004;18:1934–6. doi: 10.1096/fj.04-1805fje. [DOI] [PubMed] [Google Scholar]
- 14.Raivich G, Makwana M. The making of successful axonal regeneration: Genes, molecules and signal transduction pathways. Brain Res Rev. 2007;53:287–311. doi: 10.1016/j.brainresrev.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Hirata A, Masaki T, Motoyoshi K, Kamakura K. Intrathecal administration of nerve growth factor delays GAP 43 expression and early phase regeneration of adult rat peripheral nerve. Brain Res. 2002;944:146–56. doi: 10.1016/s0006-8993(02)02739-7. [DOI] [PubMed] [Google Scholar]
- 16.Dubový P. Wallerian degeneration and peripheral nerve conditions for both axonal regeneration and neuropathic pain induction. Ann Anat. 2011;193:267–75. doi: 10.1016/j.aanat.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Stoll G, Jander S, Myers RR. Degeneration and regeneration of the peripheral nervous system: From Augustus Waller's observations to neuroinflammation. J Peripher Nerv Syst. 2002;7:13–27. doi: 10.1046/j.1529-8027.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- 18.Webber C, Zochodne D. The nerve regenerative microenvironment: Early behavior and partnership of axons and Schwann cells. Exp Neurol. 2010;223:51–9. doi: 10.1016/j.expneurol.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 19.Uçeyler N, Tscharke A, Sommer C. Early cytokine expression in mouse sciatic nerve after chronic constriction nerve injury depends on calpain. Brain Behav Immun. 2007;21:553–60. doi: 10.1016/j.bbi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Goethals S, Ydens E, Timmerman V, Janssens S. Toll-like receptor expression in the peripheral nerve. Glia. 2010;58:1701–9. doi: 10.1002/glia.21041. [DOI] [PubMed] [Google Scholar]
- 21.Boivin A, Pineau I, Barrette B, Filali M, Vallières N, Rivest S, et al. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J Neurosci. 2007;27:12565–76. doi: 10.1523/JNEUROSCI.3027-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Perez F, Udina E, Navarro X. Extracellular matrix components in peripheral nerve regeneration. Int Rev Neurobiol. 2013;108:257–75. doi: 10.1016/B978-0-12-410499-0.00010-1. [DOI] [PubMed] [Google Scholar]
- 23.Mortimer D, Fothergill T, Pujic Z, Richards LJ, Goodhill GJ. Growth cone chemotaxis. Trends Neurosci. 2008;31:90–8. doi: 10.1016/j.tins.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Bouquet C, Nothias F. Molecular mechanisms of axonal growth. Adv Exp Med Biol. 2007;621:1–16. doi: 10.1007/978-0-387-76715-4_1. [DOI] [PubMed] [Google Scholar]
- 25.Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: Interactions at the axon level. Prog Neurobiol. 2012;98:16–37. doi: 10.1016/j.pneurobio.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Wen Z, Zheng JQ. Directional guidance of nerve growth cones. Curr Opin Neurobiol. 2006;16:52–8. doi: 10.1016/j.conb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 27.de Luca AC, Lacour SP, Raffoul W, di Summa PG. Extracellular matrix components in peripheral nerve repair: How to affect neural cellular response and nerve regeneration? Neural Regen Res. 2014;9:1943–8. doi: 10.4103/1673-5374.145366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardiner NJ. Integrins and the extracellular matrix: Key mediators of development and regeneration of the sensory nervous system. Dev Neurobiol. 2011;71:1054–72. doi: 10.1002/dneu.20950. [DOI] [PubMed] [Google Scholar]
- 29.Atkins S, Smith KG, Loescher AR, Boissonade FM, O’Kane S, Ferguson MW, et al. Scarring impedes regeneration at sites of peripheral nerve repair. Neuroreport. 2006;17:1245–9. doi: 10.1097/01.wnr.0000230519.39456.ea. [DOI] [PubMed] [Google Scholar]
- 30.Ngeow WC, Atkins S, Morgan CR, Metcalfe AD, Boissonade FM, Loescher AR, et al. Histomorphometric changes in repaired mouse sciatic nerves are unaffected by the application of a scar-reducing agent. J Anat. 2011;219:638–45. doi: 10.1111/j.1469-7580.2011.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dreesmann L, Mittnacht U, Lietz M, Schlosshauer B. Nerve fibroblast impact on Schwann cell behavior. Eur J Cell Biol. 2009;88:285–300. doi: 10.1016/j.ejcb.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Romero FJ. Antioxidants in peripheral nerve. Free Radic Biol Med. 1996;20:925–32. doi: 10.1016/0891-5849(95)02183-3. [DOI] [PubMed] [Google Scholar]
- 33.Gess B, Röhr D, Young P. Ascorbic acid and sodium-dependent Vitamin C transporters in the peripheral nervous system: From basic science to clinical trials. Antioxid Redox Signal. 2013;19:2105–14. doi: 10.1089/ars.2013.5380. [DOI] [PubMed] [Google Scholar]
- 34.Reiter RJ, Tan DX, Korkmaz A, Rosales-Corral SA. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum Reprod Update. 2014;20:293–307. doi: 10.1093/humupd/dmt054. [DOI] [PubMed] [Google Scholar]
- 35.Tan DX, Manchester LC, Liu X, Rosales-Corral SA, Acuna-Castroviejo D, Reiter RJ. Mitochondria and chloroplasts as the original sites of melatonin synthesis: A hypothesis related to melatonin's primary function and evolution in eukaryotes. J Pineal Res. 2013;54:127–38. doi: 10.1111/jpi.12026. [DOI] [PubMed] [Google Scholar]
- 36.Turgut M, Uysal A, Pehlivan M, Oktem G, Yurtseven ME. Assessment of effects of pinealectomy and exogenous melatonin administration on rat sciatic nerve suture repair: An electrophysiological, electron microscopic, and immunohistochemical study. Acta Neurochir (Wien) 2005;147:67–77. doi: 10.1007/s00701-004-0426-x. [DOI] [PubMed] [Google Scholar]
- 37.Atik B, Erkutlu I, Tercan M, Buyukhatipoglu H, Bekerecioglu M, Pence S. The effects of exogenous melatonin on peripheral nerve regeneration and collagen formation in rats. J Surg Res. 2011;166:330–6. doi: 10.1016/j.jss.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Zencirci SG, Bilgin MD, Yaraneri H. Electrophysiological and theoretical analysis of melatonin in peripheral nerve crush injury. J Neurosci Methods. 2010;191:277–82. doi: 10.1016/j.jneumeth.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Shokouhi G, Tubbs RS, Shoja MM, Hadidchi S, Ghorbanihaghjo A, Roshangar L, et al. Neuroprotective effects of high-dose vs. low-dose melatonin after blunt sciatic nerve injury. Childs Nerv Syst. 2008;24:111–7. doi: 10.1007/s00381-007-0366-x. [DOI] [PubMed] [Google Scholar]
- 40.Chang HM, Liu CH, Hsu WM, Chen LY, Wang HP, Wu TH, et al. Proliferative effects of melatonin on Schwann cells: Implication for nerve regeneration following peripheral nerve injury. J Pineal Res. 2014;56:322–32. doi: 10.1111/jpi.12125. [DOI] [PubMed] [Google Scholar]
- 41.Reid AJ, Shawcross SG, Hamilton AE, Wiberg M, Terenghi G. N-acetylcysteine alters apoptotic gene expression in axotomised primary sensory afferent subpopulations. Neurosci Res. 2009;65:148–55. doi: 10.1016/j.neures.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Zhang CG, Welin D, Novikov L, Kellerth JO, Wiberg M, Hart AM. Motorneuron protection by N-acetyl-cysteine after ventral root avulsion and ventral rhizotomy. Br J Plast Surg. 2005;58:765–73. doi: 10.1016/j.bjps.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Traina G. The neurobiology of acetyl-L-carnitine. Front Biosci (Landmark Ed) 2016;21:1314–29. doi: 10.2741/4459. [DOI] [PubMed] [Google Scholar]
- 44.Zanelli SA, Solenski NJ, Rosenthal RE, Fiskum G. Mechanisms of ischemic neuroprotection by acetyl-L-carnitine. Ann N Y Acad Sci. 2005;1053:153–61. doi: 10.1196/annals.1344.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hart AM, Wiberg M, Youle M, Terenghi G. Systemic acetyl-L-carnitine eliminates sensory neuronal loss after peripheral axotomy: A new clinical approach in the management of peripheral nerve trauma. Exp Brain Res. 2002;145:182–9. doi: 10.1007/s00221-002-1100-2. [DOI] [PubMed] [Google Scholar]
- 46.Wilson AD, Hart A, Wiberg M, Terenghi G. Acetyl-l-carnitine increases nerve regeneration and target organ reinnervation – A morphological study. J Plast Reconstr Aesthet Surg. 2010;63:1186–95. doi: 10.1016/j.bjps.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 47.Hinds TD, Stechschulte LA, Elkhairi F, Sanchez ER. Analysis of FK506, timcodar (VX-853) and FKBP51 and FKBP52 chaperones in control of glucocorticoid receptor activity and phosphorylation. Pharmacol Res Perspect. 2014;2:e00076. doi: 10.1002/prp2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gold BG, Densmore V, Shou W, Matzuk MM, Gordon HS. Immunophilin FK506-binding protein 52 (not FK506-binding protein 12) mediates the neurotrophic action of FK506. J Pharmacol Exp Ther. 1999;289:1202–10. [PubMed] [Google Scholar]
- 49.Lagoda G, Xie Y, Sezen SF, Hurt KJ, Liu L, Musicki B, et al. FK506 neuroprotection after cavernous nerve injury is mediated by thioredoxin and glutathione redox systems. J Sex Med. 2011;8:3325–34. doi: 10.1111/j.1743-6109.2011.02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Que J, Cao Q, Sui T, Du S, Zhang A, Kong D, et al. Tacrolimus reduces scar formation and promotes sciatic nerve regeneration. Neural Regen Res. 2012;7:2500–6. doi: 10.3969/j.issn.1673-5374.2012.32.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gold BG, Zhong YP. FK506 requires stimulation of the extracellular signal-regulated kinase 1/2 and the steroid receptor chaperone protein p23 for neurite elongation. Neurosignals. 2004;13:122–9. doi: 10.1159/000076565. [DOI] [PubMed] [Google Scholar]
- 52.Udina E, Voda J, Gold BG, Navarro X. Comparative dose-dependence study of FK506 on transected mouse sciatic nerve repaired by allograft or xenograft. J Peripher Nerv Syst. 2003;8:145–54. doi: 10.1046/j.1529-8027.2003.03020.x. [DOI] [PubMed] [Google Scholar]
- 53.Sano M, Yoshida M, Fukui S, Kitajima S. Radicicol potentiates neurotrophin-mediated neurite outgrowth and survival of cultured sensory neurons from chick embryo. J Neurochem. 1999;72:2256–63. doi: 10.1046/j.1471-4159.1999.0722256.x. [DOI] [PubMed] [Google Scholar]
- 54.Gold BG, Armistead DM, Wang MS. Non-FK506-binding protein-12 neuroimmunophilin ligands increase neurite elongation and accelerate nerve regeneration. J Neurosci Res. 2005;80:56–65. doi: 10.1002/jnr.20447. [DOI] [PubMed] [Google Scholar]
- 55.Wang MS, Zeleny-Pooley M, Gold BG. Comparative dose-dependence study of FK506 and cyclosporin A on the rate of axonal regeneration in the rat sciatic nerve. J Pharmacol Exp Ther. 1997;282:1084–93. [PubMed] [Google Scholar]
- 56.Albayrak BS, Ismailoglu O, Ilbay K, Yaka U, Tanriover G, Gorgulu A, et al. Doxorubicin for prevention of epineurial fibrosis in a rat sciatic nerve model: Outcome based on gross postsurgical, histopathological, and ultrastructural findings. J Neurosurg Spine. 2010;12:327–33. doi: 10.3171/2009.9.SPINE09407. [DOI] [PubMed] [Google Scholar]
- 57.Ilbay K, Etus V, Yildiz K, Ilbay G, Ceylan S. Topical application of mitomycin C prevents epineural scar formation in rats. Neurosurg Rev. 2005;28:148–53. doi: 10.1007/s10143-004-0370-5. [DOI] [PubMed] [Google Scholar]
- 58.Chan KM, Gordon T, Zochodne DW, Power HA. Improving peripheral nerve regeneration: From molecular mechanisms to potential therapeutic targets. Exp Neurol. 2014;261:826–35. doi: 10.1016/j.expneurol.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Sun HH, Saheb-Al-Zamani M, Yan Y, Hunter DA, Mackinnon SE, Johnson PJ. Geldanamycin accelerated peripheral nerve regeneration in comparison to FK-506 in vivo. Neuroscience. 2012;223:114–23. doi: 10.1016/j.neuroscience.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 60.Zhu J, Mix E, Winblad B. The antidepressant and antiinflammatory effects of rolipram in the central nervous system. CNS Drug Rev. 2001;7:387–98. doi: 10.1111/j.1527-3458.2001.tb00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Udina E, Ladak A, Furey M, Brushart T, Tyreman N, Gordon T. Rolipram-induced elevation of cAMP or chondroitinase ABC breakdown of inhibitory proteoglycans in the extracellular matrix promotes peripheral nerve regeneration. Exp Neurol. 2010;223:143–52. doi: 10.1016/j.expneurol.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karlidag T, Yildiz M, Yalcin S, Colakoglu N, Kaygusuz I, Sapmaz E. Evaluation of the effect of methylprednisolone and N-acetylcystein on anastomotic degeneration and regeneraton of the facial nerve. Auris Nasus Larynx. 2012;39:145–50. doi: 10.1016/j.anl.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Becker KW, Kienecker EW, Andrae I. Effect of locally applied corticoids on the morphology of peripheral nerves following neurotmesis and microsurgical suture. Neurochirurgia (Stuttg) 1987;30:161–7. doi: 10.1055/s-2008-1054088. [DOI] [PubMed] [Google Scholar]
- 64.Yildirim MA, Karlidag T, Akpolat N, Kaygusuz I, Keles E, Yalcin S, et al. The effect of methylprednisolone on facial nerve paralysis with different etiologies. J Craniofac Surg. 2015;26:810–5. doi: 10.1097/SCS.0000000000001502. [DOI] [PubMed] [Google Scholar]
- 65.Seth R, Revenaugh PC, Kaltenbach JA, Rajasekaran K, Meltzer NE, Ghosh D, et al. Facial nerve neurorrhaphy and the effects of glucocorticoids in a rat model. Otolaryngol Head Neck Surg. 2012;147:832–40. doi: 10.1177/0194599812451551. [DOI] [PubMed] [Google Scholar]
- 66.Xue JW, Jiao JB, Liu XF, Jiang YT, Yang G, Li CY, et al. Inhibition of peripheral nerve scarring by calcium antagonists, also known as calcium channel blockers. Artif Organs. 2016;40:514–20. doi: 10.1111/aor.12584. [DOI] [PubMed] [Google Scholar]
- 67.Longaker MT, Chiu ES, Harrison MR, Crombleholme TM, Langer JC, Duncan BW, et al. Studies in fetal wound healing. IV. Hyaluronic acid-stimulating activity distinguishes fetal wound fluid from adult wound fluid. Ann Surg. 1989;210:667–72. doi: 10.1097/00000658-198911000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ozgenel GY. Effects of hyaluronic acid on peripheral nerve scarring and regeneration in rats. Microsurgery. 2003;23:575–81. doi: 10.1002/micr.10209. [DOI] [PubMed] [Google Scholar]
- 69.Park JS, Lee JH, Han CS, Chung DW, Kim GY. Effect of hyaluronic acid-carboxymethylcellulose solution on perineural scar formation after sciatic nerve repair in rats. Clin Orthop Surg. 2011;3:315–24. doi: 10.4055/cios.2011.3.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mekaj AY, Morina AA, Manxhuka-Kerliu S, Neziri B, Duci SB, Kukaj V, et al. Electrophysiological and functional evaluation of peroneal nerve regeneration in rabbit following topical hyaluronic acid or tacrolimus application after nerve repair. Niger Postgrad Med J. 2015;22:179–84. doi: 10.4103/1117-1936.170738. [DOI] [PubMed] [Google Scholar]
- 71.Zor F, Deveci M, Kilic A, Ozdag MF, Kurt B, Sengezer M, et al. Effect of VEGF gene therapy and hyaluronic acid film sheath on peripheral nerve regeneration. Microsurgery. 2014;34:209–16. doi: 10.1002/micr.22196. [DOI] [PubMed] [Google Scholar]
- 72.Agenor A, Dvoracek L, Leu A, Hunter DA, Newton P, Yan Y, et al. Hyaluronic acid/carboxymethyl cellulose directly applied to transected nerve decreases axonal outgrowth. J Biomed Mater Res B Appl Biomater. 2017;105:568–574. doi: 10.1002/jbm.b.33576. [DOI] [PubMed] [Google Scholar]
- 73.Krupinski J, Ferrer I, Barrachina M, Secades JJ, Mercadal J, Lozano R. CDP-choline reduces pro-caspase and cleaved caspase-3 expression, nuclear DNA fragmentation, and specific PARP-cleaved products of caspase activation following middle cerebral artery occlusion in the rat. Neuropharmacology. 2002;42:846–54. doi: 10.1016/s0028-3908(02)00032-1. [DOI] [PubMed] [Google Scholar]
- 74.Ozay R, Bekar A, Kocaeli H, Karli N, Filiz G, Ulus IH. Citicoline improves functional recovery, promotes nerve regeneration, and reduces postoperative scarring after peripheral nerve surgery in rats. Surg Neurol. 2007;68:615–22. doi: 10.1016/j.surneu.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 75.Caner B, Kafa MI, Bekar A, Kurt MA, Karli N, Cansev M, et al. Intraperitoneal administration of CDP-choline or a combination of cytidine plus choline improves nerve regeneration and functional recovery in a rat model of sciatic nerve injury. Neurol Res. 2012;34:238–45. doi: 10.1179/1743132812Y.0000000003. [DOI] [PubMed] [Google Scholar]
- 76.Lodhi G, Kim YS, Hwang JW, Kim SK, Jeon YJ, Je JY, et al. Chitooligosaccharide and its derivatives: Preparation and biological applications. Biomed Res Int 2014. 2014 doi: 10.1155/2014/654913. 654913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang M, Zhuge X, Yang Y, Gu X, Ding F. The promotion of peripheral nerve regeneration by chitooligosaccharides in the rat nerve crush injury model. Neurosci Lett. 2009;454:239–43. doi: 10.1016/j.neulet.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 78.Gong Y, Gong L, Gu X, Ding F. Chitooligosaccharides promote peripheral nerve regeneration in a rabbit common peroneal nerve crush injury model. Microsurgery. 2009;29:650–6. doi: 10.1002/micr.20686. [DOI] [PubMed] [Google Scholar]
- 79.Liu J, Gao HY, Wang XF. The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system. Neural Regen Res. 2015;10:1892–6. doi: 10.4103/1673-5374.170325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jin M, Guan CB, Jiang YA, Chen G, Zhao CT, Cui K, et al. Ca2+-dependent regulation of rho GTPases triggers turning of nerve growth cones. J Neurosci. 2005;25:2338–47. doi: 10.1523/JNEUROSCI.4889-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheng C, Webber CA, Wang J, Xu Y, Martinez JA, Liu WQ, et al. Activated RHOA and peripheral axon regeneration. Exp Neurol. 2008;212:358–69. doi: 10.1016/j.expneurol.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 82.Hiraga A, Kuwabara S, Doya H, Kanai K, Fujitani M, Taniguchi J, et al. Rho-kinase inhibition enhances axonal regeneration after peripheral nerve injury. J Peripher Nerv Syst. 2006;11:217–24. doi: 10.1111/j.1529-8027.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 83.Madura T, Tomita K, Terenghi G. Ibuprofen improves functional outcome after axotomy and immediate repair in the peripheral nervous system. J Plast Reconstr Aesthet Surg. 2011;64:1641–6. doi: 10.1016/j.bjps.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 84.Camara-Lemarroy CR, Gonzalez-Moreno EI, Guzman-de la Garza FJ, Fernandez-Garza NE. Arachidonic acid derivatives and their role in peripheral nerve degeneration and regeneration. ScientificWorldJournal 2012. 2012 doi: 10.1100/2012/168953. 168953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Williams EJ, Walsh FS, Doherty P. The production of arachidonic acid can account for calcium channel activation in the second messenger pathway underlying neurite outgrowth stimulated by NCAM, N-cadherin, and L1. J Neurochem. 1994;62:1231–4. doi: 10.1046/j.1471-4159.1994.62031231.x. [DOI] [PubMed] [Google Scholar]
- 86.Fu Q, Hue J, Li S. Nonsteroidal anti-inflammatory drugs promote axon regeneration via RhoA inhibition. J Neurosci. 2007;27:4154–64. doi: 10.1523/JNEUROSCI.4353-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klegeris A, McGeer PL. Cyclooxygenase and 5-lipoxygenase inhibitors protect against mononuclear phagocyte neurotoxicity. Neurobiol Aging. 2002;23:787–94. doi: 10.1016/s0197-4580(02)00021-0. [DOI] [PubMed] [Google Scholar]
- 88.Görgülü A, Imer M, Simsek O, Sencer A, Kutlu K, Cobanoglu S. The effect of aprotinin on extraneural scarring in peripheral nerve surgery: An experimental study. Acta Neurochir (Wien) 1998;140:1303–7. doi: 10.1007/s007010050254. [DOI] [PubMed] [Google Scholar]
- 89.Petersen J, Russell L, Andrus K, MacKinnon M, Silver J, Kliot M. Reduction of extraneural scarring by ADCON-T/N after surgical intervention. Neurosurgery. 1996;38:976–83. doi: 10.1097/00006123-199605000-00025. [DOI] [PubMed] [Google Scholar]
- 90.Palatinsky EA, Maier KH, Touhalisky DK, Mock JL, Hingson MT, Coker GT. ADCON-T/N reduces in vivo perineural adhesions in a rat sciatic nerve reoperation model. J Hand Surg Br. 1997;22:331–5. doi: 10.1016/s0266-7681(97)80397-x. [DOI] [PubMed] [Google Scholar]
- 91.Isla A, Martinez JR, Perez-Lopez C, Pérez Conde C, Morales C, Budke M. A reservable antiadhesion barrier gel reduces the perineural adhesions in rats after anastomosis. J Neurosurg Sci. 2003;47:195–9. [PubMed] [Google Scholar]
- 92.Dam-Hieu P, Lacroix C, Said G, Devanz P, Liu S, Tadie M. Reduction of postoperative perineural adhesions by hyaloglide gel: An experimental study in the rat sciatic nerve. Neurosurgery. 2005;56(2 Suppl):425–33. doi: 10.1227/01.neu.0000156845.41626.e9. [DOI] [PubMed] [Google Scholar]
- 93.Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27:277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- 94.Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg Focus. 2009;26:E3. doi: 10.3171/FOC.2009.26.2.E3. [DOI] [PubMed] [Google Scholar]
- 95.Klimaschewski L, Hausott B, Angelov DN. The pros and cons of growth factors and cytokines in peripheral axon regeneration. Int Rev Neurobiol. 2013;108:137–71. doi: 10.1016/B978-0-12-410499-0.00006-X. [DOI] [PubMed] [Google Scholar]
- 96.Zhou FQ, Snider WD. Intracellular control of developmental and regenerative axon growth. Philos Trans R Soc Lond B Biol Sci. 2006;361:1575–92. doi: 10.1098/rstb.2006.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jungnickel J, Gransalke K, Timmer M, Grothe C. Fibroblast growth factor receptor 3 signaling regulates injury-related effects in the peripheral nervous system. Mol Cell Neurosci. 2004;25:21–9. doi: 10.1016/j.mcn.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 98.Subramanian A, Krishnan UM, Sethuraman S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J Biomed Sci. 2009;16:108. doi: 10.1186/1423-0127-16-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu S, Zhu Q, Liu X, Yang W, Jian Y, Zhou X, et al. Three-dimensional reconstruction of the microstructure of human acellular nerve allograft. Sci Rep. 2016;6:30694. doi: 10.1038/srep30694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Amoh Y, Kanoh M, Niiyama S, Hamada Y, Kawahara K, Sato Y, et al. Human hair follicle pluripotent stem (hfPS) cells promote regeneration of peripheral-nerve injury: An advantageous alternative to ES and iPS cells. J Cell Biochem. 2009;107:1016–20. doi: 10.1002/jcb.22204. [DOI] [PubMed] [Google Scholar]
- 101.Tos P, Ronchi G, Geuna S, Battiston B. Future perspectives in nerve repair and regeneration. Int Rev Neurobiol. 2013;109:165–92. doi: 10.1016/B978-0-12-420045-6.00008-0. [DOI] [PubMed] [Google Scholar]
- 102.Panagopoulos GN, Megaloikonomos PD, Mavrogenis AF. The present and future for peripheral nerve regeneration. Orthopedics. 2017;40:e141–56. doi: 10.3928/01477447-20161019-01. [DOI] [PubMed] [Google Scholar]
- 103.Zhao L, Lv G, Jiang S, Yan Z, Sun J, Wang L, et al. Morphological differences in skeletal muscle atrophy of rats with motor nerve and/or sensory nerve injury. Neural Regen Res. 2012;7:2507–15. doi: 10.3969/j.issn.1673-5374.2012.32.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sinis N, Geuna S, Viterbo F. Translational research in peripheral nerve repair and regeneration. Biomed Res Int 2014. 2014 doi: 10.1155/2014/381426. 381426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nagler A, Rivkind AI, Raphael J, Levi-Schaffer F, Genina O, Lavelin I, et al. Halofuginone – An inhibitor of collagen type I synthesis – Prevents postoperative formation of abdominal adhesions. Ann Surg. 1998;227:575–82. doi: 10.1097/00000658-199804000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pines M, Spector I. Halofuginone-the multifaceted molecule. Molecules. 2015;20:573–94. doi: 10.3390/molecules20010573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Järvinen TA, Prince S. Decorin: A growth factor antagonist for tumor growth inhibition. Biomed Res Int 2015. 2015 doi: 10.1155/2015/654765. 654765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Davies JE, Tang X, Denning JW, Archibald SJ, Davies SJ. Decorin suppresses neurocan, brevican, phosphacan and NG2 expression and promotes axon growth across adult rat spinal cord injuries. Eur J Neurosci. 2004;19:1226–42. doi: 10.1111/j.1460-9568.2004.03184.x. [DOI] [PubMed] [Google Scholar]


