ABSTRACT
Herpes simplex viruses 1 and 2 (HSV-1 and HSV-2) infect and establish latency in peripheral neurons, from which they can reactivate to cause recurrent disease throughout the life of the host. Stress is associated with the exacerbation of clinical symptoms and the induction of recurrences in humans and animal models. The viruses preferentially replicate and establish latency in different subtypes of sensory neurons, as well as in neurons of the autonomic nervous system that are highly responsive to stress hormones. To determine if stress-related hormones modulate productive HSV-1 and HSV-2 infections within sensory and autonomic neurons, we analyzed viral DNA and the production of viral progeny after treatment of primary adult murine neuronal cultures with the stress hormones epinephrine and corticosterone. Both sensory trigeminal ganglion (TG) and sympathetic superior cervical ganglion (SCG) neurons expressed adrenergic receptors (activated by epinephrine) and the glucocorticoid receptor (activated by corticosterone). Productive HSV infection colocalized with these receptors in SCG but not in TG neurons. In productively infected neuronal cultures, epinephrine treatment significantly increased the levels of HSV-1 DNA replication and production of viral progeny in SCG neurons, but no significant differences were found in TG neurons. In contrast, corticosterone significantly decreased the levels of HSV-2 DNA replication and production of viral progeny in SCG neurons but not in TG neurons. Thus, the stress-related hormones epinephrine and corticosterone selectively modulate acute HSV-1 and HSV-2 infections in autonomic, but not sensory, neurons.
IMPORTANCE Stress exacerbates acute disease symptoms resulting from HSV-1 and HSV-2 infections and is associated with the appearance of recurrent skin lesions in millions of people. Although stress hormones are thought to impact HSV-1 and HSV-2 through immune system suppression, sensory and autonomic neurons that become infected by HSV-1 and HSV-2 express stress hormone receptors and are responsive to hormone fluctuations. Our results show that autonomic neurons are more responsive to epinephrine and corticosterone than are sensory neurons, demonstrating that the autonomic nervous system plays a substantial role in HSV pathogenesis. Furthermore, these results suggest that stress responses have the potential to differentially impact HSV-1 and HSV-2 so as to produce divergent outcomes of infection.
KEYWORDS: herpes simplex virus, HSV-1, HSV-2, epinephrine, corticosterone, stress, reactivation
INTRODUCTION
Herpes simplex viruses 1 and 2 (HSV-1 and HSV-2) infect mucosal cells and then establish a lifelong latent infection in sensory and autonomic ganglia innervating the site of infection. HSV-1 is commonly associated with orolabial lesions (cold sores) and ocular disease (herpes simplex keratitis), while HSV-2 causes genital lesions. Both viruses can reactivate from latency to cause recurrent disease, which also tends to occur in different anatomical patterns. Although HSV-1 is diagnosed in approximately 30% of primary genital herpes cases, HSV-2 is more likely to reactivate to cause recurrent genital lesions (1). More rarely, HSV-1 causes necrotizing encephalitis with a high mortality rate, and HSV-2 can cause recurrent sacral meningitis. The mechanisms that cause these differences in the acute and recurrent disease patterns of HSV-1 and HSV-2 are not understood.
Stress is strongly correlated with the exacerbation of acute HSV disease symptoms and the appearance of recurrent disease in humans and animal models (2–7). Psychosocial stress has been correlated with oral herpes recurrences in humans (8). Psychological stress at the time of infection increases HSV-1 titers and pathology following intranasal or vaginal infection in mice (7, 9). Epinephrine (EPI), a catecholaminergic hormone secreted by the adrenal medulla to induce the “short-term” fight-or-flight stress response, is regulated by the sympathetic nervous system. Iontophoresis of epinephrine has been used to induce HSV-1 reactivation in the rabbit ocular model of infection (5), as well as in mice (10) and nonhuman primates (11), demonstrating the ability of epinephrine to impact HSV-1. Cortisol, a glucocorticoid secreted by the adrenal cortex that induces a “long-term” stress response, binds to the glucocorticoid receptor (GCR), regulating metabolism and immune system suppression. Persistent stress has been correlated with the reactivation of both HSV-1 and HSV-2 in humans (3, 4). Cold-restraint stress in rats and hyperthermic stress in mice have been reported to increase concentrations of corticosterone (CORT) (the rodent equivalent of cortisol in humans) in plasma, resulting in HSV-1 reactivation (6, 12). In addition, treatment of the immortalized neuronal cell line PC12 and human gingival fibroblasts with dexamethasone (DEX), a GCR agonist, resulted in increases in HSV-1 DNA replication and infectious virus yields during productive infection (13, 14). Furthermore, dexamethasone treatment increased the mortality of mice acutely infected with HSV-1 (15), significantly increased HSV-1 ocular shedding and corneal ulceration in latently infected rabbits (16), consistently induced the reactivation of bovine herpesvirus 1 in calves (17), and could induce HSV-1 keratitis in humans (18). Taken together, the stress hormones epinephrine and corticosterone have impacts on acute and latent infections with HSV-1 and HSV-2.
The mechanism through which these stress factors impact HSV disease severity and recurrences is thought to be suppression of the immune system, permitting the viruses to escape immune surveillance (2, 7, 12, 19). However, the receptors for the two major stress hormones, epinephrine and cortisol, are expressed selectively by the different types of neurons infected by HSV, including sensory and autonomic neurons (20, 21). Therefore, epinephrine and cortisol may contribute to the ability of the viruses to replicate efficiently in specific types of neurons, which may impact disease severity and the ability to reactivate later to cause recurrent lesions (22–29).
HSV-1 and HSV-2 preferentially establish latency in different types of sensory neurons: HSV-1 prefers sensory neurons recognized by the monoclonal antibody Fe-A5 (A5+ neurons), while HSV-2 prefers neurons bound by isolectin B4 (IB4+ neurons) (30, 31). In murine adult neuronal cultures, A5+ and IB4+ neurons are nonpermissive for productive infection with HSV-1 and HSV-2, respectively, leading to preferential establishment of latency in these neurons (32, 33). Thus, latency is established in neurons that do not support efficient productive infection. The viruses also establish latency in autonomic neurons (34–39), which are highly responsive to endocrine factors. Therefore, we hypothesized that stress hormones, acting through their cognate receptors, could potentially modulate productive HSV-1 and HSV-2 infections by acting directly on neurons rather than indirectly, through their effects on the immune system.
Although stress and the stress-related endocrine factors epinephrine and cortisol are strongly correlated with HSV-1 and HSV-2 reactivation, their effects on primary infection are not clear. Since these viruses infect sensory and autonomic neurons, the latter of which are exquisitely sensitive to endocrine factors, we sought to determine whether stress hormones differentially modulate HSV-1 and HSV-2 lytic infections at a cellular level in primary adult sensory and autonomic neurons. Characterization of stress hormone receptor expression and analysis of viral replication in primary adult sensory and autonomic neurons showed that the stress hormones epinephrine and corticosterone have differential effects on HSV-1 and HSV-2. Furthermore, these differential effects occurred only in autonomic neurons, not in sensory neurons.
RESULTS
Stress hormone receptor expression in sensory and sympathetic neurons.
Cell-type-specific differences in adrenergic (epinephrine) and glucocorticoid (cortisol) receptor expression have been reported previously (20, 21). In order to identify differences in receptor expression by different populations of sensory and autonomic neurons relevant to HSV infection, we used immunofluorescence to determine the expression profiles of the glucocorticoid receptor (GCR) and adrenergic receptors (ARs) in cultured primary adult murine neurons from sensory trigeminal ganglia (TG) and sympathetic superior cervical ganglia (SCG).
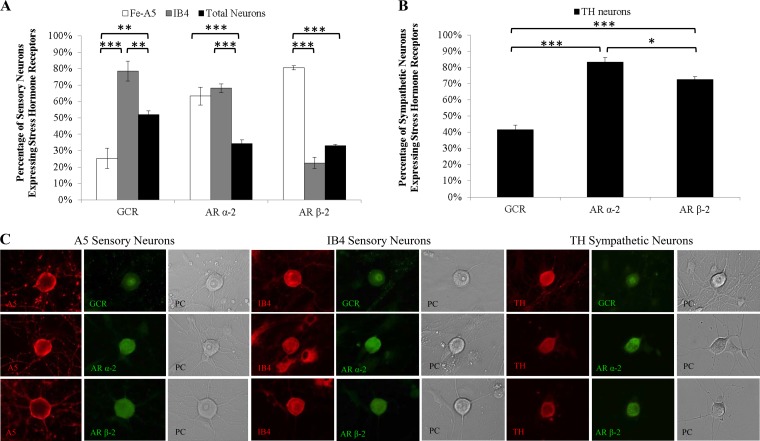
Among sensory TG neurons, a significantly greater percentage of IB4+ neurons (78.6%), which support productive HSV-1 infection, expressed the GCR (P < 0.001) than the percentage of A5+ neurons (25.3%), which support productive HSV-2 infection (Fig. 1A). Therefore, IB4+ sensory neurons are likely more responsive to glucocorticoids, including corticosterone (the rodent equivalent of human cortisol), than are A5+ neurons.
FIG 1.
Expression of stress hormone receptors in sensory and sympathetic neurons. Adult murine neuronal cultures from sensory TG and sympathetic SCG were immunostained for neuronal markers (Fe-A5, IB4, and tyrosine hydroxylase [TH]) and for the glucocorticoid receptor (GCR), the adrenergic α-2 receptor (AR α-2), and AR β-2. (A) Trigeminal ganglia. The GCR was differentially expressed in Fe-A5+ (P = 0.0023) and IB4+ (P = 0.0024) neurons relative to total neurons (n > 5). Higher percentages of Fe-A5+ (P < 0.0001) and IB4+ (P < 0.0001) neurons than of total neurons expressed AR α-2 (n > 6). A higher percentage of Fe-A5+ neurons than of IB4+ (P < 0.0001) or total (P < 0.0001) neurons expressed AR β-2 (n > 5). (B) Superior cervical ganglia. Higher percentages of neurons expressed AR α-2 (P < 0.0001) and AR β-2 (P < 0.0001) than the GCR (n > 3). (n stands for the number of cultures; all neuronal-marker-positive neurons present were counted.) *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) Representative fluorescence microscopy images showing the colocalization of neuronal markers (A5, IB4, and TH) with stress hormone receptors (GCR, AR α-2, and AR β-2) in sensory TG and sympathetic SCG. Phase-contrast (PC) microscopy was used to show neuronal morphology.
ARs α-2 and β-2 are expressed by 34.4% and 33.0% of total cultured adult sensory TG neurons, respectively (Fig. 1A). Higher percentages of A5+ neurons (63.3% and 80.7%) expressed adrenergic α-2 receptors (P < 0.0001) than the percentage expressed by the total population of TG neurons (Fig. 1A). Similarly, a higher percentage of IB4+ TG neurons (68.1%) expressed adrenergic α-2 receptors (P < 0.0001) than was expressed by the total population. However, fewer IB4+ neurons (22.5%) than other TG neurons (33.0%) expressed β-2 ARs. Therefore, while TG sensory neurons are likely responsive to epinephrine through adrenergic receptors, the neuronal populations in which HSV-1 and HSV-2 preferentially replicate express different patterns of adrenergic receptors, suggesting possible differences in responsiveness to epinephrine.
Nearly half (41.5%) of cultured sympathetic SCG neurons expressed the GCR (Fig. 1B). In contrast, 83.3% and 72.6% of SCG neurons expressed ARs α-2 and β-2, respectively (P = 0.0001) (Fig. 1B). Therefore, sympathetic SCG neurons are capable of being stimulated by both epinephrine and glucocorticoids.
Immunofluorescence detection of the sensory neuronal markers A5 and IB4, as well as the sympathetic marker tyrosine hydroxylase (TH), produced distinct staining patterns of the neuronal membrane and axons (Fig. 1C). Glucocorticoid and adrenergic receptor immunofluorescence was also detectable in distinct staining patterns, with the GCR present in the nucleus and AR α-2 and AR β-2 present on the cell surface and in the perinuclear region (Fig. 1C) (21, 40, 41). These distinct patterns were readily identifiable, enabling us to determine the percentages of neuronal subpopulations that expressed each receptor type.
To confirm that the percentages of primary adult cultured neurons expressing the adrenergic and glucocorticoid receptors were similar to the percentages expressed in vivo, ganglia from mice were cryosectioned and were immunostained for colocalization of stress hormone receptors and neuronal markers. There were no significant differences in the percentage of each neuronal subpopulation that expressed the GCR and ARs between the cultured neurons and the sectioned ganglia (Table 1). We detected no expression of stress hormone receptors in satellite glial cells present in immunostained sensory TG or sympathetic SCG primary adult murine cultures (Fig. 1C).
TABLE 1.
Expression of stress hormone receptors in sensory TG and sympathetic SCG neurons in vitro and in vivo
| Receptor | Neuron type | % of neurons of the indicated type that express stress hormone receptors: |
|
|---|---|---|---|
| In vitroa | In vivob | ||
| GCR | Fe-A5 | 25.31 (0.0622) (57/220) | 29.79 (84/282) |
| IB4 | 78.57 (0.0606) (851/1,080) | 65.88 (390/592) | |
| Total TG | 52.01 (0.0234) (536/831) | 49.05 (304/621) | |
| Total SCG | 41.50 (0.0300) (166/400) | 45.55 (225/494) | |
| AR α-2 | Fe-A5 | 63.26 (0.0536) (567/1,142) | 69.09 (509/714) |
| IB4 | 68.07 (0.0275) (1,535/2,358) | 56.97 (894/1,520) | |
| Total TG | 34.35 (0.0216) (1,331/3,883) | 37.29 (490/1,316) | |
| Total SCG | 83.33 (0.0276) (1,000/1,200) | 87.83 (758/863) | |
| AR β-2 | Fe-A5 | 80.67 (0.0125) (370/465) | 70.93 (122/172) |
| IB4 | 22.52 (0.0341) (347/1,586) | 43.99 (139/316) | |
| Total TG | 32.95 (0.0094) (997/3,234) | 39.93 (206/529) | |
| Total SCG | 72.58 (0.0171) (594/819) | 84.26 (182/216) | |
Values are percentages (standard errors of the means) (number of dually labeled neurons/number of neurons counted).
Values are percentages (number of dually labeled neurons/number of neurons counted). Counts obtained for in vivo receptor expression were from four mice, with the ganglia from all four mice pooled and not counted separately.
Neuron specificity of productive HSV infection.
To determine if HSV-1 or HSV-2 demonstrates a preference for productive infection in neurons expressing specific receptors, we coimmunostained infected adult neuronal cultures from TG and SCG for HSV antigen and the receptors AR α-2, AR β-2, and GCR (Fig. 2).
FIG 2.
Productive HSV infection in neurons expressing stress hormone receptors. Adult murine neuronal cultures from sensory TG and sympathetic SCG were immunostained at 9 hpi for HSV antigen and adrenergic α-2 receptors (AR α-2), AR β-2, or the glucocorticoid receptor (GCR). (A) AR α-2+ sensory TG neurons restricted productive HSV-1 infection (P, 0.0172 for comparison to total infected neurons), and AR α-2+ sympathetic SCG neurons supported productive HSV-1 (P < 0.0001) and HSV-2 (P < 0.0001) infection (n > 6). (B) AR β-2+ sensory TG neurons restricted productive HSV-1 (P = 0.0434) and HSV-2 (P = 0.0074) infection, and AR β-2+ sympathetic SCG neurons supported productive HSV-1 infection (P = 0.0426) (n > 3). (C) GCR+ sympathetic SCG neurons supported productive HSV-1 (P < 0.0001) and HSV-2 (P = 0.0012) infection, but there was no difference between GCR+ and GCR− sensory TG neurons (n > 3). (n stands for the number of cultures, with >200 neurons/culture counted.) *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In sensory TG neuronal cultures, neurons expressing AR α-2 restricted productive HSV-1 infection relative to total TG neurons (P = 0.0172) (Fig. 2A). In contrast, sympathetic SCG neurons that expressed AR α-2 selectively supported productive infection with HSV-1 and HSV-2, relative to other SCG neurons (P < 0.0001) (Fig. 2A).
Similarly, AR β-2+ sensory TG neurons restricted productive HSV-1 (P = 0.0434) and HSV-2 (P = 0.0074) infection, relative to other TG neurons (Fig. 2B). However, AR β-2+ SCG neurons selectively supported productive HSV-1 infection (P, 0.042 for comparison to total neurons infected with HSV-1) but not productive HSV-2 infection (Fig. 2B).
In sensory TG neurons, there were no significant differences between GCR+ neurons and total neurons infected with HSV-1 or HSV-2 (Fig. 2C), demonstrating that GCR+ sensory neurons support productive infection with HSV-1 and HSV-2 as well as sensory neurons that do not express the GCR. In SCG, however, HSV-1 and HSV-2 antigens were detected in higher percentages of GCR+ neurons than of total SCG neurons (P, <0.0001 and 0.0012, respectively).
Stress hormones modulate HSV DNA replication during productive infection in vitro.
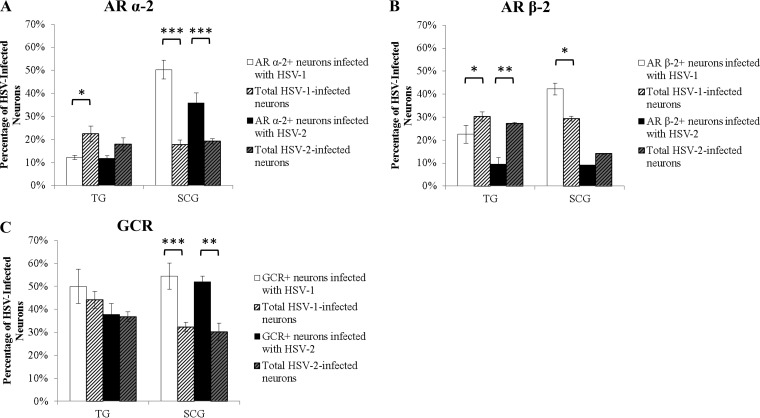
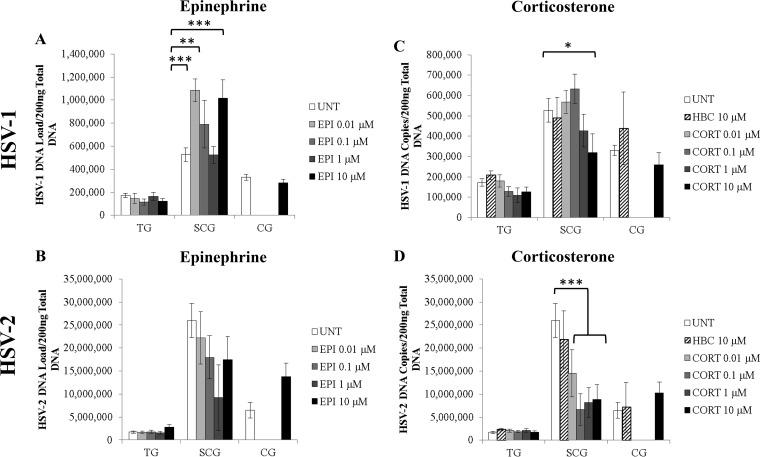
To determine if stress hormones affect HSV-1 and HSV-2 replication during productive infection, EPI or CORT [conjugated to (2-hydroxypropyl)-β-cyclodextrin (HBC) in the water-soluble form] was added to productively infected neuronal cultures at 1 h postinoculation (1 hpi). The water-soluble carrier molecule HBC was also used as a control. Viral DNA was isolated at 10 hpi, representing a single cycle of viral replication, and was quantified by quantitative PCR (qPCR) using primers and probes specific for the thymidine kinase (TK) gene.
HSV-1 DNA loads in sympathetic SCG neurons treated with 0.01, 0.1, or 10 μM EPI were significantly higher than those in untreated (UNT) control SCG neurons (P < 0.004) (Fig. 3A). In contrast, no significant differences were found in the neurons of HSV-1-infected sensory TG or parasympathetic ciliary ganglia (CG). No significant differences were detected after EPI treatment of sensory or autonomic neuronal cultures infected with HSV-2 (Fig. 3B). Therefore, epinephrine increases HSV-1 DNA replication during productive infection in sympathetic SCG neurons in a nonmonotonic dose-dependent manner but has no significant effect on HSV-2.
FIG 3.
Effects of EPI and CORT on HSV-1 and HSV-2 replication during productive infection. Primary adult murine neuronal cultures from trigeminal ganglia (TG), superior cervical ganglia (SCG), or ciliary ganglia (CG) were treated with EPI or CORT during HSV infection (10 hpi). Neurons and media were then collected for qPCR analysis for viral DNA. (A) The HSV-1 DNA load was increased by 0.01 μM (P < 0.001), 0.1 μM (P = 0.003), or 10 μM (P < 0.001) EPI in SCG neurons but not in TG or CG neurons (n > 3). (B) The HSV-2 DNA load was not significantly affected by EPI treatment, regardless of the dose (n > 3). (C) The number of HSV-1 DNA copies was decreased by 10 μM CORT (P = 0.02) in SCG neurons but not in TG or CG neurons (n > 3). (D) The number of HSV-2 DNA copies was decreased by 0.01 μM (P = 0.006), 0.1 μM (P = 1.1 × 10−5), 1 μM (P = 0.0004), or 10 μM (P = 0.0001) CORT in SCG neurons but not in TG or CG neurons (n > 3). UNT, untreated; neurons were infected with HSV-1 or HSV-2 but were not treated with stress hormones. HBC, (2-hydroxypropyl)-β-cyclodextrin (a water-soluble carrier molecule that was conjugated to CORT or was used alone as a control). In panels C and D, HBC results were not significantly different from UNT results (n > 3). (n stands for the number of cultures.) *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In contrast, the number of HSV-1 DNA copies was significantly decreased in sympathetic SCG neurons treated with 10 μM CORT (P = 0.02), showing a linear dose response to 0.1 μM to 10 μM CORT treatments (Fig. 3C). HSV-2 replication was also significantly decreased in sympathetic SCG neurons treated with CORT, regardless of the concentration, showing that HSV-2 is more sensitive to CORT treatment than HSV-1. No significant effect of CORT treatment was detected for either HSV-1 or HSV-2 DNA replication in sensory TG or parasympathetic CG neurons (Fig. 3C and D). The HBC carrier molecule also had no significant effect on HSV-1 or HSV-2 DNA replication in sensory or autonomic neurons (Fig. 3C and D).
Furthermore, both HSV-1 and HSV-2 replicated more efficiently in sympathetic SCG neurons than in sensory TG neurons, as shown by the significantly greater viral DNA loads in infected untreated SCG neurons than in infected untreated TG neurons (P < 0.0001) (Fig. 3).
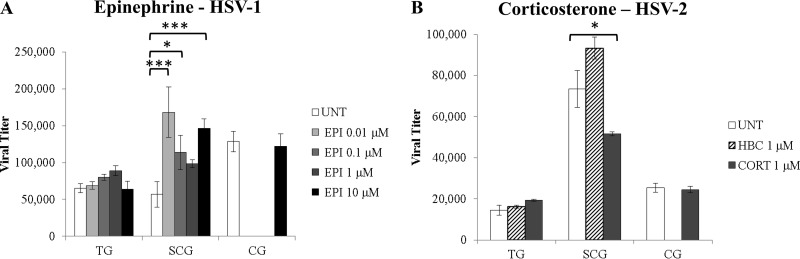
Stress hormones affect infectious HSV titers in vitro.
Since EPI increased HSV-1 DNA loads in sympathetic SCG neurons, we next determined whether EPI also increased the production of viral progeny. The same four concentrations of EPI that had been used for the determination of effects on HSV replication were used for treatment in this experiment. Neurons and media were collected 24 hpi and were analyzed for viral titers by plaque assays on Vero cells. The quantity of HSV-1 infectious virus progeny was significantly increased in sympathetic SCG neurons treated with 0.01 μM, 0.1 μM, or 10 μM EPI (P < 0.02) (Fig. 4A), correlating with the increased viral DNA loads in response to the same EPI treatments, in a similar, nonmonotonic dose response (Fig. 3A). Untreated parasympathetic CG neurons also produced significantly higher HSV-1 titers than untreated sensory TG neurons (P = 0.014), showing that autonomic neurons support more-efficient production of infectious HSV-1 progeny than sensory neurons.
FIG 4.
Effects of stress hormones on infectious virus titers during productive infection. Virus titers were determined by plaque assays on Vero cells for infected neurons and media treated with the indicated concentrations of EPI or CORT (24 hpi). (A) Epinephrine treatment of HSV-1-infected neurons increased virus titers over those in untreated HSV-1-infected control neurons (UNT). Differences were significant with 0.01 μM (P = 0.0001), 0.1 μM (P = 0.03), or 10 μM (P = 0.001) EPI (n > 3). (B) Corticosterone treatment of HSV-2-infected neurons decreased virus titers from those in untreated HSV-2-infected control neurons. Differences were significant with 1 μM CORT (P = 0.012). HBC, (2-hydroxypropyl)-β-cyclodextrin (a water-soluble carrier molecule that was conjugated to CORT or was used alone as a control). HBC results were not significantly different from UNT results (n > 3). (n, number of samples collected in which neurons were treated with stress hormones and were evaluated for infectious virus titers.) *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Since CORT decreased the number of HSV-2 DNA copies in sympathetic neurons, we determined whether CORT also decreased the production of viral progeny. CORT or its water-soluble carrier molecule, HBC, was added to HSV-2-infected neuronal cultures at 1 hpi, and infectious viral progeny were assessed by plaque assays on Vero cells. CORT treatment significantly decreased infectious HSV-2 titers in sympathetic SCG neurons from those for untreated neurons (P = 0.0115) (Fig. 4B). HBC had no significant effect on viral titers (Fig. 4B). There were no significant differences in virus titers between CORT-treated and untreated TG or CG neurons. In addition, sympathetic SCG produced significantly greater quantities of HSV-2 progeny than parasympathetic CG or sensory TG (P < 0.0001) (Fig. 4B).
DISCUSSION
Previous studies have shown that epinephrine (EPI) and corticosterone (CORT) can induce HSV-1 reactivation in animal models of infection, as well as in humans (5–7, 10–12, 18). In humans, stress is strongly correlated with the appearance of fever blisters, which are recurrent lesions caused by reactivating HSV-1, and is anecdotally correlated with recurrences of genital herpes. The neurons in which HSV establishes latency express receptors for EPI and CORT, suggesting that these endocrine factors may be capable of modulating HSV infection in the neurons directly rather than indirectly, through suppressive effects on the immune system. It is also important to consider the effects of stress hormones on primary HSV infection in neurons, since stress during primary infection could potentially affect HSV-related clinical disease in humans.
In our studies, in which we tested the effects of stress hormones on productive infection rather than reactivation from latency, EPI increased replication and the production of infectious virus progeny by HSV-1. However, the effects occurred in sympathetic neurons, not in sensory neurons. Although sensory neurons, like sympathetic neurons, express adrenergic receptors, productive HSV-1 infection was inhibited in AR+ sensory neurons but was enhanced in AR+ sympathetic neurons. While HSV-2 showed a preference for productive infection in AR α-2+ sympathetic neurons, EPI had no impact on productive HSV-2 infection. Thus, the mechanism by which EPI modulates productive infection is exclusive to HSV-1 and occurs only in sympathetic neurons.
EPI also increased replication and the release of infectious virus progeny by HSV-1 during productive infection at two different concentrations. While nonmonotonic dose-response curves are relatively common for endocrine factors, the specific mechanisms remain elusive (42). It is possible that this inverted bell curve is due to EPI's property as a nonselective agonist for the various classes of adrenergic receptors. Sympathetic neurons express two different subtypes of adrenergic receptors that differ in their intrinsic receptor properties (43, 44). α-2 ARs inhibit forskolin-mediated cyclic AMP (cAMP) accumulation at norepinephrine concentrations of 100 pM to 100 nM but potentiate cAMP accumulation at concentrations of ≥1 μM (43). This is due to the ability of α-2 ARs to recruit different G proteins (45). In contrast, the binding of EPI to AR β-2 activates a stimulatory G protein, which stimulates adenylate cyclase and causes an increase in cAMP levels in sympathetic neurons of the superior cervical ganglia (SCG) (46). An increase in cAMP production has been shown to reactivate HSV-1 from quiescence in embryonic SCG neurons (47). The adenylate cyclase enzymes are optimally stimulated by AR β-2 treated with 100 nM epinephrine (48). Therefore, the increases in HSV-1 DNA replication at 0.01 μM and 0.1 μM epinephrine most likely represent the stimulation of AR β-2, while the increase in HSV-1 DNA replication at 10 μM epinephrine results from the stimulation of AR α-2. The lack of response at 1 μM epinephrine is most likely due to the inhibition of adenylate cyclase by AR α-2. Furthermore, pretreatment with cAMP results in increases in ICP4 and ICP0 expression from an immortalized neuronal cell line (49). Therefore, it is possible that epinephrine is acting through a similar mechanism to increase HSV-1 replication and release of infectious virus progeny from sympathetic neurons during productive infection. Although further studies are necessary to determine the specific mechanism, it is clear that sympathetic neurons are significantly more responsive to EPI-induced enhancement of productive HSV-1 infection than are sensory neurons.
Corticosterone treatment had minimal effects on productive HSV-1 infection in primary adult neurons, regardless of neuron type. We observed a decreasing trend in the number of HSV-1 DNA copies at the highest concentrations of CORT, suggesting a potential effect at concentrations beyond the biologically relevant range, even under stress (50). However, CORT decreased HSV-2 DNA replication and release of infectious virus progeny. Again, these effects occurred in sympathetic neurons rather than in sensory neurons, further implicating the autonomic nervous system in HSV pathogenesis. Previous research has shown that the origin of replication in the unique long region of the HSV-1 genome (oriL) contains a glucocorticoid response element that can bind the GCR; the GCR agonist dexamethasone (DEX) was able to increase oriL-dependent HSV-1 DNA replication in rat pheochromocytoma (PC12) cells (13). In addition, pretreatment with DEX has been reported to increase infectious HSV-1 progeny in human gingival fibroblasts (14). Serum- and glucocorticoid-regulated protein kinases (SGK) induced by stress have also been implicated in the stimulation of HSV-1 replication in Vero cells (50). We observed no such increases in HSV-1 replication in response to CORT treatment, which may be due to the types of cells utilized, since PC12 cells, human gingival fibroblasts, and Vero cells are not analogous to adult, differentiated neurons (51). One report found that the effects of glucocorticoids on productive HSV-2 infection in vitro differed depending on the type of cell utilized and the characteristics of the viral strain used (52); the addition of dexamethasone and cortisol increased the infectious HSV-2 titer in 3T3 cells but decreased the plaque size of HSV-2 in embryonic mouse fibroblasts (52). The effects of glucocorticoids on HSV-2 in neurons remain largely unexplored. However, our studies show that CORT has a profound impact on HSV-2 replication in adult sympathetic neurons, but not in adult sensory neurons, during productive infection.
Because we studied productive HSV infection in primary adult sensory TG and sympathetic SCG neuronal cultures, it is possible that the satellite glial cells present within the cultured ganglia contributed to viral DNA quantities and infectious virus titers. However, there is currently no evidence to suggest that there are significant phenotypic differences between the satellite glial cells in sensory and autonomic ganglia (40, 53, 54). Satellite glial cells exhibit similar morphologies, functions, and pharmacologies in sensory and autonomic ganglia, and there has been no report that satellite glial cells from sensory or autonomic ganglia express adrenergic or glucocorticoid receptors, in agreement with our findings. One report demonstrated the uptake of radiographically tagged dexamethasone, but not corticosterone, into satellite glial cells of rat superior cervical ganglia but did not demonstrate the presence of a receptor or explain the difference in uptake for the two glucocorticoids (55). Therefore, we conclude that while HSV replication in satellite glial cells may contribute to levels of viral DNA and infectious virus titers during productive infection, the current literature and our findings suggest that the differences found between sensory and sympathetic ganglia are due to differences between the neurons, not the satellite glial cells supporting them.
In summary, the present study demonstrates that stress hormone receptors are differentially expressed on sensory and sympathetic neurons relevant to HSV-1 and HSV-2 infection. The stress hormones EPI and CORT have differential effects on HSV-1 and HSV-2 DNA replication and release of infectious virus progeny in sympathetic neurons, but not in sensory neurons, during productive infection, suggesting that autonomic neurons play a distinctive role in the stress-induced modulation of productive HSV infection.
MATERIALS AND METHODS
Virus strains.
HSV-1 strain 17+ was originally transferred from John Hay (SUNY at Buffalo, Buffalo, NY), and HSV-2 strain 333 from Gary Hayward (Johns Hopkins, Baltimore, MD), to the Krause lab (FDA, Bethesda, MD). At the Krause lab, the viruses were propagated in Vero cells (ATCC), and first-passage stocks were transferred to the Margolis lab (UCSF, San Francisco, CA). At the Margolis lab, the viruses were propagated in Vero cells, and first-passage stocks were transferred to the Bertke lab (Virginia Tech, Blacksburg, VA). At the Bertke lab, the viruses were propagated in Vero cells and were titrated in quadruplicate by plaque assays on Vero cells. Stock viruses were diluted in Neurobasal A medium supplemented with penicillin-streptomycin and B-27 supplement for the inoculation of primary adult murine neuronal cultures.
Neuronal cultures.
Sensory trigeminal ganglia (TG), sympathetic superior cervical ganglia (SCG), and parasympathetic ciliary ganglia (CG) were removed from 6-week-old female Swiss Webster mice, dissociated, and plated on Matrigel-coated Lab-Tek II chamber slides (Thermo Scientific) as described previously (32, 56). Briefly, ganglia were digested in papain, collagenase, and dispase (Worthington), followed by mechanical trituration. TG were enriched for neurons using a multistep OptiPrep gradient (BD Biosciences), while SCG and CG were plated without a gradient step, since they contain less ganglionic debris after dissociation. Cells were maintained in Complete Neuro medium, consisting of Neurobasal A medium supplemented with B-27 supplement, penicillin-streptomycin, l-glutamine, neurotrophic factors, and mitotic inhibitors (Life Technologies). All studies were approved by, and conducted in accordance with, the Virginia Tech Institutional Animal Care and Use Committee (IACUC no. 13-008-CVM and 15-237).
Infection and hormone treatment.
Four days after plating, the medium was removed, and neurons were inoculated with HSV-1 (strain 17+) or HSV-2 (strain 333) at a multiplicity of infection (MOI) of 30. After a 1-h adsorption period, the inoculum was removed and was replaced with Complete Neuro medium with no mitotic inhibitors, with or without epinephrine or corticosterone-HBC (Sigma) at the concentrations indicated in the figures. Corticosterone-HBC is a water-soluble corticosterone conjugated to (2-hydroxypropyl)-β-cyclodextrin (HBC), a carrier molecule that enables solubility in media without harming the cultured neurons. HBC alone was used as a control to ensure that any effects were due to corticosterone rather than the HBC carrier molecule.
Plaque assay.
Twenty-four hours postinfection, neurons and media were collected and were stored at −80°C until assayed. The suspension was serially diluted from 10−1 to 10−4, and dilutions were inoculated onto 70-to-80% confluent Vero cells (ATCC). After a 1-h adsorption period, the inoculum was removed and was replaced with Dulbecco's modified essential medium (DMEM) with 2% fetal bovine serum, 1% penicillin-streptomycin, and 4 μl/ml human IgG for 48 h. Vero cells were fixed and were stained with crystal violet, and plaques were counted under a light microscope (Olympus).
Immunofluorescence.
Nine hours postinoculation, neuronal cultures were fixed with 2% paraformaldehyde and were immunostained. Individual neurons positive for immunofluorescence were counted to determine the percentages of HSV-positive neurons and receptor-positive neurons. Neuronal subpopulations were labeled with isolectin IB4 conjugated to fluorescein isothiocyanate (FITC) or rhodamine (1:500; Vector) and/or the following antibodies: Fe-A5 IgM (undiluted supernatant; DSHB), FITC-conjugated anti-HSV (a polyclonal antibody that reacts with antigens common to HSV-1 and HSV-2, including all major glycoproteins in the viral envelope and at least one core protein as determined by crossed immunoelectrophoresis) (1:40; Dako) (30, 57), anti-HSV (1:750; Abcam), anti-TH (1:500; Abcam), anti-GCR (1:250; Thermo Fisher Scientific), anti-AR α-2A (1:300; Abcam), anti-AR α-2B (1:300; Sigma-Aldrich), anti-AR α-2C (1:300; Thermo Fisher Scientific), and anti-AR β-2 (1:500; Abcam). Secondary antibodies were Alexa Fluor-labeled species-specific antibodies (1:1,000; Life Technologies). The number of dually labeled neurons (receptor positive and neuronal marker positive) was compared to the total number of neurons positive for the neuronal marker in order to calculate the percentages of neuronal populations expressing specific hormone receptors (Fig. 1; Table 1). The numbers of cryosectioned murine ganglia or primary adult murine cultures (n) are given in the figure legends, and all marker-positive neurons were counted in each well. For uninfected neurons stained using immunofluorescence, both those from in vitro primary murine neuronal cultures and those from in vivo cryosectioned murine ganglia, the percentages of stress hormone receptor expression were calculated by first counting the dually labeled neurons coexpressing the stress hormone receptor and the neuronal marker and then dividing by the number of all neurons expressing the neuronal marker. For infected neurons stained using immunofluorescence in vitro, the percentages of colocalization of stress hormone receptors with HSV antigen were calculated by counting the neurons colabeled with a stress hormone receptor and HSV antigen (AR α-2+, AR β-2+, or GCR+ neurons infected with HSV-1 or HSV-2) and dividing by the number of all neurons positive for HSV antigen (Fig. 2). The percentages of infected neurons were calculated by counting the neurons positive for HSV antigen and dividing by the number of all neurons in the well (Fig. 2, total HSV-1-infected neurons or total HSV-2-infected neurons). Immunostained, uninfected adult murine neuronal cultures were imaged on an Olympus IX71 inverted fluorescence microscope using cellSens Dimension software at ×20 magnification.
Quantitation of HSV loads and gene expression.
At 10 h postinoculation (10 hpi), viral DNA was extracted from neuronal cultures with TRIzol reagent (Thermo Fisher Scientific), according to the manufacturer's instructions. Viral DNA loads were determined by quantifying viral DNA by qPCR using HSV-1 and HSV-2 thymidine kinase (TK) gene-specific primers and probes (58, 59). All assay results were normalized to 18S rRNA results (Applied Biosystems) and are reported as viral copy numbers in 200 ng of total DNA.
Statistics.
Statistical analyses were performed using parametric analyses with JMP Pro, version 12, including analysis of variance with contrast tests. Percentages of expression, viral DNA loads, and infectious virus titers were compared using analysis of variance. Error bars in figures represent standard errors of the means.
ACKNOWLEDGMENTS
This work was supported by research grant K22AI097299 from the National Institute of Allergy and Infectious Diseases (to A.S.B.) and a Graduate Research Development Program grant from Virginia Tech (to A.M.I.).
We thank Kathleen Apakupakul, Shantal Hover, and Laura Nelson for technical assistance and Rebecca Powell-Doherty for critical reviews of the manuscript.
We have no competing financial interests in the research presented.
REFERENCES
- 1.Fife KH, Schmidt O, Remington M, Corey L. 1983. Primary and recurrent concomitant genital infection with herpes simplex virus types 1 and 2. J Infect Dis 147:163. doi: 10.1093/infdis/147.1.163. [DOI] [PubMed] [Google Scholar]
- 2.Logan H, Lutgendorf SK, Hartwig A, Lilly J, Berberich SL. 1998. Immune, stress, and mood markers related to recurrent oral herpes outbreaks. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 86:48–54. doi: 10.1016/S1079-2104(98)90149-4. [DOI] [PubMed] [Google Scholar]
- 3.Cohen F, Kemeny ME, Kearney KA, Zegans LS, Neuhaus JM, Conant MA. 1999. Persistent stress as a predictor of genital herpes recurrence. Arch Intern Med 159:2430–2436. doi: 10.1001/archinte.159.20.2430. [DOI] [PubMed] [Google Scholar]
- 4.Uchakin PN, Parish DC, Dane FC, Uchakina ON, Scheetz AP, Agarwal NK, Smith BE. 2011. Fatigue in medical residents leads to reactivation of herpes virus latency. Interdiscip Perspect Infect Dis 2011:571340. doi: 10.1155/2011/571340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon BS, Gangarosa LP, Burch KD, deBack J, Hill JM. 1981. Induction of ocular herpes simplex virus shedding by iontophoresis of epinephrine into rabbit cornea. Invest Ophthalmol Vis Sci 21:442–450. [PubMed] [Google Scholar]
- 6.Blondeau JM, Aoki FY, Glavin GB. 1993. Stress-induced reactivation of latent herpes simplex virus infection in rat lumbar dorsal root ganglia. J Psychosom Res 37:843–849. doi: 10.1016/0022-3999(93)90173-D. [DOI] [PubMed] [Google Scholar]
- 7.Ashcraft KA, Bonneau RH. 2008. Psychological stress exacerbates primary vaginal herpes simplex virus type 1 (HSV-1) infection by impairing both innate and adaptive immune responses. Brain Behav Immun 22:1231–1240. doi: 10.1016/j.bbi.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chida Y, Mao X. 2009. Does psychosocial stress predict symptomatic herpes simplex virus recurrence? A meta-analytic investigation on prospective studies. Brain Behav Immun 23:917–925. doi: 10.1016/j.bbi.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Ashcraft KA, Hunzeker J, Bonneau RH. 2008. Psychological stress impairs the local CD8+ T cell response to mucosal HSV-1 infection and allows for increased pathogenicity via a glucocorticoid receptor-mediated mechanism. Psychoneuroendocrinology 33:951–963. doi: 10.1016/j.psyneuen.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willey DE, Trousdale MD, Nesburn AB. 1984. Reactivation of murine latent HSV infection by epinephrine iontophoresis. Invest Ophthalmol Vis Sci 25:945–950. [PubMed] [Google Scholar]
- 11.Rootman DS, Haruta Y, Hill JM. 1990. Reactivation of HSV-1 in primates by transcorneal iontophoresis of adrenergic agents. Invest Ophthalmol Vis Sci 31:597–600. [PubMed] [Google Scholar]
- 12.Noisakran S, Halford WP, Veress L, Carr DJ. 1998. Role of the hypothalamic pituitary adrenal axis and IL-6 in stress-induced reactivation of latent herpes simplex virus type 1. J Immunol 160:5441–5447. [PubMed] [Google Scholar]
- 13.Hardwicke MA, Schaffer PA. 1997. Differential effects of nerve growth factor and dexamethasone on herpes simplex virus type 1 oriL- and oriS-dependent DNA replication in PC12 cells. J Virol 71:3580–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erlandsson AC, Bladh LG, Stierna P, Yucel-Lindberg T, Hammarsten O, Modeer T, Harmenberg J, Wilkstrom AC. 2002. Herpes simplex virus type 1 infection and glucocorticoid treatment regulate viral yield, glucocorticoid receptor and NF-κB levels. J Endocrinol 175:165–176. doi: 10.1677/joe.0.1750165. [DOI] [PubMed] [Google Scholar]
- 15.Chucair-Elliott AJ, Carr MM, Carr DJ. 2017. Long-term consequences of topical dexamethasone treatment during acute corneal HSV-1 infection on the immune system. J Leukoc Biol doi: 10.1189/jlb.4A1116-459R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beyer CF, Arens MQ, Hill JM, Rose BT, Hill GA, Lin DT. 1989. Penetrating keratoplasty in rabbits induces latent HSV-1 reactivation when corticosteroids are used. Curr Eye Res 8:1323–1329. doi: 10.3109/02713688909013913. [DOI] [PubMed] [Google Scholar]
- 17.Kook I, Doster A, Jones C. 2015. Bovine herpesvirus 1 regulatory proteins are detected in trigeminal ganglionic neurons during the early stages of stress-induced escape from latency. J Neurovirol 21:585–591. doi: 10.1007/s13365-015-0339-x. [DOI] [PubMed] [Google Scholar]
- 18.Jusufbegovic D, Schaal S. 9 August 2016. Quiescent herpes simplex keratitis reactivation after intravitreal injection of dexamethasone implant. Retin Cases Brief Rep. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins FJ, Baum A. 1995. Stress and reactivation of latent herpes simplex virus: a fusion of behavioral medicine and molecular biology. Ann Behav Med 17:116–123. doi: 10.1007/BF02895060. [DOI] [PubMed] [Google Scholar]
- 20.Gold MS, Dastmalchi S, Levine JD. 1997. Alpha 2-adrenergic receptor subtypes in rat dorsal root and superior cervical ganglion neurons. Pain 69:179–190. doi: 10.1016/S0304-3959(96)03218-6. [DOI] [PubMed] [Google Scholar]
- 21.DeLeón M, Covenas R, Chadi G, Narvaez JA, Fuxe K, Cintra A. 1994. Subpopulations of primary sensory neurons show coexistence of neuropeptides and glucocorticoid receptors in the rat spinal and trigeminal ganglia. Brain Res 636:338–342. doi: 10.1016/0006-8993(94)91034-0. [DOI] [PubMed] [Google Scholar]
- 22.Halford WP, Gebhardt BM, Carr DJ. 1996. Mechanisms of herpes simplex virus type 1 reactivation. J Virol 70:5051–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloom DC, Stevens JG, Hill JM, Tran RK. 1997. Mutagenesis of a cAMP response element within the latency-associated transcript promoter of HSV-1 reduces adrenergic reactivation. Virology 236:202–207. doi: 10.1006/viro.1997.8723. [DOI] [PubMed] [Google Scholar]
- 24.Sinani D, Cordes E, Workman A, Thunuguntia P, Jones C. 2013. Stress-induced cellular transcription factors expressed in trigeminal ganglionic neurons stimulate the herpes simplex virus 1 ICP0 promoter. J Virol 87:13042–13047. doi: 10.1128/JVI.02476-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill JM, Garza HH, Su YH, Meegalla R, Hanna LA, Loutsch JM, Thompson HW, Varnell ED, Bloom DC, Block TM. 1997. A 437-base-pair deletion at the beginning of the latency-associated transcript promoter significantly reduced adrenergically induced herpes simplex virus type 1 ocular reactivation in latently infected rabbits. J Virol 71:6555–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarman RG, Loutsch JM, Devi-Rao GB, Marquart ME, Banaszak MP, Zheng X, Hill JM, Wagner EK, Bloom DC. 2002. The region of the HSV-1 latency-associated transcript required for epinephrine-induced reactivation in the rabbit does not include the 2.0-kb intron. Virology 292:59–69. doi: 10.1006/viro.2001.1265. [DOI] [PubMed] [Google Scholar]
- 27.Hill JM, Patel A, Bhattacharjee PS, Krause PR. 2003. An HSV-1 chimeric containing HSV-2 latency associated transcript (LAT) sequences has significantly reduced adrenergic reactivation in the rabbit eye model. Curr Eye Res 26:219–224. doi: 10.1076/ceyr.26.3.219.14896. [DOI] [PubMed] [Google Scholar]
- 28.Bhattacharjee PS, Tran RK, Myles ME, Maruyama K, Mallakin A, Bloom DC, Hill JM. 2003. Overlapping subdeletions within a 348-bp in the 5′ exon of the LAT region that facilitates epinephrine-induced reactivation of HSV-1 in the rabbit ocular model do not further define a functional element. Virology 312:151–158. doi: 10.1016/S0042-6822(03)00174-0. [DOI] [PubMed] [Google Scholar]
- 29.Zhu L, Workman A, Jones C. 2016. Potential role for a β-catenin coactivator (high-mobility group AT-hook 1 protein) during the latency-reactivation cycle of bovine herpesvirus 1. J Virol doi: 10.1128/JVI.02132-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margolis TP, Imai Y, Yang L, Vallas V, Krause PR. 2007. Herpes simplex virus type 2 (HSV-2) establishes latent infection in a different population of ganglionic neurons than HSV-1: role of latency-associated transcripts. J Virol 81:1872–1878. doi: 10.1128/JVI.02110-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imai Y, Apakupakul K, Krause PR, Halford WP, Margolis TP. 2009. Investigation of the mechanism by which herpes simplex virus type 1 LAT sequences modulate preferential establishment of latent infection in mouse trigeminal ganglia. J Virol 83:7873–7882. doi: 10.1128/JVI.00043-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertke AS, Swanson SM, Chen J, Imai Y, Kinchington PR, Margolis TP. 2011. A5-positive primary sensory neurons are nonpermissive for productive infection with herpes simplex virus 1 in vitro. J Virol 85:6669–6677. doi: 10.1128/JVI.00204-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertke AS, Ma A, Margolis MS, Margolis TP. 2013. Different mechanisms regulate productive herpes simplex virus 1 (HSV-1) and HSV-2 infections in adult trigeminal neurons. J Virol 87:6512–6516. doi: 10.1128/JVI.00383-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richter ER, Dias JK, Gilbert JE II, Atherton SS. 2009. Distribution of herpes simplex virus type 1 and varicella zoster virus in ganglia of the human head and neck. J Infect Dis 200:1901–1906. doi: 10.1086/648474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bustos DE, Atherton SS. 2002. Detection of herpes simplex virus type 1 in human ciliary ganglia. Invest Ophthalmol Vis Sci 43:2244–2249. [PubMed] [Google Scholar]
- 36.Parr MB, Parr EL. 2003. Intravaginal administration of herpes simplex virus type 2 to mice leads to infection of several neural and extraneural sites. J Neurovirol 9:594–602. doi: 10.1080/jnv.9.6.594.602. [DOI] [PubMed] [Google Scholar]
- 37.Sanjuan NA, Lascano EF. 1986. Autonomic nervous system involvement in experimental genital infection by herpes simplex virus type 2. Arch Virol 91:329–339. doi: 10.1007/BF01314291. [DOI] [PubMed] [Google Scholar]
- 38.Shimeld C, Tullo AB, Hill TJ, Blyth WA, Easty DL. 1985. Spread of herpes simplex virus and distribution of latent infection after intraocular infection of the mouse. Arch Virol 85:175–187. doi: 10.1007/BF01314229. [DOI] [PubMed] [Google Scholar]
- 39.Martin JR, Jenkins FJ, Henken DB. 1991. Targets of herpes simplex virus type 1 infection in a mouse corneal model. Acta Neuropathol 82:353–363. doi: 10.1007/BF00296546. [DOI] [PubMed] [Google Scholar]
- 40.Brum PC, Hurt CM, Shcherbakova OG, Kobilka B, Angelotti T. 2006. Differential targeting and function of α2A and α2C adrenergic receptor subtypes in cultured sympathetic neurons. Neuropharmacology 51:397–413. doi: 10.1016/j.neuropharm.2006.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sunaguchi M, Nishi M, Mizobe T, Kawata M. 2003. Real-time imaging of green fluorescent protein-tagged β2-adrenergic receptor distribution in living cells. Brain Res 984:21–32. doi: 10.1016/S0006-8993(03)03004-X. [DOI] [PubMed] [Google Scholar]
- 42.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. 2012. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jasper JR, Lesnick JD, Chang LK, Yamanishi SS, Chang TK, Hsu SAO, Daunt DA, Bonhaus DW, Eglen RM. 1998. Ligand efficacy and potency at recombinant α2 adrenergic receptors: agonist-mediated [35S]GTPγS binding. Biochem Pharmacol 55:1035–1043. doi: 10.1016/S0006-2952(97)00631-X. [DOI] [PubMed] [Google Scholar]
- 44.Baker JG. 2005. The selectivity of beta-adrenoceptor antagonists at the human β1, β2 and β3 adrenoceptors. Br J Pharmacol 144:317–322. doi: 10.1038/sj.bjp.0706048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eason MG, Kurose H, Holt BD, Raymond JR, Liggett SB. 1992. Simultaneous coupling of α2-adrenergic receptors to two G-proteins with opposing effects. Subtype-selective coupling of α2C10, α2C4, and α2C2 adrenergic receptors to Gi and Gs. J Biol Chem 267:15795–15801. [PubMed] [Google Scholar]
- 46.Vásquez C, Lewis DL. 2003. The β2-adrenergic receptor specifically sequesters Gs but signals through both Gs and Gi/o in rat sympathetic neurons. Neuroscience 118:603–610. doi: 10.1016/S0306-4522(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 47.Smith RL, Pizer LI, Johnson EM Jr, Wilcox CL. 1992. Activation of second-messenger pathways reactivates latent herpes simplex virus in neuronal cultures. Virology 188:311–318. doi: 10.1016/0042-6822(92)90760-M. [DOI] [PubMed] [Google Scholar]
- 48.Green SA, Holt BD, Liggett SB. 1992. Beta 1- and beta 2-adrenergic receptors display subtype-selective coupling to Gs. Mol Pharmacol 41:889–893. [PubMed] [Google Scholar]
- 49.Wheatley SC, Dent CL, Wood JN, Latchman DS. 1992. Elevation of cyclic AMP levels in cell lines derived from latently infectable sensory neurons increases their permissivity for herpes virus infection by activating the viral immediate-early 1 gene promoter. Mol Brain Res 12:149–154. doi: 10.1016/0169-328X(92)90078-P. [DOI] [PubMed] [Google Scholar]
- 50.Kook I, Jones C. 2016. The serum and glucocorticoid-regulated protein kinases (SGK) stimulate bovine herpesvirus 1 and herpes simplex virus 1 productive infection. Virus Res 222:106–112. doi: 10.1016/j.virusres.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 51.Shafer TJ, Atchison WD. 1991. Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: a model for neurotoxicological studies. Neurotoxicology 12:473–492. [PubMed] [Google Scholar]
- 52.Costa J, Yee C, Troost T, Rabson AS. 1974. Effect of dexamethasone on herpes simplex virus type 2 infection in vitro. Nature 252:745–746. doi: 10.1038/252745a0. [DOI] [PubMed] [Google Scholar]
- 53.Hanani M. 2005. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev 48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Hanani M. 2010. Satellite glial cells in sympathetic and parasympathetic ganglia: in search of function. Brain Res Rev 64:304–327. doi: 10.1016/j.brainresrev.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 55.Warembourg M, Otten U, Schwab ME. 1981. Labelling of Schwann and satellite cells by [3H]dexamethasone in a rat sympathetic ganglion and sciatic nerve. Neuroscience 6:1039–1043. doi: 10.1016/0306-4522(81)90078-6. [DOI] [PubMed] [Google Scholar]
- 56.Lee S, Ives AM, Bertke AS. 2015. Herpes simplex virus 1 reactivates from autonomic ciliary ganglia independently from sensory trigeminal ganglia to cause recurrent ocular disease. J Virol 89:8383–8391. doi: 10.1128/JVI.00468-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang L, Voytek CC, Margolis TP. 2000. Immunohistochemical analysis of primary sensory neurons latently infected with herpes simplex virus type 1. J Virol 74:209–217. doi: 10.1128/JVI.74.1.209-217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bertke AS, Patel A, Imai Y, Apakupakul K, Margolis TP, Krause PR. 2009. Latency-associated transcript (LAT) exon 1 controls herpes simplex virus species-specific phenotypes: reactivation in the guinea pig genital model and neuron subtype-specific latent expression of LAT. J Virol 83:10007–10015. doi: 10.1128/JVI.00559-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bertke AS, Patel A, Krause PR. 2007. Herpes simplex virus latency-associated transcript sequence downstream of the promoter influences type-specific reactivation and viral neurotropism. J Virol 81:6605–6613. doi: 10.1128/JVI.02701-06. [DOI] [PMC free article] [PubMed] [Google Scholar]