Supplemental Digital Content is Available in the Text.
Baseline nociception is higher in adult mice. Mu-opioid postoperative hypersensitivity is age-dependent; aged mice are less sensitive to DAMGO, and more to higher buprenorphine doses.
Keywords: Postoperative pain, Mu-opioids, Buprenorphine, Aged mice
Abstract
Introduction:
Suboptimal management of postoperative pain leads to increased risk of chronic opioid therapy, especially in elderly patients.
Objectives:
Although this age-dependent phenomenon has been observed clinically, basic mechanisms including baseline nociception, postoperative hypersensitivity, and mu-opioid efficiency in aged animals have never been evaluated.
Methods:
We tested these criteria using incision model on adult (3–6 months) and aged (24 months) mice to assess translatability of postoperative animal studies to clinical observations.
Results:
Thermal and mechanical testing revealed lower baseline nociception in aged vs adult mice, while behavioral assays after hind paw plantar incision showed similar hypersensitivity levels for both age groups. Efficiency of local and spinal mu-opioid injections on postoperative pain was assessed next. DAMGO, a pure mu-opioid, was effective in reducing postoperative hypersensitivity in aged and adult mice, although adult mice displayed increased sensitivity to higher doses (50 μg local; 1–15 μg spinal). Buprenorphine, a mixed mu-opioid agonist, produced dose-dependent antihypersensitivity with adult mice more sensitive to lower doses (0.1 μg local; 0.02 μg spinal), and aged mice more sensitive to higher doses (1, 10 μg local; 0.1, 1 μg spinal). Finally, exploratory locomotor activity was used to evaluate the suppression of incision-induced spontaneous pain by DAMGO. Spinal and systemic (intraperitoneal) DAMGO inhibited ongoing pain more in adults compared with aged mice.
Conclusion:
As in humans, baseline nociception was lower in aged vs adult mice, while postoperative hypersensitivity magnitudes were comparable between groups. Unlike in humans, adult mice were more sensitive to mu-opioids, although higher doses of mixed mu-opioids were more effective for postoperative antihypersensitivity in aged mice.
1. Introduction
Old age profoundly contributes to the experience of pain during a variety of pathological conditions.12,14,39 Age plays a major role in postoperative recovery and, consequently, presents a unique set of challenges in the management of postoperative complications.5,13,42 Opioids are currently first line in postoperative pain management for all age groups47; however, recent studies have shown that elderly patients are particularly vulnerable to chronic opioid use in the postoperative period.40 The reasons for chronic opioid usage by elderly patients are largely unknown. This could be the result of a changed nociceptive threshold, higher postoperative pain, and efficacy of opioids in the elderly because they often have impaired metabolism leading to altered pharmacokinetics and pharmacodynamics.10,34
Age is considered one of the predictive factors of postoperative analgesic consumption,18,40 and most (≈70%) studies have found that the younger the patients, the higher the analgesic requirement to suppress postoperative pain.2,6,15,18,23 Thus, intramuscular administration of morphine provided more postoperative pain relief in older patients.6,23 One report has also suggested that higher efficiency of opioids in the elderly does not relate to drug absorption, distribution, metabolism, or elimination.6 Alternative studies have suggested that postsurgical opioid consumption is influenced less by advanced age and more by other predictors such as chronic sleeping difficulties and history of pain.11,25 Age could also have a negative correlation with postoperative pain intensity, but this finding was less consistent.18 Multiple studies have also been undertaken to determine the effect of aging on baseline nociception threshold, and a meta-analysis of over 50 studies indicates that there is definitive evidence of an increase in baseline nociceptive threshold with advancing age.21
Despite abundant information on age dependency in relation to nociceptive threshold, postoperative pain and the efficacy of opioids, these effects have not been tested in aged animal systems. In this study, we chose to examine the age dependency of baseline nociceptive threshold, thermal and mechanical postoperative hypersensitivity, and the efficacy of opioid analgesia in an aged vs adult postoperative mouse model. We chose to test 2 routes of drug administration: locally at the site of surgery and spinal administration. We selected DAMGO and buprenorphine for this study. Selection of the drugs was dictated by the idea that oxycodone, which is often used to treat postoperative pain in the elderly, metabolizes into oxymorphone, a mu-opioid receptor (MOR) agonist.20 Additionally, buprenorphine is emerging as an alternative medication for chronic postoperative pain control in elderly patients.32,43 Because a majority of clinical studies evaluated nonevoked postoperative pain in elderly patients, we employed the exploratory locomotor activity approach along with thermal and mechanical testing to assess postoperative pain in adult and aged mice.26
2. Methods
2.1. Animals
Animal experiments conformed to the American Pain Society Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training, to protocols approved by University Texas Health Science Center at San Antonio Institutional Animal Care and Use Committee, and followed guidelines issued by NIH and SfN to minimize the number of animals used.
Animals used in these experiments were males. Adult and aged mice were from a C57BL/6 background. Adult mice were 3- to 6-month-old (20–25 g) and purchased at Jackson Laboratories (Bar Harbor, ME). Aged mice were 18- and 24-month-old and were kindly donated by NIH/NIA, or were purchased at 17 to 18 months of age from Jackson Laboratories and grown to 24-month-old in University Texas Health Science Center at San Antonio Division of Laboratory Animal Resources facilities.
2.2. Acute postoperative pain model
All behavioral experiments were conducted by a blinded observer. The plantar incision in adult and aged mice was performed as previously described.33 Briefly, a 5 mm incision, beginning 2 mm from the proximal edge of the right heel, was made. Curved forceps were used to elevate the underlying muscle. A mattress suture of 8-0 nylon on a TG175-8 needle (ophthalmic, 1716G; Ethicon; Cincinnati, OH) was used to close the incision. An antibiotic ointment (Bacitracin Zinc Ointment USP; Nycomed US Inc., Melville, NY) was applied to the wound. Sham-treated mice received anesthesia, antiseptic preparation, and topical antibiotic without an incision.
2.3. Thermal (ie, heat) hypersensitivity
Thermal (ie, heat) nociception was assessed as previously described.30 In brief, mice were placed on a glass surface with temperature held constant at ≈20°C. After habituation, thermal/heat withdrawal latencies to a radiant heat beam focused on the plantar area of the hind paw were recorded at each time point (3× measurements at each time point, averaged to obtain the data value used in analyses). The stimulus was terminated after ≈20 seconds if the animal did not withdraw the hind paw.
2.4. Mechanical hypersensitivity
Mechanical nociception was assessed as previously described.30 After habituation to the testing environment, the baseline readings (3 readings per animal) were taken on the right hind paw using the Dynamic Plantar Aesthesiometer (Ugo Basile; Varese, Italy) to record withdrawal thresholds for mechanical stimulation (in grams) of the hind paw plantar areas. The instrument applies a constant ramp of increasing mechanical pressure to the paw (0–15 g over 10 seconds intervals).
2.5. The exploratory locomotor activity
The exploratory locomotor activity was used as a model to assess nonreflexive pain, which has an ongoing postoperative pain component, as previously described.26 The exploratory locomotor activity was measured as previously described.22 We used four 30 × 15 × 15 cm customized acrylic boxes that were separately enclosed in commercially available sound-attenuating chambers (ENV-022M; MED Associates; Fairfax, VT). Four infrared light beams were spaced 6 cm apart and located 2 cm above the floor of each box. Software (Multi-Varimex V1; Columbus Instruments; Columbus, OH) calculated total breaks of beams and ambulation defined by software as movements consisting of 6 cm or greater.22 On the first day, the exploratory locomotor activity after administration of saline (intrathecal or intraperitoneal [i.p.]) was measured for 2 hours. Seventy-two hours later, naive or 1 day postoperated animals received a drug, and activity was measured for 2 hours.
2.6. Drug administration
Fifty milligram per milliliter DAMGO and 15 mg/mL buprenorphine stocks were prepared in phosphate-buffered saline. DAMGO and buprenorphine were dissolved/diluted in sterile 0.9% saline. 0.9% saline does not produce hypersensitivity or antihypersensitivity.19 For local drug administration, operated or contralateral mouse hind paws were injected with 20 μL solution. For all spinal injections, mice were anesthetized with isoflurane for no longer than 3 minutes, and drugs were delivered in 10 μL solution into a lumbar (L3–L5) intrathecal space as described.31 Systemic administration was achieved through i.p. injection of drugs.
2.7. Data analyses
GraphPad Prism 7.0 (GraphPad, La Jolla, CA) was used for statistical analyses. Data in the figures are mean ± (SEM), with “n” referring to the number of mice per group. Some experiments were performed at least in duplicate. Some data are presented as area under the curve (post-drug time dependency). Differences between groups were assessed by regular 2-way analysis of variance (ANOVA; drug dose and mice age factors) with multiple comparisons to the Tukey posthoc test. In 1-way ANOVA with Tukey post hoc tests, each column was compared with all other columns. A difference was accepted as statistically significant when P < 0.05. Interaction F ratios and the associated P values were reported.
3. Results
3.1. Baseline nociception and postoperative hypersensitivity in adult and aged mice
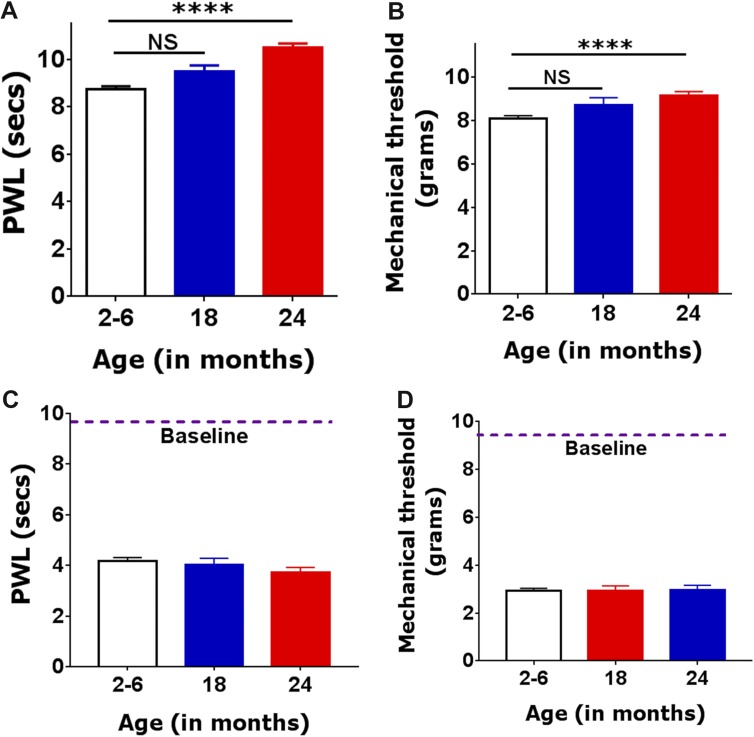
A 3- to 6-month-old mouse (corresponding to a 20- to 30-year-old human) is considered a mature adult; whereas an 18- to 24-month-old mouse (corresponding to a 56- to 69-year-old human) is referred to as “aged.”1 The literature indicates that morbidity related to surgery, preoperative morbidity, and other age-related complications are somewhat more prevalent in 69- vs 56-year-old patients7,12,39,41,42,46; therefore, we initially accessed basal nociceptive threshold and postoperative hypersensitivity for both 18- and 24-month-old mice. Twenty-four-month-old mice showed significantly longer paw withdrawal latencies than adult mice (1-way ANOVA; F(2,120) = 34.75; P < 0.0001; Fig. 1A). Eighteen-month-old mice exhibited the same trend, but it was statistically insignificant (Fig. 1A). Basal mechanical responses to a probe were insignificantly higher in aged 24-month-old, but not 18-month-old, compared with adult mouse groups (1-way ANOVA; F(2,84) = 11.54; P < 0.0001; Fig. 1B). The magnitude of postoperative thermal (Fig. 1C) and mechanical (Fig. 1D) hypersensitivity at 1 day postincision was not significantly different for 3- to 6-, 18- and 24-month-old mice (1-way ANOVA; F(2,99) = 2.109; P = 0.1268 for Fig. 1C; and F(2,100) = 0.01419; P = 0.9859 for Fig. 1D). In summary, basal thermal and mechanical nociceptive thresholds in aged compared with adult mice are higher. Similar increases in basal nociceptive thresholds were reported in elderly individuals.21 Thresholds of postoperative thermal and mechanical hypersensitivity were age independent, as some clinical studies on postoperative pain in elderly patients indicate.11,25 Importantly, because the 24-month-old animals seemed to differ more from adults than the 18-month-old animals, the remaining studies were conducted on 3- to 6- and 24 month-old mice.
Figure 1.
Basal nociception and postoperative thermal and mechanical hypersensitivity levels in adult and aged mice. (A) Basal thermal nociception was measured as paw withdrawal latency (PWL) in seconds in 2- to 6-, 18- and 24-month-old mice (1-way ANOVA; NS nonsignificant; ****P < 0.0001; n = 61 for adults; n = 15 for 18-month-old and n = 52 for 24-month-old). (B) Basal mechanical nociception is measured as paw withdrawal threshold in grams (statistic and n are the same as for A-panel). Postoperative thermal (C) and mechanical (D) hypersensitivity at 1 day after incision in 2- to 6-, 18- and 24-month-old mice (1-way ANOVA; NS nonsignificant; n = 48 for adults; n = 12 for 18-month-old and n = 45 for 24-month-old). Mouse ages are indicated below x-axis. Approximate baseline nociception levels (thermal on panel C and mechanical on panel D) are indicated as dashed lines and marker “Baseline.”
3.2. Local DAMGO injection and postoperative hypersensitivity in adult and aged mice
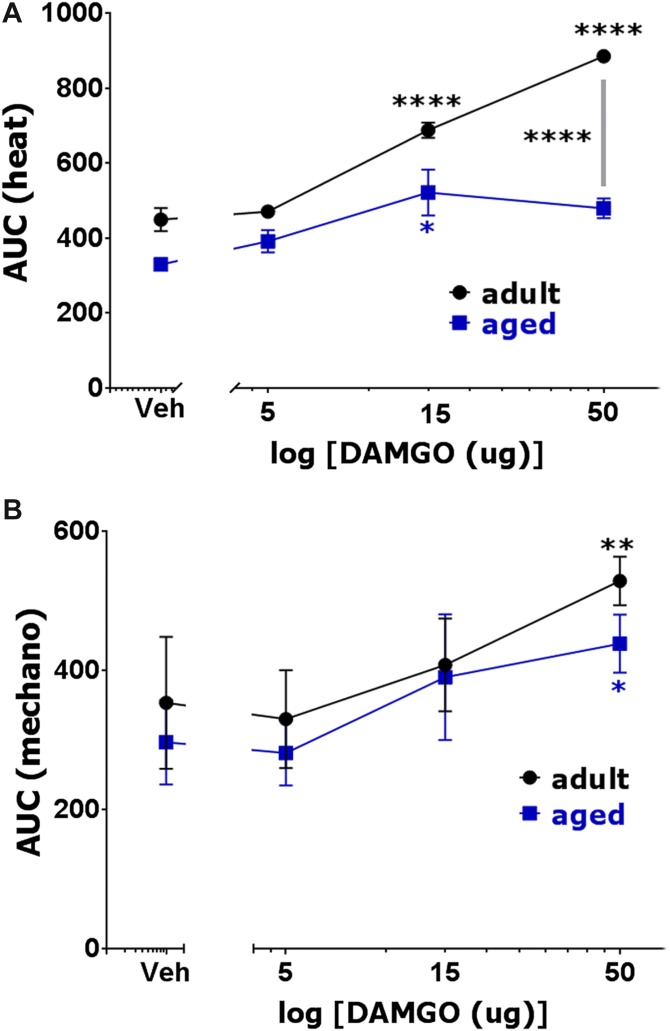
Initially, we examined whether locally (at incision site) administrated DAMGO produces postoperative antihypersensitivity in adult and aged mice. DAMGO-induced antihypersensitivity was monitored for 2 hours postinjection. Fifteen microgram and 50 μg, but not 5 μg, of DAMGO produced robust thermal (1-way ANOVA; F(3,16) = 89.67; P < 0.0001; Fig. 2A) and, to a lesser extent, mechanical (1-way ANOVA; F(3,16) = 7.995; P = 0.0018; Fig. 2B), postoperative antihypersensitivity in adult mice. In aged mice, local administration of 5 to 50 μg DAMGO was either ineffective or produced slight postoperative hypersensitivity (Fig. 2A, B). Fifty microgram, but not 5 and 15 μg DAMGO made more effective thermal antihypersensitivity in adult compared with aged mice (2-way ANOVA; F(3,32) = 11.51; P < 0.0001; Fig. 2A). In mechanical postoperative antihypersensitivity, 5 to 50 μg DAMGO was equally ineffective in adult and aged mice (2-way ANOVA; F(3,32) = 0.5058; P = 0.6810; Fig. 2B). Raw data on development of DAMGO-induced postoperative antihypersensitivity is shown in Supplementary Figure 1 (http://links.lww.com/PR9/A3). Altogether, only high (50 μg) dosage of locally injected DAMGO showed preference for thermal antihypersensitivity in adult compared with aged mice. Other DAMGO dosages administered at surgical sites produced similar effects in adult and aged mice.
Figure 2.
Effects of locally (at surgical site) administrated DAMGO on postoperative hypersensitivity in adult and aged mice. Incision-induced thermal (A) and mechanical (B) hypersensitivity were generated in adult and aged mice. At 1 day (for adults) and 1 to 3 days (for aged) postsurgery, operated hind paws were injected with different dosages of DAMGO, and thermal (A) and mechanical (B) hypersensitivity were measured at 30, 60 (for some dosages) and 120 minutes post-DAMGO injection. Data are presented as area under the curves (AUC). Significance within age groups for DAMGO-produced antihypersensitivity was compared against vehicle treatment (1-way ANOVA; *P < 0.05; **P < 0.01; ****P < 0.0001; n = 5–8). Significance between age groups was assessed by 2-way ANOVA. DAMGO dosages are indicated below log x-axis. Veh is vehicle treated conditions. Mouse ages are indicated.
3.3. Ipsilateral and contralateral DAMGO effect on postoperative antihypersensitivity
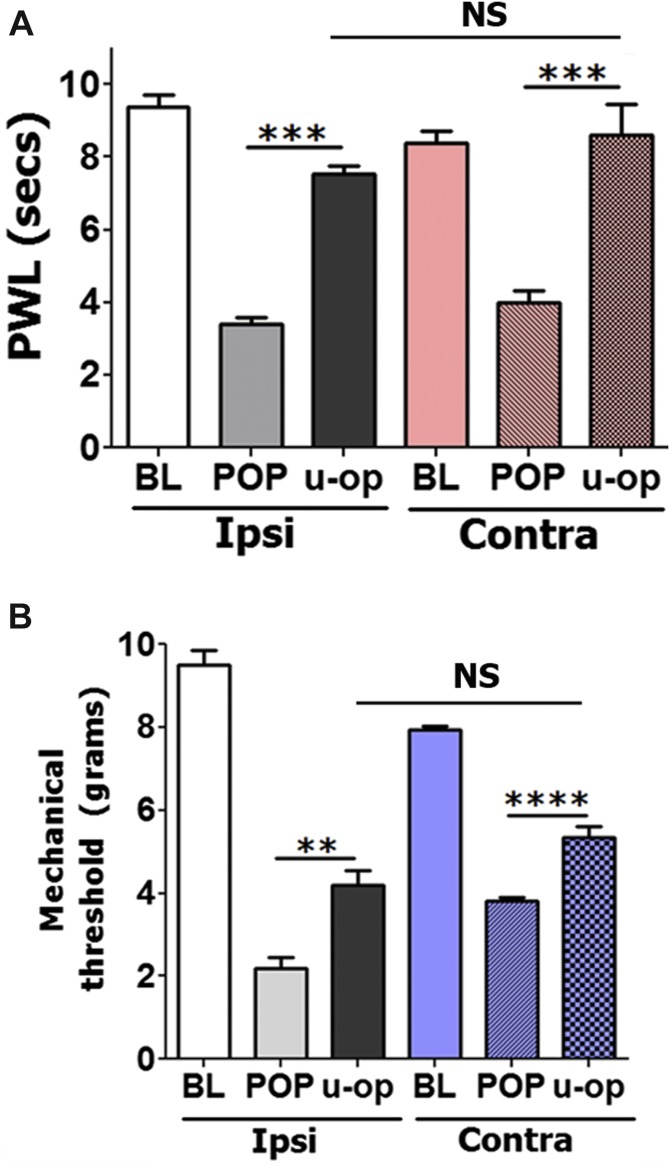
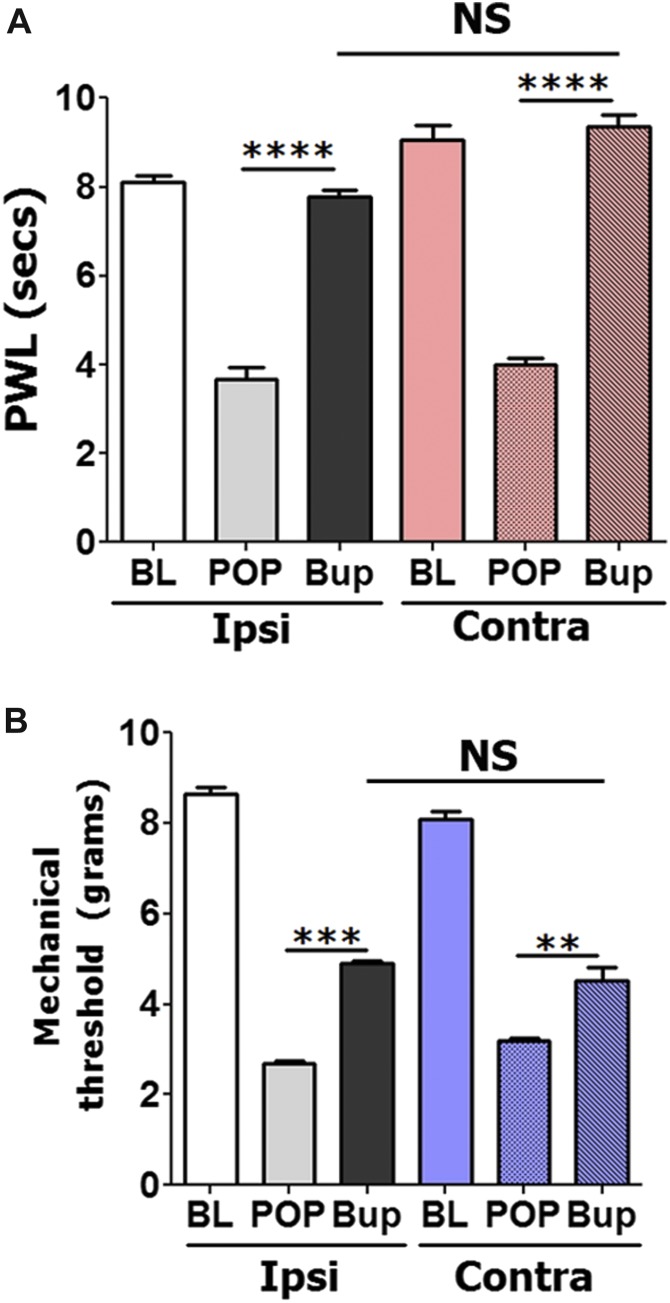
To evaluate the possibility that locally administrated DAMGO could induce antihypersensitivity through central mechanism at systemic dosages, we attempted to reverse postoperative hypersensitivity by injection of an antihypersensitivity-producing dose of DAMGO (15 μg) in contralateral (nonoperated) hind paws. Figure 3A, B show that thermal and mechanical postoperative antihypersensitivity can effectively be achieved by injection of DAMGO in either ipsilateral or contralateral hind paws (1-way ANOVA; F(5,27) = 30.75; P < 0.0001 for Fig. 3A; and F(5,27) = 117.6; P < 0.0001 for Fig. 3B). In summary, these data demonstrate that locally injected DAMGO (15 μg) in adult and aged mice distributes systemically and generates thermal and mechanical antihypersensitivity only through central mechanisms.
Figure 3.
Effects of contralateral injection of DAMGO on postoperative hypersensitivity in adult and aged mice. Thermal (A) and mechanical (B) postoperative antihypersensitivity produced at 2 hours after injection of 15 μg DAMGO in operated (ipsi) or intact (contra) hind paws of adult mice. DAMGO was administrated 1 day postincision surgery. Statistical analysis is 1-way ANOVA comparing all groups (NS, nonsignificant; **P < 0.05; ***P < 0.001; ****P < 0.0001; n = 5–6).
3.4. Spinal DAMGO injection and postoperative hypersensitivity in adult and aged mice
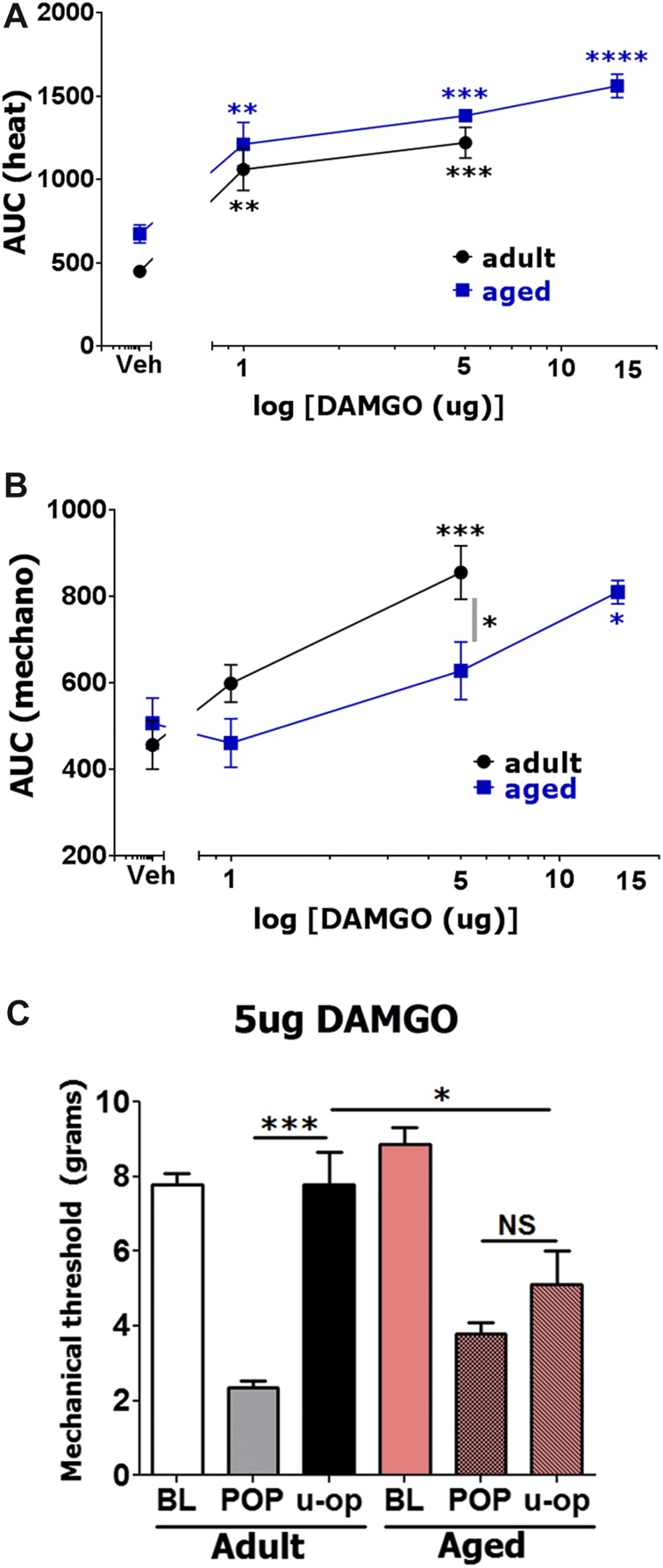
As expected, spinally administrated DAMGO produced robust thermal (1-way ANOVA; F(2,14) = 18.63; P < 0.0001; Fig. 4A) and mechanical (1-way ANOVA; F(2,15) = 12.5; P = 0.0006; Fig. 4B) postoperative antihypersensitivity in adult mice.49 Thus, 15 μg DAMGO paralyzed the movement of hind legs of some (but not all) animals, an effect that lasted 90 minutes after intrathecal injection (see Supplement Fig. 2B, http://links.lww.com/PR9/A3). Therefore, these data are not taken into account in Figure 4A, B. In addition to antihypersensitivity, thermal antinociception was detected even with DAMGO doses as low as 1 μg (1-way ANOVA; Fig. 4A; Supplement Fig. 2, http://links.lww.com/PR9/A3). For each dosage, spinally injected DAMGO was equally effective in thermal antihypersensitivity in both adult and aged mice (2-way ANOVA; F(2,23) = 0.0873; P = 0.9167; Fig. 4A). By contrast, postoperative mechanical antihypersensitivity by spinally administrated DAMGO was not as robust in aged compared with adult mice (2-way ANOVA for 1 and 5 μg doses; F(2,24) = 2.545; P = 0.0495; Fig. 4B). Thus, we observed that 5 μg DAMGO did not significantly reverse mechanical hypersensitivity in aged mice (Fig. 4B, C). Moreover, 15 μg DAMGO did not cause paralysis of hind legs of tested aged mice, and did not show mechanical antinociception (ie, threshold above baseline) in aged mice (Fig. 4B; Supplement Fig. 2, http://links.lww.com/PR9/A3). Overall, spinally injected DAMGO generally produced more effective thermal than mechanical postoperative antihypersensitivity in adult and aged mice (Fig. 4A, B). Furthermore, a higher dosage of spinal DAMGO is required for aged compared with adult mice to achieve mechanical postoperative antihypersensitivity.
Figure 4.
Effects of spinal injection of DAMGO on postoperative hypersensitivity in adult and aged mice. Incision-induced thermal (A) and mechanical (B) hypersensitivity were generated in adult and aged mice. At 1 day (for adults) and 1 to 3 days (for aged) postsurgery, different dosages of DAMGO were administrated intrathecally, and thermal (A) and mechanical (B) hypersensitivity were measured at 30 and 120 minutes post-DAMGO injection. Data are presented as area under the curve (AUC). Significance within age groups for DAMGO-produced antihypersensitivity was compared against vehicle treatment (1-way ANOVA; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; n = 5–8). Significance between age groups was assessed by 2-way ANOVA. DAMGO dosages are indicated below log x-axis. Veh is vehicle treated conditions. Mouse ages are indicated. (C) Mechanical antihypersensitivity (at 30 minutes post-opioid injection) by spinal administration of 5 μg DAMGO for adult and aged mice (1-way ANOVA; separately for adult and aged mice and 2-way ANOVA between adult and aged mice; NS, nonsignificant; *P < 0.05; ***P < 0.001; n = 5–8). U-op marking below x-axis is DAMGO produced effect.
3.5. Local buprenorphine injection and postoperative hypersensitivity in adult and aged mice
Buprenorphine is a partial MOR agonist and a kappa and delta-opioid receptor antagonist.29 Buprenorphine is also capable of inhibiting and modulating voltage-gated sodium channels.24 The mu-agonist effect of buprenorphine models a bell-shaped distribution,43 and it is reported to be at least 100 times more potent than morphine.37 We monitored buprenorphine-induced antihypersensitivity for 2 hours after local injection. Local administration of relatively low dosages of buprenorphine (0.1 μg) produced thermal (1-way ANOVA; F(3,16) = 93.56; P < 0.0001; Fig. 5A) and mechanical (1-way ANOVA; F(3,16) = 27.1; P < 0.0001; Fig. 5B) postoperative antihypersensitivity in adult mice. Moreover, maximal antihypersensitivity effects were at around 0.1 μg buprenorphine, whereas higher dosages were not as effective in reducing postoperative hypersensitivity, especially mechanical hypersensitivity (Fig. 5B). This effect was not due to a peripheral mechanism, as 0.1 μg buprenorphine injected contralaterally produced similar to ipsilaterally injected thermal (1-way ANOVA; F(5,27) = 119.2; P < 0.0001; Fig. 6A) and mechanical (1-way ANOVA; F(5,27) = 204.5; P < 0.0001; Fig. 6B) postoperative antihypersensitivity at 30 (data not shown) and 120 minutes after opioid injections (Fig. 6A, B).
Figure 5.
Effects of locally (at surgical site) administrated buprenorphine on postoperative hypersensitivity in adult and aged mice. Incision-induced thermal (A) and mechanical (B) hypersensitivity were generated in adult and aged mice. At 1 day (for adults) and 1 to 3 days (for aged) postsurgery, operated hind paws were injected with different dosages of buprenorphine, and thermal (A) and mechanical (B) hypersensitivity were measured at 30 and 120 minutes after buprenorphine injection. Data are presented as area under the curve (AUC). Significance within age groups for buprenorphine-produced antihypersensitivity was compared against vehicle treatment (1-way ANOVA; *P < 0.05; **P < 0.01; ****P < 0.0001; n = 5–8). Significance between age groups was assessed by 2-way ANOVA. Buprenorphine dosages are indicated below log x-axis. Veh is vehicle treated conditions. Mouse ages are indicated.
Figure 6.
Effects of contralateral injection of buprenorphine on postoperative hypersensitivity in adult and aged mice. Thermal (A) and mechanical (B) postoperative antihypersensitivity produced at 2 hours after injection of 0.1 μg buprenorphine in operated (ipsi) or intact (contra) hind paws of adult mice. Readings were made from surgery affected hind paws (1-way ANOVA; NS, nonspecific; **P < 0.05; ***P < 0.001; ****P < 0.0001; n = 5–6).
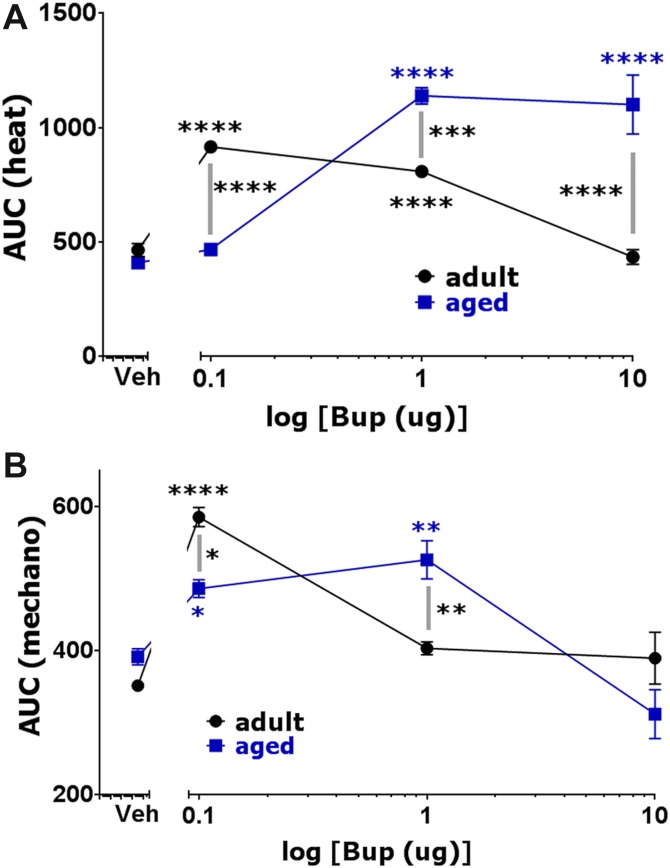
Unlike DAMGO, age-dependent efficiency of buprenorphine was dictated by drug dosage: lower dosages were more effective in adult mice, whereas higher dosages were more effective in aged mice. Thus, 1 and 10 μg of buprenorphine were more efficacious in reducing postoperative thermal hypersensitivity in aged mice (2-way ANOVA; F(3,30) = 59.99; P < 0.0001; Fig. 5A). Similarly, 1 μg buprenorphine made more postoperative mechanical antihypersensitivity in aged mice (2-way ANOVA; F(3,30) = 12.57; P < 0.0001; Fig. 5B). However, maximal thermal and mechanical postoperative antihypersensitivity was achieved at a dose of 0.1 μg buprenorphine in adults (Fig. 5A, B). Raw data on development of buprenorphine-induced postoperative antihypersensitivity are shown in Supplementary Figure 3 (http://links.lww.com/PR9/A3). Overall, the presented results show that locally injected buprenorphine is effective only at systemic dosages. Moreover, it seems that adult mice are more sensitive to lower dosages of buprenorphine, whereas aged mice are more sensitive to higher dosages.
3.6. Spinal buprenorphine injection and postoperative hypersensitivity in adult and aged mice
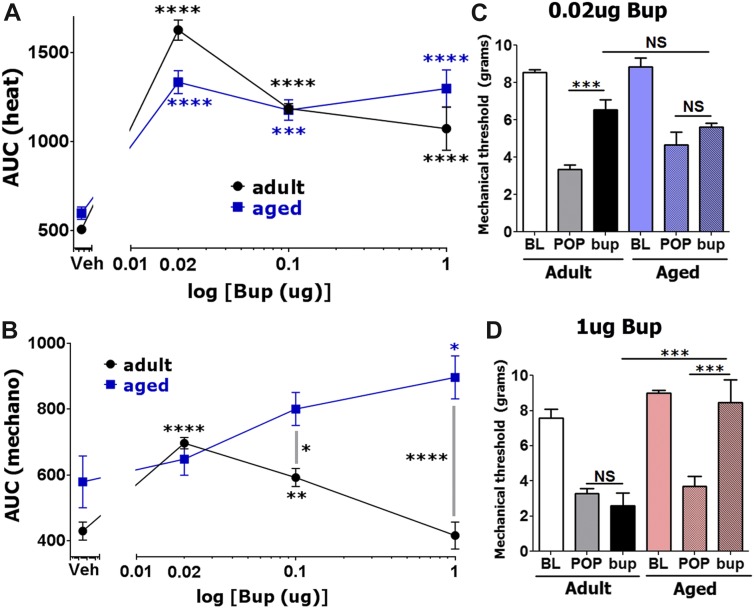
We monitored buprenorphine-induced postoperative antihypersensitivity for 2 hours after intrathecal injection. Similar to local administration, adult mice were more or equally sensitive to lower doses of spinally injected buprenorphine, whereas higher buprenorphine dosages produced pronounced postoperative thermal (1-way ANOVA; F(3,12) = 23.68; P < 0.0001; Fig. 7A) and mechanical (1-way ANOVA; F(3,12) = 5.381; P = 0.0140; Fig. 7B) antihypersensitivity in aged mice. Thus, 0.02 μg buprenorphine generated the highest antihypersensitivity in adult mice, and it was slightly (but statistically insignificant) more effective than in aged mice (Fig. 7A, B). Usually, statistical difference between buprenorphine-induced inhibition of thermal postoperative hypersensitivity between aged and adult mice was not statistically significant (2-way ANOVA; F(3,27) = 5.543; P = 0.0043; Fig. 7A). Raw data on development of buprenorphine-induced postoperative antihypersensitivity are shown in Supplementary Figure 4 (http://links.lww.com/PR9/A3). The difference between adult and aged mice for buprenorphine-induced mechanical postoperative antihypersensitivity was clearer (2-way ANOVA; F(3,27) = 11.17; P < 0.0001; Fig. 7B). Statistical difference between aged and adult mice in 0.02 μg buprenorphine-produced mechanical postoperative antihypersensitivity was also not observed (Fig. 7B, C). However, 0.1 and 1 μg buprenorphine inhibited postoperative mechanical hypersensitivity more in aged mice (Fig. 7B, D). Overall, spinal buprenorphine-induced postoperative antihypersensitivity is substantially more potent (>100 fold), but differs from spinal DAMGO-produced mechanical postoperative antihypersensitivity. At certain doses, buprenorphine, unlike DAMGO, is more effective in aged mice. The bell-shaped dose response curve for buprenorphine could explain some aspects of locally and spinally administrated drug on this phenomenon.43
Figure 7.
Effects of spinal injection of buprenorphine on postoperative hypersensitivity in adult and aged mice. Incision-induced thermal (A) and mechanical (B) hypersensitivity were generated in adult and aged mice. At 1 day (for adults) and 1 to 3 days (for aged) postsurgery, different dosages of buprenorphine were intrathecally administrated, and thermal (A) and mechanical (B) hypersensitivity were evaluated at 30 and 120 minutes after buprenorphine injection. Data are presented as area under the curve (AUC). Significance within age groups for buprenorphine-produced antihypersensitivity was compared against vehicle treatment (1-way ANOVA; *P < 0.05; **P < 0.01; ****P < 0.0001; n = 5–8). Significance between age groups was assessed by 2-way ANOVA. Buprenorphine dosages are indicated below log x-axis. Veh is vehicle treated conditions. Mouse ages are indicated. Mechanical antihypersensitivity (30 minutes postinjection) by spinal administration of 0.02 μg (C) and 1 μg (D) buprenorphine for adult and aged mice (1-way ANOVA; separately for adult and aged mice and 2-way ANOVA between adult and aged mice; NS, nonsignificant; ***P < 0.001; n = 5–8). Bup marked below x-axis is the buprenorphine-produced effect.
3.7. Spinally and systemically mu-opioid injection and incision-induced ongoing pain in adult and aged mice
The exploratory locomotor studies measured ongoing (ie, spontaneous) pain behaviors of animals that were largely motivated by exploration of a novel environment.26 Such behavior requires intact cognitive function and motivation, which could be affected by ongoing pain. We measured total beam breaks and ambulation, which well correlated with ongoing postoperative pain.26 We noted that spinal injection of certain buprenorphine dosages produced undesirable stimulatory effects in adult mice that were manifested by increases in locomotor activities. Thus, direct measurement of total exploratory locomotor activity (beam breaks) showed that higher dosages of spinally administrated buprenorphine (1 μg), which are ineffective in postoperative mechanical antihypersensitivity in adult mice (Fig. 7B, D), elevated locomotor activity in these animals (Supplement Fig. 5A, B, http://links.lww.com/PR9/A3). Ten microgram buprenorphine produced an even greater increase in the locomotor activity of adult mice (data not shown). By contrast, 1 μg buprenorphine, which is effective in thermal and mechanical antihypersensitivity in aged mice (Fig. 7), had no stimulatory effects in these animals (Supplement Fig. 5A, B, http://links.lww.com/PR9/A3; for 1 μg buprenorphine adult vs aged, 2-way ANOVA; ****P < 0.0001 first 30 minutes; **P < 0.01 second 30 minutes; *P < 0.05 third and fourth 30 minutes). Experimental observers did not notice stimulatory effects of 0.1 μg buprenorphine in aged mice.
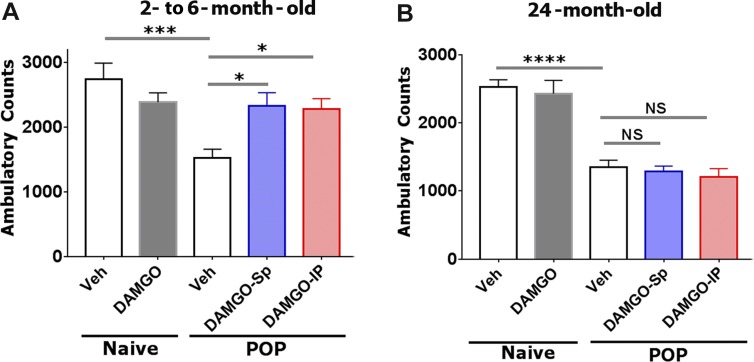
Based on these results, we selected DAMGO and used the exploratory locomotor approach to assess ongoing pain in adult and aged mice after incision surgery.26 Figures 8A, B shows spinal administration of 5 μg DAMGO did not substantially affect ambulatory counts in naive adult and aged mice. Incision surgery significantly decreased ambulation compared with naive adult (1-way ANOVA; F(4,29) = 6.749; P = 0.0006; Fig. 8A) and aged mice (1-way ANOVA; F(4,30) = 26.11; P < 0.0001; Fig. 8B). Spinal administration of 5 μg DAMGO significantly reversed ambulatory behavior in adult (Fig. 8A) but not aged mice (Fig. 8B). Similarly, systemic i.p. injection of DAMGO (1 mg/kg) affected ambulatory behavior only in adult animals (Fig. 8). In summary, mu-opioids were more successful at inhibiting incision-induced ongoing pain in adult compared with aged mice.
Figure 8.
Effect of spinally and systemically injected DAMGO on surgery-induced exploratory locomotor activity in adult and aged mice: Reversal effects of DAMGO on incision-induced ambulatory behavior in adult (A) and aged mice (B). The ambulatory counts are shown on naive animals (column 1) and naive animals injected spinally with 5 ìg DAMGO (column 2). For incision surgery (POP), the ambulatory counts are shown 1 day after surgical intervention (column 3), after spinal injection of 5 ìg DAMGO (column DAMGO-Sp) or after intraperitoneal injection of DAMGO (1 mg/kg; column DAMGO-IP). Locomotor activities were measured on individual animals for a 2 hours period. Statistic is 1-way ANOVA; NS, nonsignificant; *P < 0.05; ***P < 0.001 and ****P < 0.0001.
4. Discussion
In this study, we examined basal nociception, postoperative hypersensitivity levels, and effects of local, contralateral paw and spinal administration of mu-opioids, DAMGO and buprenorphine on postoperative hypersensitivity in aged and adult mice. Our data indicate that, as in human volunteers, thresholds of baseline thermal and mechanical nociception in naive aged mice are higher than in naive adult mice. Most publications also indicate that postoperative pain in adult and aged patients has comparable magnitude.18 Similarly, postoperative thermal and mechanical hypersensitivity has almost the same magnitude in aged and adult mice. However, aged compared with adult mice are less sensitive to pure mu-opioid DAMGO in postoperative antihypersensitivity and suppression of ongoing pain. By contrast, postoperative antihypersensitivity by mixed mu-opioid, buprenorphine depends on dosage; aged mice are more sensitive to higher doses. Hence, pure mu-opioid pharmacology in relation to postoperative pain suppression in aged mice is different from reported mu-opioid pharmacology (such as morphine) in managing postoperative pain in elderly individuals.18 Data on buprenorphine cannot be compared, because there are no systematic studies on effects of buprenorphine in adult vs aged patients.
There is still a discussion about the analgesic sites of action for mu-opioids. This is an important point, as plasticity of nociceptive pathways at the opioid action sites may be responsible for altering the efficacy of opioids in aged subjects. Four main sites of action for mu-opioids have been suggested: peripheral17 and central terminals of sensory neurons,3,4 intrinsic spinal cord neurons,35 and periaqueductal gray (PAG) neurons.27
Locally, at the surgical site, injected opioids produced effects which were centrally mediated, as antihypersensitivity dosages of opioids (ie, 15 μg DAMGO or 0.1 μg buprenorphine) injected into the contralateral hind paw inhibited postoperative hypersensitivity. In a variety of inflammatory pain models, peripheral dosages of mu-opioids produce some antihypersensitivity17 and inhibit sensitization of primary afferent fibers.44 For instance, carrageenan-induced thermal hypersensitivity can be reversed by ≈30% with mu-opioids such as fentanyl, ethylketocyclazocine, and levorphanol.19 Complete Freund's Adjuvant-induced inflammatory mechanical hypersensitivity can also be partially reversed by opioids.38 Interestingly, peripheral effects of mu-opioids in reduction of CFA inflammatory pain peaked at 5 minutes after administration of opioids and expired within 30 minutes.38 In this respect, it is possible that we missed the peripherally mediated effect of DAMGO, because the first reading was taken 30 minutes after DAMGO injection. But, in general, peripheral mechanisms do not account for sufficiently strong19 and prolonged38 opioid-induced antihyperalgesia/analgesia. Another explanation for the absence of peripherally mediated postoperative antihypersensitivity by opioids is that surgery triggers the release of a distinct set of inflammatory mediators that may not be effective in priming the MOR, which, according to reports, is necessary for activity of MOR in sensory neurons.8 The mechanisms of postoperative and inflammatory pain have similarities, as well as many notable differences.9 For example, the wound healing process after surgery recruits a unique subset of inflammatory cells to the injury site, which is substantially different from a set of immune cells generated by CFA injection.16 It also can be noted that mu-opioid–induced thermal (but not mechanical) antinociception can be registered without the presence of inflammation, as soon as opioids are administrated spinally.36
In a majority, if not all clinical studies, mu-opioids were administrated either parenteral or enteral at dosages that produced a central effect.6,15,18 In ≈70% of all studies on postoperative efficacy of opioids in adult and aged patients, mu-opioids reduced postoperative pain by ≈15% to 25% more in elderly patients compared with adults.18 Postoperative pain levels were tested with the sum of the pain intensity differences6 or the visual analogues scale.15,18 Evoked pain was not accessed in these patients.6,15,18 sum of the pain intensity differences and visual analogues scale reflect ongoing pain and, to a lesser extent, mechanical pain. Furthermore, results on spinally delivered opioids also cannot be directly compared with published clinical data, because systemic and spinally delivered opioids produce different results.49 Therefore, we examined effects of opioids on both evoked and nonevoked postoperative pain in adult and aged mice. Overall, it appears that mu-opioid pharmacology on postoperative pain in aged mice compared with aged patients is quite different. It could be noted that the effect of buprenorphine cannot be compared with clinical data because there are not sufficient data for the mixed mu-opioid agonist in adult vs aged patients. Nevertheless, it is difficult to draw conclusions on whether this difference is due to pharmacokinetic/pharmacodynamics or distinct neuronal plasticity of the pain pathways in elderly humans vs aged mice. The possible difference in age-dependent neuronal plasticity between mice and humans could be either in PAG, intrinsic spinal cord neuronal or sensory neuronal circuitries. The highest behavioral analgesia was achieved on administration of mu-opioids in spinal cord or PAG,27 and in the spinal cord, sensory neuronal central terminals or intrinsic neurons could produce strong mu-opioid–mediated analgesia.
The consensus reached in this and previous studies is that age alters basal nociception by possibly modifying neuronal circuitries within pain pathways (Fig. 1; Ref. 21). However, age-dependent plasticity in nociceptive pathways is not well characterized and still remains an open issue, because little basic pain research has been conducted on aged animals. Moreover, results sometimes seem to be contradictory.45,48 For example, there is little information on whether aging alters neuronal circuitries in spinal cord and sensory neurons28; and whether surgical procedures affect sensory neuronal and spinal plasticity in age-dependent fashion. Better understanding these questions in adult and aged mice can help explain the differences of opioid pharmacology in postoperative pain management in the elderly.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Funding received for the work: American Heart Association 44081 (M.J.P.); NIH/NIGMS R01 GM112747 (A.N.A.); UTHSCSA IIMS-CTSA Pilot Project (A.N.A.) and School of Dentistry URC pilot project (A.N.A.).
Supplementary Material
Acknowledgments
The authors thank Michael Henry, DDS, PhD (Professor, Department of Endodontics, UTHSCSA, San Antonio, TX) for setting up and training of acute postoperative pain model in mice, (ie, incision model); and Dr Nadon and NIH/NIA for kindly providing 24-month old mice via “one-time exception” program.
Appendix A. Supplemental Digital Content
Supplemental Digital Content associated with this article can be found online at http://links.lww.com/PR9/A3.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Ackert-Bicknell CL, Anderson LC, Sheehan S, Hill WG, Chang B, Churchill GA, Chesler EJ, Korstanje R, Peters LL. Aging research using mouse models. Curr Protoc Mouse Biol 2015;5:95–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aubrun F, Bunge D, Langeron O, Saillant G, Coriat P, Riou B. Postoperative morphine consumption in the elderly patient. Anesthesiology 2003;99:160–5. [DOI] [PubMed] [Google Scholar]
- [3].Baillie LD, Schmidhammer H, Mulligan SJ. Peripheral mu-opioid receptor mediated inhibition of calcium signaling and action potential-evoked calcium fluorescent transients in primary afferent CGRP nociceptive terminals. Neuropharmacology 2015;93:267–73. [DOI] [PubMed] [Google Scholar]
- [4].Bardoni R, Tawfik VL, Wang D, Francois A, Solorzano C, Shuster SA, Choudhury P, Betelli C, Cassidy C, Smith K, de Nooij JC, Mennicken F, O'Donnell D, Kieffer BL, Woodbury J, Basbaum AI, MacDermott AB, Scherrer G. Delta opioid receptors presynaptically regulate cutaneous mechanosensory neuron input to the spinal cord dorsal horn (vol 81, pg 1312, 2014). Neuron 2014;81:1443. [DOI] [PubMed] [Google Scholar]
- [5].Beale BS. Orthopedic problems in geriatric dogs and cats. Vet Clin North Am Small Anim Pract 2005;35:655–74. [DOI] [PubMed] [Google Scholar]
- [6].Bellvill JW, Forrest WH, Miller E, Browen BW. Influence of age on pain relief from analgesics—study of postoperative patients. JAMA 1971;217:1835. [PubMed] [Google Scholar]
- [7].Bentov I, Reed MJ. Anesthesia, microcirculation, and wound repair in aging. Anesthesiology 2014;120:760–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Berg KA, Patwardhan AM, Sanchez TA, Silva YM, Hargreaves KM, Clarke WP. Rapid modulation of mu-opioid receptor signaling in primary sensory neurons. J Pharmacol Exp Ther 2007;321:839–47. [DOI] [PubMed] [Google Scholar]
- [9].Brennan TJ. Pathophysiology of postoperative pain. PAIN 2011;152(3 suppl):S33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chau DL, Walker V, Pai L, Cho LM. Opiates and elderly: use and side effects. Clin Interv Aging 2008;3:273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chia YY, Chow LH, Hung CC, Liu K, Ger LP, Wang PN. Gender and pain upon movement are associated with the requirements for postoperative patient-controlled iv analgesia: a prospective survey of 2,298 Chinese patients. Can J Anaesth 2002;49:249–55. [DOI] [PubMed] [Google Scholar]
- [12].Crook J, Rideout E, Browne G. The prevalence of pain complaints in a general-population. PAIN 1984;18:299–314. [DOI] [PubMed] [Google Scholar]
- [13].Dixon V. Effects of vascular surgery on the elderly vascular patient. J Vasc Nurs 1999;17:86–8. [DOI] [PubMed] [Google Scholar]
- [14].Ferrell B, Casarett D, Epplin J, Fine P, Gloth FM, Herr K, Katz P, Keefe F, Koo PJS, O'Grady M, Szwabo P, Vallerand AH, Weiner D, Older APPP. The management of persistent pain in older persons. J Am Geriatr Soc 2002;50:S205–24.12067390 [Google Scholar]
- [15].Gagliese L, Gauthier LR, Macpherson AK, Jovellanos M, Chan VWS. Correlates of postoperative pain and intravenous patient-controlled analgesia use in younger and older surgical patients. Pain Med 2008;9:299–314. [DOI] [PubMed] [Google Scholar]
- [16].Ghasemlou N, Chiu IM, Julien JP, Woolf CJ. CD11b+Ly6G- myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A 2015;112:E6808–6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hargreaves KM. Peripheral opioid regulation of nociceptors. Focus on “morphine directly inhibits nociceptors in inflamed skin.” J Neurophysiol 2006;95:2031. [DOI] [PubMed] [Google Scholar]
- [18].Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption A qualitative systematic review. Anesthesiology 2009;111:657–77. [DOI] [PubMed] [Google Scholar]
- [19].Joris JL, Dubner R, Hargreaves KM. Opioid analgesia at peripheral sites—a target for opioids released during stress and inflammation. Anesth Analg 1987;66:1277–81. [PubMed] [Google Scholar]
- [20].Kalso E. How different is oxycodone from morphine? PAIN 2007;132:227–8. [DOI] [PubMed] [Google Scholar]
- [21].Kaye AD, Baluch A, Scott JT. Pain management in the elderly population: a review. Ochsner J 2010;10:179–87. [PMC free article] [PubMed] [Google Scholar]
- [22].Koek W, France CP, Javors MA. Morphine-induced motor stimulation, motor incoordination, and hypothermia in adolescent and adult mice. Psychopharmacology (Berl) 2012;219:1027–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lasagna L, Mosteller F, Vonfelsinger JM, Beecher HK. A study of the placebo response. Am J Med 1954;16:770–9. [DOI] [PubMed] [Google Scholar]
- [24].Leffler A, Frank G, Kistner K, Niedermirtl F, Koppert W, Reeh PW, Nau C. Local anesthetic-like inhibition of voltage-gated Na(+) channels by the partial mu-opioid receptor agonist buprenorphine. Anesthesiology 2012;116:1335–46. [DOI] [PubMed] [Google Scholar]
- [25].Mamie C, Bernstein M, Morabia A, Klopfenstein CE, Sloutskis D, Forster A. Are there reliable predictors of postoperative pain? Acta Anaesthesiol Scand 2004;48:234–42. [DOI] [PubMed] [Google Scholar]
- [26].Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology 2004;101:191–203. [DOI] [PubMed] [Google Scholar]
- [27].Mates FF, Rollema H, Taiwo YO, Levine JD, Basbaum AI. Relationship between analgesia and extracellular morphine in brain and spinal-cord in awake rats. Brain Res 1995;693:187–95. [DOI] [PubMed] [Google Scholar]
- [28].Namer B, Barta B, Orstavik K, Schmidt R, Carr R, Schmelz M, Handwerker HO. Microneurographic assessment of C-fibre function in aged healthy subjects. J Physiol 2009;587:419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Negus SS, Mello NK, Linsenmayer DC, Jones RM, Portoghese PS. Kappa opioid antagonist effects of the novel kappa antagonist 5'-guanidinonaltrindole (GNTI) in an assay of schedule-controlled behavior in rhesus monkeys. Psychopharmacology (Berl) 2002;163:412–19. [DOI] [PubMed] [Google Scholar]
- [30].Patil MJ, Green DP, Henry MA, Akopian AN. Sex-dependent roles of prolactin and prolactin receptor in postoperative pain and hyperalgesia in mice. Neuroscience 2013;253:132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci U S A 2009;106:18820–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Plosker GL. Buprenorphine 5, 10 and 20 mug/h transdermal patch: a review of its use in the management of chronic non-malignant pain. Drugs 2011;71:2491–509. [DOI] [PubMed] [Google Scholar]
- [33].Pogatzki EM, Raja SN. A mouse model of incisional pain. Anesthesiology 2003;99:1023–7. [DOI] [PubMed] [Google Scholar]
- [34].Priano L, Gasco MR, Mauro A. Transdermal treatment options for neurological disorders: impact on the elderly. Drugs Aging 2006;23:357–75. [DOI] [PubMed] [Google Scholar]
- [35].Santos SFA, Melnick IV, Safronov BV. Selective postsynaptic inhibition of tonic-firing neurons in substantia gelatinosa by mu-opioid agonist. Anesthesiology 2004;101:1177–83. [DOI] [PubMed] [Google Scholar]
- [36].Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 2009;137:1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sittl R, Nuijten M, Nautrup BP. Changes in the prescribed daily doses of transdermal fentanyl and transdermal buprenorphine during treatment of patients with cancer and noncancer pain in Germany: results of a retrospective cohort study. Clin Ther 2005;27:1022–31. [DOI] [PubMed] [Google Scholar]
- [38].Stein C, Millan MJ, Shippenberg TS, Peter K, Herz A. Peripheral opioid receptors mediating antinociception in inflammation—evidence for involvement of mu-receptors, delta-receptors and kappa-receptors. J Pharmacol Exp Ther 1989;248:1269–75. [PubMed] [Google Scholar]
- [39].Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA 1992;267:64–9. [PubMed] [Google Scholar]
- [40].Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med 2016;176:1286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol 2001;117:1027–35. [DOI] [PubMed] [Google Scholar]
- [42].Tashjian RZ. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med 2012;31:589–604. [DOI] [PubMed] [Google Scholar]
- [43].Vadivelu N, Hines RL. Management of chronic pain in the elderly: focus on transdermal buprenorphine. Clin Interv Aging 2008;3:421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wenk HN, Brederson JD, Honda CN. Morphine directly inhibits nociceptors in inflamed skin. J Neurophysiol 2006;95:2083–97. [DOI] [PubMed] [Google Scholar]
- [45].Weyer AD, Zappia KJ, Garrison SR, O'Hara CL, Dodge AK, Stucky CL. Nociceptor sensitization depends on age and pain chronicity(1,2,3). eNeuro 2016;3 ENEURO.0115–15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Woo J, Ho SC, Lau J, Leung PC. Musculoskeletal complaints and associated consequences in elderly Chinese aged 70 years and over. J Rheumatol 1994;21:1927–31. [PubMed] [Google Scholar]
- [47].Wunsch H, Wijeysundera DN, Passarella MA, Neuman MD. Opioids prescribed after low-risk surgical procedures in the United States, 2004-2012. JAMA 2016;315:1654–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yezierski RP. The effects of age on pain sensitivity: preclinical studies. Pain Med 2012;13(suppl 2):S27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zahn PK, Gysbers D, Brennan TJ. Effect of systemic and intrathecal morphine in a rat model of postoperative pain. Anesthesiology 1997;86:1066–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.