Abstract
Human infectious diseases have been studied in pigs because the two species have common microbial, parasitic, and zoonotic organisms, but there has been no systematic evaluation of cytokine gene expression in response to infectious agents in porcine species. In this study, pigs were inoculated with two clinically and economically important parasites, Toxoplasma gondii and Ascaris suum, and gene expression in 11 different tissues for 20 different swine Th1/Th2-related cytokines, cytokine receptors, and markers of immune activation were evaluated by real-time PCR. A generalized Th1-like pattern of gene expression was evident in pigs infected with T. gondii, along with an increased anti-inflammatory gene expression pattern during the recovery phase of the infection. In contrast, an elevated Th2-like pattern was expressed during the period of expulsion of A. suum fourth-stage larvae from the small intestine of pigs, along with low-level Th1-like and anti-inflammatory cytokine gene expression. Prototypical immune and physiological markers of infection were observed in bronchial alveolar lavage cells, small intestinal smooth muscle, and epithelial cells. This study validated the use of a robust quantitative gene expression assay to detect immune and inflammatory markers at multiple host tissue sites, enhanced the definition of two important swine diseases, and supported the use of swine as an experimental model for the study of immunity to infectious agents relevant to humans.
Although experimental protozoan and helminth infections of rodents have provided critical information on the Th1/Th2 response paradigm, these models lack critical evaluation of the responses in outbreed host species and often do not involve natural host-parasite interactions that are relevant to humans. Furthermore, the phylogenetic distance from mice to humans tempers the extrapolation of results between these species. Pigs that are used as a model of infectious disease can address these limitations because of the similarity between their immune and physiological functions and those of humans. To test this assumption, pigs were infected with two important human zoonotic agents, Toxoplasma gondii and Ascaris suum, and their responses to the infections were evaluated. T. gondii is an intracellular protozoan parasite that causes severe, sometimes lethal disease in animals and humans (27). Felids are the definitive host; however, 300 mammalian species, including pigs, and 20 avian species have been identified as intermediate hosts (16). Recent data indicate that about 25% of the United States population has detectable antibodies to T. gondii, whereas estimates of the exposure in other areas of the world range from 15 to 80% (27). Most humans that are exposed are asymptomatic, but immunocompromised and congenitally infected humans are at risk of developing severe disease, and acute infections in susceptible women during pregnancy cause birth defects and recurrent disease in children (27). Pigs, like humans, can acquire a transplacental T. gondii infection (19), but they are typically infected by ingestion of tissue-derived cysts or infective oocysts, which makes them a potential source of food-borne infection for humans. Most of what is known about the immune response to T. gondii infection has been derived from murine models; however, pigs also develop protective immunity (17). Infection of mice with T. gondii elicits a dominant Th1 response (63, 64); gamma interferon (IFN-γ), interleukin-12 (IL-12), and tumor necrosis factor alpha (TNF-α) are protective, whereas IL-18 and TNF-α contribute to pathology. Th2-associated cytokines, such as IL-4 and IL-10, appear relatively late after infection and may limit immune pathology (32). Most strains of mice are classified as sensitive to clinical toxoplasmosis, while humans and pigs are asymptomatic except when the infection levels are high or the host is immune deficient (10).
Ascaris lumbricoides is an extracellular gastrointestinal nematode parasite that affects more than one-quarter of humans worldwide, including an estimated 4 million people in the United States (12, 59). A closely related species, Ascaris suum, infects more than 50% of pigs raised for food globally, and infective eggs can be transmitted to humans (61). Migrating larvae produce focal liver lesions and eosinophilic pneumonitis in both humans and pigs (45). The development and persistence of a bolus of adult worms in the small intestine is associated with reduced growth and cognitive development in children and intestinal obstructions and aberrant worm migration that often require surgical interventions (13). Rodents do not support the development of the late larval and adult stages of Ascaris spp. in the intestine; these later stages are important in human morbidity and epidemiological studies. The protective immune response to Ascaris in pigs or humans is dominated by a Th2 response based on evidence of Ascaris-induced immunoglobulin E (IgE) production, peripheral eosinophilia, and increased ex vivo lymphocyte Th2 cytokine production (2, 9, 40). There is self-curing of fourth-stage larvae from the small intestine of swine between 2 and 3 weeks after inoculation that is associated with a localized mast cell-dependent immediate-type hypersensitivity response to parasite antigens (3, 44). Study of self-curing in humans is not feasible, but the relationship between high levels of Ascaris allergen-specific IgE antibodies in children and low adult worm reinfestation levels is supportive of a similar mechanism of clearance (40).
One limitation of pigs as a model of human infectious disease is the relative lack of defined immunological reagents. Competitive PCR assays have been developed for porcine IFN-γ (33), IL-10, IL-12p35, IL-12p40 (39), and IL-12Rβ2 (51). These assays are accurate but time- and labor-intensive. The recent development of real-time fluorogenic PCR technology permits rapid, high-throughput analysis of gene expression. Real-time PCR assays have been described for a panel of bovine (8, 34), equine (35), human (24, 53), and murine (24) cytokines. To date, there have been few reports of studies in which real-time PCR was used to analyze the expression of cytokines in pigs (1, 11, 36, 52). The objective of the present study was to characterize the immunobiology of polarized cytokine responses to experimental infection with A. suum and T. gondii by using molecular, cellular, and physiological markers of immunity and inflammation. Our hypothesis is that T. gondii elicits a prototypical Th1-associated response and A. suum elicits a prototypical Th2-associated response that contributes to disease resolution and immunity to infection.
MATERIALS AND METHODS
Animal infection models.
Eight- to 14-week-old Poland China X Landrace pigs were obtained from the experimental farm at the Beltsville Agricultural Research Center. They were housed two pigs per pen in stalls with a nonabsorptive concrete floor surface and had access to water and feed ad libitum. The diet was a corn-soybean formulation containing 16% crude protein and vitamins and minerals that exceeded National Research Council guidelines (57). Group I pigs (n = 4) were noninfected controls. Group II pigs (n = 4) were inoculated per os with 4.5 × 105 oocysts of the VEG strain of T. gondii (a type III genotype originally obtained from a patient expressing AIDS) (18, 51). The pigs were examined 7 days after inoculation (DAI) after an overdose of pentobarbital. Group III pigs (n = 3) were given the same inoculum and were examined 14 DAI. A second set of pigs was segregated into group A noninfected control pigs (n = 4) and group B pigs (n = 4) and group C pigs (n = 4) that were inoculated per os with infective A. suum eggs (57) and examined at 14 and 21 DAI, respectively. The inoculation dose of eggs was based on a test infection that yielded between 1,500 and 2,000 migrating larvae in the lungs and small intestines of test pigs at 7 and 14 DAI, respectively (57). Both infections were replicated twice, and all procedures were conducted in accordance with approvals and recommendations made by the Beltsville Area Animal Care and Use Committee.
Tissue preparation.
Whole blood was obtained by venipuncture in EDTA Vacutainers and mixed 1:2 with phosphate-buffered saline (PBS) (52). Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation by using 1.077d Histopaque (Sigma). The isolated cells were washed twice in PBS, resuspended in TriZol (Invitrogen, Gaithersburg, Md.), and vigorously mixed to lyse all of the cells. Solid tissues were excised with scalpels and forceps and cut into 1-mm cubes; all samples were immediately flash frozen and stored at −80°C until they were processed for RNA isolation.
RNA isolation, cDNA synthesis, and real-time PCR assays.
All probes and primers were designed by using the Primer Express software (Applied Biosystems, Foster City, Calif.) with sequences obtained from GenBank or The Institute for Genome Research porcine expressed sequence tag database and were produced by Biosource (Camarillo, Calif.) (Table 1). Primers and fluorescent probes were designed across adjacent exons when possible; however, the intron-exon structures for most of the genes assayed have not been described, and it was presumed that the assays amplified genomic DNA. Therefore, extracted RNA was treated with DNase in the presence of an RNase inhibitor (11). RNA integrity and quantity and genomic DNA contamination were assessed by using an Agilent Bioanalyzer 2100 and an RNA 6000 Labchip kit (Agilent Technologies, Palo Alto, Calif.). cDNA was synthesized by using Superscript RT (Invitrogen) and oligo(dT) (11), and 100 ng of this cDNA per well was used for PCR amplification.
TABLE 1.
Primers and probes
| Gene | Forward primer
|
Reverse primer
|
Probe
|
||||
|---|---|---|---|---|---|---|---|
| Concn (nM) | Sequence | Concn (nM) | Sequence (5′ to 3′) | Concn (nM) | Sequence (5′ to 3′)a | Accession no.b | |
| IFN-α | 300 | TCAGCTGCAATGCCATCTG | 300 | AGGGAGAGATTCTCCTCATTTGTG | 200 | 6FAM-TGACCTGCCTCAGACCCACAGCC-TAMRA | X57191 |
| IFN-γ | 300 | TGGTAGCTCTGGGAAACTGAATG | 300 | GGCTTTGCGCTGGATCTG | 200 | 6FAM-CTTCGAAAAGCTGATTAAAATTCCGGTAGATAATCTGC-TAMRA | X53085 |
| IL-2 | 300 | ACAGTTGCTTTTGAAGGAAGTTAAGAA | 300 | CCTGCTTGGGCATGTAAAATTT | 150 | 6FAM-CGAGAATGCTGATCTCTCCAGGATGCTC- TAMRA | X56750 |
| IL-12p35 | 300 | GCCCAGGAATGTTCAAATGC | 300 | GGGTTTGTTTGGCCTTCTGA | 200 | 6FAM-CAACCACTCCCAAAATCTGCTGAAGGC- TAMRA | L35765 |
| IL-12 p40 | 300 | AAGCTGTTCACAAGCTCAAGTATGA | 300 | TCTTGGGAGGGTCTGGTTTG | 200 | 6FAM-ACCAGCAGCTTCTTCATCAGGGACATCA- TAMRA | U08317 |
| IL-12Rβ2 | 300 | GGCCAGGAAAGGGACAAAG | 300 | CCCCAGCACCTTGTACAGATC | 100 | 6FAM-AAGTCCACCACCTCCAAGGGCTCTCAC- TAMRA | AF448143 |
| IL-15 | 300 | GATGCCACATTGTATACTGAAAGTGA | 300 | GCGTAACTCCAGGAGAAAGCA | 150 | 6FAM-CATCCCAATTGCAAAGTAACAGCGATGA- TAMRA | U58142 |
| IL-18 | 300 | CGTGTTTGAGGATATGCCTGATT | 300 | TGGTTACTGCCAGACCTCTAGTGA | 100 | 6FAM-TGACTGTTCAGATAATGCACCTCAGACCGT-TAMRA | AF176949 |
| IL-23 | 300 | GCCCTGCTTGGGCTCAA | 300 | GTAGATCCACATGTCCCATTGGT | 200 | TET-CACGCTGGCCTGGACTGCACATC-BHQ | AB030002 |
| iNOS | 300 | CGTTATGCCACCAACAATGG | 300 | AGACCCGGAAGTCGTGCTT | 200 | 6FAM-CATCAGGTCGGCCATCACCGTG-TAMRA | U59390 |
| STAT4 | 300 | GAAAACCCTCTGAAGTACCTCTATCCT | 300 | TCACATGGCTGGGAGCTGTA | 200 | TET-TGCTGCCTCCCACTGAACAGGACCT-BHQ | AB020984 |
| TNF-α | 300 | TGGCCCCTTGAGCATCA | 300 | CGGGCTTATCTGAGGTTTGAGA | 150 | 6FAM-CCCTCTGGCCCAAGGACTCAGATCA-TAMRA | X54001 |
| IL-4 | 300 | GCCGGGCCTCGACTGT | 300 | TCCGCTCAGGAGGCTCTTC | 200 | 6FAM-CTTCGGCACATCTACAGACACCACACG- TAMRA | X68330 |
| IL-5 | 300 | GACTGGTGGCAGAGACCTTGAC | 300 | CTTCAATGCATAGTTGGTGATTTGT | 200 | 6FAM-CTGCTCTCCATTCATCGAACTCTGCTGAT- TAMRA | AJ010088 |
| IL-10 | 300 | TGAGAACAGCTGCATCCACTTC | 300 | TCTGGTCCTTCGTTTGAAAGAAA | 150 | 6FAM-CAACCAGCCTGCCCCACATGC-TAMRA | L20001 |
| IL-13 | 50 | CTGACCACCAGCATGCAGTACT | 900 | GCTGCAGTCGGAGATGTTGA | 150 | 6FAM-TGCCGCCCTGGAATCCCTCA-TAMRA | AF385626 |
| Mast cell tryptase | 300 | ACCGGCCTGGCATCTACAC | 300 | AGGAGGTGACTGGCTTTGCA | 200 | 6FAM-CGCGTCACCCACTACTTGGACTGGAT- TAMRA | AB038652 |
| STAT6 | 300 | CCTGGGTTGGTGAAGACATGT | 300 | GCCCCTCCAAGAGAAGCTTAG | 200 | TET-TGCTGCCTCCCACTGAACAGGACCT-BHQ | TC52764 |
| TGF-β1 | 300 | AGGGCTACCATGCCAATTTCT | 300 | CCGGGTTGTGCTGGTTGT | 200 | 6FAM-CACTCAGTACAGCAAGGTCCTGGCTCTGTA-TAMRA | M23703 |
| CD3ɛ | 300 | ACCTGCACAGTCGGAGAGAAG | 300 | CTCCACGCAGTTCTCACACACT | 150 | 6FAM-CGAGCCATAGGCTCTACCTGAAAGCCA- TAMRA | U08270 |
6FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine; TET, 6-carboxy-2′,4,7,7′-tetrachlorofluorescein; BHQ, black hole quencher.
The STAT6 sequence was obtained from the Institute for Genome Research porcine EST database; all other sequences were obtained from the GenBank database.
PCR was performed with a Brilliant quantitative PCR core reagent kit (Stratagene, La Jolla, Calif.) and an ABI PRISM 7700 sequence detector system (Applied Biosystems, Foster City, Calif.). The amplification conditions were as follows: 50°C for two min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. Fluorescence signals measured during amplification were processed postamplification and were considered positive if the fluorescence intensity was >20-fold greater than the standard deviation of the baseline fluorescence. This level was defined as the Ct value. Assays were optimized as follows. The forward and reverse primer concentrations (900, 300 and 50 nM) were tested with a fixed concentration of probe to determine the primer set with the highest signal-to-noise intensity and lowest Ct. The probe concentration was then varied from 250 to 50 nM (in increments of 50 nM), and the concentration with the highest signal-to-noise intensity and lowest Ct was chosen. To determine the linearity and slope for each optimized assay, the cDNA template was serially diluted, and the Ct was measured. In order to assess the reproducibility of each assay, a pool of cDNA obtained from A. suum- and T. gondii-infected mesenteric lymph node (MLN) tissue was assayed on four consecutive days to establish the coefficient of variation. Because variation in the expression of housekeeping genes (L32, HPRT, EF1-a, and RPL13A) was observed during infection with A. suum and T. gondii, as well as in other parasite infection studies in which Trichuris suis and Oesophagostomum dentatum were used (data not shown), we normalized gene expression based upon the constant amount of RNA and cDNA amplified. This method has recently been proposed to be the most reliable means of standardization of quantitative measurements of mRNA expression by real-time PCR provided an accurate estimate of the total RNA can be made (e.g., by use of an Agilent Bioanalyzer) (5).
Real-time PCR assay characterization.
The coefficient of variation of all assays was <2%, with a linearity of >0.950 to >0.990 (data not shown). For genes such as the IL-12Rβ2 gene, the range of the linear response was >4 logs. The slope of the Ct value line was used as the ordinate, and the value for the log of the input of RNA was used as the abscissa. This described the efficiency of the reaction in which a slope of −3.4 represented 100% efficient PCR; the slopes of the great majority of the assays were within ±0.2 of 3.4 (data not shown).
Immune staining and phenotypic analysis of BAL cells.
Bronchial alveolar lavage (BAL) was obtained from the lungs of pigs infected with either A. suum or T. gondii. Briefly, the large right lobe of a lung was gravity filled with 500 ml of PBS, and this was followed by massaging for 30 s and draining of the cell suspension into 50-ml polypropylene tubes. The cells were washed and resuspended in RPMI 1640 medium with 5% heat-inactivated fetal bovine serum, and cell counts and viability were determined after trypan blue staining. Aliquots of BAL cells were centrifuged onto glass slides, air dried, and stained with Giemsa stain after methanol fixation, and differential counting was performed with more than 100 cells.
The expression of surface antigen on immune cells isolated from BAL was determined by flow cytometry (52). The BAL cells were stained with specific monoclonal antibodies against porcine macrophages (SWC9-IgG1) (clone 18-5 provided by Y. B. Kim), porcine granulocytes (SWC3-IgG2b) (hybridoma 74-22-15), and isotype controls (Serotec, Raleigh, N.C.). An isotype-specific (Fab′2) antibody labeled with fluorescein isothiocyanate or R-phycoerythrin (Southern Biotechnology, Birmingham, Ala.) was added. After 15 min on ice, the cells were washed and resuspended in 1% formaldehyde (Polysciences, Warrington, Pa.). Independent gates for monocytes/macrophages and granular cells were used for data collection from more than 10,000 events. The percentage of bound secondary antibody was determined with a Becton Dickinson FACSCalibur flow cytometer and was used as a measurement to determine the relative percentage of SWC3−/SWC9+ cells (macrophages) and SWC3+/SWC9− cells (eosinophils); CellQuest software (Becton Dickinson, San Jose, Calif.) was used to analyze data files.
In vitro muscle contractility of intestinal circular smooth muscle.
Mucosa-free segments of jejunum (A. suum infected) or ileum (T. gondii infected) were suspended in organ baths containing Krebs' buffer solution. Concentration-dependent responses to acetylcholine (1 nM to 1 mM) and frequency-dependent responses to electrical field stimulation (1, 2.5, 5, 10, 20 Hz; duration, 1 ms; 80 V) were determined with separate muscle strips, and tension was recorded as the amount of force per cross-sectional area by using a Grass model 79 polygraph (Grass Medical Instruments, Quincy, Mass.) (65).
Ussing chamber measurements of intestinal epithelial cell function.
Segments of mucosae from either uninfected or parasite-infected jejunum (A. suum infected) or ileum (T. gondii infected) were stripped of muscle and mounted in Ussing chambers. The basal net ion flux across the mucosa (Isc) and the tissue resistance, a measure of tissue permeability, were determined for concentration-dependent changes in Isc to histamine added to the serosal side and, in separate tissues, concentration-dependent changes in Isc to the cumulative addition of glucose to the mucosal side (49).
Plasma cytokine and nitrite assays.
Matched enzyme-linked immunosorbent assay antibody pairs were used to measure porcine IFN-γ and IL-4 (Biosource); the results were expressed in picograms per milliliter. Plasma nitrite was determined (Promega, Madison, Wis.) after dilution with an equal volume of sterile distilled H2O and filtration through a 3,000-molecular-weight-cutoff filter (Centricon; Millipore, Bedford, Mass.).
Statistical analysis.
All statistical analyses were performed by using Statview 5.0 for Macintosh (Abacus Concepts, Berkeley, Calif.). Data were analyzed for equality of variance by using Fisher's F test. If the variance was heterogeneous, the appropriate transformation of the data was performed. Plasma cytokine or nitrite levels or tissue mRNA expression (Ct values) was evaluated by one-way analysis of variance to examine the effect of infection. A P value of <0.05 was considered statistically significant for all analyses.
RESULTS
Characterization of the pig response to T. gondii.
All pigs fed T. gondii oocysts developed an acute clinical toxoplasmosis during the first week after inoculation. The body temperature was elevated 4 to 7 DAI, and the pigs exhibited inappetence, lethargy, and erythematic ears, nose, and extremities. Pigs in group II that were examined at 7 DAI showed mild enteritis characterized by a pseudomembrane over the ileum mucosa, edematous mesenteries, and fluid in the peritoneum. The MLN were edematous, had pale areas representing necrosis, and were hyperplastic, as were the colonic lymph nodes (CLN) and tracheal-bronchial lymph nodes (TBLN). These clinical features were not apparent in pigs in group III at 14 DAI, indicating that there was resolution of overt disease. Mice fed suspensions of pig ileum, liver, and lungs taken 7 DAI died within 3 weeks of inoculation, and the brains of these mice contained microscopically detectable tachyzoites (15, 18).
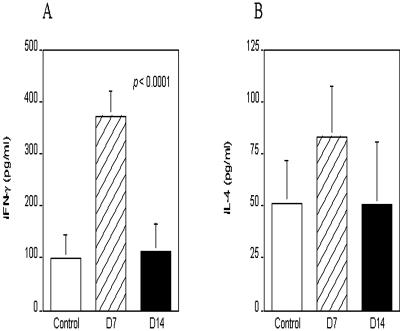
There was a significant increase (P < 0.0001) in the level of IFN-γ in the serum of T. gondii-infected pigs at 7 DAI (group II pigs), and the levels fell to control levels at 14 DAI (group III pigs) (Fig. 1A). Plasma nitrite, which is induced by IFN-γ and is known to accompany T. gondii infection in mice, was measured as a surrogate marker of tissue inducible nitric oxide synthase (iNOS) activity. All plasma samples contained measurable amounts of nitrite, but these levels did not vary in response to T. gondii infection (data not shown). There was a mean 25% increase in serum IL-4 levels in pigs in group II (7 DAI) compared to uninfected pigs and pigs at 14 DAI that was not statistically significant (Fig. 1B).
FIG. 1.
Plasma IFN-γ and IL-4 levels in T. gondii-infected pigs at 7 and 14 DAI. Whole blood was obtained from pigs by venipuncture and mixed with EDTA; the isolated plasma was used to determine IFN-γ levels (A) and IL-4 levels (B) with matched enzyme-linked immunosorbent assay antibody pairs, and the results were expressed in picograms per milliliter. The data are representative of two replicate experiments, and a minimum of three samples were used to obtain each mean value. The differences in IFN-γ were statistically significant (P < 0.0001). D7, 7 days after infection; D14, 14 days after infection.
Local responses to T. gondii in the lung and small intestine.
Flow cytometric analysis of BAL cells indicated that there was no change in the relative percentage of macrophages in the experimental groups. The BAL from control uninfected pigs contained 97.4% ± 0.90% macrophages, while the BAL from pigs at 7 and 14 DAI contained 98.2% ± 0.58% macrophages (group II) and 94.5% ± 2.96% macrophages (group III), respectively.
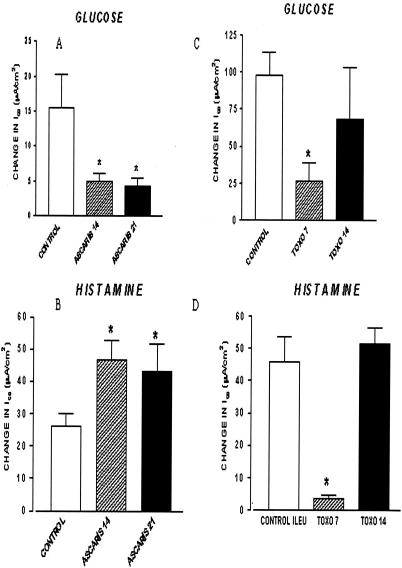
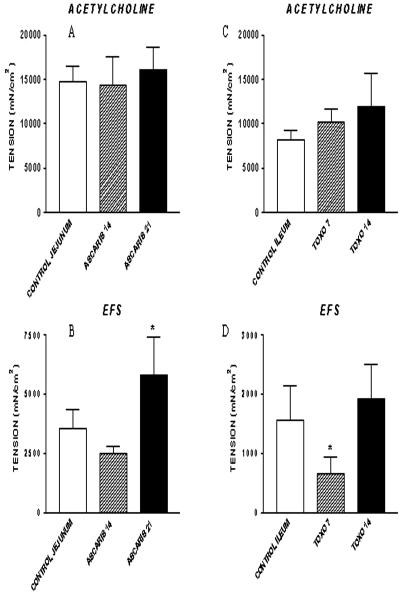
There was a clear time-dependent and selective effect on epithelial cell and smooth muscle function in the ileum of T. gondii-infected pigs, indicating that there were periods of acute local response to infection followed by recovery. There was no change in epithelial cell resistance in the ileum of either the uninfected or T. gondii-infected pigs, demonstrating that tissue permeability was not impaired by the infection (data not shown). There were, however, statistically significant decreases in Na+-linked glucose absorption (Fig. 2C) and chloride secretion in response to histamine (Fig. 2D) in pigs in group II at 7 DAI, which returned to normal at 14 DAI. Likewise, electrical field stimulation of smooth muscle contractility decreased significantly in pigs at 7 DAI but not at 14 DAI (Fig. 3D). There was no generalized difference in smooth muscle contractility between infected and uninfected pigs, however, because there was no response to the cholinergic neurotransmitter acetylcholine (Fig. 3C).
FIG. 2.
Ussing chamber measurements of intestinal epithelial cell function in A. suum- and T. gondii-infected pigs at different times after inoculation. Segments of mucosae from either uninfected or parasite-infected jejunum (A. suum-infected pigs at 14 and 21 DAI) (A and B) or ileum (T. gondii-infected pigs at 7 and 14 DAI) (C and D) were stripped of muscle and mounted in Ussing chambers. Concentration-dependent changes in Isc with histamine added to the serosal side (B and D) or, in separate tissues, with cumulative addition of glucose to the mucosal side (A and C) were determined. ASCARIS 14, A. suum-infected pigs at 14 DAI; ASCARIS 21, A. suum-infected pigs at 21 DAI; TOXO 7, T. gondii-infected pigs at 7 DAI; TOXO 14, T. gondii-infected pigs at 14 DAI; CONTROL ILEU, control ileum.
FIG. 3.
In vitro muscle contractility of intestinal circular smooth muscle from pigs infected with A. suum and T. gondii. Mucosa-free segments of jejunum (A. suum-infected pigs at 14 and 21 DAI) (A and B) or ileum (T. gondii-infected pigs at 7 and 14 DAI) (C and D) were suspended in organ baths containing Krebs' buffer solution; similar tissues were obtained from uninfected control pigs. Concentration-dependent responses to acetylcholine (1 nM to 1 mM) (A and C) and frequency-dependent responses to electrical field stimulation (EFS) (1, 2.5, 5, 10, and 20 Hz; duration, 1 ms; 80 V) (B and D) were determined with separate muscle strips. Tension was recorded on a Grass model 79 polygraph and was expressed as force per square centimeter of cross-sectional area. ASCARIS 14, A. suum-infected pigs at 14 DAI; ASCARIS 21, A. suum-infected pigs at 21 DAI; TOXO 7, T. gondii-infected pigs at 7 DAI; TOXO 14, T. gondii-infected pigs at 14 DAI.
mRNA expression following infection with T. gondii.
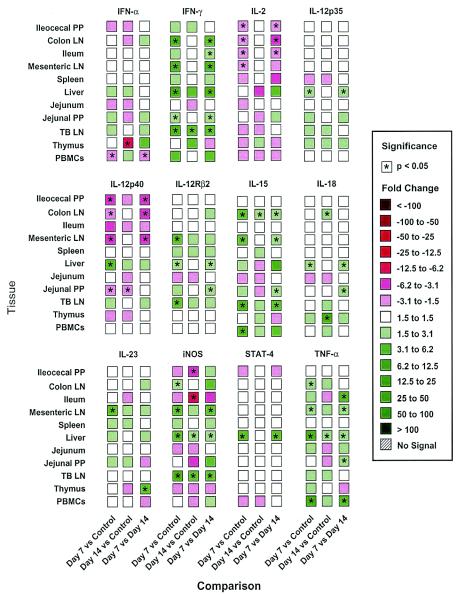
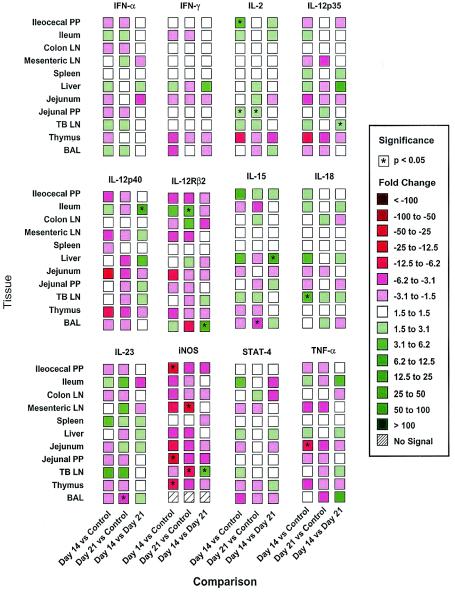
There was a statistically significant increase in Th1-derived type 1 and proinflammatory cytokine gene expression in lymph nodes draining sites of infection during acute disease at 7 DAI (Fig. 4). The levels of IL-15, TNF-α, and iNOS were elevated in the TBLN, CLN, and MLN, as well as in the liver, and the levels of the signature type 1 cytokine marker IFN-γ were increased in 9 of 11 tissues at 7 DAI compared with control tissues; statistically significant differences in expression in the CLN, jejunal Peyer's patches (PP), liver, MLN, and TBLN were observed. The most dramatic change was an 83.7-fold difference observed in the liver. In contrast, the levels of IFN-γ mRNA were increased in 4 of 11 tissues 14 DAI compared to control tissues, but only the TBLN data were significantly different from the control data. The level of IFN-γ mRNA was also higher at 7 DAI than at 14 DAI in nine tissues, and significant increases were observed for the CLN, ileum, liver, MLN, and TBLN. It is notable that the expression of CD3ɛ increased ninefold in the liver 7 DAI and was also elevated at 14 DAI, suggesting that there was T-cell involvement in the cytokine patterns observed. There were more selective changes in the expression of other type 1-associated cytokines, including IL-2, IL-18, IL-23p19, IL-12p35, and IL-12p40; indeed, the level of IL-12p40 mRNA was significantly decreased in 4 or 5 of 11 tissues at 7 DAI. Nevertheless, the prominence of significant increases in the levels of many markers in the liver at 7 DAI followed by reductions in expression at 14 DAI was consistent with a period of rapid response to infection, followed by recovery.
FIG. 4.
Real-time PCR assay characterization of 11 different tissues from pigs infected with T. gondii and measurement of a cluster of 12 type 1 cytokines and related gene expression products. PCR was performed by using a Brilliant quantitative PCR core reagent kit and an ABI PRISM 7700 sequence detector system. Fluorescence signals measured during amplification were processed after amplification and were considered positive if the fluorescence intensity was >20-fold greater than the standard deviation of the baseline fluorescence. This level was defined as the Ct value. To determine the linearity and slope for each optimized assay, the cDNA template was serially diluted, and the Ct value was determined. Gene expression was normalized based upon the constant amount of RNA and cDNA amplified. A statistical analysis was performed by using Statview 5.0 for Macintosh. Data were analyzed for equality of variance by using Fisher's F test. Tissue mRNA expression (Ct values) was evaluated by one-way analysis of variance to examine the effect of infection. Fold changes are indicated by different colors, and statistical significance is indicated by asterisks (P < 0.05). Tissues were obtained from four uninfected control pigs and from pigs infected with T. gondii for 7 days (four pigs) and 14 days (three pigs); the data are representative of the data from two separate experiments. LN, lymph nodes.
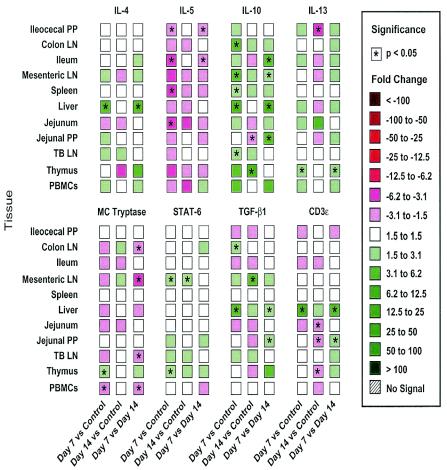
Commensurate with recovery from overt disease was the elevation of anti-inflammatory cytokine gene expression (Fig. 5). The IL-10 mRNA levels, in particular, increased in 10 of 11 tissues at 7 DAI, and there were significant differences in the CLN, MLN, liver, spleen, and TBLN. The IL-10 mRNA levels increased in five tissues at 14 DAI, but only the increase in the thymus was statistically significant. The expression of transforming growth factor β1 (TGF-β1) significantly increased in the CLN and liver at 7 DAI and increased in the MLN at 14 DAI. There was also significantly increased expression of IL-4 mRNA in the liver at 7 DAI that was not observed at 14 DAI, and there were statistically significant increases in the STAT6 levels in the MLN and thymus at 7 DAI and in the MLN at 14 DAI.
FIG. 5.
Real-time PCR assay characterization of 11 different tissues from pigs infected with T. gondii and measurement of a cluster of eight type 2 and anti-inflammatory cytokines. PCR was performed as described in the legend to Fig. 4. Tissues were obtained from four uninfected control pigs and from pigs infected with T. gondii for 7 days (four pigs) and 14 days (three pigs); the data are representative of the data from two separate experiments. LN, lymph nodes.
Increases in IFN-γ, IL-12p35, iNOS, TNF-α, and IL-12Rβ2 levels should signal a decrease in the expression of type 2 cytokines. This hypothesis was supported by the decrease in IL-5 mRNA expression in 8 of the 10 tissues examined 7 DAI compared with the control; significant differences were observed for the CLN, ileocecal PP, jejunum, and spleen, and decreases were observed in seven tissues at 14 DAI (Fig. 5). However, IL-13 mRNA expression increased in six tissues at 7 DAI, with statistical significance only for the thymus, but decreased in five tissues at 14 DAI. Only the deceased expression in ileocecal PP was statistically significant.
Characterization of host response to A. suum.
Pigs generally tolerated infection with A. suum without overt signs of disease. The only gross indicator of infection was the appearance of fibrotic lesions or white spots that covered the surface and parenchyma of the livers of pigs in groups B and C as a result of migrating A. suum larvae. The lesions on the livers of pigs in group C at 21 DAI were less intense than those on the livers of pigs in group B at 14 DAI (data not shown), indicating that there was disease resolution and recovery in the absence of subsequent larval migration. There was no significant increase in the plasma levels of IFN-γ, IL-4, or nitrite in A. suum-infected pigs at either 14 or 21 DAI compared to the levels in uninfected pigs (data not shown).
Local responses to A. suum in the lung and small intestine.
The relative percentages of macrophages and granulocytes in the BAL determined by flow cytometry showed that in control pigs 93.3% ± 1.1% of the cells were alveolar macrophages (group A). There was a significant reduction in the percentage of macrophages at 14 DAI (average ± standard deviation, 43.1% ± 8.5%; P < 0.01) compared to the percentage in uninfected pigs. The relative number of macrophages was also reduced at 21 DAI (78.0% ± 5.0%), but the difference did not reach full statistical significance (P = 0.05). In contrast, there were significant increases in the percentages of granulocytes at 14 DAI (50.6% ± 9.5%) (P < 0.01) (group B) and at 21 DAI (18.9% ± 5.5%) (group C) compared to the percentage in control animals (0%) (group A). Differential histochemical staining of fixed BAL cells (data not shown) confirmed both the relative differences in alveolar macrophages between groups and that the majority of the granulocytes were eosinophils. Staining also showed that for all infected pigs, other cell types, such as lymphocytes and neutrophils, were present at levels of <5%.
There was a clear time-dependent but selective effect on epithelial cell and smooth muscle function in the jejunum of A. suum-infected pigs, indicating that there was a period of change in intestinal physiology that was coincidentally related to self-curing of the fourth-stage larvae (44) and increased mucosal mast cell hyperplasia (3). There was no change in epithelial cell resistance in the jejunum in either the uninfected or A. suum-infected pigs, indicating that tissue permeability was not generally impaired by the infection (data not shown). There was, however, a significant decrease in Na+-linked glucose absorption (Fig. 2A) and a statistically significant (P < 0.05) increase in Cl− secretion in response to histamine in pigs in groups B and C at 14 and 21 DAI compared to the values for uninfected pigs (group A) (Fig. 2B), which supported the hypothesis that there was a net increase in fluid in the intestinal lumen during the period of self-curing. In addition, electrical field stimulation of smooth muscle contractility increased significantly in pigs in group C at 21 DAI compared to uninfected pigs (group A) and pigs infected with A. suum for 14 days (group B) (Fig. 3B). There was, however, no change in smooth muscle contractility with the cholinergic neurotransmitter acetylcholine at either time after infection with A. suum compared to the contractility in uninfected pigs (Fig. 3A).
mRNA expression after A. suum infection.
The cytokine gene expression pattern induced by A. suum was much more polarized toward a Th2-like response than the pattern observed in T. gondii-infected pigs, especially in the small intestine, lungs, and draining lymph nodes (Fig. 5 and 6). Gene expression for the Th1-associated molecules (Fig. 7), including IFN-γ, IL-12p35, IL-12Rβ2, IL-23p19, STAT4, and TNF-α, was mostly decreased or only modestly increased in A. suum-infected pigs compared to the expression in the controls, but the differences were not statistically significant. IL-2, however, was significantly upregulated in ileocecal PP and jejunal PP at 14 DAI and in jejunal PP at 21 DAI. Gene expression for the type 1 cytokine effector molecule that contributes to control of T. gondii, iNOS, was downregulated by A. suum infection in 9 of 10 tissues examined at 14 and 21 DAI; however, statistical significance was observed only in ileocecal PP, jejunal PP, and thymus at 14 DAI and in the MLN and TBLN at 21 DAI.
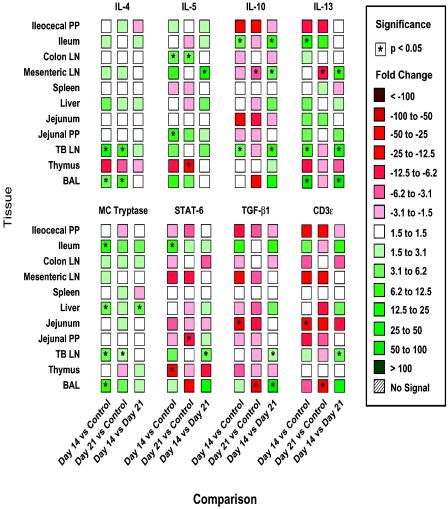
FIG. 6.
Real-time PCR assay characterization of 11 different tissues from pigs infected with A. suum and measurement of a cluster of eight type 2 and anti-inflammatory cytokines. PCR was performed as described in the legend to Fig. 4. Tissues were obtained from four uninfected control pigs and from pigs infected with A. suum for 14 days (four pigs) and 21 days (four pigs); the data are representative of the data from two separate experiments. LN, lymph nodes.
FIG. 7.
Real-time PCR assay characterization of 11 different tissues from pigs infected with A. suum and measurement of a cluster of 12 type 1 cytokines and related gene expression products. PCR was performed as described in the legend to Fig. 4. Tissues were obtained from four uninfected control pigs and from pigs infected with A. suum for 14 days (four pigs) and 21 days (four pigs); the data are representative of the data from two separate experiments. LN, lymph nodes.
The levels of Th2-associated cytokines (Fig. 6), in contrast, were increased by A. suum infection. IL-4 gene expression was significantly increased in BAL and TBLN at 14 and 21 DAI. The level of IL-5 was significantly increased in CLN and jejunal PP at 14 DAI and in the CLN at 21 DAI. General induction of IL-13 mRNA expression was observed in most tissues at 14 DAI along with significant increases in the ileum, BAL, and TBLN, but these changes did not persist at 21 DAI, when significantly decreased IL-13 gene expression was observed in MLN. It is notable that the level of IL-18, which is purported to regulate IL-13 expression, significantly increased in TBLN at 14 DAI. Mast cell tryptase gene expression, a marker of mucosal mast cell activity, was induced in most tissues at 14 and 21 DAI, and there was significant induction in the ileum, TBLN, liver, and BAL. IL-10 expression was increased in the ileum and TBLN at 14 DAI and was decreased in MLN at 21 DAI. STAT6 expression, TGF-β1 expression, and CD3ɛ expression were generally decreased by A. suum infection at the times examined.
DISCUSSION
Large-animal models can arguably provide a proof of principle for the broad evolutionary theories of immune function. They can be especially useful in the analysis of natural host-pathogen interactions in outbred populations for the design of control strategies (25). There are, however, limitations on the technical tools that are necessary to evaluate the immune responses of diverse large-animal species. The present study demonstrated that the technology for quantitative determination of multiple-target gene expression by real-time PCR analysis can test hypotheses concerning the immune responses of pigs to infectious agents. This study is novel, first because of the inherent flexibility of the multiwell PCR array format to analyze different sets of genes of interest in various combinations in pigs (in this case Th1- and Th2-associated gene expression) and second because the model uses zoonotic infections that evoke immunological and physiological responses in a large-animal species that cannot be systematically evaluated in humans or inbred mouse strains. Thus, it provides insight into immune and inflammatory protective mechanisms. The results indicate that a prototypical Th1 response to T. gondii is expressed in pigs. Furthermore, the remarkable resilience of pigs to infection was evidenced by the rapid resolution of disease and the elaboration of some of the gene expression pathways responsible for host protective and antiparasite mechanisms. Likewise, the expression of a largely Th2-derived response to the parasitic nematode A. suum fit the concept of a type 2 cytokine response orchestrating an immediate hypersensitivity response and facilitating intestinal worm expulsion.
During infection of pigs with T. gondii, the magnitude of changes in proinflammatory gene expression, IFN-γ, IL-15, iNOS, and TNF-α was greatest at 7 DAI and was reduced at 14 DAI, supporting the observed clinical changes that indicated that there was partial resolution of overt disease at 14 DAI. IFN-γ mRNA was induced in all tissues analyzed except the jejunum and thymus and was reflected in an increase in the level of the circulating plasma IFN-γ protein. Multiple studies have established that IFN-γ production is associated with a protective immune response to T. gondii infection in murine models (30). TNF-α is induced early after T. gondii infection in murine models and is important for resistance to infection, as well as in host tissue pathology (63). Although there have been reports that IL-2 production is decreased in mice infected with T. gondii (7), IL-2-knockout (KO) mice are susceptible to infection (60), and treatment with recombinant murine IL-2 increases survival (48). Thus, the generalized decrease in IL-2 mRNA in the gut-associated lymphoid tissues in our pig model of T. gondii infection was unexpected, but it may reflect the enteritis and necrosis in the MLN at 7 DAI and contribute to the immune suppression that is often seen during infection with T. gondii (32).
We previously observed a decrease in IL-12p35 and IL-12Rβ2 expression in PBMC isolated from pigs infected with T. gondii for >10 DAI (52). Here, we observed upregulation of IL-12Rβ2 in selected tissues at 7 DAI that was reduced at 14 DAI. IL-12Rβ2 is expressed on activated murine T cells (55), is maintained in an IFN-γ-dependent manner during Th1 differentiation, and is lost in an IL-4-dependent fashion during Th2 differentiation (50, 55). In the present study, IL-12p35 and IL-12p40 were significantly induced only in the liver at 7 DAI. In murine models, IL-12 p35 is produced early after acute T. gondii infection (46). There was a more generalized downregulation of swine IL-12p40 at multiple tissues sites during the infection that is difficult to reconcile with current knowledge of the biology of murine or human IL-12p40. Solano-Aguilar et al. (52), however, showed that biologically active recombinant swine IL-12 did not induce marked IL-12Rβ2 gene expression or IFN-γ production in naïve pig PBMC, suggesting that it may not be a major Th1 activator. Alternately, IL-12p40 is the heterodimeric partner of IL-12p35 and IL-23p19 (42) that also forms homodimers that inhibit IL-12p70 signaling through the IL-12 receptor when it is produced in excess (26). Thus, the role of IL-15, IL-18, and IL-23p19 in the development of pig Th1 responses, particularly those in mucosal tissues, needs to be evaluated further.
IL-15 was significantly induced in several tissues in response to T. gondii infection. It is synthesized by murine macrophages in response to in vitro exposure to T. gondii (14), and it is partially responsible for the protective responses induced by memory CD8+ T cells (28, 29, 31). IL-18 was significantly induced by T. gondii infection only in swine liver at 7 DAI. The role of IL-18 in the murine response to T. gondii is complex because it can enhance IL-12-mediated resistance to T. gondii in SCID mice, but overproduction can lead to pathology and mortality (41), which may explain the tighter regulation of generalized expression of IL-18 in pigs.
iNOS was upregulated in multiple tissues following T. gondii infection of pigs, but the plasma nitrite levels did not change. This suggests that infection in pigs leads to strict local regulation of NO-dependent effector mechanisms which may be necessary to control the multiple downstream effects of NO on many immune and nonimmune cell types. Inappropriately regulated iNOS has consequences in mice infected with T. gondii because iNOS KO mice have greater parasite burdens but survive longer than wild-type controls (63). Nitric oxide has not been associated with antiparasitic effects during infection with gastrointestinal nematodes, but it has been implicated in the accompanying tissue pathology (22); it was significantly downregulated in most tissues from A. suum-infected pigs.
The overall lack of correlation between STAT4 mRNA expression and a Th1 response in most infected pig tissues was surprising because STAT4 KO mice rapidly succumb to acute T. gondii infection (6). The increase in STAT4 mRNA in liver after infection with T. gondii may reflect the presence of activated T cells or macrophages (4). The overall lack of correlation between STAT4 and Th1 cytokine mRNA expression may mean that phosphorylation of STAT4, not regulation of mRNA levels, is required for the induction or maintenance of Th1 responses (62).
IL-4 was significantly induced by T. gondii only in the livers of pigs at 7 DAI. Previous reports showed that IL-4 is induced in the lung and spleen late after infection of mice with T. gondii (20, 32). The role of IL-4 in the resolution of T. gondii infection in mice is confounding because infection of IL-4 KO mice with T. gondii leads to increased mortality but a lower number of cysts in the brains of the surviving mice (43). IL-5 was broadly downregulated in most tissues examined at 7 DAI, while the levels of IL-4, IL-10, and IL-13 were reduced at 14 DAI, suggesting that there was a generalized reduction in Th2-derived cytokines as a consequence of increased levels of IFN-γ, IL-12p35, and TNF-α. IL-10 levels were significantly increased at 7 DAI in most pig tissues by T. gondii infection. This interleukin is produced early after murine T. gondii infection (41), and IL-10R KO mice rapidly succumb to doses of T. gondii that are not lethal to the wild-type strain (54); death is associated with increased tissue destruction due to uncontrolled effects of proinflammatory cytokines. Studies in mice suggest that the liver is a major organ affected by T. gondii infection (23, 41). Our study confirmed and extended these data. The magnitude of the changes in IFN-γ and iNOS gene expression was greatest in the liver, and expression of CD3ɛ was elevated 10-fold, suggesting that infiltrating or expanding T cells play a role. In fact, recent studies have shown that the positive regulators of Th1 responses, IRF1, MYD88, and STAT1, were found to be expressed in the livers of T. gondii-infected pigs prior to the simultaneous upregulation of IFN-γ, HLX1, and TBX21 gene expression (11). The concomitant and significant increases in the levels of the anti-inflammatory cytokines IL-4, IL-10, and TGF-β in the liver at 7 DAI were predictive of the decreases in the levels of proinflammatory cytokines at 14 DAI and improved clinical features.
The resolution of overt disease expression during infection with T. gondii in pigs is supported by the physiological data for intestinal function in the ileum at 7 and 14 DAI. The epithelial cell resistance, a measure of mucosal permeability, was unchanged during the infection, but Na+-coupled glucose absorption and responses to histamine were significantly reduced at 7 DAI and returned to normal levels at 14 DAI. This suggests that the barrier function is maintained at the expense of lost responsiveness to selected secretagogues and glucose metabolism but that the deficiency is quickly normalized. This was also reflected in a reduced response to electrical field stimulation of intestinal smooth muscle at 7 DAI that returned to normal at 14 DAI, suggesting that there was significant, but transient, suppression of intestinal function.
The cellular changes in the livers and lungs of A. suum-infected pigs and the physiological changes in the small intestine are characteristic of the effects of enhanced type 2 cytokine expression. Intestinal mucosal resistance in the jejunum was not altered during the infection, while Na+-coupled glucose absorption and Cl− secretion in response to histamine were increased at 14 and 21 DAI compared to the data for uninfected control pigs. Smooth muscle contractility increased significantly from 14 to 21 DAI, indicating that there was a local response to fourth-stage A. suum larvae in the jejunum, and the increase was correlated with increases in IL-4, IL-5, and IL-13 gene expression. This mimics the IL-4-, IL-13-, and STAT6-dependent weep and sweep response that supports clearance or self-curing of murine gastrointestinal nematode parasites like Nippostrongylus brasiliensis and Heligmosomoides polygyrus (38, 49, 65). The net effect of increased fluid in the intestinal lumen and enhanced smooth muscle contractility between 14 and 21 DAI coincides with the expulsion of fourth-stage larvae from their predilection site in the jejunum to the ileum during expulsion from the intestine (44). A histamine-dependent increase in epithelial cell secretion during this period is also consistent with the mucosal mast cell hyperplasia in vivo, the increase in mast cell tryptase gene expression shown here, and the parasite antigen-specific induction of histamine release from isolated porcine mucosal mast cells in vitro between 14 and 21 DAI (3).
We observed significant increases in gene expression for IL-4, IL-5, IL-13, and mast cell tryptase in specific tissues and draining lymph nodes at 14 and 21 DAI with A. suum, which were commensurate with the development of type 2 effector pathways that coordinate protective responses to nematode parasites (21). The lack of a more generalized and sustained type 2 cytokine response may have been due to the rapid migration of A. suum larvae in the host or to the fact that regulation of these cytokines by T cells is dependent on different activation stimuli, kinetics, transcription factors, and cell types (37). Increased STAT6 gene expression in the pig ileum at 7 DAI is consistent with its role in murine intestinal responses to nematode infection (2, 56, 58), but STAT6 activity, like STAT4 activity in T. gondii-infected pigs, may be more a function of the state of phosphorylation than a function of gene expression (62).
The notable absence of gene expression for type 1 cytokines and the anti-inflammatory cytokine TGF-β1 in A. suum-infected pigs compared to pigs infected with T. gondii suggests that there is a definitive skewing toward a Th2-like response. There were also significant increases in IL-10 gene expression in the ileum and TBLN at 14 DAI that were indicative of the activity of IL-10 as a type 2-related cytokine that regulates resistance and local immune pathology during intestinal nematode infections in mice (47). There apparently is not a need, however, for a more generalized increase in the levels of IL-10 and other anti-inflammatory cytokines to control tissue responses like those observed during infection with T. gondii.
In summary, we established that T. gondii infection elicits a prototypical Th1-associated response, including generalized increases in IFN-γ, iNOS, and TNF-α levels, as well as localized increases in the liver that include increases in IL-12 levels. In contrast, infection with A. suum induces a prototypical Th2-associated response that includes increased IL-4, IL-5, IL-13, and mast cell tryptase mRNA expression and is supported by physiological and cellular responses that are characteristic of immunity to gastrointestinal nematode parasites. The pig can model specific molecular and physiological aspects of these infections that cannot be easily studied in rodents, such as the rapid emergence of anti-inflammatory pathways to control overt infection with T. gondii and the role of intestinal stages of A. suum in mucosal immune activation and modulation.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Allen, D. G., J. E. Riviere, and N. A. Monteiro-Riviere. 2001. Cytokine induction as a measure of cutaneous toxicity in primary and immortalized porcine keratinocytes exposed to jet fuels, and their relationship to normal human epidermal keratinocytes. Toxicol. Lett. 119:209-217. [DOI] [PubMed] [Google Scholar]
- 2.Artis, D., and R. K. Grencis. 2001. T helper responses during intestinal nematode infection: induction, regulation and effector function, p. 331-371. In M. W. Kennedy and W. Harnett (ed.), Parasitic nematodes: molecular biology, biochemistry and immunology. CAB International, Wallingford, Oxon, United Kingdom.
- 3.Ashraf, M., J. F. Urban, Jr., T. D. Lee, and C. M. Lee. 1988. Characterization of isolated porcine intestinal mucosal mast cells following infection with Ascaris suum. Vet. Parasitol. 29:143-158. [DOI] [PubMed] [Google Scholar]
- 4.Bacon, C. M., E. F. Petricoin, 3rd, J. R. Ortaldo, R. C. Rees, A. C. Larner, J. A. Johnston, and J. J. O'Shea. 1995. Interleukin 12 induces tyrosine phosphorylation and activation of STAT4 in human lymphocytes. Proc. Natl. Acad. Sci. USA 92:7307-7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bustin, S. A. 2002. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J. Mol. Endocrinol. 29:23-39. [DOI] [PubMed] [Google Scholar]
- 6.Cai, G., T. Radzanowski, E. N. Villegas, R. Kastelein, and C. A. Hunter. 2000. Identification of STAT4-dependent and independent mechanisms of resistance to Toxoplasma gondii. J. Immunol. 165:2619-2627. [DOI] [PubMed] [Google Scholar]
- 7.Candolfi, E., C. A. Hunter, and J. S. Remington. 1995. Roles of gamma interferon and other cytokines in suppression of the spleen cell proliferative response to concanavalin A and toxoplasma antigen during acute toxoplasmosis. Infect. Immun. 63:751-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins, R. A., C. J. Howard, S. E. Duggan, and D. Werling. 1999. Bovine interleukin-12 and modulation of IFNgamma production. Vet. Immunol. Immunopathol. 68:193-207. [DOI] [PubMed] [Google Scholar]
- 9.Cooper, P. J., M. E. Chico, C. Sandoval, I. Espinel, A. Guevara, M. W. Kennedy, J. F. Urban, Jr., G. E. Griffin, and T. B. Nutman. 2000. Human infection with Ascaris lumbricoides is associated with a polarized cytokine response. J. Infect. Dis. 182:1207-1213. [DOI] [PubMed] [Google Scholar]
- 10.Darcy, F., and L. Zenner. 1993. Experimental models of toxoplasmosis. Res. Immunol. 144:16-23. [DOI] [PubMed] [Google Scholar]
- 11.Dawson, H. D., A. R. Royaee, S. Nishi, D. Kuhar, W. M. Schnitzlein, F. Zuckermann, J. Urban, Jr., and J. K. Lunney. 2004. Identification of key immune mediators regulating T helper 1 responses in swine. Vet. Immunol. Immunopathol. 100:105-111. [DOI] [PubMed] [Google Scholar]
- 12.de Silva, N. R., M. S. Chan, and D. A. Bundy. 1997. Morbidity and mortality due to ascariasis: re-estimation and sensitivity analysis of global numbers at risk. Trop. Med. Int. Health 2:519-528. [DOI] [PubMed] [Google Scholar]
- 13.de Silva, N. R., H. L. Guyatt, and D. A. Bundy. 1997. Morbidity and mortality due to Ascaris-induced intestinal obstruction. Trans. R. Soc. Trop. Med. Hyg. 91:31-36. [DOI] [PubMed] [Google Scholar]
- 14.Doherty, T. M., R. A. Seder, and A. Sher. 1996. Induction and regulation of IL-15 expression in murine macrophages. J. Immunol. 156:735-741. [PubMed] [Google Scholar]
- 15.Dubey, J. P. 1997. Bradyzoite-induced murine toxoplasmosis: stage conversion, pathogenesis, and tissue cyst formation in mice fed bradyzoites of different strains of Toxoplasma gondii. J. Eukaryot. Microbiol. 44:592-602. [DOI] [PubMed] [Google Scholar]
- 16.Dubey, J. P. 1994. Toxoplasmosis. J. Am. Vet. Med. Assoc. 205:1593-1598. [PubMed] [Google Scholar]
- 17.Dubey, J. P., D. G. Baker, S. W. Davis, J. F. Urban, Jr., and S. K. Shen. 1994. Persistence of immunity to toxoplasmosis in pigs vaccinated with a nonpersistent strain of Toxoplasma gondii. Am. J. Vet. Res. 55:982-987. [PubMed] [Google Scholar]
- 18.Dubey, J. P., C. A. Speer, S. K. Shen, O. C. Kwok, and J. A. Blixt. 1997. Oocyst-induced murine toxoplasmosis: life cycle, pathogenicity, and stage conversion in mice fed Toxoplasma gondii oocysts. J. Parasitol. 83:870-882. [PubMed] [Google Scholar]
- 19.Dubey, J. P., and J. F. Urban, Jr. 1990. Diagnosis of transplacentally induced toxoplasmosis in pigs. Am. J. Vet. Res 51:1295-1299. [PubMed] [Google Scholar]
- 20.Filice, G. A., C. R. Clabots, P. E. Riciputi, O. Goni-Laguardia, and C. Pomeroy. 1999. Changes in cytokine levels during reactivation of Toxoplasma gondii infection in lungs. Infect. Immun. 67:2082-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkelman, F. D., and J. F. Urban, Jr. 2001. The other side of the coin: the protective role of the TH2 cytokines. J. Allergy Clin. Immunol. 107:772-780. [DOI] [PubMed] [Google Scholar]
- 22.Garside, P., M. W. Kennedy, D. Wakelin, and C. E. Lawrence. 2000. Immunopathology of intestinal helminth infection. Parasite Immunol. 22:605-612. [DOI] [PubMed] [Google Scholar]
- 23.Gazzinelli, R. T., M. Wysocka, S. Hieny, T. Scharton-Kersten, A. Cheever, R. Kuhn, W. Muller, G. Trinchieri, and A. Sher. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J. Immunol. 157:798-805. [PubMed] [Google Scholar]
- 24.Giulietti, A., L. Overbergh, D. Valckx, B. Decallonne, R. Bouillon, and C. Mathieu. 2001. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25:386-401. [DOI] [PubMed] [Google Scholar]
- 25.Hein, W. R., and P. J. Griebel. 2003. A road less travelled: large animal models in immunological research. Nat. Rev. Immunol. 3:79-84. [DOI] [PubMed] [Google Scholar]
- 26.Heinzel, F. P., A. M. Hujer, F. N. Ahmed, and R. M. Rerko. 1997. In vivo production and function of IL-12 p40 homodimers. J. Immunol. 158:4381-4388. [PubMed] [Google Scholar]
- 27.Jones, J. L., D. Kruszon-Moran, M. Wilson, G. McQuillan, T. Navin, and J. B. McAuley. 2001. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am. J. Epidemiol. 154:357-365. [DOI] [PubMed] [Google Scholar]
- 28.Khan, I. A., and L. Casciotti. 1999. IL-15 prolongs the duration of CD8+ T cell-mediated immunity in mice infected with a vaccine strain of Toxoplasma gondii. J. Immunol. 163:4503-4509. [PubMed] [Google Scholar]
- 29.Khan, I. A., and L. H. Kasper. 1996. IL-15 augments CD8+ T cell-mediated immunity against Toxoplasma gondii infection in mice. J. Immunol. 157:2103-2108. [PubMed] [Google Scholar]
- 30.Khan, I. A., T. Matsuura, and L. H. Kasper. 1994. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect. Immun. 62:1639-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan, I. A., M. Moretto, X. Q. Wei, M. Williams, J. D. Schwartzman, and F. Y. Liew. 2002. Treatment with soluble interleukin-15Ralpha exacerbates intracellular parasitic infection by blocking the development of memory CD8+ T cell response. J. Exp. Med. 195:1463-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, Y. H., J. Y. Channon, T. Matsuura, J. D. Schwartzman, D. W. Shin, and L. H. Kasper. 1999. Functional and quantitative analysis of splenic T cell immune responses following oral Toxoplasma gondii infection in mice. Exp. Parasitol. 91:212-221. [DOI] [PubMed] [Google Scholar]
- 33.Lessard, M., S. He, and B. Benkel. 1998. A quantitative competitive reverse transcription-polymerase chain reaction technique to measure porcine interferon-gamma. J. Anim. Sci. 76:2155-2161. [DOI] [PubMed] [Google Scholar]
- 34.Leutenegger, C. M., A. M. Alluwaimi, W. L. Smith, L. Perani, and J. S. Cullor. 2000. Quantitation of bovine cytokine mRNA in milk cells of healthy cattle by real-time TaqMan polymerase chain reaction. Vet. Immunol. Immunopathol. 77:275-287. [DOI] [PubMed] [Google Scholar]
- 35.Leutenegger, C. M., B. von Rechenberg, J. B. Huder, K. Zlinsky, C. Mislin, M. K. Akens, J. Auer, and H. Lutz. 1999. Quantitative real-time PCR for equine cytokine mRNA in nondecalcified bone tissue embedded in methyl methacrylate. Calcif. Tissue Int. 65:378-383. [DOI] [PubMed] [Google Scholar]
- 36.Liu, F., Y. Abiko, M. Nishimura, K. Kusano, S. Shi, and T. Kaku. 2001. Expression of inflammatory cytokines and beta-defensin 1 mRNAs in porcine epithelial rests of Malassez in vitro. Med. Electron Microsc. 34:174-178. [DOI] [PubMed] [Google Scholar]
- 37.Loots, G. G., R. M. Locksley, C. M. Blankespoor, Z. E. Wang, W. Miller, E. M. Rubin, and K. A. Frazer. 2000. Identification of a coordinate regulator of interleukins 4, 13, and 5 by cross-species sequence comparisons. Science 288:136-140. [DOI] [PubMed] [Google Scholar]
- 38.Madden, K. B., L. Whitman, C. Sullivan, W. C. Gause, J. F. Urban, Jr., I. M. Katona, F. D. Finkelman, and T. Shea-Donohue. 2002. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J. Immunol. 169:4417-4422. [DOI] [PubMed] [Google Scholar]
- 39.Mansfield, L. S., J. F. Urban, R. R. Holley-Shanks, M. P. Murtaugh, D. S. Zarlenga, D. Foss, A. Canals, W. Gause, and J. K. Lunney. 1998. Construction of internal cDNA competitors for measuring IL-10 and IL-12 cytokine gene expression in swine. Vet. Immunol. Immunopathol. 65:63-74. [DOI] [PubMed] [Google Scholar]
- 40.McSharry, C., Y. Xia, C. V. Holland, and M. W. Kennedy. 1999. Natural immunity to Ascaris lumbricoides associated with immunoglobulin E antibody to ABA-1 allergen and inflammation indicators in children. Infect. Immun. 67:484-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mordue, D. G., F. Monroy, M. La Regina, C. A. Dinarello, and L. D. Sibley. 2001. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J. Immunol. 167:4574-4584. [DOI] [PubMed] [Google Scholar]
- 42.Oppmann, B., R. Lesley, B. Blom, J. C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, F. Zonin, E. Vaisberg, T. Churakova, M. Liu, D. Gorman, J. Wagner, S. Zurawski, Y. Liu, J. S. Abrams, K. W. Moore, D. Rennick, R. de Waal-Malefyt, C. Hannum, J. F. Bazan, and R. A. Kastelein. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715-725. [DOI] [PubMed] [Google Scholar]
- 43.Roberts, C. W., D. J. Ferguson, H. Jebbari, A. Satoskar, H. Bluethmann, and J. Alexander. 1996. Different roles for interleukin-4 during the course of Toxoplasma gondii infection. Infect. Immun. 64:897-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roepstorff, A., L. Eriksen, H. C. Slotved, and P. Nansen. 1997. Experimental Ascaris suum infection in the pig: worm population kinetics following single inoculations with three doses of infective eggs. Parasitology 115:443-452. [DOI] [PubMed] [Google Scholar]
- 45.Sakakibara, A., K. Baba, S. Niwa, T. Yagi, H. Wakayama, K. Yoshida, T. Kobayashi, T. Yokoi, K. Hara, M. Itoh, and E. Kimura. 2002. Visceral larva migrans due to Ascaris suum which presented with eosinophilic pneumonia and multiple intra-hepatic lesions with severe eosinophil infiltration—outbreak in a Japanese area other than Kyushu. Intern. Med. 41:574-579. [DOI] [PubMed] [Google Scholar]
- 46.Scharton-Kersten, T., E. Y. Denkers, R. Gazzinelli, and A. Sher. 1995. Role of IL12 in induction of cell-mediated immunity to Toxoplasma gondii. Res. Immunol. 146:539-545. [DOI] [PubMed] [Google Scholar]
- 47.Schopf, L. R., K. F. Hoffmann, A. W. Cheever, J. F. Urban, Jr., and T. A. Wynn. 2002. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J. Immunol. 168:2383-2392. [DOI] [PubMed] [Google Scholar]
- 48.Sharma, S. D., J. M. Hofflin, and J. S. Remington. 1985. In vivo recombinant interleukin 2 administration enhances survival against a lethal challenge with Toxoplasma gondii. J. Immunol. 135:4160-4163. [PubMed] [Google Scholar]
- 49.Shea-Donohue, T., C. Sullivan, F. D. Finkelman, K. B. Madden, S. C. Morris, J. Goldhill, V. Pineiro-Carrero, and J. F. Urban, Jr. 2001. The role of IL-4 in Heligmosomoides polygyrus-induced alterations in murine intestinal epithelial cell function. J. Immunol. 167:2234-2239. [DOI] [PubMed] [Google Scholar]
- 50.Smeltz, R. B., J. Chen, R. Ehrhardt, and E. M. Shevach. 2002. Role of IFN-gamma in Th1 differentiation: IFN-gamma regulates IL-18R alpha expression by preventing the negative effects of IL-4 and by inducing/maintaining IL-12 receptor beta 2 expression. J. Immunol. 168:6165-6172. [DOI] [PubMed] [Google Scholar]
- 51.Solano Aguilar, G. I., E. Beshah, K. G. Vengroski, D. Zarlenga, L. Jauregui, M. Cosio, L. W. Douglass, J. P. Dubey, and J. K. Lunney. 2001. Cytokine and lymphocyte profiles in miniature swine after oral infection with Toxoplasma gondii oocysts. Int. J. Parasitol. 31:187-195. [DOI] [PubMed] [Google Scholar]
- 52.Solano-Aguilar, G. I., D. Zarlenga, E. Beshah, K. Vengroski, L. Gasbarre, D. Junker, M. Cochran, C. Weston, D. Valencia, C. Chiang, H. Dawson, J. F. Urban, and J. K. Lunney. 2002. Limited effect of recombinant porcine interleukin-12 on porcine lymphocytes due to a low level of IL-12 beta2 receptor. Vet. Immunol. Immunopathol. 89:133-148. [DOI] [PubMed] [Google Scholar]
- 53.Stordeur, P., L. F. Poulin, L. Craciun, L. Zhou, L. Schandene, A. de Lavareille, S. Goriely, and M. Goldman. 2002. Cytokine mRNA quantification by real-time PCR. J. Immunol. Methods 259:55-64. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki, Y., A. Sher, G. Yap, D. Park, L. E. Neyer, O. Liesenfeld, M. Fort, H. Kang, and E. Gufwoli. 2000. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J. Immunol. 164:5375-5382. [DOI] [PubMed] [Google Scholar]
- 55.Szabo, S. J., A. S. Dighe, U. Gubler, and K. M. Murphy. 1997. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med. 185:817-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Urban, J. F., Jr., N. Noben-Trauth, D. D. Donaldson, K. B. Madden, S. C. Morris, M. Collins, and F. D. Finkelman. 1998. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8:255-264. [DOI] [PubMed] [Google Scholar]
- 57.Urban, J. F., Jr., R. D. Romanowski, and N. C. Steele. 1989. Influence of helminth parasite exposure and strategic application of anthelmintics on the development of immunity and growth of swine. J. Anim. Sci. 67:1668-1677. [DOI] [PubMed] [Google Scholar]
- 58.Urban, J. F., Jr., L. Schopf, S. C. Morris, T. Orekhova, K. B. Madden, C. J. Betts, H. R. Gamble, C. Byrd, D. Donaldson, K. Else, and F. D. Finkelman. 2000. Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism. J. Immunol. 164:2046-2052. [DOI] [PubMed] [Google Scholar]
- 59.Valentine, C. C., R. J. Hoffner, and S. O. Henderson. 2001. Three common presentations of ascariasis infection in an urban emergency department. J. Emerg. Med. 20:135-139. [DOI] [PubMed] [Google Scholar]
- 60.Villegas, E. N., L. A. Lieberman, S. R. Carding, and C. A. Hunter. 2002. Susceptibility of interleukin-2-deficient mice to Toxoplasma gondii is associated with a defect in the production of gamma interferon. Infect. Immun. 70:4757-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wagner, B., and L. Polley. 1997. Ascaris suum prevalence and intensity: an abattoir survey of market hogs in Saskatchewan. Vet. Parasitol. 73:309-313. [DOI] [PubMed] [Google Scholar]
- 62.Wurster, A. L., T. Tanaka, and M. J. Grusby. 2000. The biology of Stat4 and Stat6. Oncogene 19:2577-2584. [DOI] [PubMed] [Google Scholar]
- 63.Yap, G. S., and A. Sher. 1999. Cell-mediated immunity to Toxoplasma gondii: initiation, regulation and effector function. Immunobiology 201:240-247. [DOI] [PubMed] [Google Scholar]
- 64.Zenner, L., F. Darcy, A. Capron, and M. F. Cesbron-Delauw. 1998. Toxoplasma gondii: kinetics of the dissemination in the host tissues during the acute phase of infection of mice and rats. Exp. Parasitol. 90:86-94. [DOI] [PubMed] [Google Scholar]
- 65.Zhao, A., J. McDermott, J. F. Urban, Jr., W. Gause, K. B. Madden, K. A. Yeung, S. C. Morris, F. D. Finkelman, and T. Shea-Donohue. 2003. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J. Immunol. 171:948-954. [DOI] [PubMed] [Google Scholar]