Introduction
Key Teaching Points.
-
•
Our case illustrates a novel mechanism of premature ventricular complexes (PVCs), ie, a dual intraventricular response, caused by slow conduction in scar tissue.
-
•
Different PVC morphologies can be cured by transecting a single isthmus of conduction after previous myocardial infarction and does not require extended ablation procedures.
-
•
The recognition of this PVC pattern is important because a straightforward ablation strategy can eliminate these PVCs and improve cardiac resynchronization therapy and prognosis.
Frequent premature ventricular complexes (PVCs) are a clinical challenge in heart failure, especially after resynchronization therapy. This case illustrates a unique mechanism of premature beats caused by a dual intraventricular response after cardiac resynchronization.
Case report
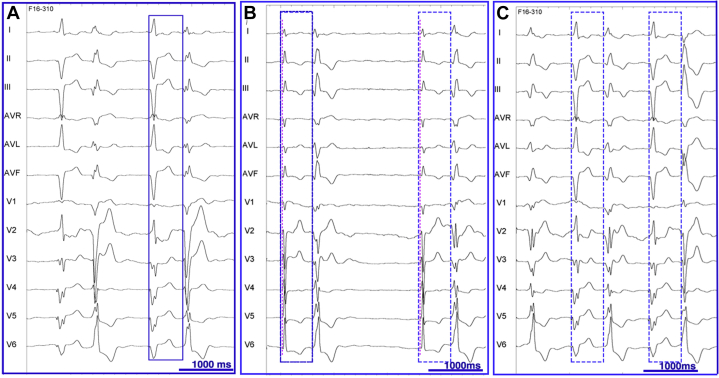
A 72-year-old woman with a history of heart failure secondary to ischemic cardiomyopathy, coronary artery bypass graft surgery, and cardiac resynchronization therapy (CRT) presented with functional deterioration (NYHA class III). She was treated with apixaban, aspirin, bisoprolol, candesartan, bumetanide, pravastatin, pantoprazole, and sertraline. There was no indication for additional coronary revascularization. Ablation of the PVCs was planned for symptomatic and prognostic reasons. Her electrocardiogram (ECG) demonstrated PVCs with a single morphology in bigeminy during biventricular pacing at 60 beats per minute with underlying left atrial flutter (Figure 1A). Interrogation of her CRT device revealed 50% biventricular pacing due to continuous bigeminy. Bigeminy was also present during atrioventricular conducted beats (Figure 1B), during right or left ventricular–only pacing, and during biventricular pacing at 80 beats per minute (Figure 1C). Two different PVCs were identified with a similar coupling interval of 630 ms.
Figure 1.
A: Baseline electrocardiogram (ECG) during biventricular pacing at 60 beats per minute showing an underlying left atrial flutter and bigeminy. The premature ventricular complexes have the same morphology and a coupling interval of 630 ms, indicated by the blue rectangle. B: Baseline ECG, after reprograming the lower pacing rate at 30 beats per minute, showing poor residual atrioventricular conduction with narrow QRS complexes and 2 different morphologies of premature ventricular complexes (PVCs) with the same coupling interval (580 ms). The red line corresponds to the beginning of the narrow QRS complexes. The blue rectangles indicate a longer PVC coupling interval (630 ms) during biventricular pacing at 60 beats per minute. C: Biventricular pacing at 80 beats per minute showing bigeminy with the same 2 different PVC morphologies. PVCs alternate and have a similar coupling interval compared with biventricular pacing at 60 beats per minute (blue rectangle).
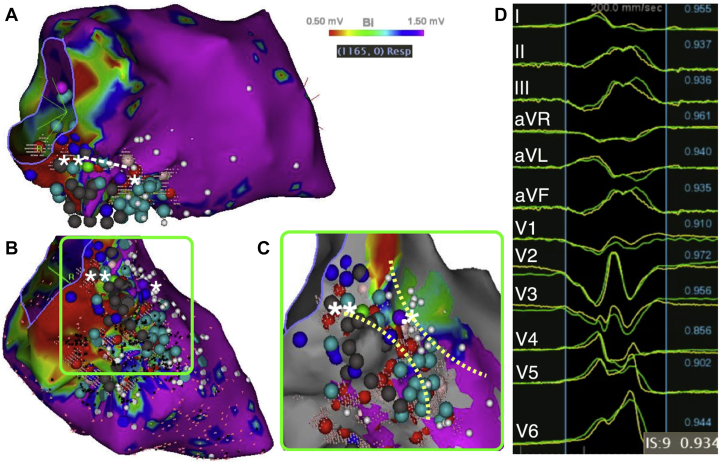
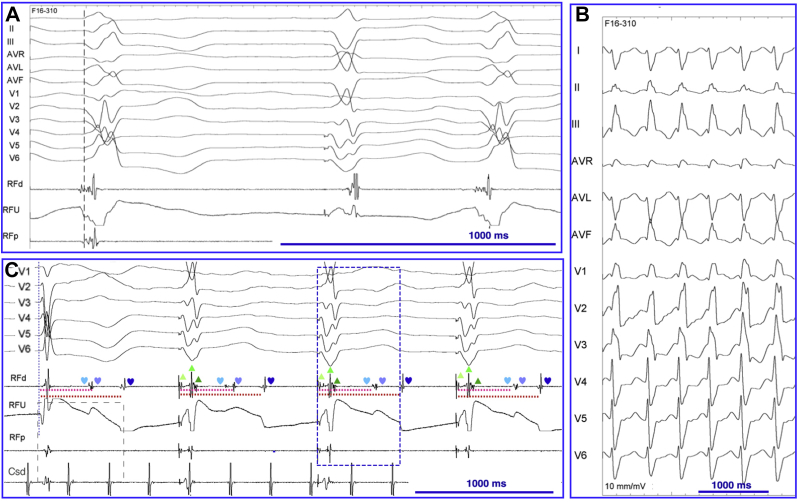
Under general anesthesia, bigeminy was replaced by quadrigeminy, the second PVC morphology disappeared, and the coupling interval of the first PVC morphology increased toward 700 ms. A deflectable decapolar catheter was placed in the coronary sinus and a transesophageal electrocardiogram–guided transseptal puncture was performed. A multielectrode mapping catheter (PentaRay; Biosense Webster, Diamond Bar, CA) was inserted into the steerable sheath; mapping of the left ventricle was performed in sinus rhythm using the Confidense module of the CARTO system (Biosense Webster) and hence excluded the PVCs from the voltage map. An inferior infarct zone was delineated. Late abnormal ventricular activations and late potentials (LPs) were annotated (Figure 2A–C). A limited pace mapping, using PaSo software (Biosense Webster), revealed a good match with the spontaneous PVCs at the basal and septal site of the infarcted area (Figure 2C and D) and identified a conduction isthmus (Figure 2C). A conventional activation map demonstrated low-amplitude and fractionated electrograms 37 ms before the PVC at the basal site of this isthmus (Figure 3A). Slow monomorphic ventricular tachycardia (VT) was induced with a cycle length of 496 ms and a QRS morphology different from that of the PVCs (Figure 3B).
Figure 2.
A: Voltage map in right anterior oblique 30° view. The white dotted line (from * to **) indicates where the initial ablation line abolishing the premature ventricular complexes (PVCs) has been performed. B: Voltage map in a modified inferior view. Late abnormal ventricular activations: light blue dots; late potentials: dark blue dots; points with no ventricular capture: gray dots. C: Enlarged view of the pace map within the infarcted area. The borders of a conduction isthmus containing the point with the best pace map (*) are indicated by the yellow dotted lines. PVCs disappeared after completion of the line through the conduction isthmus (**). D: Match (93%) between the PVCs and the paced complex at the initial ablation site (*).
Figure 3.
A: Conventional mapping at the conduction isthmus exit site showing a fragmented signal 37 ms before QRS, with a QS pattern in the unipolar lead. B: Monomorphic ventricular tachycardia with a different QRS morphology compared with the premature ventricular complexes (PVCs). C: Electrograms within the QRS (▲) and nonconducted late potentials (♥) after ablation. The coupling interval of the latest late potential after ablation fits the coupling interval of the PVCs (700 ms) after anesthesia. The blue rectangle indicates the coupling interval of the PVCs before ablation (630 ms).
Based on the hypothesis discussed in the next section, we decided to transect the isthmus shared by the 2 PVCs under the pacing site with the best correlation with the PVC still present under general anesthesia. The occurrence of PVCs was abolished after completion of the line through the conduction isthmus (Figure 2C). After this discrete ablation, nonconducted LPs fitting the coupling interval of the PVC during general anesthesia were identified within the isthmus (Figure 3C). LPs and late abnormal ventricular activations were additionally ablated. VT was no longer inducible. No residual PVCs could be detected during continuous 12-hour cardiac monitoring after ablation. Five months later, the patient's functional status was improved (NYHA class I) and device interrogation revealed 99% biventricular pacing.
Discussion
Intermittent PVCs can be due to ventricular ectopy, intermittent aberrancy, or intermittent preexcitation. In the present case, the R-R interval of the PVCs showed a regular irregular pattern determined by the previous ventricular complexes. This finding did not favor enhanced automaticity as the mechanism of the PVCs but was somewhat compatible with a “dual atrioventricular nodal response” phenomenon associated with aberrancy.1 Nevertheless, both the severe atrioventricular nodal conduction disturbances and the PVC morphology excluded this hypothesis. PVC morphology was not compatible with intermittent preexcitation. We hypothesized that the systematic coupling of the PVC with previous ventricular complexes was explained by a slow conducting pathway located within the intramyocardial scar. This hypothesis is supported by 3 findings. First, the fact that the timing of the PVCs was solely determined by the previous QRS is nearly diagnostic. The 1:1 ratio between paced complexes and PVCs and the very long coupling interval are not in favor of a trigger activity mechanism. We are not aware of any other putative mechanism that could explain this pattern. This uncommon and characteristic pattern led us to generate our hypothesis before performing the electrophysiology study. Second, the PVC did not disappear while ablating the distal isthmus adjacent to the site with the best pace map, despite excellent electrogram characteristics (Figure 3A) but rather after completion of the line across the isthmus. Third, the identification of LPs with a coupling interval fitting the coupling interval of the PVC at the distal portion of the identified isthmus is also in line with our hypothesis.
To allow a second ventricular contraction or an “intraventricular echo,” the intra-scar conduction time had to be longer than the refractory period of the left ventricle. A changing of conduction properties of the “slow myocardial pathway” occurred under general anesthesia, as bigeminy was replaced by quadrigeminy. This could be owing to a lower catecholaminergic status. The 2 different but somewhat similar PVCs could be explained by 2 different exit points within the myocardial infarction border zone. The pace map showing a conduction isthmus in the infarcted area was in favor of this hypothesis.
While a reentrant mechanism can explain ventricular couplets, ventricular triplets, nonsustained VT, or sustained VT, isolated PVCs can be explained by an intramyocardial dual response owing to slow conduction zones protected by a conduction isthmus.
CRT confers a mortality benefit and a reduction in hospitalizations, and improves functionality in patients with systolic heart failure and left bundle branch block. A high percentage of biventricular pacing is crucial for a favorable outcome of CRT.2 PVCs account for 16.6% of the loss of CRT.3 An association between the frequency of PVCs and outcome in patients with CRT has been demonstrated.4 Successful ablation of PVCs promotes reverse remodeling in CRT nonresponders.5
To our knowledge, this is the first case showing how ‘dual intraventricular response’ can produce PVCs. We are aware that this phenomenon is probably rare compared with enhanced automaticity as a mechanism of ventricular ectopy. Although we have no data to support this statement, we suspect that this mechanism may occur in some patients with a high level of PVCs presenting with a regular irregular pattern. As this condition can be very symptomatic and/or affect the prognosis of patients and can easily be cured by discrete ablation, it is important to recognize this ECG pattern.
Conclusions
Our case describes a novel mechanism of PVCs (ie, a dual intraventricular response) caused by slow conduction in scar tissue. Frequent PVCs in patients with heart failure may interfere with resynchronization therapy and lead to worsening of left ventricular systolic dysfunction. The recognition of this ECG pattern is important because a straightforward ablation strategy can eliminate these PVCs and improve patient prognosis.
References
- 1.Debruyne P., Vankelecom B., Janssens L. Who is guilty? J Cardiovasc Electrophysiol. 2009;20:942–943. doi: 10.1111/j.1540-8167.2008.01399.x. [DOI] [PubMed] [Google Scholar]
- 2.Hayes D.L., Boehmer J.P., Day J.D., Gilliam F.R., 3rd, Heidenreich P.A., Seth M., Jones P.W., Saxon L.A. Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart Rhythm. 2011;8:1469–1475. doi: 10.1016/j.hrthm.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Cheng A., Landman S.R., Stadler R.W. Reasons for loss of cardiac resynchronization therapy pacing: insights from 32 844 patients. Circ Arrhythm Electrophysiol. 2012;5:884–888. doi: 10.1161/CIRCEP.112.973776. [DOI] [PubMed] [Google Scholar]
- 4.Ruwald M.H., Mittal S., Ruwald A.C. Association between frequency of atrial and ventricular ectopic beats and biventricular pacing percentage and outcomes in patients with cardiac resynchronization therapy. J Am Coll Cardiol. 2014;64:971–981. doi: 10.1016/j.jacc.2014.06.1177. [DOI] [PubMed] [Google Scholar]
- 5.Lakkireddy D., Di Biase L., Ryschon K. Radiofrequency ablation of premature ventricular ectopy improves the efficacy of cardiac resynchronization therapy in nonresponders. J Am Coll Cardiol. 2012;60:1531–1539. doi: 10.1016/j.jacc.2012.06.035. [DOI] [PubMed] [Google Scholar]