Abstract
Background
Recently, some studies have found that retinoblastoma-binding protein 2 (RBP2) is involved in the development and progression of many kinds of malignant tumors. This study aimed to explore the expression level of RBP2 in hepatocellular carcinoma (HCC) and its prognostic significance.
Material/Methods
Immunohistochemical analysis was used to evaluate the RBP2 expression level in 130 HCC patients and adjacent normal tissues. Tumor angiogenesis was marked by CD31 and vascular endothelial growth factor (VEGF) staining. Kaplan-Meier and Cox regression analyses were performed to examine the relationship between RBP2 expression and prognosis of HCC patients.
Results
RBP2 expression was significantly higher in HCC tissues (positive expression rate: 72.3%, 94/130). Increased RBP2 expression was dramatically associated with AFP level (P=0.016), degree of differentiation (P=0.000), and TNM stage (P=0.035). Moreover, tumors with RBP2-positive expression showed significantly higher intratumoral MVD than those with RBP2-negative expression (P=0.000). Kaplan-Meier analysis revealed RBP2-positive expression was related to decreased disease-free survival (DFS) (P=0.000) and overall survival (OS) (P=0.000). Furthermore, RBP2 was an independent poor prognostic factor of DFS and OS (P=0.029 and 0.010, respectively) as demonstrated by multivariate analysis.
Conclusions
Increased RBP2 expression, as an independent poor prognostic factor for DFS and OS of HCC patients, is closely related to tumor angiogenesis. RBP2 is expected to become a new potential therapeutic target for HCC.
MeSH Keywords: Angiogenesis Inducing Agents; Carcinoma, Hepatocellular; Prognosis; Retinoblastoma-Binding Protein 2
Background
Hepatocellular carcinoma (HCC), a common malignant tumor of the digestive tract, is the third-leading cause of cancer death in the world [1] and ranks second in malignant tumor mortality in China [2]. Liver cancer has obvious vascular characteristics, the tumor cells of which can produce a variety of vascular growth factor to promote angiogenesis. Based on the above characteristics, anti-tumor angiogenesis strategy research and exploration in HCC patients is particularly necessary and has important clinical significance.
The emergence of targeted drugs presents new hope for the treatment of cancer patients, with higher specificity and relatively minor adverse effects [3]. In liver cancer, sorafenib [4] and regorafenib [5] are clinically proven to be effective oral agents, but the effect is still very limited. At present, there are a large number of molecular-targeted drugs. One such drug is apatinib, which is still in clinical trials and its efficacy is uncertain. In view of this, a currently popular liver cancer research focus is the molecular mechanism underlying the development of HCC and establishing a more effective targeted therapy.
Histone modification plays a key role in tumor progression, including angiogenesis [6]. For example, mixed-lineage leukemia 1 (MLL1), as the histone methylase, plays an important role in tumor growth and angiogenesis. Histone deacetylase 3 (HDAC3) can act as a negative regulator of angiogenesis factor. Retinoblastoma-binding protein 2 (RBP2) is a newly discovered histone demethylase that can participate in the development and progression of cancer [7,8]. Recently, studies have shown that RBP2 also plays an important role in the angiogenesis of cancer [9,10]. However, the biological and clinical significance of RBP2 in HCC patients remain largely unknown.
Therefore, in the present study, immunohistochemical staining was done to examine the expressions of RBP2, VEGF, and CD31-labeled microvessel density (MVD) in HCC and corresponding adjacent normal tissues. We also investigated RBP2 expression in HCC and its relationships with patient clinicopathological features, prognosis, and angiogenesis.
Material and Methods
Patients and samples
The tissue samples were collected from 130 patients diagnosed with HCC after curative operation at Renmin Hospital of Wuhan University from August 2009 to December 2012. Tumor staging was established on the basis of the sixth edition of the tumor-node-metastasis (TNM) classification of the Union for International Cancer Control (UICC). All patients’ clinicopathological parameters are summarized in Table 1. The study was authorized by the Ethics Committee of Renmin Hospital of Wuhan University and abided by the Declaration of Helsinki. Written informed consent was provided by all patients.
Table 1.
Relationships between RBP2 protein expression in HCC tissues and clinicopathological variables.
| Variables | Total | RBP2 expression | |||
|---|---|---|---|---|---|
| Negative (n=36) | Positive (n=94) | χ2 | P | ||
| Gender | |||||
| Male | 106 | 33 | 73 | 3.393 | 0.065 |
| Female | 24 | 3 | 21 | ||
| Age at surgery (yeas) | |||||
| ≤60 | 90 | 24 | 66 | 0.154 | 0.695 |
| >60 | 40 | 12 | 28 | ||
| Tumor size (cm) | |||||
| ≤5 | 62 | 21 | 41 | 2.260 | 0.133 |
| >5 | 68 | 15 | 53 | ||
| HbsAg | |||||
| Negative | 19 | 5 | 14 | 0.021 | 0.885 |
| Positive | 111 | 31 | 80 | ||
| Cirrhosis | |||||
| No | 10 | 2 | 8 | 0.320 | 0.572 |
| Yes | 120 | 34 | 86 | ||
| Child-Pugh | |||||
| A | 124 | 36 | 88 | 2.409 | 0.121 |
| B | 6 | 0 | 6 | ||
| AFP (ng/ml) | |||||
| ≤20 | 44 | 18 | 26 | 5.802 | 0.016 |
| >20 | 86 | 18 | 68 | ||
| Degree of differentiation | |||||
| Well/moderate | 71 | 35 | 36 | 36.463 | 0.000 |
| Poor and not | 59 | 1 | 58 | ||
| TNM stage | |||||
| I/II | 99 | 32 | 67 | 4.446 | 0.035 |
| III/IV | 31 | 4 | 27 | ||
Immunohistochemical protocol and analysis
Immunohistochemical staining was done by a two-step method. RBP2, VEGF, and CD31 antibodies were used (the concentration of all antibodies was 1: 100). In brief, the procedure was: (1) Fix tumor tissues with 10% formalin at room temperature; (2) Rinse the tissue with running tap water to eliminate the formaldehyde; (3) Dehydrate the tissues in EtOH baths; (4) Clear the tissue twice in xylene; (5) Melt the paraffin prior to adding the tissue; (6) Pour melted paraffin into a paraffin block mold; (7) Section the paraffin-embedded tissue block in 4-μm-thick slices; (8) Float the tissue sections onto clean glass slides and microwave at 65°C for 15 min, and then store overnight at room temperature; and (9) Establish a negative control by using PBS to replace the primary antibody. Immunohistochemical scores were classified according to a published report [11].
MVD counts
MVD counts were labeled by CD31-positive staining vascular endothelial cells. After scanning an immunostained section at low magnification (×40), the regions with maximum number of dramatically marked microvessels stained with anti-CD31 were selected, and microvessels were counted at higher power (×100). All sections were evaluated by 2 pathologists independently.
Statistics and data analysis
Statistical analysis was done using SPSS 19.0 software. The relationship between RBP2 expression and clinicopathological characteristics was examined by Pearson X2 test or Fisher test. Kaplan-Meier and Cox regression models were used to determine the survival rates and for multivariate analysis. Statistical significance was defined as P < 0.05.
Results
RBP2 expression and correlation with clinicopathological parameters in HCC
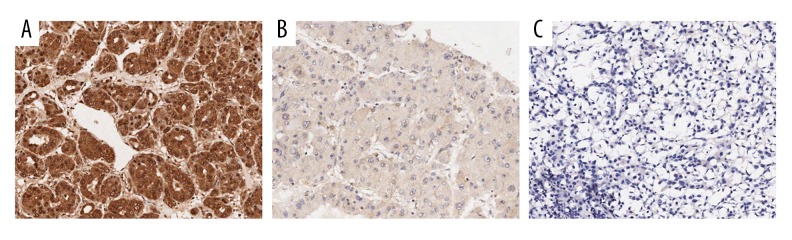
Immunohistochemistry was used in 130 cases of HCC and corresponding adjacent normal tissues to detect the clinicopathological and prognostic values of RBP2 in HCC. RBP2 protein staining was mainly located in the cytoplasm (Figures 1A, 2A). RBP2 positive expression rate was 72.3% (94/130) in HCC tissues (Figure 1). The correlations of RBP2 expression with clinicopathological factors are summarized in Table 1. Elevated RBP2 expression was dramatically related to AFP level (P=0.016), degree of differentiation (P=0.000) and TNM stage (P=0.035).
Figure 1.
Immunochemical staining of RBP2 in HCC tissues. (A) High positive expression of RBP2; (B) Low positive expression of RBP2; (C) Negative expression of RBP2. (with 100× magnification).
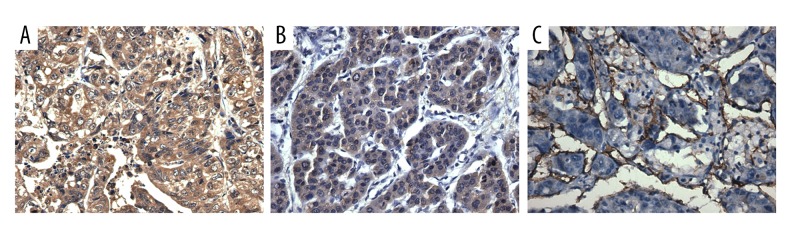
Figure 2.
(A–C) Positive co-expression of RBP2, VEGF, and CD31 in HCC tissues, confirmed by immunochemical staining (400× magnification).
Relationship between RBP2 and VEGF protein expression
VEGF staining was mainly located in the cytoplasm (Figure 2B); 93 of the 130 HCC tissues were VEGF-positive (71.5%), and the positive rate of both RBP2 and VEGF was 57.7% (75/130). Furthermore, Pearson’s test showed a significant relationship between expression of RBP2 and VEGF in tumor tissues (r=0.295, P=0.001; Table 2).
Table 2.
Expression correlation of RBP2 and VEGF in HCC tissues.
| Group | RBP2 expression | r | P-value | |
|---|---|---|---|---|
| Positive | Negative | |||
| VEGF expression | 0.295 | 0.001 | ||
| Positive | 75 | 18 | ||
| Negative | 19 | 18 | ||
Correlation between RBP2 and MVD in HCC
MVD was counted by examining the CD31 staining to assess the correlation between RBP2 and angiogenesis (Figure 2C). RBP2-positive HCC tissues had an evidently higher MVD than in RBP2-negative tissues (P=0.000; Figure 3).
Figure 3.
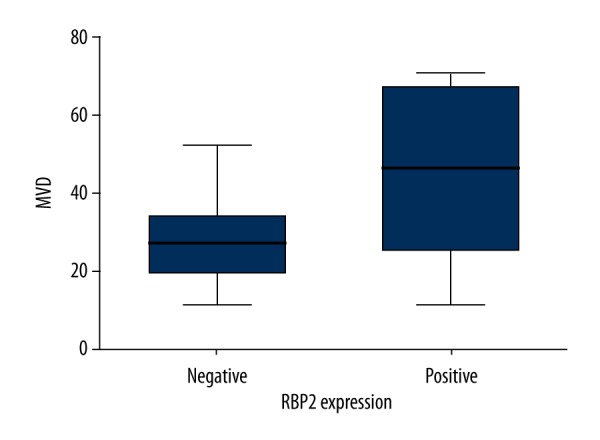
Intratumoral microvessel density (MVD) in relation to RBP2 protein immunoreactivity. HCC patients with RBP2-positive expression showed significantly higher intratumoral MVD than in patients with RBP2-negative expression (P=0.000).
Relationship between RBP2 and prognosis
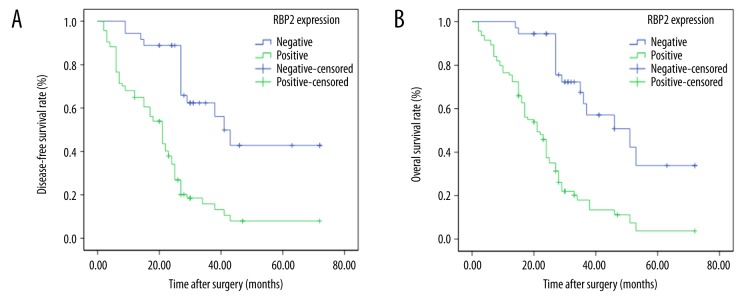
Survival analysis showed that RBP2 expression was inversely related to the survival of HCC patients. In comparison to those with negative RBP2 expression, DFS and OS times were significantly decreased in RBP2-positive patients (P=0.000, Figure 4A and P=0.000, Figure 4B, respectively).
Figure 4.
Kaplan-Meier analysis of disease-free survival (DFS) and overall survival (OS) curves of HCC patients based on RBP2 expression as positive or negative. (A) DFS curve of HCC patients based on RBP2 expression; (B) OS curve of HCC patients based on RBP2 expression.
Prognostic values in HCC patients
Univariate analysis was performed to reveal that RBP2 expression, degree of differentiation, and TNM stage had significant prognostic impacts on DFS and OS (Tables 3, 4). Furthermore, Cox analysis showed that RBP2 expression was an independent prognostic parameter for DFS (P=0.029) and OS (P=0.010) (Tables 5, 6).
Table 3.
Univariate analysis of the correlation between clinicopathological parameters and disease free survival time of HCC patients.
| Variable | Mean survival time (m) | 95% CI | P |
|---|---|---|---|
| Gender | |||
| Male | 32.256 | 20.793–43.719 | 0.843 |
| Female | 28.854 | 24.038–33.669 | |
| Age at surgery (yeas) | |||
| ≤60 | 30.910 | 25.438–36.382 | 0. 319 |
| >60 | 22.316 | 17.729–26.902 | |
| Tumor size (cm) | |||
| ≤5 | 33.435 | 26.636–40.234 | 0.120 |
| >5 | 24.766 | 19.587–29.945 | |
| HbsAg | |||
| Negative | 29.906 | 25.081–34.731 | 0. 307 |
| Positive | 20.632 | 15.972–25.291 | |
| Cirrhosis | |||
| No | 29.291 | 24.689–33.893 | 0.755 |
| Yes | 23.600 | 18.195–29.005 | |
| Child-Pugh | |||
| A | 29.791 | 25.287–34.294 | 0.034 |
| B | 12.667 | 4.671–20.662 | |
| AFP (ng/ml) | |||
| ≤20 | 32.007 | 30.650–43.410 | 0.127 |
| >20 | 28.649 | 23.388–33.910 | |
| Degree of differentiation | |||
| Well/moderate | 32.825 | 27.433–38.217 | 0.013 |
| Poor and not | 18.965 | 13.862–24.069 | |
| TNM stage | |||
| I/II | 39.352 | 32.666–46.038 | 0.000 |
| III/IV | 16.549 | 13.397–19.701 | |
| RBP2 expression | |||
| Negative | 47.323 | 38.148–56.228 | 0.000 |
| Positive | 22.140 | 17.965–26.315 | |
Table 4.
Univariate analysis of the correlation between clinicopathological parameters and overall survival time of HCC patients.
| Variable | Mean survival time (m) | 95% CI | P |
|---|---|---|---|
| Gender | |||
| Male | 32.341 | 21.511–43.171 | 0.943 |
| Female | 30.505 | 26.228–34.781 | |
| Age at surgery (yeas) | |||
| ≤60 | 31.999 | 26.799–37.199 | 0. 389 |
| >60 | 27.550 | 22.750–32.350 | |
| Tumor size (cm) | |||
| ≤5 | 34.738 | 28.539–40.938 | 0.082 |
| >5 | 27.087 | 22.083–32.092 | |
| HbsAg | |||
| Negative | 31.652 | 27.185–36.119 | 0. 361 |
| Positive | 23.592 | 17.896–29.288 | |
| Cirrhosis | |||
| No | 30.650 | 26.405–34.894 | 0.665 |
| Yes | 28.700 | 21.558–35.842 | |
| Child-Pugh | |||
| A | 31.220 | 27.112–35.328 | 0.104 |
| B | 15.667 | 5.529–25.804 | |
| AFP (ng/ml) | |||
| ≤20 | 37.030 | 30.650–43.410 | 0.016 |
| >20 | 28.649 | 23.388–33.910 | |
| Degree of differentiation | |||
| Well/moderate | 33.340 | 28.324–38.356 | 0.034 |
| Poor and not | 23.492 | 17.595–29.389 | |
| TNM stage | |||
| I/II | 40.293 | 34.167–46.420 | 0.000 |
| III/IV | 19.677 | 16.115–23.239 | |
| RBP2 expression | |||
| Negative | 48.395 | 40.477–56.344 | 0.000 |
| Positive | 23.670 | 20.000–27.341 | |
Table 5.
Multivariate analysis of the correlation between clinicopathological parameters and disease free survival time of HCC patients.
| Covariates | HR | 95% CI for HR | P |
|---|---|---|---|
| Gender (Male vs. Female) | 0.739 | 0.413–1.324 | 0.310 |
| Age (≤60 vs. >60 cm) | 0.622 | 0.385–1.005 | 0.052 |
| Tumor size (≤5 vs. >5 cm) | 0.610 | 0.378–1.985 | 0.043 |
| HbsAg (negative vs. positive) | 1.654 | 0.813–3.362 | 0.165 |
| Cirrhosis (No vs. Yes) | 1.127 | 0.410–3.097 | 0.817 |
| Child-Pugh (A vs. B) | 0.547 | 0.185–1.618 | 0.276 |
| AFP (≤20 vs. >20 ng/ml) | 0.906 | 0.547–1.500 | 0.700 |
| Differentiation (Well/moderate vs. Poor and not) | 0.835 | 0.494–1.411 | 0.501 |
| TNM stage (stage I/II vs. III/IV) | 0.309 | 0.168–0.569 | 0.000 |
| RBP2 expression (negative vs. positive) | 0.476 | 0.244–0.925 | 0.029 |
Table 6.
Multivariate analysis of the correlation between clinicopathological parameters and overall survival time of HCC patients.
| Covariates | HR | 95% CI for HR | P |
|---|---|---|---|
| Gender (Male vs. Female) | 0.828 | 0.460–1.491 | 0.529 |
| Age (≤60 vs. >60 cm) | 0.722 | 0.718–1.624 | 0.177 |
| Tumor size (≤5 vs. >5 cm) | 0.592 | 0.365–0.960 | 0.034 |
| HbsAg (negative vs. positive) | 1.998 | 1.000–3.989 | 0.050 |
| Cirrhosis (No vs. Yes) | 0.531 | 0.192–1.466 | 0.222 |
| Child-Pugh (A vs. B) | 0.745 | 0.253–2.192 | 0.593 |
| AFP (≤20 vs. >20 ng/ml) | 0.707 | 0.425–1.176 | 0.182 |
| Differentiation (Well/moderate vs. Poor and not) | 0.945 | 0.554–1.611 | 0.835 |
| TNM stage (stage I/II vs. III/IV) | 0.377 | 0.208–0.681 | 0.001 |
| RBP2 expression (negative vs. positive) | 0.414 | 0.211–0.812 | 0.010 |
Discussion
RBP2 belongs to the JARID family and can remarkably demethylate H3K4me2 and H3K4me3 [12]. Accumulating evidence demonstrated that RBP2 is abnormally expressed in many kinds of malignant tumors such as gastric cancer [9], non-small cell lung cancer (NSCLC) [10], and liver cancer [13]. These findings show that the function of RBP2 is mainly associated with the epithelial-mesenchymal transition (EMT), migration, invasion, and cell proliferation of cancer. However, whether RBP2 expression is related to HCC angiogenesis and its prognostic value still remain unclear. In the present study, our preliminary findings demonstrated that RBP2 was highly expressed in HCC tissues. Moreover, further results showed that RBP2-positive expression was remarkably related to the AFP level, degree of differentiation, and TNM stage. The above data suggest a pivotal role for RBP2 in progression and development of HCC.
Accumulating research demonstrates that overexpression of VEGF is associated with aggressive behavior and unfavorable prognosis of cancer [14,15]. Moreover, several studies have demonstrated that increased VEGF expression and MVD are significantly correlated with poorer prognosis in HCC [16,17]. In our study, a remarkable positive relationship between expression of RBP2 and VEGF was found. In comparison to those with negative RBP2 expression, patients with positive RBP2 expression had a significantly higher MVD, suggesting that RBP2 is involved in HCC tumor angiogenesis, possibly in cooperation with VEGF. Recently, Li et al. [9] found that RBP2 can directly bind to the promoter of VEGF to regulate its expression and promote the angiogenesis of gastric cancer by histone H3K4 demethylation. Qi et al. [10] found that RBP2 can promote HIF-1α-VEGF-induced angiogenesis of NSCLC via the AKT pathway. The AKT signaling pathway plays an important regulatory role in many cellular survival pathways, primarily in angiogenesis and tumorigenesis, through regulation of VEGF [18]. These results suggest that RBP2 may be engaged in promoting VEGF expression through PI3K/AKT/HIF-1α signaling. Furthermore, Fan et al. [19] recently reported that miR-34a promotes the osteogenic differentiation of hASCs via the RBP2/NOTCH1/CYCLIN D1 coregulatory network. Therefore, further detailed research is needed to elucidate the role of RBP2 in angiogenesis of HCC.
Next, we explored the clinical significance in prognosis of RBP2 in HCC. Compared to those with RBP2-negative expression, patients with RBP2-positive expression have decreased DFS and OS, as shown by Kaplan-Meir analysis. Univariate and multivariate analyses showed that RBP2 was an independent unfavorable predictor of DFS and OS in HCC patients.
There are several limitations in the present study. Firstly, it was a relatively small-sample, retrospective study, possibly leading to a selective bias. Secondly, we only used immunohistochemical staining, which is a semi-quantitative method, to examine the expression of relative antibodies. Finally, the detailed underlying molecular mechanisms were not explored, which needs to be elucidated in our further studies.
Conclusions
Our preliminary findings demonstrated that increased RBP2 expression is closely related to HCC angiogenesis and is an independent adverse prognostic factor. RBP2 is expected to become a new potential therapeutic target for HCC.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zuo T, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res. 2016;28:1–11. doi: 10.3978/j.issn.1000-9604.2016.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12:408–24. doi: 10.1038/nrclinonc.2015.103. [DOI] [PubMed] [Google Scholar]
- 4.Colagrande S, Inghilesi AL, Aburas S, et al. Challenges of advanced hepatocellular carcinoma. World J Gastroenterol. 2016;22:7645–59. doi: 10.3748/wjg.v22.i34.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J, Qin S, Merle P, et al. RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 6.Gołąbek K, Strzelczyk JK, Wiczkowski A, Michalski M. Potential use of histone deacetylase inhibitors in cancer therapy. Contemp Oncol (Pozn) 2015;19:436–40. doi: 10.5114/wo.2015.51824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maggi EC, Trillo-Tinoco J, Struckhoff AP, et al. Retinoblastoma-binding protein 2 (RBP2) is frequently expressed in neuroendocrine tumors and promotes the neoplastic phenotype. Oncogenesis. 2016;5:e257. doi: 10.1038/oncsis.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang X, Zeng J, Wang L, et al. Histone demethylase RBP2 promotes malignant progression of gastric cancer through TGF-β1-(p-Smad3)-RBP2-E-cadherin-Smad3 feedback circuit. Oncotarget. 2015;6:17661–74. doi: 10.18632/oncotarget.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Wang L, Song P, et al. Critical role of histone demethylase RBP2 in human gastric cancer angiogenesis. Mol Cancer. 2014;13:81. doi: 10.1186/1476-4598-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi L, Zhu F, Li SH, et al. Retinoblastoma binding protein 2 (RBP2) promotes HIF-1α-VEGF-induced angiogenesis of non-small cell lung cancer via the Akt pathway. PLoS One. 2014;9:e106032. doi: 10.1371/journal.pone.0106032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudo Y, Ogawa I, Kitajima S, et al. Periostin promotes invasion and anchorage-independent growth in the metastatic process of head and neck cancer. Cancer Res. 2006;66:6928–35. doi: 10.1158/0008-5472.CAN-05-4540. [DOI] [PubMed] [Google Scholar]
- 12.Ge W, Shi L, Zhou Y, et al. Inhibition of osteogenic differentiation of human adipose-derived stromal cells by retinoblastoma binding protein 2 repression of RUNX2-activated transcription. Stem Cells. 2011;29:1112–25. doi: 10.1002/stem.663. [DOI] [PubMed] [Google Scholar]
- 13.Liang X, Zeng J, Wang L, et al. Histone demethylase retinoblastoma binding protein 2 is overexpressed in hepatocellular carcinoma and negatively regulated by hsa-miR-212. PLoS One. 2013;8:e69784. doi: 10.1371/journal.pone.0069784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia H, Shen J, Chen S, et al. Overexpression of VEGF-C correlates with a poor prognosis in esophageal cancer patients. Cancer Biomark. 2016;17:165–70. doi: 10.3233/CBM-160627. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Guo Y, Wang B, et al. Lymphatic microvessel density and vascular endothelial growth factor-C and -D as prognostic factors in breast cancer: A systematic review and meta-analysis of the literature. Mol Biol Rep. 2012;39:11153–65. doi: 10.1007/s11033-012-2024-y. [DOI] [PubMed] [Google Scholar]
- 16.Qin LX, Tang ZY. Recent progress in predictive biomarkers for metastatic recurrence of human hepatocellular carcinoma: A review of the literature. J Cancer Res Clin Oncol. 2004;130:497–513. doi: 10.1007/s00432-004-0572-9. [DOI] [PubMed] [Google Scholar]
- 17.Tseng PL, Tai MH, Huang CC, et al. Overexpression of VEGF is associated with positive p53 immunostaining in hepatocellular carcinoma (HCC) and adverse outcome of HCC patients. J Surg Oncol. 2008;98:349–57. doi: 10.1002/jso.21109. [DOI] [PubMed] [Google Scholar]
- 18.Moeini A, Cornellà H, Villanueva A. Emerging signaling pathways in hepatocellular carcinoma. Liver Cancer. 2012;1:83–93. doi: 10.1159/000342405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan C, Jia L, Zheng Y, et al. MiR-34a promotes osteogenic differentiation of human adipose-derived stem cells via the RBP2/NOTCH1/CYCLIN D1 coregulatory network. Stem Cell Reports. 2016;7:236–48. doi: 10.1016/j.stemcr.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]