Abstract
To investigate the roles of plasma miR-21 in the pathogenic process of Type 2 diabetes (T2D) with diabetic retinopathy (DR). T2D patients included patients without DR (NDR) group, patients with non-proliferative/background DR (BDR) group and patients with proliferative DR (PDR) group. Healthy individuals served as control group. Fasting plasma glucose (FPG), glycosylated haemoglobin (HbA1c), triacylglycerol (TG), total cholesterol (TC), urine creatinine (Cr), fasting blood glucose (FBG), blood urea nitrogen (BUN), low-density lipoprotein cholesterol (LDL-C), fasting insulin (FINS) and plasma miR-21 expression were measured. Quantitative real-time PCR (qRT-PCR) was applied to detect miR-21 expression. Pearson analysis was used to conduct correlation analysis and receiver operating characteristic (ROC) curve was used to analyse the diagnostic value of miR-21 in T2D with DR. Compared with the control group, FBG and HbA1c increased in the NDR group; compared with the control and NDR groups, disease course, HbA1c, FPG levels and homoeostasis model assessment of insulin resistance (HOMA-IR) were increased in the BDR and PDR groups; and compared with the BDR group, disease course, HbA1c and FPG levels were higher in the PDR group. miR-21 expression was higher in the BDR group than the control group, and higher in the PDR group than the BDR group. miR-21 expression was positively related with disease course, HbA1C, FPG and HOMA-IR, and had diagnostic value for T2D with DR and PDR. The plasma miR-21 expression was increased in the development of T2D with DR and can be used as an indicator for the severity of T2D with DR.
Keywords: Background diabetic retinopathy, Diabetic retinopathy, Linear regression, Plasma microRNA-21, Proliferative diabetic retinopathy, Type 2 diabetes
Introduction
More than 6% of the world’s population is affected by Type 2 diabetes (T2D) and the prevalence across worldwide is estimated to double by 2025 [1]. Diabetic retinopathy (DR) is one of the major complications in diabetic patients and considered as the major cause of new-onset blindness [2]. Hyperglycaemia promotes vascular damage in T2D patients and the presence of DR is associated with an over 2-fold higher risk of coronary events [3]. DR remains the most common cause of blindness among people aged 30–69 years in several countries [4]. The prevalence of DR ranges from 15.3 to 42.4% in different epidemiologic studies and both modifiable risk factors (blood glucose, pressure and lipids) and non-modifiable risk factors (duration, age and genetic predisposition) are responsible for the progression of DR [5]. Proliferative DR (PDR) is a serious diabetic microvascular complication and a leading cause of visual loss affecting the quality of life of diabetic patients, and neovascularization plays a pivotal role in the development of PDR [6].
miR-21 is an important member of miRNAs and located on chromosome 17q23-2 overlapping with the TMEM49 gene [7]. miR-21 played a critical role in the development of tumours including cell proliferation, migration and metastasis, angiogenesis and anti-apoptosis via a variety of molecular mechanisms [8,9]. miR-21 is involved in the development of the endocrine pancreas and in the regulation of insulin secretion, and may contribute to β-cell dysfunction in T2D [10]. Besides, miRNAs also implicated in various physiological and pathophysiological processes, including glucose homoeostasis, angiogenesis, inflammatory response modulation and the pathogenesis of diabetes and the related micro- and macrovascular complications [11]. miR-21 inhibition might be an effective treatment for diabetic nephropathy [12]. However, insufficient data demonstrated the role of miR-21 in T2D patients with DR.
In the present study, we aim to investigate the role of plasma miR-21 in the pathogenic process of T2D with DR, especially in diagnosing the severity of DR in T2D. Our study might provide a valuable reference for the clinical diagnosis of T2D patients with DR.
Materials and methods
Study subjects
A total of 189 patients diagnosed with T2D were selected from outpatient service and inpatient of endocrinology department during May 2013 and March 2015, including 94 cases of males and 95 cases of females with an age range from 20 to 80 years and a disease course of 0.2 to 24.5 years. The diagnosis was in line with the diagnostic criteria and classification of diabetes of World Health Organization (WHO) in 1999 [13]. All the patients were subjected to a detailed medical examination and fundus ophthalmoscope examination by using a Canon non-mydriasis retinal camera (Canon, Tokyo, Japan). According to the designated staging criteria in the fundi disease academic conference in 2002 [14], T2D patients were divided into three groups: 65 cases without DR (NDR) group including 34 males and 31 females with a mean age of 47.76 ± 8.05 years and a mean duration of 4.16 ± 1.31 years; 73 cases with non-proliferative/background DR (BDR/NPDR) group including 32 males and 41 females with a mean age of 48.83 ± 7.14 years and a mean duration of 9.13 ± 3.05 years; and 51 cases with PDR group including 28 males and 23 females with a mean age of 50.75 ± 10.18 years and a mean duration of 13.58 ± 3.82 years. Exclusion criteria were as follows: patients accompanied by diabetic ketoacidosis, diabetic hyperosmolar coma and other acute complications of diabetes; patients in severe stress such as recent cardiovascular events, trauma surgery etc.; patient suffering from acute or chronic infection; patients with liver disease; and patients combined with other endocrine and metabolic diseases.
A total of 115 non-T2D healthy individuals (60 males and 55 females) were enrolled as control group with matched age and gender proportions and body mass index (BMI) and a mean age of 48.53 ± 7.26 years. An oral glucose tolerance test confirmed that the healthy individuals had no diabetes and a healthy examination excluded the control cases from high blood pressure, heart, liver, kidney diseases and pituitary, thyroid and other endocrine and metabolic diseases. The Canon non-mydriasis retinal camera fundus ophthalmoscope examination revealed no intraocular diseases in the healthy controls. All the cases or their families signed an informed consent form and the experiment was approved by China-Japan Union Hospital of Jilin University and complied with the guidelines and principles of the Declaration of Helsinki [15].
Data collection
When the patients were admitted, their height and weight were measured and BMI was calculated as weight/height2 (kg/m2). The disease history, age, gender, disease course of diabetes, glycaemic control etc. were recorded.
Plasma separation and laboratory testing
All the selected subjects were fasted for 8–12 h. Approximately 6 ml of venous blood sample was extracted at 6:00 the next morning in an EDTA anticoagulant tube at room temperature. The venous blood sample was centrifuged at 3000 rev/min for 10 min. The upper supernatant was taken and sub-packaged in different Eppendorf (EP) tubes, and the sub-packaged plasma was frozen at −80°C for the standby use. Fasting plasma glucose (FPG) was detected by glucose oxidase; triacylglycerol (TG) and total cholesterol (TC) were measured by an enzymatic colorimetric test (GPO-PAP method); glycosylated haemoglobin (HbA1c), blood urea nitrogen (BUN), creatinine (Cr), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and fasting insulin (FINS) levels were detected by a Beckman automatic biochemical analyzer (Roche, Germany). Homoeostasis model assessment of insulin resistance (HOMA-IR) was consequently calculated. All steps were in strict accordance with the kit instructions.
Quantitative real-time PCR
The total plasma RNA of patients was extracted according to kit instructions (Qiagen Company, Valencia, CA). After extraction, the optical density (OD) 260 and OD280 values of each RNA sample were measured using a spectrophotometer (Thermo Fisher Scientific, San Jose, CA). The RNA purity was calculated as OD260/OD280 and RNA concentration was calculated. The purity of RNA was ensured between 1.8 and 2.0. The extracted RNA was preserved at −80°C for use. The cDNA was conducted according to the kit instructions (Qiagen Company, Valencia, CA). According to the gene sequences in the GenBank database and miRBase database, primer 5.0 software was used to design primers. The specific primers with stem–loop structures for miR-21 and U6 were as follows: miR-21: forward: 5'-AAAGGATCCGCCATAGAAACCCAGTTTC-3' and reverse: 5'-GTGCAGGGTCCGAGGT-3'; U6: forward: 5'-CTCGCTTCGGCAGCACA-3' and reverse: 5'-AACGCTTCACGAATTTGCGT-3'. The primers were designed and synthesized by Shanghai Sangon Company (Shanghai, China). PCR reaction system (20 μl): 10 μl of SYBR Premix Ex Taq II (2×), 0.4 μl of forward primer, 0.4 μl of reverse primer, 0.4 μl of ROX Reference Dye II, 2 μl of DNA template and 6.8 μl of dH2O. The reaction conditions were 45 cycles of 95°C for 5 s and 60°C for 30 s. U6 was used as an internal control. Melting curves were used to evaluate the reliability of PCR results. Ct value (amplification power curve inflection) was recorded: ΔCt = CtmiR-21 − CtU6, ΔΔCt = (CtmiR-21 − CtU6)experimental group − (CtmiR-21 − CtU6)control group and 2−ΔΔCt was used to calculate the relative expression of the target gene. The high expression was defined as the relative expression of miR-21 larger than the mean value and the low expression was defined as the relative expression of miR-21 smaller than the mean value.
Statistical analysis
SPSS 21.0 software (SPSS Inc., Chiago, IL) was used for statistical analysis and measurement data were expressed as mean ± standard deviation. Comparisons between two groups were conducted using t-test. Correlation analysis was conducted using Pearson correlation analysis. Multiple linear regression analysis (two-sided α = 0.05) was conducted to analyse the main factors influencing the expression of miR-21 with related indicators as independent variables. A receiver operating characteristic (ROC) curve was used to analyse the diagnostic value of miR-21 in T2D with DR. P<0.05 was considered statistically significant.
Results
Comparisons of general data and laboratory indicators of patients in each group
No significant difference was observed in the general data (except for disease course, fasting blood glucose (FBG) and HbA1c between the NDR group and the control group (all P>0.05). Besides, there were no significant difference in gender, age, BMI, FBG, Cr, BUN, FINS, TC, TG and LDL-C levels among the NDR, BDR, PDR and control groups (all P>0.05). Disease course, HbA1c, FPG levels and HOMA-IR levels were significantly increased in the NDR group compared with those in the control group (all P<0.05). Disease course, HbA1c and FPG were significantly increased in the PDR group compared with the BDR group (all P<0.05), showing a gradually increasing trend from the NDR group to the BDR group to the PDR group (Table 1).
Table 1.
Comparisons of general data and laboratory indicators of patients in each group (x ± S.D.)
| Group | Gender (male/ female) | Age (year) | Disease course (year) | BMI (kg/m2) | FBG (mmol/l) | HbA1c (%) | TC (mmol/l) | LDL-C (mmol/l) | FPG (mmol/l) |
TG (mmol/l) | BUN (mmol/l) | FINS (mIU/l) | Cr (μmol/l) | HOMA-IR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 60/55 | 48.53 ± 7.26 | / | 22.82 ± 2.33 | 4.74 ± 0.75 | 4.30 ± 0.42 | 4.13 ± 0.88 | 3.21 ± 0.55 | 5.02 ± 0.68 | 1.45 ± 0.44 | 4.89 ± 1.17 | 5.50 ± 0.88 | 58.82 ± 7.65 | 1.25 ± 0.37 |
| NDR | 34/31 | 47.76 ± 8.05 | 4.16 ± 1.21* | 22.67 ± 1.98 | 7.12 ± 0.68* | 6.25 ± 0.58* | 4.25 ± 1.02 | 3.26 ± 0.61 | 5.31 ± 0.73 | 1.48 ± 0.32 | 5.26 ± 1.34 | 5.66 ± 1.02 | 59.07 ± 7.88 | 1.37 ± 0.43 |
| BDR | 32/41 | 48.83 ± 7.13 | 9.13 ± 2.54*† | 23.06 ± 1.85 | 7.25 ± 0.84* | 7.02 ± 0.66*† | 4.32 ± 0.94 | 3.30 ± 0.63 | 6.17 ± 0.75*† | 1.51 ± 0.34 | 5.33 ± 1.38 | 5.71 ± 1.14 | 61.55 ± 8.39 | 1.60 ± 0.50*† |
| PDR | 28/23 | 50.75 ± 10.18 | 13.58 ± 3.82*†‡ | 23.13 ± 2.02 | 7.33 ± 1.05* | 8.16 ± 0.79*†‡ | 4.43 ± 1.08 | 3.38 ± 0.72 | 6.86 ± 0.96*†‡ | 1.55 ± 0.45 | 5.41 ± 1.71 | 5.78 ± 1.21 | 62.43 ± 10.06 | 1.81 ± 0.62*† |
*, compared with the control group, P<0.05; †, compared with the NDR group, P<0.05; ‡, compared with the BDR group, P<0.05.
Comparisons of plasma miR-21 expression of patients in each group
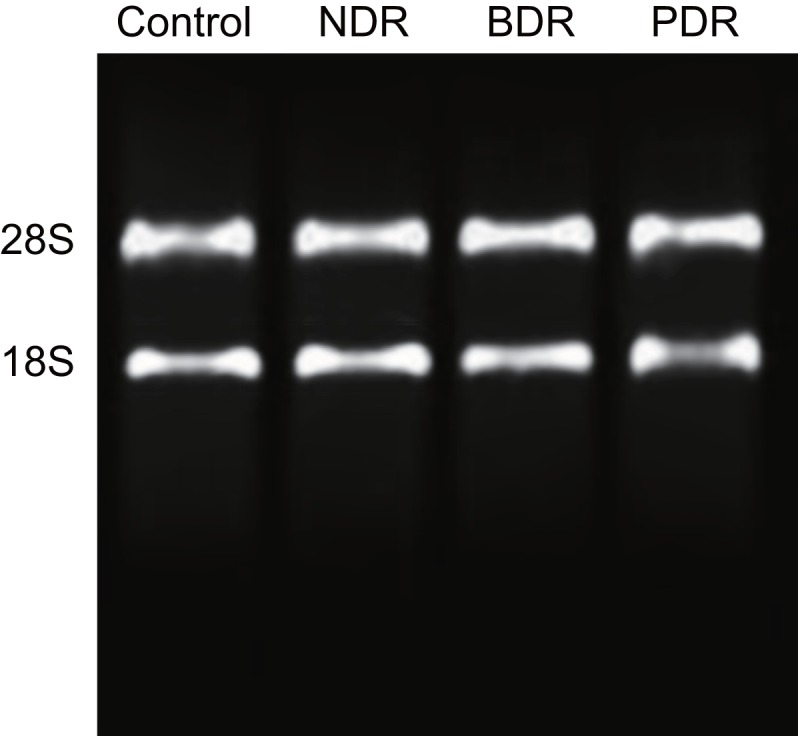
Agarose gel electrophoresis showed that total RNA was successfully extracted from the control, NDR, BDR and PDR groups (Figure 1) and the results of quantitative real-time PCR (qRT-PCR) reaction showed that the Ct values of the amplification curve were all less than 32, indicating that the experimental data were valid.
Figure 1. Expressions of plasma miR-21 of patients in each group.
The melting curves were all with single peak, indicating good reaction specificity without the interference factors such as non-specific amplification and primer dimers. 2−ΔΔCt relative quantification method was used and the relative expression of miR-21 in the control group was set as 1. Compared with the control group, the miR-21 expression in the NDR group (1.06 ± 0.01) showed no significant increase (P>0.05) and the miR-21 expression was significantly increased in the BDR and PDR groups (both P<0.05). Besides, the miR-21 expression in the PDR group was significantly higher than those in the BDR group (P<0.05) (Table 2).
Table 2.
Comparisons of expression of plasma miR-21 of patients in each group
| Variable | Control | NDR | BDR | PDR |
|---|---|---|---|---|
| CtU6 | 27.05 ± 0.21 | 27.01 ± 0.17 | 27.36 ± 0.15 | 27.38 ± 0.16 |
| CtmiR-21 | 29.27 ± 0.16 | 29.15 ± 0.18 | 28.82 ± 0.23*† | 28.63 ± 0.20*†‡ |
| 2−ΔΔCt | 1.00 | 1.06 ± 0.01 | 1.70 ± 0.09*† | 1.96 ± 0.05*†‡ |
*, compared with the control group, P<0.05; †, compared with the NDR group, P<0.05; ‡, compared with the BDR group, P < 0.05.
Correlation between plasma miR-21 expression and indicators of patients in each group
Pearson correlation analysis showed that the miR-21 expression in each group was positively correlated with disease course (r=0.794), HOMA-IR (r=0.326), HbA1c (r=0.693) and FPG (r=0.598) (all P<0.001). However, there was no significant correlation between miR-21 and TG, TC, Cr, BUN, HDL-C, FINS or LDL-C (all P>0.05) (Table 3).
Table 3.
Correlation between expression of plasma miR-21 and indicators of patients in each group
| Indicators | Disease course | TG | TC | LDL-C | HbA1C | Cr | BUN | FINS | FPG | HOMA-IR |
|---|---|---|---|---|---|---|---|---|---|---|
| r | 0.794 | 0.082 | 0.076 | 0.078 | 0.693 | 0.134 | 0.051 | 0.052 | 0.598 | 0.326 |
| P | <0.001 | 0.265 | 0.296 | 0.285 | <0.001 | 0.065 | 0.486 | 0.478 | <0.001 | <0.001 |
Cr, creatinine.
Multivariate linear regression analysis of miR-21-related factors
Multiple linear regression analysis showed that the disease course, HbA1c, FPG and HOMA-IR were all main factors influencing the plasma miR-21 expression (all P<0.05). The regression equation was as follows: Y = 1.424 + 0.02X1 + 0.74X2 + 0.21X3 − 0.413X4 (X1, disease course; X2, FPG; X3, HOMA-IR; X4, HbA1c; F=13.727, P<0.001). Multiple correlation coefficient R2=0.379, and the independent variables of the equation and related parameter values were shown in Table 4.
Table 4.
Multivariate linear regression analysis of miR-21-related factors
| Variable | Regression coefficient | S.E.M. | B | t | P |
|---|---|---|---|---|---|
| Constant | 1.424 | 0.413 | – | 3.450 | 0.001 |
| Disease course | 0.020 | 0.006 | 0.245 | 3.535 | 0.001 |
| FPG | 0.740 | 0.206 | 1.975 | 3.594 | <0.001 |
| HOMA-IR | 0.210 | 0.068 | 0.300 | 3.107 | 0.002 |
| HbA1c | −0.413 | 0.159 | −1.097 | −2.597 | 0.010 |
B, standardized regression coefficient.
Diagnostic value of miR-21 in DR
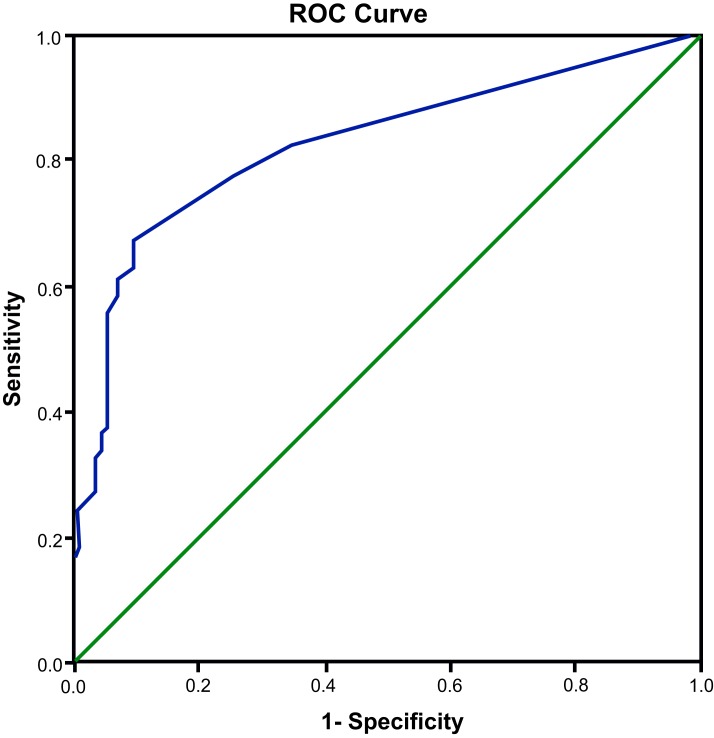
ROC curve analysis was applied to measure the diagnostic value of miR-21 in DR with the control and NDR groups (−) and the BDR and PDR groups (+) as the two categorical variables. The results showed an area under the curve (AUC) of 0.825 (95% confidence interval (95% CI) = 0.778−0.872; P<0.001), a sensitivity of 66.1% and a specificity of 90.4%, which indicated that plasma miR-21 expression had a relative predictive value for T2D with DR (Figure 2).
Figure 2. ROC curve of plasma miR-21 in diagnosing DR.
Diagnostic value of miR-21 in PDR
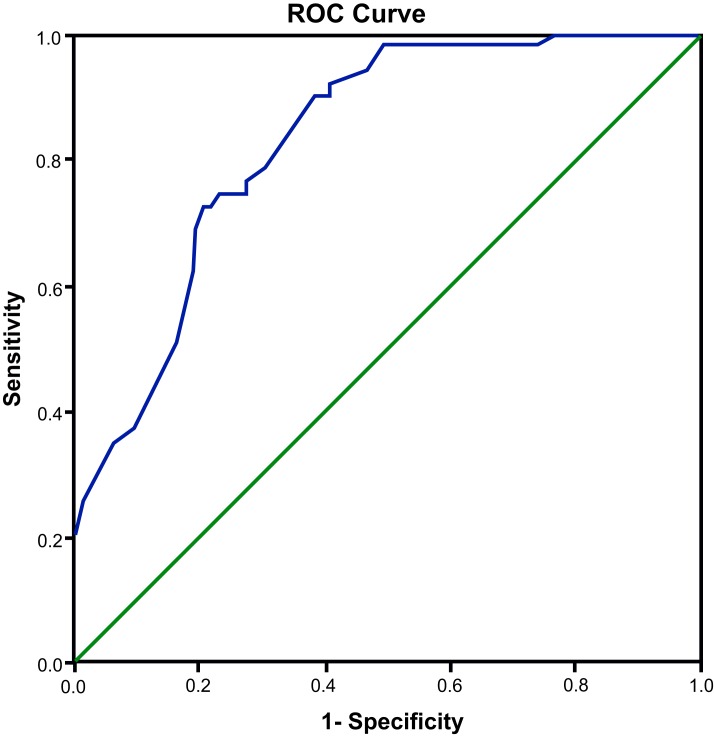
ROC curve analysis was applied to measure the diagnostic value of miR-21 in PDR. The results showed that using miR-21 to diagnose PDR yield an AUC of 0.830 (95% CI = 0.761−0.900; P<0.001), with a sensitivity of 72.5% and a specificity of 79.5%, indicating that plasma miR-21 expression had a relative predictive value for PDR (Figure 3).
Figure 3. ROC curve of plasma miR-21 in diagnosing PDR.
Discussion
In the present study, we investigated the role of plasma miR-21 in the pathogenic process of T2D with DR. One of our main results showed that compared with the control group, the miR-21 expression was relatively increased in the BDR and PDR groups. Besides, the miR-21 expression in the PDR group was significantly higher than that in the BDR group. The results indicated that miR-21 was associated with the pathogenic process of DR in T2D and the severity of DR. Aberrant miRNA expression profiles were associated with the DR development, and modulation of retinal miRNA expression may provide a potential treatment for DR [16]. The miR-126 was associated with sight-threatening DR, suggesting that the miR-126 would be a potential therapeutic target for inhibiting DR development [17]. MiR-23b-3p regulates high glucose-induced cellular metabolic memory in DR via a SIRT1-dependent signalling pathway [18]. The expressions of several miRNAs related to angiogenesis and fibrosis were expressed significantly higher in PDR [19].
As for the mechanism of miR-21 in the development of T2D with DR, we investigated the relationships between miR-21 and some clinical indicators. Our correlation analysis showed that miR-21 expression was positively correlated with disease course, HbA1c, FPG and HOMA-IR. Besides, the multiple linear regression analysis confirmed that the disease course, HbA1c, FPG and HOMA-IR were all main factors influencing the plasma miR-21 expression. HOMA-IR is a robust tool for the assessment of insulin resistance (IR) and is most widely used in a large population [20]. Moderate and large increases in HOMA-IR had a strong impact on the development of T2D with impaired insulin secretion [21]. HbA1c monitoring is often needed for patients with variable glycaemic control and receiving intensive insulin treatment, and is used as a diagnostic marker for T2D and T2D associated complications [22,23]. The HbA1c variability increases to the risk of DR independently of average metabolic control [24]. A threshold of an FPG of 7.0 mmol/l defines the presence of diabetes provided by the rationale for American Diabetes Association’s recommendation and DR has served as the basis for diagnostic criteria of T2D [25].
Another important result of our study showed that plasma miR-21 level had a relative predictive value for the existence and the severity of T2D with DR. DR was an ocular disease and governed by systemic and local ocular factors, including primarily chronic levels of blood glucose [26]. DR results in progressive visual deterioration from mild, moderate, severe to very severe NPDR with a risk of developing PDR [27]. NPDR is a first stage of DR, marked by gradual capillary dropout, and associated with a set of clinically observable changes in the microcirculation, which are focal disruptions of the capillary topology [28]. PDR is a serious T2D complication of diabetes and a leading cause of legal blindness and visual impairment [29]. Combination of miR-21, miR-181c and miR-1179 had a moderate ability to discriminate between PDR and NPDR and the accuracy rate of the three miRNA profiles was 82.6%, suggesting that serum miRNAs had the potential to be sensitive, cost-effective biomarkers for PDR detection from NPDR [30].
Taken together, our results demonstrated that plasma miR-21 expression was increased in the development of DR and can be used as an indicator for the severity of DR in T2D patients. The role of miR-21 in the development of T2D with DR was related with disease course, HbA1c, FPG and HOMA-IR. Our study provided valuable references for diagnosis of T2D with BDR and PDR. However, a clear description of the relationship between miR-21 and T2D with DR is needed more data to support the relative importance of each part. It is likely that deepening our understanding in this field, and hopefully, more experiments to prove evidence for the correlation between miRNAs and T2D with DR are needed in the future.
Acknowledgments
We would like to acknowledge the helpful comments on the present study received from our reviewers.
Abbreviations
- AUC
area under the curve
- BDR
T2D patients with background DR group
- BMI
body mass index
- BUN
blood urea nitrogen
- CI
confidence interval
- Cr
creatinine
- DR
diabetic retinopathy
- FBG
fasting blood glucose
- FINS
fasting insulin
- FPG
fasting plasma glucose
- GPO
glycerophosphate-oxidase
- HbA1c
glycosylated haemoglobin
- HDL-C
high-density lipoprotein cholesterol
- HOMA-IR
homoeostasis model assessment of insulin resistance
- IR
insulin resistance
- LDL-C
low-density lipoprotein cholesterol
- NDR
T2D patients without DR
- NPDR
T2D patients with non-proliferative DR
- OD
optical density
- PDR
T2D patients with proliferative DR
- qRT-PCR
quantitative real-time PCR
- SYBR
synergy brands
- TC
total cholesterol
- T2D
Type 2 diabetes
- TG
triacylglycerol
- TMEM
transmembrane protein 4
Funding
The authors declare that there are no sources of funding to be acknowledged.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Author contribution
Q.J. designed the study, collated the data, designed and developed the database, carried out data analyses and produced the initial draft of the manuscript. X.-M.L. carried out data analyses and produced the initial draft of the manuscript. Y.Y. and L.W. contributed to drafting the manuscript. All authors have read and approved the final version of the manuscript.
References
- 1.Tsai F.J., Yang C.F., Chen C.C., Chuang L.M., Lu C.H., Chang C.T. et al. (2010) A genome-wide association study identifies susceptibility variants for type 2 diabetes in Han Chinese. PLos Genet. 6, e1000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porojan M.D., Catana A., Popp R.A., Dumitrascu D.L. and Bala C. (2015) The role of NOS2A -954G/C and vascular endothelial growth factor +936C/T polymorphisms in type 2 diabetes mellitus and diabetic nonproliferative retinopathy risk management. Ther. Clin. Risk Manag. 11, 1743–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morita K., Saruwatari J., Miyagawa H., Uchiyashiki Y., Oniki K., Sakata M. et al. (2013) Association between aldehyde dehydrogenase 2 polymorphisms and the incidence of diabetic retinopathy among Japanese subjects with type 2 diabetes mellitus. Cardiovasc. Diabetol. 12, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin P., Peng J., Zou H., Wang W., Fu J., Shen B. et al. (2014) The 5-year onset and regression of diabetic retinopathy in Chinese type 2 diabetes patients. PLoS ONE 9, e113359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao C., Wang W., Xu D., Li H., Li M. and Wang F. (2014) Insulin and risk of diabetic retinopathy in patients with type 2 diabetes mellitus: data from a meta-analysis of seven cohort studies. Diagn. Pathol. 9, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeon H.J., Choi H.J., Park B.H., Lee Y.H. and Oh T. (2013) Association of monocyte chemoattractant protein-1 (MCP-1) 2518A/G polymorphism with proliferative diabetic retinopathy in Korean type 2 diabetes. Yonsei Med. J. 54, 621–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu H., Ng R., Chen X., Steer C.J. and Song G. (2015) MicroRNA-21 is a potential link between non-alcoholic fatty liver disease and hepatocellular carcinoma via modulation of the HBP1-p53-Srebp1c pathway. Gut 65, 1850–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao Y.J., Li Y.J., Zheng W., Zhao J.J., Guo M.M., Zhou Y. et al. (2015) Antisense oligonucleotides against microRNA-21 reduced the proliferation and migration of human colon carcinoma cells. Cancer Cell Int. 15, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding Y., Wu M., Liu J., Wu C., Huang R., Zhu R. et al. (2014) Seed-targeting anti-miR-21 inhibiting malignant progression of retinoblastoma and analysis of their phosphorylation signaling pathways. Exp. Eye Res. 122, 1–8 [DOI] [PubMed] [Google Scholar]
- 10.Sekar D., Venugopal B., Sekar P. and Ramalingam K. (2016) Role of microRNA 21 in diabetes and associated/related diseases. Gene 582, 14–18 [DOI] [PubMed] [Google Scholar]
- 11.Mastropasqua R., Toto L., Cipollone F., Santovito D., Carpineto P. and Mastropasqua L. (2014) Role of microRNAs in the modulation of diabetic retinopathy. Prog. Retin. Eye Res. 43, 92–107 [DOI] [PubMed] [Google Scholar]
- 12.Zhong X., Chung A.C., Chen H.Y., Dong Y., Meng X.M., Li R. et al. (2013) miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia 56, 663–674 [DOI] [PubMed] [Google Scholar]
- 13.Bonfrate L., Procino G., Wang D.Q., Svelto M. and Portincasa P. (2015) A novel therapeutic effect of statins on nephrogenic diabetes insipidus. J. Cell. Mol. Med. 19, 265–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilkinson C.P., Ferris F.L. III, Klein R.E., Lee P.P., Agardh C.D., Davis M. et al. (2003) Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 110, 1677–1682 [DOI] [PubMed] [Google Scholar]
- 15.Mastroleo I. (2016) Post-trial obligations in the Declaration of Helsinki 2013: classification, reconstruction and interpretation. Dev. World Bioeth. 16, 80–90 [DOI] [PubMed] [Google Scholar]
- 16.Wu J.H., Gao Y., Ren A.J., Zhao S.H., Zhong M., Peng Y.J. et al. (2012) Altered microRNA expression profiles in retinas with diabetic retinopathy. Ophthalmic Res. 47, 195–201 [DOI] [PubMed] [Google Scholar]
- 17.McAuley A.K., Dirani M., Wang J.J., Connell P.P., Lamoureux E.L. and Hewitt A.W. (2015) A genetic variant regulating miR-126 is associated with sight threatening diabetic retinopathy. Diab. Vasc. Dis. Res. 12, 133–138 [DOI] [PubMed] [Google Scholar]
- 18.Zhao S., Li T., Li J., Lu Q., Han C., Wang N. et al. (2016) miR-23b-3p induces the cellular metabolic memory of high glucose in diabetic retinopathy through a SIRT1-dependent signalling pathway. Diabetologia 59, 644–654 [DOI] [PubMed] [Google Scholar]
- 19.Hirota K., Keino H., Inoue M., Ishida H. and Hirakata A. (2015) Comparisons of microRNA expression profiles in vitreous humor between eyes with macular hole and eyes with proliferative diabetic retinopathy. Graefes. Arch. Clin. Exp. Ophthalmol. 253, 335–342 [DOI] [PubMed] [Google Scholar]
- 20.Tang Q., Li X., Song P. and Xu L. (2015) Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: Developments in research and prospects for the future. Drug Discov. Ther. 9, 380–385 [DOI] [PubMed] [Google Scholar]
- 21.Morimoto A., Tatsumi Y., Soyano F., Miyamatsu N., Sonoda N., Godai K. et al. (2014) Increase in homeostasis model assessment of insulin resistance (HOMA-IR) had a strong impact on the development of type 2 diabetes in Japanese individuals with impaired insulin secretion: the Saku study. PLoS ONE 9, e105827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCoy R.G., Van Houten H.K., Ross J.S., Montori V.M. and Shah N.D. (2015) HbA1c overtesting and overtreatment among US adults with controlled type 2 diabetes, 2001-13: observational population based study. BMJ 351, h6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen P., Ong R.T., Tay W.T., Sim X., Ali M., Xu H. et al. (2013) A study assessing the association of glycated hemoglobin A1C (HbA1C) associated variants with HbA1C, chronic kidney disease and diabetic retinopathy in populations of Asian ancestry. PLoS ONE 8, e79767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermann J.M., Hammes H.P., Rami-Merhar B., Rosenbauer J., Schutt M., Siegel E. et al. (2014) HbA1c variability as an independent risk factor for diabetic retinopathy in type 1 diabetes: a German/Austrian multicenter analysis on 35,891 patients. PLoS ONE 9, e91137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng Y.J., Gregg E.W., Geiss L.S., Imperatore G., Williams D.E., Zhang X. et al. (2009) Association of A1C and fasting plasma glucose levels with diabetic retinopathy prevalence in the U.S. population: implications for diabetes diagnostic thresholds. Diabetes Care 32, 2027–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank R.N. (2015) Diabetic retinopathy and systemic factors. Middle East Afr. J. Ophthalmol. 22, 151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Carrizalez A.D., Castellanos-Gonzalez J.A., Martinez-Romero E.C., Miller-Arrevillaga G., Villa-Hernandez D., Hernandez-Godinez P.P. et al. (2014) Oxidants, antioxidants and mitochondrial function in non-proliferative diabetic retinopathy. J. Diabetes 6, 167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tam J., Dhamdhere K.P., Tiruveedhula P., Lujan B.J., Johnson R.N., Bearse M.A. Jr et al. (2012) Subclinical capillary changes in non-proliferative diabetic retinopathy. Optom. Vis. Sci. 89, E692–E703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nittala M.G., Keane P.A., Zhang K. and Sadda S.R. (2014) Risk factors for proliferative diabetic retinopathy in a Latino American population. Retina 34, 1594–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qing S., Yuan S., Yun C., Hui H., Mao P., Wen F. et al. (2014) Serum miRNA biomarkers serve as a fingerprint for proliferative diabetic retinopathy. Cell. Physiol. Biochem. 34, 1733–1740 [DOI] [PubMed] [Google Scholar]