Abstract
Microsporidia are obligate intracellular opportunistic protists that infect a wide variety of animals, including humans, via environmentally resistant spores. Infection requires that spores be in close proximity to host cells so that the hollow polar tube can pierce the cell membrane and inject the spore contents into the cell cytoplasm. Like other eukaryotic microbes, microsporidia may use specific mechanisms for adherence in order to achieve target cell proximity and increase the likelihood of successful infection. Our data show that Encephalitozoon intestinalis exploits sulfated glycans such as the cell surface glycosaminoglycans (GAGs) in selection of and attachment to host cells. When exogenous sulfated glycans are used as inhibitors in spore adherence assays, E. intestinalis spore adherence is reduced by as much as 88%. However, there is no inhibition when nonsulfated glycans are used, suggesting that E. intestinalis spores utilize sulfated host cell glycans in adherence. These studies were confirmed by exposure of host cells to xylopyranoside, which limits host cell surface GAGs, and sodium chlorate, which decreases surface sulfation. Spore adherence studies with CHO mutant cell lines that are deficient in either surface GAGs or surface heparan sulfate also confirmed the necessity of sulfated glycans. Furthermore, when spore adherence is inhibited, host cell infection is reduced, indicating a direct association between spore adherence and infectivity. These data show that E. intestinalis specifically adheres to target cells by way of sulfated host cell surface GAGs and that this mechanism serves to enhance infectivity.
Microsporidia are obligate intracellular opportunistic protists that infect vertebrates and invertebrates alike. Although microsporidia were identified as agents of disease in animals more than 150 years ago, it was during the AIDS epidemic that microsporidia were implicated as a cause of severe diarrhea and systemic infections in some human immunodeficiency virus-infected individuals. However, microsporidiosis is not limited to the immunosuppressed, as there are numerous reports of immunocompetent persons becoming infected (29, 39, 40). Most human microsporidiosis is due to infection with Enterocytozoon bieneusi, while the second most common is Encephalitozoon intestinalis (27). An efficient long-term in vitro culturing method for Enterocytozoon bieneusi has not been established, and therefore, E. intestinalis is commonly used to study microsporidiosis (41).
Microsporidia are transmitted via an environmentally stable spore and infect host cells by a unique mechanism. It is thought that when an ingested spore comes in close association with a host cell in the gastrointestinal tract, it encounters the optimal conditions for spore activation, and this encounter triggers a cascade of events leading to the extrusion of a hollow polar filament that pierces the host cell plasma membrane (8). The infectious sporoplasm is then injected into the host cell cytoplasm, where the parasite subsequently propagates. When developing spores mature, the host cell ruptures, releasing them into the lumen of the gut for excretion back into the environment, infection of nearby cells, or, as in the case of E. intestinalis, dissemination to other tissues and organs throughout the body (7). Microsporidial spores may also be phagocytosed by both professional and nonprofessional phagocytes via an actin-based mechanism (11, 43). Nevertheless, whether spores are internalized or extracellular, host cells are not known to become infected without spore polar filament extrusion.
Because of the obligate intracellular nature of microsporidia, most are routinely cultured and propagated in host cells in vitro (41). During in vitro cultivation, microsporidial spores adhere to host cell surfaces, and adherent spores cannot be removed by routine washing. Although this adherence seems to occur spontaneously, the mechanism of adherence has not been described. Therefore, this study examines spore adherence to host cells to determine if a specific mechanism is involved.
In studies of other eukaryotic microbes, glycosaminoglycans (GAGs), proteoglycans containing linear disaccharide repeating units that are found on almost all cell types, have been shown to play important roles in selection of and attachment to host cells. Plasmodium falciparum sporozoites, for example, bind heparin and heparan sulfate, allowing them to target hepatocytes and the placenta (17, 42). In addition, the broad host and tissue recognition of Toxoplasma gondii is attributed to the ability of the parasite to bind a variety of host GAGs (9). Therefore, it is possible that host cell GAGs may play an important role in the adherence of E. intestinalis spores.
MATERIALS AND METHODS
Microsporidia and host cell cultivation.
The adherent host cell lines used for cultivation of microsporidial spores and as substrates for spore adherence assays included African green monkey kidney cells (Vero; ATCC CCL-81), rabbit kidney cells (RK-13; ATCC CCL-37), human epithelial colorectal cells (Caco-2; ATCC HTB-37), Chinese hamster ovary cells (CHO; ATCC CCL-61), mutant CHO pgsA-745 cells (ATCC CRL-2242), and mutant CHO pgsD-677 cells (ATCC CRL-2244). All cell lines were grown as previously described (22) in Dulbecco's modified Eagle's medium (BioWhittaker, Walkersville, Md.) supplemented with l-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), amphotericin B (0.25 μg/ml), and 10% fetal bovine serum (BioWhittaker) in 5% CO2 at 37°C. For maintenance of Vero and RK cells, 10% fetal bovine serum was replaced with 2% fetal bovine serum in the medium.
For microsporidial spore propagation, subconfluent Vero monolayers in 75-cm2 flasks were infected with E. intestinalis spores as previously described (21). Briefly, spores were incubated with the adherent host cells for 12 to 15 days, with medium replacement every 2 or 3 days. Spores were then harvested from the flasks daily until most host cells were dead. The spores were purified from host cell debris by washing once with 0.25% sodium dodecyl sulfate, followed by several washes with sterile water. Spore stocks that were free of host cell debris were counted and stored in H2O at 4°C.
Spore adherence assays.
Host cells were seeded on circular glass coverslips (18 mm) at 5 × 105 in 12-well plates with normal growth medium and allowed to grow for a minimum of 16 h. Then 1 million or 10 million E. intestinalis spores were added to each well with 1 ml of fresh medium supplemented with 1 mM MnCl2 for 4 h either on ice or at 37°C. Incubation at 37°C resulted in an approximately 30 to 50% increase in bound spores compared to incubation on ice. Assays were performed on ice in an effort to minimize host cell surface protein turnover. The addition of MnCl2 augments spore adherence to host cells as much as eightfold (unpublished data).
The spore adherence time course experiment was conducted in a similar manner except that the incubation period ranged from 2 to 48 h at 37°C. The spore inocula represent approximate multiplicities of infection of 2 and 20. The coverslips were then harvested and washed with PBS to remove any nonadherent spores. Dried coverslips were fixed with acetone-methanol, and an immunofluorescent assay was performed to quantitate the number of bound spores. Mouse monoclonal antibody 7G7 (31), which recognizes SWP2 of E. intestinalis (21), was used as the primary antibody (at 1:1,000), and a fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin was used as the secondary antibody (1:500) (Rockland Immunochemicals).
To identify intracellular versus extracellular spores, host cells with bound spores were fixed with 0.25% paraformaldehyde and reacted with mouse monoclonal antibody 7G7 (1:1,000) prior to permeabilization with either 0.1% NP-40 or 0.05% saponin in phosphate-buffered saline. The intracellular spores were then reacted with a rabbit polyclonal antiserum at a 1:500 dilution. The secondary antibody was a combination of a fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin antibody and a tetramethylrhodamine isothiocyanate-conjugated anti-rabbit immunoglobulin antibody (both at a 1:500 dilution). Fluorescent microscopy (Zeiss; Axiovert S100) was employed to count the number of bound spores per field of magnification at 630×. The results were expressed as the mean ± standard deviation of 20 fields of magnification counted in a blinded fashion. In some experiments, the results are shown as the percentage of adherent spores relative to control samples.
The glycans tested as potential inhibitors of spore adherence included heparin (Sigma), chondroitin sulfate A (Sigma), chondroitin sulfate B (Sigma), hyaluronic acid (Sigma), type II porcine stomach mucin (Sigma), dextran sulfate (Sigma), and dextran (Sigma). Each glycan was dissolved in maintenance medium supplemented with 1 mM MnCl2 at the maximal indicated concentration and serially diluted. Diluted glycans were added with the spores to Vero host cell grown on glass coverslips as described above. Spore adherence was quantitated by immunofluorescence assay, and the percentage of adherent spores was calculated. The 50% effective concentration values (EC50), defined as the concentration of inhibitor that produced a 50% decrease in spore adherence from 100% to an asymptotic value, were calculated by generating standard curves from the spore inhibition data. Statistical analysis was performed with the assistance of the SAS/PC statistical software (SAS Institute, Inc.) and Student's t test.
To confirm the role of host cell surface GAGs in spore adherence, Vero host cells were grown on glass coverslip in the presence of 1 or 10 mM p-nitrophenyl-β-d-xylopyranoside for 24 h prior to the addition of 10 million spores per well. After spore incubation at 37°C for 4 h, the coverslips were washed to remove unbound spores, and the number of bound spores was quantified as described above. In control samples, p-nitrophenyl-α-d-galactopyranoside, which does not affect GAG assembly, was used.
The adherence assay was also performed with the CHO mutant cell lines pgsA-745 (ATCC CRL-2242) and pgsD-677 (ATCC CRL-2244) and the nonmutated CHO-K1 parent cell line (ATCC CCL-61). These mutant cell lines are deficient in or have reduced levels of surface GAGs (13, 14). After seeding the cells on glass coverslips to confluence, 10 million spores were added to the mutant and parent cell lines in 1 ml of medium for 4 h on ice. The coverslips were removed, washed, and fixed, and an immunofluorescence assay was performed as described above. Adherence assays with subconfluent monolayers did not show the same degree of deficient adherence as controls.
The role of host cell surface GAG sulfation was confirmed by treating Vero host cells with 10, 20, 40, or 60 mM NaClO3 for 24 or 48 h prior to the addition of 10 million E. intestinalis spores. Similar concentrations of NaCl2 were used for the control. The standard adherence assay was performed, and the percentage of adherent spores were calculated relative to untreated control samples.
Rate of infection in the presence of inhibitor.
With Vero epithelial cells grown on glass coverslips, the spore adherence assay was performed in the presence of 10 μg of chondroitin sulfate A per ml for 4 h. The inocula ranged from 1 to 100 million spores per well of a 12-well culture plate. Control coverslips were removed after 4 h and processed by immunofluorescence assay to measure the percentage of inhibition of spore adherence in the presence and absence of chondroitin sulfate A. The remaining coverslips were washed with PBS to remove unbound spores and placed back in culture for about 30 h to allow infection and intracellular spore propagation to occur. The developing intracellular spore clusters were visualized by the addition of propidium iodide, which stains both the host and microsporidial nuclei. An infected cell was identified by visualization of spore clusters within the host cell cytoplasm. The percentage of infected cells was determined by dividing the number of infected cells by the total number of host cells per field of 630× magnification. The CHO mutant cell lines could not be used for this experiment because they tended to overgrow during the extended incubation period, making infected-cell quantification difficult. These experiments were repeated three times with similar results.
Transmission electron microscopy.
Caco-2 intestinal epithelial host cells were seeded at 105 on 8-μm porous Transwell inserts (BD Biosciences) in growth medium and maintained for at least 7 days prior to experimentation; 50 million E. intestinalis spores were added to the upper chamber and allowed to adhere to the host cell surfaces for 8 h. The unbound spores were removed by washing, and the host cells were prepared for transmission electron microscopy as previously described (20). Briefly, the host cell-spore sample was fixed in 2% glutaraldehyde-0.5% paraformaldehyde in 0.1 M cacodylate buffer for 2 h at 37°C. The host cells were then scraped from the inserts, pelleted, and enrobed in 3% SeaKem agarose. After washing the host cell pellet in 0.2 M cacodylate buffer containing 0.3 M sucrose, it was postfixed for 1 h at 25°C in 1% osmium tetroxide in 0.2 M cacodylate buffer. The sample was sequentially dehydrated in ethanol, infiltrated with Epon-Araldite 812 resin, and embedded in fresh Epon. Ultrathin sections were cut on a Reichert Ultracut (Leica) microtome and subsequently mounted and viewed with a Tecnai-10 electron microscope.
RESULTS
Microsporidial spore adherence to host cells.
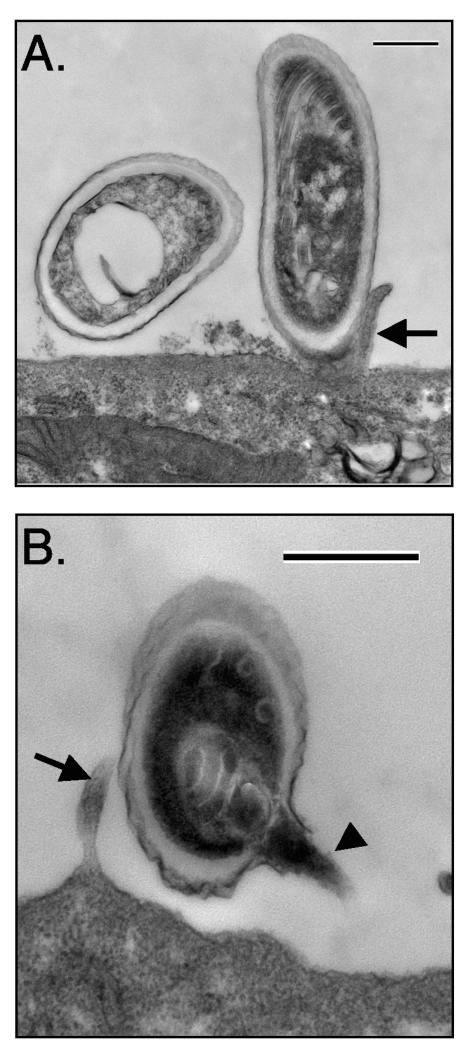
E. intestinalis can be cultured easily in vitro with a variety of adherent cell lines (41). Once in culture, the spores adhere to the surface of host cells, as observed by light microscopy. Adherent spores cannot be removed by routine washing. To examine spore adherence more closely, transmission electron microscopy was performed with E. intestinalis spores and the human intestinal epithelial cell line Caco-2 (Fig. 1). Many adherent spores were observed in direct physical contact with both the host cell surface and microvilli (Fig. 1A). Although it is sometimes difficult to determine the orientation of adherent spores because of the ultrathin sections used in transmission electron microscopy, some spores were clearly attached longitudinally to the cell surface. Other spores were oriented so that the apical end of the spore, where the anchoring disk is located, was in close proximity to the host cell (Fig. 1B). Orientation in this manner would allow extrusion of the polar filament directly into the host cell. However, many retained the polar filament in the characteristic coil within the spore despite attachment in this orientation. The presence of attached spores with unfired polar filaments may indicate that spore adherence to host cell surfaces is a precursor to spore activation and polar filament extrusion.
FIG. 1.
Transmission electron microscopy of E. intestinalis spores attached to Caco-2 cell surfaces. E. intestinalis spores were allowed to adhere to the apical surface of confluent Caco-2 cells grown on porous Transwell inserts. After a 4-h incubation of host cells with 107 spores, the unbound spores were removed by washing, and the Transwell inserts were fixed and processed for transmission electron microscopy as described in Materials and Methods. The attached spores appear to be in direct contact with the cell surface or microvilli and have either intact, unextruded polar filaments (A) or extruded polar tubes (B). Bars, 500 nm. Arrows show host cell microvilli, and the arrowhead points to the E. intestinalis polar tube.
Rate of E. intestinalis spore adherence to host cells.
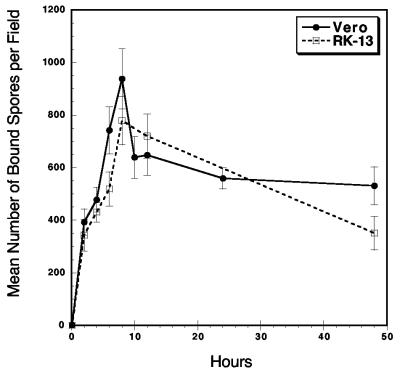
An adherence assay was developed to quantitate spore adherence to host cells. The rate of E. intestinalis spore adherence to the surface of the host cell was measured. The peak in spore adherence occurred about 8 h postinoculation and was followed by a slight decrease and plateau in adherence over the next 40 h (Fig. 2). Although it is not clear why peak adherence takes 8 h, it is possible that this time is required for the putative spore ligand to recognize the host cell receptor. At the peak of adherence to either Vero or RK cells, approximately 800 spores were attached per field of magnification. This is approximately 10 spores per cell, indicating that about 1/10th of the inoculum was attached. Whether this represents a saturation of spore adherence to host cell surfaces or the limit of adherence-capable spores in the inoculum has yet to be determined.
FIG. 2.
Rate of E. intestinalis spore adherence to Vero and RK host cells. An adherence assay was performed with 107 spores and either Vero or RK cells grown on glass coverslips in 12-well plates. The coverslips were removed at the indicated times and washed. The bound spores were quantitated by immunofluorescence as described in Materials and Methods. Twenty random magnification fields were counted for each data point, and the mean number of attached spores was determined at each indicated time point. These data represent one of three experiments that were performed with similar results.
It is possible that the plateau seen in Fig. 2 may indicate attachment and release of spores from the host cell surface. Alternatively, the decrease in spore adherence beginning after 8 h of incubation may indicate phagocytosis and subsequent degradation by the host cell. Differential immunofluorescent staining assays were performed to discriminate intracellular and extracellular spores and to determine if phagocytosis was occurring. With the nonprofessional phagocytic RK and Vero cells used in these experiments, less than one internalized spore per field of magnification was found to be intracellular (data not shown).
Inhibition of spore adherence by exogenous sulfated glycans.
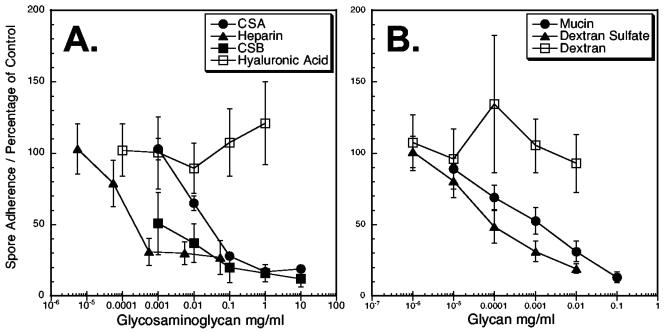
GAGs are glycans found on the surface of almost all vertebrate cell lineages (4, 44). The involvement of GAGs in adherence of other pathogens to host cells, leading to invasion and infection, has been well documented (3). To determine if GAGs and other glycans play a role in microsporidial spore adherence to host cells, various exogenous glycans were used as inhibitors of adherence. Heparin, chondroitin sulfate A, chondroitin sulfate B, mucin, and dextran sulfate inhibited E. intestinalis adherence to host cells, implicating the involvement of host cell surface glycans in E. intestinalis spore adherence to host cell surfaces (Fig. 3A and B). Inhibition was dose dependent, resulting in a 73 to 88% reduction in spore adherence when higher concentrations of the exogenous proteoglycans were used compared to control samples without exogenous proteoglycans.
FIG. 3.
E. intestinalis spore adherence is inhibited by exogenous sulfated glycans. An adherence assay was performed as described in Materials and Methods in the presence or absence of various exogenous glycans in 10-fold serial dilutions. The glycans tested as potential inhibitors included the GAGs heparin, chondroitin sulfate A (CSA), chondroitin sulfate B (CSB), and hyaluronic acid (A). The non-GAG glycans tested included type II porcine stomach mucin, dextran sulfate, and dextran (B). After 4 h of incubation of 107 spores with and without the glycan inhibitor, the host cells grown on coverslips were removed and washed to remove unbound spores. The mean number of attached spores was determined for each concentration of potential inhibitor. The percentage of spores inhibited from adherence is shown relative to that of control samples without exogenous glycans. These data represent one of three experiments that were performed with similar results.
To determine if sulfated glycans played a role in adherence, dextran sulfate and dextran were tested in the adherence assay (Fig. 3B). Dextran sulfate inhibited spore adherence, and the nonsulfated dextran did not. Thus, these results suggest that spore adherence to host cells involves a specific sulfated-glycan-dependent mechanism. In addition, the nonsulfated but negatively charged hyaluronic acid (12, 33) also failed to inhibit adherence, suggesting that the negative charge of the sulfate groups was likely not a contributing factor (Fig. 3A).
To compare the effective concentrations of glycan inhibitors, standard curves were generated for each glycan and the EC50 values were determined (Table 1). The EC50 is defined as the inhibitor concentration that results in a 50% reduction from 100% to the asymptotic value. While heparin had the lowest EC50 value, it also had the lowest maximal inhibition level (73%; Table 1) and required the highest concentration to achieve this inhibition (28.4 μg/ml). On the other hand, only 11.2 μg of mucin per ml was required to reach a maximal inhibition of 88%. The other sulfated glycans fell between these two.
TABLE 1.
EC50 and maximal inhibition values for exogenous glycan inhibitors of spore adherence
| Glycana | EC50 (μg/ml) | 95% Confidence intervals (μg/ml)
|
Maximal inhibition (% of control) | Mean estimated peak reductionb (μg/ml) ± SE | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Heparin | 0.097 | 0.059 | 0.157 | 73 | 28.4 ± 1.38 |
| CSA | 14.08 | 6.44 | 30.78 | 83 | 18.0 ± 2.72 |
| CSB | 0.641 | 0.193 | 2.13 | 88 | 11.2 ± 2.99 |
| Mucin | 0.119 | 0.003 | 4.87 | 87 | 1.5 ± 3.33 |
| Dextran sulfate | 53.67 | 19.45 | 414.11 | 81 | 15.3 ± 4.46 |
| Dextran | No response curve | ||||
| Hyaluronic acid | No response curve | ||||
CSA, chondroitin sulfate A; CSB, chondroitin sulfate B.
Peak reduction is the lowest concentration of inhibitor calculated from standard curves to achieve maximal inhibition.
Confirmation of sulfated glycosaminoglycan-mediated spore adherence.
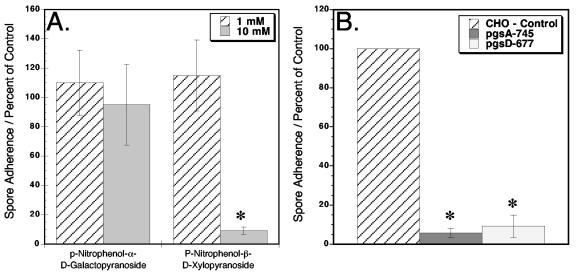
To confirm that E. intestinalis spore adherence involves surface GAGs, host cells were grown in the presence of p-nitrophenyl-β-d-xylopyranoside for 24 h prior to performing the adherence assay (Fig. 4A). p-Nitrophenyl-β-d-xylopyranoside acts as a soluble receptor for GAG polymerization and will compete with endogenous proteoglycan core assemblies, resulting in an absent or diminished amount of surface proteoglycans (32). As a control, host cells were treated with p-nitrophenyl-α-d-galactopyranoside, which does not affect GAG assembly (32), and the host cells have a full complement of surface GAGs. Following host cell treatment with xylopyranoside, E. intestinalis spore adherence was reduced by 90% compared to untreated host cells (Fig. 4A), confirming the involvement of host cell surface GAGs in spore adherence. The cells treated with galactopyranoside showed no significant difference from untreated host cells.
FIG. 4.
E. intestinalis spore adherence to host cells with reduced surface proteoglycans. The standard adherence assay was performed with Vero host cells that were treated with normal medium supplemented with either 1 or 10 mM p-nitrophenyl-β-d-xylopyranoside or control p-nitrophenyl-α-d-galactopyranoside for 24 h prior to the assay (A). This experiment was repeated three times with similar results. The spore adherence assay was also performed with CHO pgsA-745 and pgsD-677 mutant cell lines in addition to the control nonmutated CHO parent cell line (B). This experiment was repeated four times with similar results. The data are shown as the percentage of spore adherence relative to that of control samples without xylopyranoside or galactopyranoside (A) or control samples of nonmutated parent CHO cells (B). Twenty random fields were counted for each data point. The asterisks indicate significant differences (P < 0.0001).
The spore adherence assay was also performed with confluent monolayers of nonmutant parent CHO cells and mutant CHO cell lines that lack specific GAG assemblies (13, 14). Spore adherence to pgsA-745 mutant cells, which are completely surface GAG deficient, was reduced 94% in comparison to the control (Fig. 4B). Interestingly, the mutant cell line pgsD-677, which lacks surface heparan sulfate but has threefold higher levels of chondroitin sulfate, also showed a 91% reduction in adherence. These data may indicate that E. intestinalis preferentially binds host cell surface heparan sulfate and confirm that E. intestinalis spore adherence is dependent on host cell surface GAGs.
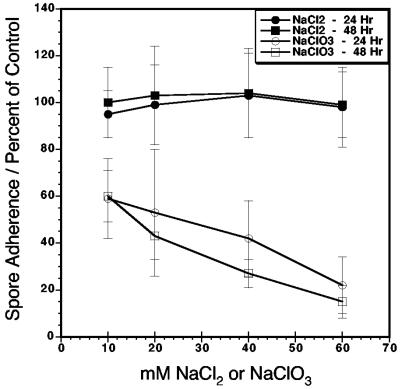
In additional experiments, host cells were treated with sodium chlorate, which significantly reduces sulfation of surface chondroitin and heparan without significantly altering host cell growth (25) (Fig. 5). When host cells are treated with 10 to 60 mM sodium chlorate for 24 or 48 h prior to the adherence assay, E. intestinalis spore adherence is reduced approximately 40 and 80%, respectively. Host cells treated with equivalent concentrations of sodium chloride showed no inhibition of spore adherence compared to untreated host cells. These data confirm that microsporidial spore adherence to host cells is dependent on sulfated surface GAGs.
FIG. 5.
E. intestinalis spore adherence to host cells with reduced sulfated surface proteoglycans by sodium chlorate treatment. Vero host cells grown on glass coverslips in 12-well plates were treated with 10, 20, 40, or 60 mM sodium chlorate or control sodium chloride for 24 or 48 h prior to the standard spore adherence assay. The percentage of attached spores relative to spore adherence on nontreated host cells is shown. The values for all sodium chlorate-treated samples are significantly different from those for either sodium chloride-treated or nontreated controls (P < 0.0001). The data presented are from one experiment of three that were performed with similar results.
Inhibition of E. intestinalis spore adherence results in decreased infection.
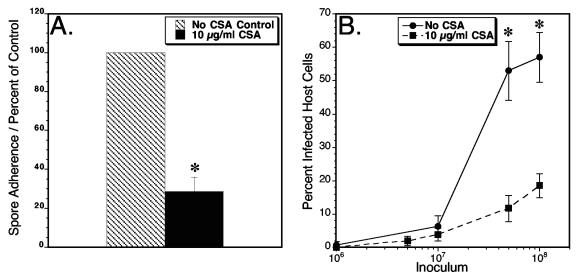
To determine the role of spore adherence in infection, the number of infected host cells was quantified after E. intestinalis spores were allowed to adhere to host cell surfaces in the presence or absence of the glycan inhibitor chondroitin sulfate A. Following adherence in the presence or absence of chondroitin sulfate A, the unbound spores were removed by washing. Chondroitin sulfate A inhibited spore adherence to host cells by 72% (Fig. 6A). Additional chondroitin sulfate A-treated and nontreated, washed coverslips were placed back in culture without the inhibitor, and the infection was allowed to progress for about 30 h to a stage at which developing immature and mature spores can be visualized by immunofluorescence and the percentage of host cell infection can be calculated. When 1 or 10 million spores were used as the inocula, there was no significant difference in the percentage of infected host cells in chondroitin sulfate A-treated samples compared to untreated samples (Fig. 6B). However, when an inoculum of 50 or 100 million was used, there was a significant decrease in the percentage of infected host cells in the presence of the inhibitor. The reduction in infected cells was 78% when 50 million spores were used and 68% when 100 million spores were used. These data therefore indicate a direct relationship between E. intestinalis spore adherence and host cell infection.
FIG. 6.
Effect of spore adherence inhibition on host cell infection. E. intestinalis spores (1, 5, 10, 50, or 100 million) were allowed to adhere to Vero host cells grown on glass coverslips in 12-well plates in the presence or absence of the adherence inhibitor chondroitin sulfate A (CSA). After 4 h of incubation, one set of coverslips from the 10-million-spore inoculum was removed to quantitate the inhibition of adherence (A). The remaining coverslips were washed to remove unattached spores and placed back in culture with normal medium for 30 h (B). Infected host cells were determined by visualizing developing intracellular spore clusters stained with propidium iodide as described in Materials and Methods. The percentage of infected host cells was calculated by dividing the mean number of infected cells per field of magnification by the mean total number of cells per field of magnification. Twenty random fields were counted for each data point. The asterisks indicate significant differences between chondroitin sulfate A-treated samples and nontreated samples (P < 0.0001). This experiment was repeated three times with similar results.
DISCUSSION
Our data indicate that microsporidial spore adherence to host cell surfaces involves a specific mechanism that is mediated by host cell surface sulfated GAGs. In these studies, all sulfated glycans tested inhibited spore binding to host cell surfaces. This mechanism of adherence was confirmed by treating host cells with xylopyranoside to reduce the amount of surface GAGs, by using host cells deficient in surface GAGs, and by treating host cells with sodium chlorate to reduce the level of GAG sulfation. In each of these studies, E. intestinalis spore adherence to host cells was reduced compared to that of untreated or nonmutated host cells. Collectively, these data give strong evidence for the involvement of host cell surface sulfated proteoglycans in a specific spore adherence mechanism.
GAGs are found on the surface of almost all cell types (4, 44) and are used by many intracellular pathogens to attach and gain entry to host cells. Microsporidia are known to have a wide host range, and a variety of host cell lines can support E. intestinalis growth in vitro (41). In our studies, E. intestinalis spore adherence was inhibited by five different sulfated glycans, some of which are present on a wide variety of cells from many species. It is possible that the ability of microsporidia to infect a wide range of hosts and tissues correlates with their ability to utilize multiple GAGs for adherence in a fashion similar to that proposed for Toxoplasma gondii (9).
Our experimentation with mutant CHO cell lines reveals that microsporidial spores have a preference for sulfated heparan proteoglycans. The CHO mutant cell line deficient in heparan sulfate showed roughly the same reduction in spore adherence as the CHO mutant cell line, which is deficient in both heparan sultate and chondroitin sulfate. Other intracellular microbial pathogens specifically utilize host cell surface heparan sulfate to attach and gain entry. The bacterial pathogens Listeria monocytogenes (1) and Mycobacterium spp. (34) produce a surface heparin binding protein that is involved in adherence to epithelial cells. The dengue (10) and foot-and-mouth disease viruses also interact with cell surface heparan sulfate, but these interactions alone may not in themselves be sufficient for infection (26). Moreover, the eukaryotic parasites Trypanosoma cruzi (24), Plasmodium spp. (17), and Leishmania spp. (6, 30) have been reported to utilize heparan sulfate in host cell adhesion. Although the CHO mutant cell line data indicate a preference for heparin and the EC50 values show that heparin is the most efficient inhibitor tested, heparin is not the only exogenous sulfated glycan that can inhibit adherence. In fact, all other sulfated glycans tested resulted in higher maximal inhibitions. Consequently, additional studies are needed to conclusively determine preference.
Of the sulfated proteoglycans tested for microsporidial adherence inhibition, mucin may be the most physiologically relevant since, during natural infections, the spores are exposed to mucin in the intestine. A combination of mucin with a change in pH has been shown to induce the extrusion of the polar filament in spores in the absence of cells (37), but we can only speculate about the conditions for spore activation in vivo. Once spores are ingested by a suitable host, it is possible that the microsporidia adhere by attaching to intestinal mucus first, subsequently gaining access to host cell surfaces. This method would allow microsporidia to circumvent host cell protective measures by using intestinal mucus to their advantage. Interestingly, another gastrointestinal eukaryotic pathogen, Entamoeba histolytica, recognizes the terminal galactose/N-acetyl-d-galactosamine residues of target glycoproteins that are found both on intestinal host cells and in colonic mucin (36). Invasive amebiasis initiates by attachment of the amoebae to the mucus layer, followed by amebic adherence to mucosal epithelial cells (35). The studies presented here indicate that microsporidia may act in a similar fashion.
Complete ablation of spore adherence to host cells is not achieved with the inhibitors tested; however, the maximal level of spore inhibition achieved is roughly 70 to 90% of the control. It is possible that another mechanism of spore adherence exists that is independent of the sulfated proteoglycan mechanism. Several viruses are known to use a combination of host cell receptors to attach and gain entry. For example, herpesviruses initially bind to host cell heparan sulfate via a virion glycoprotein (15, 18). The virions then fuse with the host cell membrane with other viral glycoproteins. Furthermore, the foot-and-mouth disease viruses use cell surface heparan sulfate to concentrate virus particles for subsequent integrin receptor binding (26, 38). Additional studies are under way to determine if microsporidia utilize an additional mechanism for spore adherence to host cells.
Microsporidial spore adherence to certain host cell types could be viewed as a host defense mechanism. It has been shown that nonprofessional phagocytic cells, such as those lining the intestines, can take up both viable and nonviable Encephalitozoon spp. spores through traditional phagocytic mechanisms involving host cell actin polymerization (11). These phagocytic mechanisms do not involve host cell membrane ruffling characteristic of macropinocytosis (11, 16). Once inside the host cell, the phagocytosed spores may be degraded. However, with the change in environmental conditions in degradation centers within host cells, microsporidial spores could still activate and extrude the polar filament into the host cell cytoplasm and cause an infection (11). Alternatively, the internalized spores may theoretically pass through the host cell and emerge into the subepithelial layer. In this case, spore adherence may be more relative to dissemination than infection. Encephalitozoon spp. and others are known to disseminate to other tissues and organs (7, 27). Other pathogens such as Neisseria spp. and Toxoplasma gondii use a similar mechanism to transverse epithelial barriers to enter the bloodstream (2, 19). Nonetheless, further study is required to determine if there is a relationship between spore adherence, internalization of spores, and dissemination.
The role of microsporidial spore adherence is unknown. Spore germination is a multistep process involving spore activation, the buildup of internal pressure, polar filament extrusion, and passage of the infectious sporoplasm. Microsporidial spore adherence to host cell surfaces may be the initial event that signals the spore to activate and sets into motion the cascade of events leading to germination and infection. In support of this hypothesis, we clearly show that inhibition of spore adherence results in decreased host cell infection, indicating a direct association between spore adherence and infection. If spores are activated by contact with the host cell surface, calcium influx may play an initial role in the following cascade of events (23, 28, 37). On the other hand, if spore-cell contact is not signaling activation of the spore, the anchoring of a nonactivated spore to the host cell would be beneficial to the physical piercing of host cells by the polar tube when the spore becomes activated.
It is difficult to speculate on the spore surface ligand involved in host cell adherence. To date, only two spore wall proteins have been identified for E. intestinalis (21) and one for E. cuniculi (5). These spore wall proteins are very similar at the amino terminus but differ significantly at the C terminus. The SWP2 of E. intestinalis has a unique 12- or 15-amino-acid repeating motif that has no matches in the databases. The function of this motif is unknown, but the hydrophilic and repeating nature of these motifs suggest that they form a unique external repeating structure.
In summary, a specific mechanism of microsporidial spore adherence to host cells that involves host cell surface sulfated GAGs has been identified. These studies show that a variety of glycans can inhibit spores from attaching to host cells, suggesting that the ability of microsporidia to infect a wide range of hosts and tissues may correlate with their ability to utilize multiple GAGs for adherence. These studies also show a direct association between spore adherence and host cell infection because reduced adherence due to exogenous proteoglycans in an in vitro assay results in reduced host cell infection. Identifying and characterizing the mechanism of spore adherence may lead to understanding of how spores become activated. This may ultimately lead to the development of novel therapies.
Acknowledgments
We thank Pricilla Wyrick and Judy Whittimore for consultation and technical assistance with transmission electron microscopy, Melissa E. Lester for laboratory technical assistance, John Kalbfleishch for assistance with statistical analyses, and Sara Davis-Hayman for critical reading of the manuscript.
This study was supported by Public Health Service grant AI055267 (to J.R.H.) from the National Institute of Allergy and Infectious Diseases.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Alvarez-Dominguez, C., J. A. Vazquez-Boland, E. Carrasco-Marin, P. Lopez-Mato, and F. Leyva-Cobian. 1997. Host cell heparan sulfate proteoglycans mediate attachment and entry of Listeria monocytogenes, and the listerial surface protein ActA is involved in heparan sulfate receptor recognition. Infect. Immun. 65:78-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barragan, A., and L. D. Sibley. 2002. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J. Exp. Med. 195:1625-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernfield, M., M. Gotte, P. W. Park, O. Reizes, M. L. Fitzgerald, J. Lincecum, and M. Zako. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68:729-777. [DOI] [PubMed] [Google Scholar]
- 4.Bernfield, M., R. Kokenyesi, M. Kato, M. T. Hinkes, J. Spring, R. L. Gallo, and E. J. Lose. 1992. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu. Rev. Cell Biol. 8:365-393. [DOI] [PubMed] [Google Scholar]
- 5.Bohne, W., D. J. Ferguson, K. Kohler, and U. Gross. 2000. Developmental expression of a tandemly repeated, glycine- and serine-rich spore wall protein in the microsporidian pathogen Encephalitozoon cuniculi. Infect. Immun. 68:2268-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butcher, B. A., L. A. Sklar, L. C. Seamer, and R. H. Glew. 1992. Heparin enhances the interaction of infective Leishmania donovani promastigotes with mouse peritoneal macrophages. A fluorescence flow cytometric analysis. J. Immunol. 148:2879-2886. [PubMed] [Google Scholar]
- 7.Cali, A., D. P. Kotler, and J. M. Orenstein. 1993. Septata intestinalis N. G., N. Sp., an intestinal microsporidian associated with chronic diarrhea and dissemination in AIDS patients. J. Eukaryot. Microbiol. 40:101-112. [DOI] [PubMed] [Google Scholar]
- 8.Cali, A., and P. M. Takvorian. 1999. Developmental morphology and life cycles of the microsporidia, p. 85-128. In L. M. Weiss and M. Wittner (ed.), The microsporidia and microsporidiosis, 1st ed. American Society for Microbiology, Washington, D.C.
- 9.Carruthers, V. B., S. Hakansson, O. K. Giddings, and L. D. Sibley. 2000. Toxoplasma gondii uses sulfated proteoglycans for substrate and host cell attachment. Infect. Immun. 68:4005-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Y., T. Maguire, R. E. Hileman, J. R. Fromm, J. D. Esko, R. J. Linhardt, and R. M. Marks. 1997. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 3:866-871. [DOI] [PubMed] [Google Scholar]
- 11.Couzinet, S., E. Cejas, J. Schittny, P. Deplazes, R. Weber, and S. Zimmerli. 2000. Phagocytic uptake of Encephalitozoon cuniculi by nonprofessional phagocytes. Infect. Immun. 68:6939-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeAngelis, P. L., and C. G. Glabe. 1987. Polysaccharide structural features that are critical for the binding of sulfated fucans to bindin, the adhesive protein from sea urchin sperm. J. Biol. Chem. 262:13946-13952. [PubMed] [Google Scholar]
- 13.Esko, J. D., K. S. Rostand, and J. L. Weinke. 1988. Tumor formation dependent on proteoglycan biosynthesis. Science 241:1092-1096. [DOI] [PubMed] [Google Scholar]
- 14.Esko, J. D., T. E. Stewart, and W. H. Taylor. 1985. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 82:3197-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feyzi, E., E. Trybala, T. Bergstrom, U. Lindahl, and D. Spillmann. 1997. Structural requirement of heparan sulfate for interaction with herpes simplex virus type 1 virions and isolated glycoprotein C. J. Biol. Chem. 272:24850-24857. [DOI] [PubMed] [Google Scholar]
- 16.Foucault, C., and M. Drancourt. 2000. Actin mediates Encephalitozoon intestinalis entry into the human enterocyte-like cell line, Caco-2. Microb. Pathog. 28:51-58. [DOI] [PubMed] [Google Scholar]
- 17.Frevert, U., P. Sinnis, C. Cerami, W. Shreffler, B. Takacs, and V. Nussenzweig. 1993. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. J. Exp. Med. 177:1287-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 19.Gray-Owen, S. D. 2003. Neisserial Opa proteins: impact on colonization, dissemination and immunity. Scand. J. Infect. Dis. 35:614-618. [DOI] [PubMed] [Google Scholar]
- 20.Guseva, N. V., S. T. Knight, J. D. Whittimore, and P. B. Wyrick. 2003. Primary cultures of female swine genital epithelial cells in vitro: a new approach for the study of hormonal modulation of Chlamydia infection. Infect. Immun. 71:4700-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayman, J. R., S. F. Hayes, J. Amon, and T. E. Nash. 2001. Developmental expression of two spore wall proteins during maturation of the microsporidian Encephalitozoon intestinalis. Infect. Immun. 69:7057-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayman, J. R., and T. E. Nash. 1999. Isolating expressed microsporidial genes using a cDNA subtractive hybridization approach. J. Eukaryot. Microbiol. 46:21S-24S. [PubMed] [Google Scholar]
- 23.He, Q., G. J. Leitch, G. S. Visvesvara, and S. Wallace. 1996. Effects of nifedipine, metronidazole, and nitric oxide donors on spore germination and cell culture infection of the microsporidia Encephalitozoon hellem and Encephalitozoon intestinalis. Antimicrob. Agents Chemother. 40:179-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrera, E. M., M. Ming, E. Ortega-Barria, and M. E. Pereira. 1994. Mediation of Trypanosoma cruzi invasion by heparan sulfate receptors on host cells and penetrin counter-receptors on the trypanosomes. Mol. Biochem. Parasitol. 65:73-83. [DOI] [PubMed] [Google Scholar]
- 25.Humphries, D. E., and J. E. Silbert. 1988. Chlorate: a reversible inhibitor of proteoglycan sulfation. Biochem. Biophys. Res. Commun. 154:365-371. [DOI] [PubMed] [Google Scholar]
- 26.Jackson, T., F. M. Ellard, R. A. Ghazaleh, S. M. Brookes, W. E. Blakemore, A. H. Corteyn, D. I. Stuart, J. W. Newman, and A. M. King. 1996. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J. Virol. 70:5282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotler, D. P., and J. M. Orenstein. 1999. Clinical syndromes associated with microsporidiosis, p. 258-292. In L. M. Weiss and M. Wittner (ed.), The microsporidia and microsporidiosis, 1st ed. American Society for Microbiology, Washington, D.C.
- 28.Leitch, G. J., Q. He, S. Wallace, and G. S. Visvesvara. 1993. Inhibition of the spore polar filament extrusion of the microsporidium Encephalitozoon hellem isolated from an AIDS patient. J. Eukaryot. Microbiol. 40:711-717. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Velez, R., M. C. Turrientes, C. Garron, P. Montilla, R. Navajas, S. Fenoy, and C. del Aguila. 1999. Microsporidiosis in travelers with diarrhea from the tropics. J. Travel Med. 6:223-227. [DOI] [PubMed] [Google Scholar]
- 30.Love, D. C., J. D. Esko, and D. M. Mosser. 1993. A heparin-binding activity on Leishmania amastigotes which mediates adhesion to cellular proteoglycans. J. Cell Biol. 123:759-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lujan, H. D., J. T. Conrad, C. G. Clark, M. C. Touz, F. Delbac, C. P. Vivares, and T. E. Nash. 1998. Detection of microsporidia spore-specific antigens by monoclonal antibodies. Hybridoma 17:237-243. [DOI] [PubMed] [Google Scholar]
- 32.Mark, M. P., V. Karcher-Djuricic, J. R. Baker, and J. V. Ruch. 1990. Effects of beta-D-xyloside on morphogenesis and cytodifferentiation in cultured embryonic mouse molars. Cell Differ. Dev. 32:1-16. [DOI] [PubMed] [Google Scholar]
- 33.Ortega-Barria, E., and J. C. Boothroyd. 1999. A Toxoplasma lectin-like activity specific for sulfated polysaccharides is involved in host cell infection. J. Biol. Chem. 274:1267-1276. [DOI] [PubMed] [Google Scholar]
- 34.Pethe, K., M. Aumercier, E. Fort, C. Gatot, C. Locht, and F. D. Menozzi. 2000. Characterization of the heparin-binding site of the mycobacterial heparin-binding hemagglutinin adhesin. J. Biol. Chem. 275:14273-14280. [DOI] [PubMed] [Google Scholar]
- 35.Petri, W. A., Jr., and B. J. Mann. 1993. Molecular mechanisms of invasion by Entamoeba histolytica. Semin. Cell Biol. 4:305-313. [DOI] [PubMed] [Google Scholar]
- 36.Petri, W. A., Jr., R. D. Smith, P. H. Schlesinger, C. F. Murphy, and J. I. Ravdin. 1987. Isolation of the galactose-binding lectin that mediates the in vitro adherence of Entamoeba histolytica. J. Clin. Investig. 80:1238-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pleshinger, J., and E. Weidner. 1985. The microsporidian spore invasion tube. IV. Discharge activation begins with pH-triggered Ca2+ influx. J. Cell Biol. 100:1834-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Putnak, J. R., N. Kanesa-Thasan, and B. L. Innis. 1997. A putative cellular receptor for dengue viruses. Nat. Med. 3:828-829. [DOI] [PubMed] [Google Scholar]
- 39.Svenungsson, B., T. Capraru, B. Evengard, R. Larsson, and M. Lebbad. 1998. Intestinal microsporidiosis in a HIV-seronegative patient. Scand. J. Infect. Dis. 30:314-316. [DOI] [PubMed] [Google Scholar]
- 40.Visvesvara, G. S., M. Belloso, H. Moura, A. J. Da Silva, I. N. Moura, G. J. Leitch, D. A. Schwartz, P. Chevez-Barrios, S. Wallace, N. J. Pieniazek, and J. D. Goosey. 1999. Isolation of Nosema algerae from the cornea of an immunocompetent patient. J. Eukaryot. Microbiol. 46:10S. [PubMed] [Google Scholar]
- 41.Visvesvara, G. S., H. Moura, G. J. Leitch, and D. A. Schwartz. 1999. Culture and propagation of microsporidia, p. 363-392. In L. M. Weiss and M. Wittner (ed.), The microsporidia and microsporidiosis, 1st ed. American Society for Microbiology, Washington, D.C.
- 42.Wadstrom, T., and A. Ljungh. 1999. Glycosaminoglycan-binding microbial proteins in tissue adhesion and invasion: key events in microbial pathogenicity. J. Med. Microbiol. 48:223-233. [DOI] [PubMed] [Google Scholar]
- 43.Weidner, E., and L. D. Sibley. 1985. Phagocytized intracellular microsporidian blocks phagosome acidification and phagosome-lysosome fusion. J. Protozool. 32:311-317. [DOI] [PubMed] [Google Scholar]
- 44.Yanagishita, M., and V. C. Hascall. 1992. Cell surface heparan sulfate proteoglycans. J. Biol. Chem. 267:9451-9454. [PubMed] [Google Scholar]