Abstract
Chest wall strapping (CWS) induces breathing at low lung volumes. Mild to moderate obesity can lead to similar changes in lung volumes, due to chest wall and abdominal restriction. Chest wall strapping is also conceptually similar to a mismatch between significantly oversized donor lungs transplanted into a recipient with a smaller chest cavity. Chest wall strapping increases lung elastic recoil, reduces pulmonary compliance, and substantially increases maximal expiratory flows. The interactions between elastic properties of the lung parenchyma and small airways are critical for pulmonary function. Chest wall strapping lowers residual volume and closing volume, likely from the interdependence between increased elastic recoil and airways, leading to greater radial distending forces on small airways and small airway dilation. Chronic obstructive pulmonary disease (COPD) and chronic rejection of the transplanted lung, bronchiolitis obliterans syndrome (BOS), are primarily diseases of the small airways, and are characterized by progressive obstruction and subsequent loss of small airways. In COPD, higher body mass index (BMI) (conceptually like being more tightly strapped) is associated with lower lung volumes, increased airway conductance, and lower risk of progression to emphysema or death. Likewise, in lung transplantation, oversized donor lungs have been linked to higher expiratory airflows, lower risk of bronchiolitis obliterans syndrome, and improved survival. This article reviews the physiology of chest wall strapping and explores how it could enhance the understanding or even the treatment of small airway diseases, such as COPD and bronchiolitis obliterans syndrome.
Keywords: chest wall strapping, expiratory airflow, elastic recoil, small airway disease
Chest wall strapping (CWS) is a procedure that involves restricting the thorax and abdomen, forcing the subject to breathe at low lung volumes (1–10). CWS has been used to understand basic mechanisms of pulmonary physiology. In particular, it has long served as a model for studying the physiology of restrictive chest wall diseases, respiratory muscle weakness, and the effects of general anesthesia and paralytics on lung physiology (2, 3, 8). It now holds the additional promise of providing insights and potential treatments for diseases as diverse as chronic obstructive pulmonary disease (COPD) and post-transplant bronchiolitis obliterans syndrome (BOS).
The aim of this Perspective is to provide a concise overview of the physiology of CWS, explore analogies to respiratory effects of obesity and transplantation of oversized lungs, and demonstrate how the physiology of CWS can enhance our understanding of diseases of small airways, and possibly lead to novel treatments.
The Physiology of Chest Wall Strapping
Lung Volumes, Expiratory Airflows, and Airway Conductance
Lung volumes are decreased by the application of external CWS (1–10). The magnitude of these decreases in lung volumes depends on the stiffness of the restricting device, the force with which it is applied, and the extent to which it restricts the abdomen (Figure 1 and the online supplement). In studies of normal subjects, CWS reduced total lung capacity (TLC) by a mean of 34% (range, 28–43%), vital capacity (VC) by 41% (range, 30–50%), and FRC by 31% (range, 22–38%) (Table 1). Residual volumes (RVs) decreased by 15% (range, 6–24%). The expiratory reserve volume (ERV) consistently showed the greatest decrease, averaging 51% (range, 36–60%). In three studies reporting on closing volume it was uniformly reduced by CWS, on average 18% (range, 14–20%) (1, 5, 9).
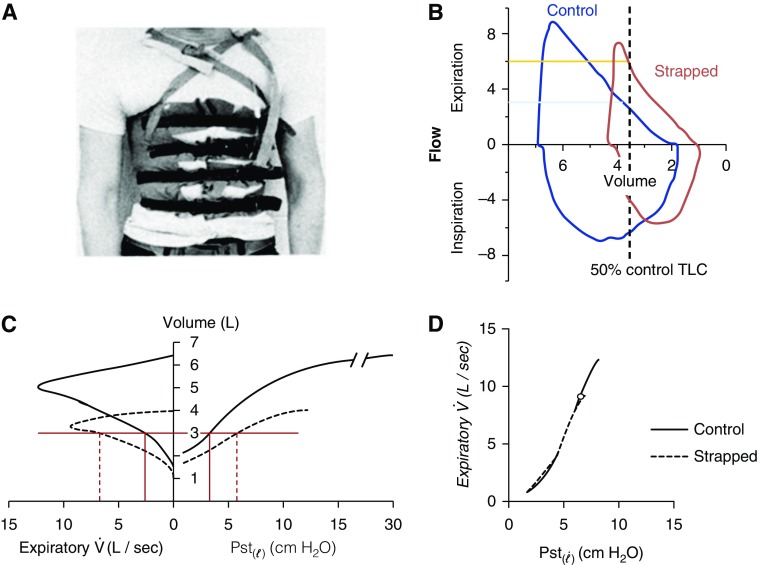
Figure 1.
Chest wall strapping (CWS) and its physiological effect. (A) CWS as performed by Sybrecht and colleagues (9) (reproduced by permission from Reference 9). (B) Flow–volume loops in control (blue) and CWS (orange) condition of author M.E. plotted against absolute lung volume. Vertical dashed line shows absolute lung volume at 50% of control total lung capacity (TLC). The blue horizontal line shows that expiratory flow at 50% of control TLC in control state is 3.2 L/s, whereas, in the strapped state at the same absolute lung volume (50% of control TLC), expiratory flow is 5.9 L/s. (C) Presentation of all the basic data from a typical subject with CWS (reproduced by permission from Reference 8). Control (solid) and strapped flow–volume (dashed) curves on the left and corresponding static lung elastic recoil pressures (Pst()) on the right of the ordinate. At the same absolute lung volume (horizontal red line), the CWS shows higher expiratory flows () and higher static lung elastic recoil pressure (Pst()). (D) Relating expiratory flows () and static lung elastic recoil pressures (Pst()) from the same data as shown in (C) (reproduced by permission from Reference 8). The expiratory flows and static lung elastic recoil pressure (Pst()) curves from control and strapped state overlie each other, suggesting that the increased elastic recoil with CWS is the explanation for the increase expiratory airflows.
Table 1.
Chest wall strapping and lung volumes in healthy subjects
| First Author, Year (Ref.) | n | TLC | VC | FRC | ERV | RV | CV |
|---|---|---|---|---|---|---|---|
| Caro, 1960 (3) | 25 | −36% | −45% | −35% | −60% | −24% | — |
| Butler, 1960 (2) | 10 | — | — | — | — | — | — |
| Stubbs, 1972 (8) | 8 | −33% | −40% | −36% | −57% | −11% | |
| Sybrecht, 1975 (9) | 5 | −28% | −30% | −27% | −44% | −20% | −20% |
| Bradley, 1980 (1) | 8 | −43% | −50% | −38% | −60% | −9% | −14% |
| Douglas, 1981 (4) | 6 | Variable | Variable | Variable | 0 to −70% | Variable. | — |
| Klineberg, 1981 (5) | 7 | −36% | −44% | −32% | −58% | −15% | −20% |
| Scheidt, 1981 (7) | 10 | −36% | −46% | −24% | −48% | −6% | |
| Noord, 1986 (10) | 14 | −31% | −36% | −28% | −40% | −12% | |
| O’Donnell, 2000 (6) | 12 | −29% | −35% | −22% | −36% | −10% | |
| Weighted mean, % (SE) | −34% (0.5) | −41% (0.7) | −31% (0.6) | −51% (1.0) | −15% (0.7) | −18% (0.7) |
Definition of abbreviations: CV = closing volume; ERV = expiratory reserve volume; FRC = functional residual capacity; RV = residual volume; TLC = total lung capacity; VC = vital capacity.
Somewhat more surprisingly, CWS enhances forced expiratory airflows. Maximal expiratory airflows (for the same absolute lung volumes) were consistently and substantially increased to averaging 159% (range, 147–188%) of the prestrapped flow rates (Table 2, Figure 1C). Douglas and colleagues (4) described the relationship between CWS-induced changes in lung volumes (represented by reductions in ERV) and raised expiratory flow (Figure 2A). After a threshold reduction in ERV of 20–30%, further reductions were associated with higher maximal expiratory airflows (represented by the slope of the flow–volume curve from 30 to 10% of the unstrapped VC). This study also showed that voluntarily breathing at low lung volume leads to changes in lung function identical to those of CWS. Enhanced expiratory airflow could be measured within 30 seconds of low–lung volume breathing, and increased further up to 2 minutes (4). Six studies reported on airway conductance (reciprocal of airway resistance), showing a consistent increase after CWS (1, 3–5, 7–10). Specific conductance (conductance adjusted for lung volume) increased by an average of 32% (range, 24–43%) (6, 8, 10).
Table 2.
Chest wall strapping, expiratory flows, and lung mechanics in healthy subjects
| First Author, Year (Ref.) | n | FEF at 50% TLC | Conductance | Lung Compliance 50% TLC | Lung Elastic Recoil 50% TLC |
|---|---|---|---|---|---|
| Caro, 1960 (3) | 25 | — | +9% (overall) | −38%* | +185* |
| Butler, 1960 (2) | 10 | — | ↑↑† | ↓↓† | — |
| Stubbs, 1972 (8) | 8 | +188% | +43% (50% TLC) | −33% | +164% |
| Sybrecht, 1975 (9) | 5 | +133% | −43% | ||
| Bradley, 1980 (1) | 8 | +150%‡ | −59% | +244% | |
| Douglas, 1981 (4) | 6 | +148%§ | ↑↑ | — | +139%‡ |
| Klineberg, 1981 (5) | 7 | +181% | −48% | +151% | |
| Scheidt, 1981 (7) | 10 | +167%‡ | +188% | ||
| Noord, 1986 (10) | 14 | +147% | +24% (50% TLC) | ||
| O’Donnell, 2000 (6) | 12 | — | +35% (sGaw) | ||
| Weighted mean, % (SE) | +159% (2.3) | +32% (1.3) | −42% (1.1) | +182% (3.6) |
Definition of abbreviations: FEF = forced expiratory airflow; FRC = functional residual capacity; sGaw = specific conductance; TLC = total lung capacity.
Measured at control FRC.
Graphic display of conductance and compliance over the entire lung volume range. At each lung volume, conductance and compliance were significantly higher/lower, respectively.
At 40% TLC.
At 30% of vital capacity.
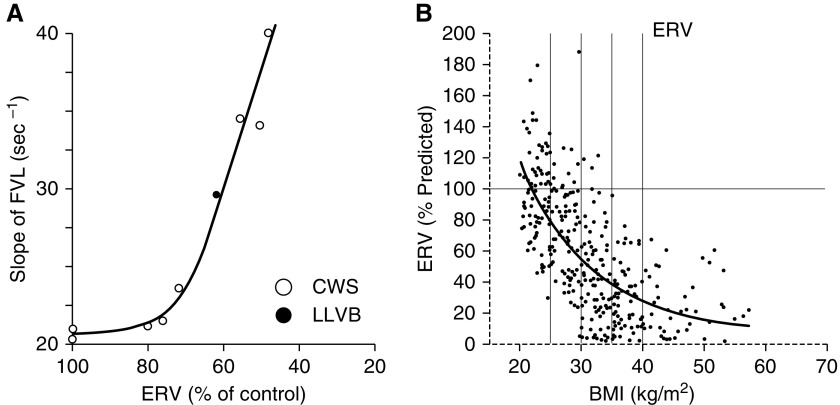
Figure 2.
Chest wall strapping (CWS) and obesity induced breathing at low lung volumes. (A) The degree of expiratory reserve volume (ERV) reduction via CWS (open circles) or voluntary low–lung volume breathing (LLVB) for 2 minutes (solid circles) is correlated the slope of the 30–10% (of control vital capacity) portion of the flow–volume curve (as a surrogate for expiratory airflow—greater slope correlates with higher airflows). Reproduced with permission from Douglas and colleagues (4). (B) The exponential regressions for ERV as a function of body mass index (BMI). Reproduced by permission from Reference 22. FVL = flow-volume loop.
Chest Wall Strapping and Lung Mechanics
CWS decreases compliance of the respiratory system. However, studies of CWS have not only shown decreased chest wall compliance, but have consistently shown reduced lung compliance (slope of the expiratory limb of the static pressure–volume curve) and an increase in lung elastic recoil (static lung recoil pressure measured at 50% of control TLC) (Table 2, Figure 1C) (1–5, 7–10). Increased lung elastic recoil is similarly seen in voluntary low–lung volume breathing (4).
The key determinants of maximal expiratory airflow are lung elastic recoil, airway resistance, and the “tube law” (the relationship between airway cross-sectional area and transmural pressure) (11). Thus, the increased expiratory airflows with CWS can be explained, in part, by the elevated lung elastic recoil (Figure 1D) (8). The elastic properties of the lung parenchyma interact with small airways to impact pulmonary function. Increased lung elastic recoil due to CWS would be expected to dilate small airways, altering the tube law (12). This is the likely explanation for the significant decrease in residual and closing volume and the increase in airway conductance that attend CWS (1–10). This is supported by preliminary data from computerized tomography (CT) airway structure analysis comparing CT scans at 50% of control TLC in the strapped and control states (Figure 3). In the strapped condition, there were greater numbers of detectable airways (129 versus 107; P < 0.05), and this increase occurred only in airways less than 5-mm diameter. Some airways of very small diameter in the control condition may have been dilated to larger diameters in the strapped condition, and thus reached the detection threshold of the automated airway segmentation algorithm.
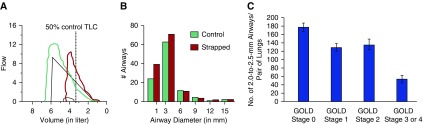
Figure 3.
The association of chest wall strapping (CWS) and chronic obstructive pulmonary disease (COPD) with small airway structure. (A) Flow–volume loop in control (green) and CWS (red) state of a healthy study subject. (B) Computerized tomography (CT) scans were obtained at 50% of the control total lung capacity (TLC) in control and CWS state under spirometric control (same study subject as in [A]). The airway tree was analyzed via an automated airway segmentation algorithm. The airway histogram is comparing the number of detectable airways by size group (mm) in the CWS and control states. CWS increased the number of detectable small airways (<5 mm) via an automated CT airway segmentation algorithm (M.E., unpublished data). (C) The number of airways measuring 2.0–2.5 mm in diameter per lung pair according to various stages of COPD. Reproduced by permission from Reference 39. In that study, multidetector CT scans from 78 patients with various stages of COPD were analyzed. As compared with the control group, there was a reduced number of small airways per lung pair in patients with stage 1 disease on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) (P = 0.001), GOLD stage 2 disease (P = 0.02), and GOLD stages 3 or 4 disease (P < 0.001). Data presented are means ± SE.
What Is the Mechanism for Chest Wall Strapping–induced Lung Function Changes?
The mechanisms of increased lung elastic recoil with CWS have not been conclusively defined. Overall lung elastic recoil is determined by tissue forces and surface forces at the air–liquid interface (13), both of which could be affected by CWS. Possible mechanisms for the increased lung elastic recoil with CWS could include airway closure and atelectasis, distortion of the lung, and decreased alveolar surface compliance from changes in surfactant function. Of these, decreased alveolar surface compliance is the most likely mechanism, supported by the following observations.
From animal studies.
When isolated rat (14) and dog (15) lungs were ventilated at low volumes, lung compliance decreased by approximately 40%. There was no histological evidence of airway occlusion, atelectasis, or change in alveolar ducts or alveoli. In other experiments, after a period of ventilation at low volumes, lungs were filled with saline to nullify the effect of surface forces and reveal that of tissue forces. There was no difference in the pressure–volume relationship of control and low–lung volume–ventilated lungs. This suggested that ventilation at low volumes reduces lung compliance via a change in surface forces, not tissue forces related to atelectasis or distortion. There was a significant negative correlation between the minimum surface tension in the lung extracts and the compliance changes. Higher surface forces were associated with lower lung compliance.
From human studies.
Several studies demonstrated that, after CWS, only one breath to the unstrapped TLC was necessary to normalize lung compliance (1–5, 7–9). On the other hand, when CWS was released, but no deep breaths were taken, the decrease in lung compliance persisted (1, 4). This latter observation makes lung distortion via CWS unlikely to be a significant mechanism. Regional ventilation, as assessed by xenon breathing, showed no inhomogeneities during CWS (9). Furthermore, in patients with low-volume breathing because of respiratory muscle weakness, there was no imaging evidence of atelectasis on CT scans of the chest (16). These findings provide further evidence that atelectasis is unlikely to be a significant mechanism underlying elevated elastic recoil due to CWS.
The role of surface compliance in the increased elastic recoil seen with CWS draws attention to pulmonary surfactant. Massaro and colleagues (17, 18) provide insight into how surfactant function might change with ventilation at low volumes. Low-volume ventilation without periodic large inflations led to a decrease in lung compliance in rats. Disaturated phosphatidylcholine (DSPC) is the only recognized surface-active constituent of surfactant present in a sufficient amount to form a monomolecular film over the entire alveolar surface. The total amount of DSPC after low-volume ventilation was unchanged compared with the control condition. However, large aggregates of DSPC (that sediment at 1,000 × g) accumulated in the lungs of low-volume–ventilated animals. Surfactant in this form lowered surface tension less rapidly than surfactant in the 1,000 × g supernatant fraction. As with the CWS data, a large inflation after low-volume ventilation returned the amount of DSPC sedimenting at 1,000 × g to control levels (17, 18).
The Analogy of Chest Wall Strapping and Obesity
The effects of obesity on the respiratory function of healthy subjects have been reviewed recently (19–21). This section focuses only on possible analogies to and differences from CWS. The greatest proportional changes in lung volumes with obesity occur in FRC and ERV. With increasing body mass index (BMI), FRC and ERV decrease exponentially (Figure 2B) (22). ERV is reduced in obesity secondary to reduction in FRC, as RV usually decreases only modestly. CWS of healthy subjects reduces FRC by 30%, on average, resembling the lung volume impact on FRC and ERV of mild to moderate obesity (Table 3). However, with CWS, TLC and VC are reduced to a similar proportion as ERV and FRC, whereas, in mild to moderate obesity, TLC and VC are, in general, normal or only modestly reduced (22, 23). Reductions in TLC and VC often only occur with morbid obesity.
Table 3.
Comparison of respiratory effects of chest wall strapping, obesity, and transplantation of oversized lungs
| Obesity (22) |
|||||
|---|---|---|---|---|---|
| Lung Function | CWS (1–10) | Mild, BMI 30–35 | Moderate, BMI 35–40 | Morbid, BMI >40 | Oversized Transplant (36) |
| Lung Volumes | |||||
| TLC, % predicted (SD) | 66% (5) | 93% (9) | 92% (11) | 88% (11) | 65% (13) |
| FRC, % predicted (SD) | 70% (6) | 78% (13) | 72% (13) | 66% (12) | 81% (22) |
| ERV, % predicted (SD) | 50% (9) | 42% (29) | 29% (19) | 24% (19) | 55% (18) |
| Expiratory airflow | ↑↑ | ↑ (33, 34) | ↑ (33, 34) | variable | ↑↑ |
| Slope of expiratory flow–volume loop | ↑↑ | ↑ (34) | ↑ (34) | variable | ↑↑ (37) |
| Lung elastic recoil | ↑↑ | ↑ (24, 27, 28) | ↑↑ (24, 27, 28) | ↑↑ (24, 27, 28) | ↑ (38) |
Definition of abbreviations: BMI = body mass index; CWS = chest wall strapping; ERV = expiratory reserve volume; FRC = functional residual capacity; TLC = total lung capacity.
Supporting references are shown in parentheses, except SD where indicated.
The mass loading effects of excess adipose tissue on the chest wall and abdomen reduce compliance (increase stiffness) of the relaxed respiratory system (24–28). Although early physiological studies emphasized the contribution of the chest wall to lowered respiratory system compliance in obesity (26), later investigations repeatedly highlight the significant contribution of the lung (24, 27, 28), as in CWS. Reduced lung compliance is consistently shown in obese subjects undergoing anesthesia (24, 27, 28). In awake, otherwise healthy obese subjects, reduced lung compliance is reported (29, 30); however, there are also reports on normal lung mechanics in obese subjects (31). Almost all studies on the respiratory effects of obesity rely on BMI. However, for the same change in BMI, different patterns of adipose tissue distribution likely associate with different alterations in respiratory mechanics (32). In CWS, the location of the strapping is also important. Strapping the thorax only does not reduce lung volumes sufficiently to affect expiratory airflows or lung compliance (7, 9). The chest straps need to restrict at least the upper portion of the abdomen also to cause a decrease in FRC and ERV in normal subjects. Similarly, isolated abdominal strapping does not reduce lung volumes significantly.
In several studies, higher BMIs are associated with higher maximal midexpiratory airflows and higher FEV1/FVC ratios in otherwise healthy, obese, nonsmoking subjects (33). In addition, the effect of obesity on FRC correlates significantly with increases in maximal expiratory flows (as measured by the slope of the expiratory flow–volume relationship) (34). The observation that flow at moderate to low lung volumes is increased in obesity is best explained by an increase in lung elastic recoil from breathing at low lung volumes. These findings in obese subjects are analogous to those in healthy subjects exposed to CWS. The authors state “this analogy (of CWS) with our findings makes us confident that until the decrease in lung volumes does not exceed a given threshold in obesity, the increase in lung stiffness can protect ventilation from becoming more heterogeneous and worsen gas exchange” (34). This threshold was approximately 65% of FRC or an ERV below 0.6 L, where an exponential increase in ventilation heterogeneities, suggestive of airway closures, was observed (34).
The Analogy of Chest Wall Strapping and Transplanting Oversized Lungs
The FRC of a lung transplant recipient is determined by both the recipient’s chest wall mechanics and those of the donor lung. A patient given an oversized allograft will likely have an FRC that is lower than the donor’s FRC, because of the mechanics of the relatively smaller recipient thorax. In adults, absolute RV is determined by intrinsic characteristics of the lung (airway closure), rather than the chest wall (35). Thus, the RV of an oversized allograft is likely large relative to the recipient’s thorax. As a consequence, a patient with an oversized allograft will likely breathe at relatively low lung volumes that are closer to the RV of the allograft (that is, ERV is reduced). In fact, in a cohort of recipients of oversized lungs, the pulmonary function pattern resembled that of CWS (Table 3) (36). In another group of bilateral lung transplant patients, an oversized allograft was associated with higher expiratory airflows, FEV1/FVC ratio, and flow–volume loop slope estimates (37). To evaluate the physiology of the transplanted lung, it is helpful to analyze post–lung transplant allograft function in relation to donor predicted function. When flow–volume loops are analyzed in this way, oversized allografts resemble those of CWS (Figure 4) (37).
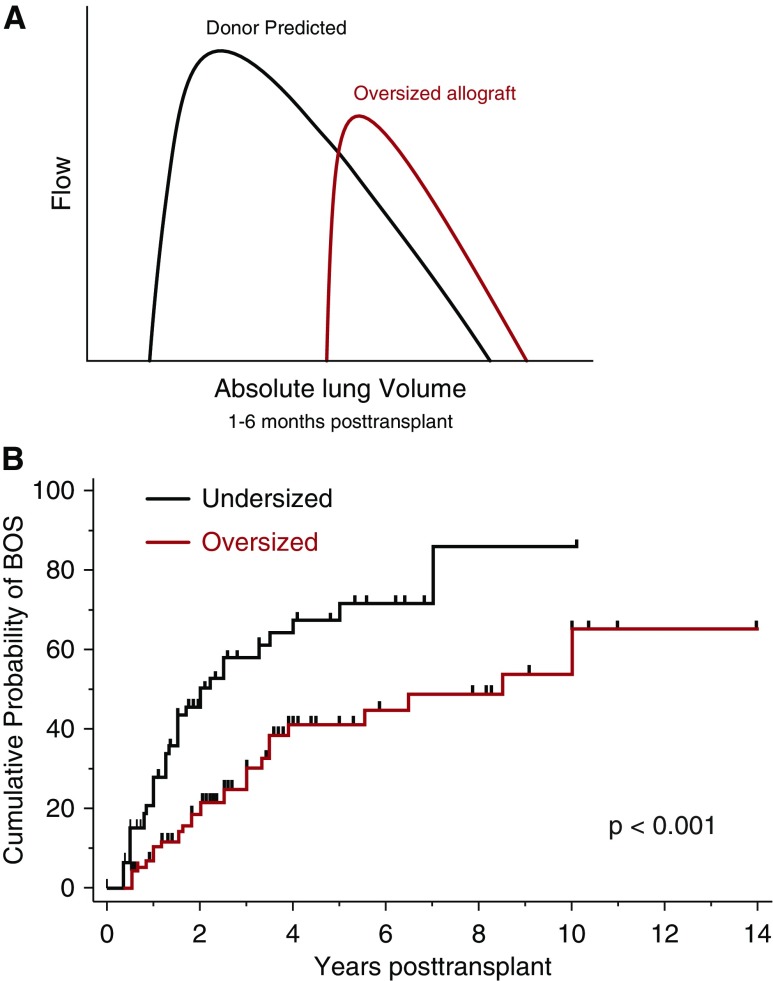
Figure 4.
Analogy of oversized allograft at lung transplantation to chest wall strapping (CWS). (A) Schematic flow–volume loops according to donor predicted values (black line) and measured mean values of recipients of oversized allografts (red line) during the early post-transplant period (1–6 mo). Flows are plotted against absolute lung volume. Adapted by permission from Reference 37. (B) Kaplan-Meier estimates of proportion of patients with bronchiolitis obliterans syndrome (BOS) stratified by recipients of undersized or oversized donor lungs. Oversized was defined as a donor-to-recipient predicted total lung capacity (pTLC) ratio greater than 1.0, and undersized was defined as a pTLC ratio of 1.0 or less. Comparison between over- and undersized cohorts was via log-rank test. Adapted by permission from Reference 37.
There is very limited information on lung compliance and lung elastic recoil pressure after lung transplantation in relation to donor–recipient size matching. In 15 patients who had undergone bilateral lung transplantation whose donor lungs were, on average, mildly oversized, elastic recoil of the transplanted lungs was mildly increased (38).
Thus, the respiratory effects of mild to moderate obesity in healthy subjects and transplantation of oversized allografts show significant analogies to CWS. Differences are that mild to moderate obesity in general does not reduce TLC and that morbid obesity can lead to even greater reductions in FRC and ERV than are typically achievable by CWS. These greater reductions in ERV and FRC with morbid obesity might exhaust the “compensatory mechanism” of increased lung stiffness, instead compromising airway patency, effective ventilation, and gas exchange at low lung volumes.
Chest Wall Strapping and Implications for Diseases of the Small Airways
This section explores how conditions that are conceptually similar to CWS (obesity and oversized lung transplantation) associate with the occurrence and progression of diseases of the small airways.
COPD
CT and micro-CT studies in COPD have demonstrated that small conducting airways narrow and disappear before the onset of emphysematous disease, drawing attention to these airways in its pathogenesis (Figure 3C) (39). In COPD, obesity lowers lung volumes much as it does when the lungs are healthy (40). As such, the impact of obesity on the respiratory system in patients with COPD could be considered a “CWS experiment.” For the same degree of COPD severity, obesity is associated with lower lung volumes, decreased airflow resistance, and higher inspiratory capacity (20, 40). Ora and colleagues (41) performed a study matching obese and normal-weight patients with COPD based on disease severity. In the obese patients with COPD, TLC was reduced by 15%, FRC by 20%, and ERV by 45%. Lung static recoil pressure was significantly increased by 29% in the obese group. If it is correct that widespread narrowing and loss of smaller conducting airways precedes the onset of emphysematous destruction, then the “CWS effect of obesity” could protect small airway function or reduce the risk for progression to emphysema. Interestingly, in the Multiethnic Study of Atherosclerosis lung study, the higher the BMI, the lower the risk for emphysema (42). In a risk-adjusted analysis, obesity was associated with improved survival in COPD when compared with normal weight (43). The above observations form the basis for the “obesity paradox of COPD,” as this is contrary to data from the general population, where obesity is associated with adverse health effects (44).
Bronchiolitis Obliterans Syndrome after Lung Transplantation
BOS is a disease that primarily affects small airways (45), and represents the main cause of long-term mortality after lung transplantation. Oversized allografts have been associated with a decreased risk for the occurrence of BOS (Figure 4) (37). Furthermore, an oversized allograft is associated with improved short-term and long-term survival (46–49). The mechanism(s) for these associations remains unclear. In the transplanted lung, immune- and nonimmune-mediated injuries to the small airways are risk factors for BOS (50). In injured small airways, repetitive opening and closing is associated with accelerated airway epithelial cell damage (51), inflammation, and, ultimately, fibrosis.
The likely increased elastic recoil of oversized lungs could have a beneficial effect on small airway function via greater radial distending traction on small airways (12). A possible mechanistic explanation for the physiology of CWS relates to the surfactant system. The associations between the surfactant system and risk factors for BOS are summarized in Table 4. The surfactant system shows adaptive responses to changes in lung compliance. In a model of decreased lung compliance, increases in surfactant protein and phospholipid content mediated a compensatory reduction in surface tension (52). Furthermore, compared with normal inflation state in the donor chest, an oversized allograft would operate at lower lung volumes in the recipient, and thus alveolar size would, on average, be reduced. Surfactant fills in the regions adjacent to infolding of the alveoli as the lung deflates to maintain a spherical inner surface (53). Thus, a chronically underinflated lung could be expected to accumulate more surfactant.
Table 4.
The surfactant system and its relation to risk factors for bronchiolitis obliterans syndrome
| BOS Risk factor | Effect on Surfactant System | Reference |
|---|---|---|
| Primary graft dysfunction | Successful treatment with surfactant | (59) |
| Acute rejection | • Type II pneumocyte destruction and surfactant disruption | (60) |
| • Rejection is associated with surfactant dysfunction | (61) | |
| • Immunosupression preserves surfactant function | (62) | |
| GERD—aspiration | Inactivation of surfactant | (63) |
| Pulmonary infection | Inactivation of surfactant | (64) |
Definition of abbreviations: BOS = bronchiolitis obliterans syndrome; GERD = gastroesophageal reflux disease.
Adapted by permission from Reference 37.
Chest Wall Strapping as a (Preventive) Therapy for COPD or Bronchiolitis Obliterans Syndrome?
So, does the normal or underweight subject with COPD have a greater risk of developing emphysema because his chest wall is not restricted? On the contrary, is the “strapped” obese patient with COPD protected from emphysema because increased lung elastic recoil benefits small airway function? Instead of treating end-stage emphysema with lung volume reduction surgery, can “chest cavity reduction” (i.e., CWS) limit progression to emphysema?
Abdominal binding or strapping in patients with COPD has produced variable results (54, 55). However, even these studies may not be informative, because strapping failed to affect either TLC or FRC (55).
When thinking about CWS as a possible nonpharmacologic approach to COPD or BOS, several issues need additional clarification. First, it is unclear if the response to CWS in subjects with COPD or other pulmonary diseases will be similar to that in healthy subjects, although the effect of obesity in COPD would suggest so. However, the possible effect of obesity on COPD can be confounded by similar airflow reduction from pathology of a emphysema and one of small airway disease with little emphysematous destruction. Then, in the latter case, elastic recoil would be greater, TLC may not be increased, and the clinical course may differ, because of the difference in disease pathology rather than as a function of obesity.
Furthermore, the duration of CWS needed in order to have a potentially beneficial impact is completely speculative.
Potential Negative Effects of Chest Wall Strapping
In general, CWS in healthy subjects is well tolerated and not associated with serious adverse events (1–10). However, CWS can increase airways hyperresponsiveness to a methacholine challenge (56). Furthermore, during exercise in healthy subjects, CWS is associated with a greater dyspnea intensity at any given workload (6, 57), and reduced breath-to-breath variability (58). In subjects with COPD, abdominal strapping was well tolerated at rest, but impaired exercise performance (55). However, it is possible that CWS is poorly tolerated in subjects with lung disease.
Conclusions
Breathing at low lung volumes is associated with increased lung stiffness (increased elastic recoil). Increased lung stiffness produces radial traction on small airways, which seems to maintain airway patency at low lung volumes and increase expiratory airflows. The similarities of CWS to respiratory effects of mild to moderate obesity and transplantation of oversized lungs bring new relevance to this old physiology experiment. Further studies of CWS could provide novel insights and potential treatments for diseases as diverse as COPD and BOS.
Acknowledgments
Acknowledgment
The seed for the topic of this Perspective was planted by Solbert Permutt, M.D. (March 6, 1925 to May 23, 2012), who, in discussions on the physiology of an oversized lung allograft transplanted into a recipient with a smaller chest cavity (36, 37), mentioned the similarities to the effects of chest wall strapping as reported by Stubbs and Hyatt (8). The authors are and will forever be grateful for these inspiring discussions.
Footnotes
M.E. is supported by a PILOT grant from the Institute for Clinical and Translational Science (ICTS) at the University of Iowa via the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, grant 2 UL1 TR000442-06.
Supported by a PILOT grant from the Institute for Clinical and Translational Science (ICTS) at the University of Iowa (M.E.). The ICTS at the University of Iowa is supported by the NIH Clinical and Translational Science Award (CTSA) program, grant 2 UL1 TR000442-06. The CTSA program is led by the NIH’s National Center for Advancing Translational Sciences.
The contents of this article are solely the responsibility of the author, and do not necessarily represent the official views of the NIH.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bradley CA, Anthonisen NR. Rib cage and abdominal restrictions have different effects on lung mechanics. J Appl Physiol. 1980;49:946–952. doi: 10.1152/jappl.1980.49.6.946. [DOI] [PubMed] [Google Scholar]
- 2.Butler J, Caro CG, Alcala R, Dubois AB. Physiological factors affecting airway resistance in normal subjects and in patients with obstructive respiratory disease. J Clin Invest. 1960;39:584–591. doi: 10.1172/JCI104071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caro CG, Butler J, Dubois AB. Some effects of restriction of chest cage expansion on pulmonary function in man: an experimental study. J Clin Invest. 1960;39:573–583. doi: 10.1172/JCI104070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douglas NJ, Drummond GB, Sudlow MF. Breathing at low lung volumes and chest strapping: a comparison of lung mechanics. J Appl Physiol. 1981;50:650–657. doi: 10.1152/jappl.1981.50.3.650. [DOI] [PubMed] [Google Scholar]
- 5.Klineberg PL, Rehder K, Hyatt RE. Pulmonary mechanics and gas exchange in seated normal men with chest restriction. J Appl Physiol. 1981;51:26–32. doi: 10.1152/jappl.1981.51.1.26. [DOI] [PubMed] [Google Scholar]
- 6.O’Donnell DE, Hong HH, Webb KA. Respiratory sensation during chest wall restriction and dead space loading in exercising men. J Appl Physiol (1985) 2000;88:1859–1869. doi: 10.1152/jappl.2000.88.5.1859. [DOI] [PubMed] [Google Scholar]
- 7.Scheidt M, Hyatt RE, Rehder K. Effects of rib cage or abdominal restriction on lung mechanics. J Appl Physiol. 1981;51:1115–1121. doi: 10.1152/jappl.1981.51.5.1115. [DOI] [PubMed] [Google Scholar]
- 8.Stubbs SE, Hyatt RE. Effect of increased lung recoil pressure on maximal expiratory flow in normal subjects. J Appl Physiol. 1972;32:325–331. doi: 10.1152/jappl.1972.32.3.325. [DOI] [PubMed] [Google Scholar]
- 9.Sybrecht GW, Garrett L, Anthonisen NR. Effect of chest strapping on regional lung function. J Appl Physiol. 1975;39:707–713. doi: 10.1152/jappl.1975.39.5.707. [DOI] [PubMed] [Google Scholar]
- 10.van Noord JA, Demedts M, Clément J, Cauberghs M, Van de Woestijne KP. Effect of rib cage and abdominal restriction on total respiratory resistance and reactance. J Appl Physiol (1985) 1986;61:1736–1740. doi: 10.1152/jappl.1986.61.5.1736. [DOI] [PubMed] [Google Scholar]
- 11.Pride NB, Permutt S, Riley RL, Bromberger-Barnea B. Determinants of maximal expiratory flow from the lungs. J Appl Physiol. 1967;23:646–662. doi: 10.1152/jappl.1967.23.5.646. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura M, Sasaki H, Takishima T. Effect of lung surface tension on bronchial collapsibility in excised dog lungs. J Appl Physiol. 1979;47:692–700. doi: 10.1152/jappl.1979.47.4.692. [DOI] [PubMed] [Google Scholar]
- 13.Bachofen H, Schürch S, Urbinelli M, Weibel ER. Relations among alveolar surface tension, surface area, volume, and recoil pressure. J Appl Physiol (1985) 1987;62:1878–1887. doi: 10.1152/jappl.1987.62.5.1878. [DOI] [PubMed] [Google Scholar]
- 14.Young SL, Tierney DF, Clements JA. Mechanism of compliance change in excised rat lungs at low transpulmonary pressure. J Appl Physiol. 1970;29:780–785. doi: 10.1152/jappl.1970.29.6.780. [DOI] [PubMed] [Google Scholar]
- 15.Faridy EE, Permutt S, Riley RL. Effect of ventilation on surface forces in excised dogs’ lungs. J Appl Physiol. 1966;21:1453–1462. doi: 10.1152/jappl.1966.21.5.1453. [DOI] [PubMed] [Google Scholar]
- 16.Estenne M, Gevenois PA, Kinnear W, Soudon P, Heilporn A, De Troyer A. Lung volume restriction in patients with chronic respiratory muscle weakness: the role of microatelectasis. Thorax. 1993;48:698–701. doi: 10.1136/thx.48.7.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massaro D, Clerch L, Massaro GD. Surfactant aggregation in rat lungs: influence of temperature and ventilation. J Appl Physiol. 1981;51:646–653. doi: 10.1152/jappl.1981.51.3.646. [DOI] [PubMed] [Google Scholar]
- 18.Massaro D, Clerch L, Temple D, Baier H. Surfactant deficiency in rats without a decreased amount of extracellular surfactant. J Clin Invest. 1983;71:1536–1543. doi: 10.1172/JCI110909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Littleton SW. Impact of obesity on respiratory function. Respirology. 2012;17:43–49. doi: 10.1111/j.1440-1843.2011.02096.x. [DOI] [PubMed] [Google Scholar]
- 20.O’Donnell DE, Ciavaglia CE, Neder JA. When obesity and chronic obstructive pulmonary disease collide. Physiological and clinical consequences. Ann Am Thorac Soc. 2014;11:635–644. doi: 10.1513/AnnalsATS.201312-438FR. [DOI] [PubMed] [Google Scholar]
- 21.Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol (1985) 2010;108:206–211. doi: 10.1152/japplphysiol.00694.2009. [DOI] [PubMed] [Google Scholar]
- 22.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130:827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 23.Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K. Effects of obesity on respiratory function. Am Rev Respir Dis. 1983;128:501–506. doi: 10.1164/arrd.1983.128.3.501. [DOI] [PubMed] [Google Scholar]
- 24.Behazin N, Jones SB, Cohen RI, Loring SH. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol (1985) 2010;108:212–218. doi: 10.1152/japplphysiol.91356.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedenstierna G, Santesson J. Breathing mechanics, dead space and gas exchange in the extremely obese, breathing spontaneously and during anaesthesia with intermittent positive pressure ventilation. Acta Anaesthesiol Scand. 1976;20:248–254. doi: 10.1111/j.1399-6576.1976.tb05036.x. [DOI] [PubMed] [Google Scholar]
- 26.Naimark A, Cherniack RM. Compliance of the respiratory system and its components in health and obesity. J Appl Physiol. 1960;15:377–382. doi: 10.1152/jappl.1960.15.3.377. [DOI] [PubMed] [Google Scholar]
- 27.Pelosi P, Croci M, Ravagnan I, Tredici S, Pedoto A, Lissoni A, Gattinoni L. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg. 1998;87:654–660. doi: 10.1097/00000539-199809000-00031. [DOI] [PubMed] [Google Scholar]
- 28.Pelosi P, Croci M, Ravagnan I, Vicardi P, Gattinoni L. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest. 1996;109:144–151. doi: 10.1378/chest.109.1.144. [DOI] [PubMed] [Google Scholar]
- 29.Sharp JT, Henry JP, Sweany SK, Meadows WR, Pietras RJ. The total work of breathing in normal and obese men. J Clin Invest. 1964;43:728–739. doi: 10.1172/JCI104957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharp JT, Henry JP, Sweany SK, Meadows WR, Pietras RJ. Effects of mass loading the respiratory system in man. J Appl Physiol. 1964;19:959–966. doi: 10.1152/jappl.1964.19.5.959. [DOI] [PubMed] [Google Scholar]
- 31.Douglas FG, Chong PY. Influence of obesity on peripheral airways patency. J Appl Physiol. 1972;33:559–563. doi: 10.1152/jappl.1972.33.5.559. [DOI] [PubMed] [Google Scholar]
- 32.Collins LC, Hoberty PD, Walker JF, Fletcher EC, Peiris AN. The effect of body fat distribution on pulmonary function tests. Chest. 1995;107:1298–1302. doi: 10.1378/chest.107.5.1298. [DOI] [PubMed] [Google Scholar]
- 33.Lazarus R, Sparrow D, Weiss ST. Effects of obesity and fat distribution on ventilatory function: the normative aging study. Chest. 1997;111:891–898. doi: 10.1378/chest.111.4.891. [DOI] [PubMed] [Google Scholar]
- 34.Pellegrino RG, Gobbi A, Antonelli A, Torchio R, Gulotta C, Pellegrino GM, Dellacà R, Hyatt RE, Brusasco V. Ventilation heterogeneity in obesity. J Appl Physiol (1985) 2014;116:1175–1181. doi: 10.1152/japplphysiol.01339.2013. [DOI] [PubMed] [Google Scholar]
- 35.Leith DE, Mead J. Mechanisms determining residual volume of the lungs in normal subjects. J Appl Physiol. 1967;23:221–227. doi: 10.1152/jappl.1967.23.2.221. [DOI] [PubMed] [Google Scholar]
- 36.Eberlein M, Permutt S, Brown RH, Brooker A, Chahla MF, Bolukbas S, Nathan SD, Pearse DB, Orens JB, Brower RG. Supranormal expiratory airflow after bilateral lung transplantation is associated with improved survival. Am J Respir Crit Care Med. 2011;183:79–87. doi: 10.1164/rccm.201004-0593OC. [DOI] [PubMed] [Google Scholar]
- 37.Eberlein M, Permutt S, Chahla MF, Bolukbas S, Nathan SD, Shlobin OA, Shelhamer JH, Reed RM, Pearse DB, Orens JB, et al. Lung size mismatch in bilateral lung transplantation is associated with allograft function and bronchiolitis obliterans syndrome. Chest. 2012;141:451–460. doi: 10.1378/chest.11-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chacon RA, Corris PA, Dark JH, Gibson GJ. Respiratory mechanics after heart-lung and bilateral lung transplantation. Thorax. 1997;52:718–722. doi: 10.1136/thx.52.8.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, Wright AC, Gefter WB, Litzky L, Coxson HO, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365:1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Donnell DE, Deesomchok A, Lam YM, Guenette JA, Amornputtisathaporn N, Forkert L, Webb KA. Effects of BMI on static lung volumes in patients with airway obstruction. Chest. 2011;140:461–468. doi: 10.1378/chest.10-2582. [DOI] [PubMed] [Google Scholar]
- 41.Ora J, Laveneziana P, Wadell K, Preston M, Webb KA, O’Donnell DE. Effect of obesity on respiratory mechanics during rest and exercise in COPD. J Appl Physiol (1985) 2011;111:10–19. doi: 10.1152/japplphysiol.01131.2010. [DOI] [PubMed] [Google Scholar]
- 42.Barr RG, Ahmed FS, Carr JJ, Hoffman EA, Jiang R, Kawut SM, Watson K. Subclinical atherosclerosis, airflow obstruction and emphysema: the MESA Lung Study. Eur Respir J. 2012;39:846–854. doi: 10.1183/09031936.00165410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao C, Wang R, Wang J, Bunjhoo H, Xu Y, Xiong W. Body mass index and mortality in chronic obstructive pulmonary disease: a meta-analysis. PLoS ONE. 2012;7:e43892. doi: 10.1371/journal.pone.0043892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guenette JA, Jensen D, O’Donnell DE. Respiratory function and the obesity paradox. Curr Opin Clin Nutr Metab Care. 2010;13:618–624. doi: 10.1097/MCO.0b013e32833e3453. [DOI] [PubMed] [Google Scholar]
- 45.Verleden SE, Vasilescu DM, Willems S, Ruttens D, Vos R, Vandermeulen E, Hostens J, McDonough JE, Verbeken EK, Verschakelen J, et al. The site and nature of airway obstruction after lung transplantation. Am J Respir Crit Care Med. 2014;189:292–300. doi: 10.1164/rccm.201310-1894OC. [DOI] [PubMed] [Google Scholar]
- 46.Eberlein M, Reed RM, Permutt S, Chahla MF, Bolukbas S, Nathan SD, Iacono A, Pearse DB, Fessler HE, Shah AS, et al. Parameters of donor-recipient size mismatch and survival after bilateral lung transplantation J Heart Lung Transplant 2012311207–1213.e1207 [DOI] [PubMed] [Google Scholar]
- 47.Eberlein M, Diehl E, Bolukbas S, Merlo CA, Reed RM. An oversized allograft is associated with improved survival after lung transplantation for idiopathic pulmonary arterial hypertension. J Heart Lung Transplant. 2013;32:1172–1178. doi: 10.1016/j.healun.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 48.Eberlein M, Reed RM, Bolukbas S, Parekh KR, Arnaoutakis GJ, Orens JB, Brower RG, Shah AS, Hunsicker L, Merlo CA. Lung size mismatch and survival after single and bilateral lung transplantation. Ann Thorac Surg. 2013;96:457–463. doi: 10.1016/j.athoracsur.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 49.Eberlein M, Reed RM, Maidaa M, Bolukbas S, Arnaoutakis GJ, Orens JB, Brower RG, Merlo CA, Hunsicker LG. Donor-recipient size matching and survival after lung transplantation: a cohort study. Ann Am Thorac Soc. 2013;10:418–425. doi: 10.1513/AnnalsATS.201301-008OC. [DOI] [PubMed] [Google Scholar]
- 50.Weigt SS, DerHovanessian A, Wallace WD, Lynch JP, III, Belperio JA. Bronchiolitis obliterans syndrome: the Achilles’ heel of lung transplantation. Semin Respir Crit Care Med. 2013;34:336–351. doi: 10.1055/s-0033-1348467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yalcin HC, Perry SF, Ghadiali SN. Influence of airway diameter and cell confluence on epithelial cell injury in an in vitro model of airway reopening. J Appl Physiol (1985) 2007;103:1796–1807. doi: 10.1152/japplphysiol.00164.2007. [DOI] [PubMed] [Google Scholar]
- 52.Dixon DL, De Pasquale CG, De Smet HR, Klebe S, Orgeig S, Bersten AD. Reduced surface tension normalizes static lung mechanics in a rodent chronic heart failure model. Am J Respir Crit Care Med. 2009;180:181–187. doi: 10.1164/rccm.200809-1506OC. [DOI] [PubMed] [Google Scholar]
- 53.Haber PS, Colebatch HJ, Ng CK, Greaves IA. Alveolar size as a determinant of pulmonary distensibility in mammalian lungs. J Appl Physiol. 1983;54:837–845. doi: 10.1152/jappl.1983.54.3.837. [DOI] [PubMed] [Google Scholar]
- 54.Alexander HLKW. Symptomatic relief of emphysema by an abdominal belt. Am J Med Sci. 1934;187:687–692. [Google Scholar]
- 55.Dodd DS, Brancatisano TP, Engel LA. Effect of abdominal strapping on chest wall mechanics during exercise in patients with severe chronic air-flow obstruction. Am Rev Respir Dis. 1985;131:816–821. doi: 10.1164/arrd.1985.131.6.816. [DOI] [PubMed] [Google Scholar]
- 56.Torchio R, Gulotta C, Ciacco C, Perboni A, Guglielmo M, Crosa F, Zerbini M, Brusasco V, Hyatt RE, Pellegrino R. Effects of chest wall strapping on mechanical response to methacholine in humans. J Appl Physiol (1985) 2006;101:430–438. doi: 10.1152/japplphysiol.00379.2005. [DOI] [PubMed] [Google Scholar]
- 57.Harty HR, Corfield DR, Schwartzstein RM, Adams L. External thoracic restriction, respiratory sensation, and ventilation during exercise in men. J Appl Physiol (1985) 1999;86:1142–1150. doi: 10.1152/jappl.1999.86.4.1142. [DOI] [PubMed] [Google Scholar]
- 58.Brack T, Jubran A, Tobin MJ. Effect of elastic loading on variational activity of breathing. Am J Respir Crit Care Med. 1997;155:1341–1348. doi: 10.1164/ajrccm.155.4.9105077. [DOI] [PubMed] [Google Scholar]
- 59.van der Kaaij NP, Kluin J, Haitsma JJ, den Bakker MA, Lambrecht BN, Lachmann B, de Bruin RW, Bogers AJ.Surfactant pretreatment decreases long-term damage after ischemia-reperfusion injury of the lung Eur J Cardiothorac Surg 200935304–312.discussion 312 [DOI] [PubMed] [Google Scholar]
- 60.Semik M, Schnabel R, Bruske T, Lange V, Wottge H, Morgenrot K, Toomes H. Ultrastructural studies of acute rejection following single lung transplantation in the rat—histological and immunohistological findings. Thorac Cardiovasc Surg. 1994;42:290–297. doi: 10.1055/s-2007-1016507. [DOI] [PubMed] [Google Scholar]
- 61.Waldhausen JA, Giammona ST, Kilman JW, Daly WJ. Effect of transplantation of canine lung on pulmonary compliance and surfactant. JAMA. 1965;191:1002–1005. doi: 10.1001/jama.1965.03080120036009. [DOI] [PubMed] [Google Scholar]
- 62.Thomas PA, Jolly PC. Preservation of pulmonary surfactant activity in canine lung allografts by immune suppressive therapy. J Thorac Cardiovasc Surg. 1968;55:405–410. [PubMed] [Google Scholar]
- 63.D’Ovidio F, Mura M, Ridsdale R, Takahashi H, Waddell TK, Hutcheon M, Hadjiliadis D, Singer LG, Pierre A, Chaparro C, et al. The effect of reflux and bile acid aspiration on the lung allograft and its surfactant and innate immunity molecules SP-A and SP-D. Am J Transplant. 2006;6:1930–1938. doi: 10.1111/j.1600-6143.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- 64.Ray NB, Durairaj L, Chen BB, McVerry BJ, Ryan AJ, Donahoe M, Waltenbaugh AK, O’Donnell CP, Henderson FC, Etscheidt CA, et al. Dynamic regulation of cardiolipin by the lipid pump Atp8b1 determines the severity of lung injury in experimental pneumonia. Nat Med. 2010;16:1120–1127. doi: 10.1038/nm.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]