Abstract
Rationale: Rehabilitation started early during an intensive care unit (ICU) stay is associated with improved outcomes and is the basis for many quality improvement (QI) projects showing important changes in practice. However, little evidence exists regarding whether such changes are sustainable in real-world practice.
Objectives: To evaluate the sustained effect of a quality improvement project on the timing of initiation of active physical therapy intervention in patients with acute lung injury (ALI).
Methods: This was a pre–post evaluation using prospectively collected data involving consecutive patients with ALI admitted pre–quality improvement (October 2004–April 2007, n = 120) versus post–quality improvement (July 2009–July 2012, n = 123) from a single medical ICU.
Measurements and Main Results: The primary outcome was time to first active physical therapy intervention, defined as strengthening, mobility, or cycle ergometry exercises. Among ICU survivors, more patients in the post–quality improvement versus pre–quality improvement group received physical therapy in the ICU (89% vs. 24%, P < 0.001) and were able to stand, transfer, or ambulate during physical therapy in the ICU (64% vs. 7%, P < 0.001). Among all patients in the post–quality improvement versus pre–quality improvement group, there was a shorter median (interquartile range) time to first physical therapy (4 [2, 6] vs. 11 d [6, 29], P < 0.001) and a greater median (interquartile range) proportion of ICU days with physical therapy after initiation (50% [33, 67%] vs. 18% [4, 47%], P = 0.003). In multivariable regression analysis, the post–quality improvement period was associated with shorter time to physical therapy (adjusted hazard ratio [95% confidence interval], 8.38 [4.98, 14.11], P < 0.001), with this association significant for each of the 5 years during the post–quality improvement period. The following variables were independently associated with a longer time to physical therapy: higher Sequential Organ Failure Assessment score (0.93 [0.89, 0.97]), higher FiO2 (0.86 [0.75, 0.99] for each 10% increase), use of an opioid infusion (0.47 [0.25, 0.89]), and deep sedation (0.24 [0.12, 0.46]).
Conclusions: In this single-site, pre–post analysis of patients with ALI, an early rehabilitation quality improvement project was independently associated with a substantial decrease in the time to initiation of active physical therapy intervention that was sustained over 5 years. Over the entire pre–post period, severity of illness and sedation were independently associated with a longer time to initiation of active physical therapy intervention in the ICU.
Keywords: rehabilitation, acute lung injury, critical illness, intensive care unit, quality improvement
As short-term mortality for patients with acute lung injury (ALI) decreases (1–4), there is a growing population of survivors who frequently experience long-lasting physical impairments (5–10). Patients with ALI are often exposed to prolonged immobilization, contributing to neuromuscular weakness that has a negative impact on the survivors’ physical function and quality of life for years after discharge from the intensive care unit (ICU) (6–11). Early rehabilitation, including physical therapy in the ICU, reduces neuromuscular weakness and improves physical function and quality of life in ALI survivors (12–20).
Despite evidence showing improved outcomes with early physical therapy in the ICU, many critically ill patients remain immobilized for prolonged periods, with initiation of physical therapy delayed until mechanical ventilation is discontinued and the patient is discharged from the ICU (14, 19, 21–25). There are several potentially modifiable barriers to incorporating early physical therapy into routine clinical practice, including: inadequate multidisciplinary education, staffing, and collaboration; insufficient knowledge; and deep sedation (16, 21, 24, 26, 27). Multiple centers have addressed such potential barriers to early physical therapy by successfully implementing structured quality improvement (QI) projects (28–36). These projects, along with clinical trials and observational studies, have demonstrated that early physical therapy in critically ill patients is safe and feasible (12–16, 18, 26, 27, 37–41). However, there is little published evidence assessing whether QI projects can be sustained beyond the period of their initial implementation and evaluation. Sustainability is an important aspect in evaluating QI interventions because, over time, clinician practice may revert to earlier routines when there is a focus on new areas of practice (42).
Hence, our objective is to evaluate the sustainability of an early rehabilitation QI project in a single medical ICU (MICU) and to evaluate how the QI project and other patient- and ICU-related factors are associated with the timing of initiation of active physical therapy intervention in the MICU.
Some of the results of these studies have been previously reported in the form of an abstract (43).
Methods
Study Design
An early rehabilitation QI project was conducted from May to August 2007 at the Johns Hopkins Hospital MICU. This project aimed to reduce modifiable barriers to early rehabilitation interventions for all MICU patients (28, 44). Details of this QI project are provided elsewhere (28, 44) and summarized herein. The QI project followed a preestablished structured methodology (44, 45) and included the following components: (1) changing the default MICU activity order from “bed rest” to “as tolerated,” (2) encouraging a change in sedation practice from continuous infusions to “as-needed” boluses, (3) establishing simple guidelines for consultation to rehabilitation therapy, (4) establishing safety guidelines for initiating early rehabilitation, and (5) obtaining full-time dedicated MICU rehabilitation therapist staffing (28). The project was planned and implemented via a multidisciplinary team that met weekly to plan and evaluate the project.
After evaluation of the QI project, the hospital administration funded an ongoing, early rehabilitation program starting the next fiscal year, July 2008 onward. In addition to maintaining the QI project components described above, a new protocol for sedation management and delirium screening was implemented to formalize changes made during the QI project starting July 2009 (46). Sedation management during the both the pre- and post-QI periods included daily sedation interruption and goal-directed sedation, but in the post-QI period the following refinements were made: emphasizing sedation goal of Richmond Agitation Sedation Scale (RASS) = 0 (alert and calm), using “as-needed” boluses of sedative medications rather than continuous infusions whenever possible, and training bedside nurses to recognize delirium and anticipate agitated delirium as patients “awoke” from sedation (46). Moreover, to further promote sustainability, the multidisciplinary weekly meetings continued with a focus on identifying and addressing new barriers, evaluating individual patient needs, inspiring innovation in early rehabilitation practices (47–49), and continuing interdisciplinary education and collaboration with more than 20 physical therapists who worked in the MICU during the post-QI period. Moreover, safety events (37, 39) and mobility milestones continued to be evaluated with feedback provided, at least monthly, in the multidisciplinary meetings. Meetings with hospital administrators, highlighting the program’s successes and sustained reduction in ICU length of stay (50), were scheduled regularly.
To evaluate the sustainability of a change in the timing of initiation of active physical therapy intervention, we conducted a pre–post comparison study using prospectively collected data. Data for the pre-QI control period was obtained from a prospective cohort study of consecutive patients meeting the 1994 American-European Consensus Conference criteria for ALI admitted to the Johns Hopkins MICU from October 2004 to April 2007 (51). To evaluate the sustainability of the QI project, data for the post-QI period started 1 year after initiation of the early rehabilitation program (i.e., July 2009 onward). These data were obtained from a preexisting clinical registry of consecutive patients admitted to the Johns Hopkins MICU from July 2009 until July 2012. For both the pre- and post-QI groups, all patients evaluated in this analysis were consecutive mechanically ventilated patients meeting the American-European Consensus Conference criteria for ALI (52). The prospective cohort study providing the pre-QI control group data excluded patients with any of the following characteristics: (1) preexisting cognitive impairment, (2) non-English speaking, (3) life expectancy less than 6 months due to a preexisting illness, (4) limitations in care (e.g., an order for no vasopressors) at time of meeting ALI criteria, and (5) fewer than 5 days of mechanical ventilation before ALI or transfer from another ICU with preexisting ALI (>24 h). To ensure comparability, these same exclusion criteria were applied to the post-QI group.
Primary Outcome
The primary outcome is the time (in days) from ALI onset to initiation of active physical therapy intervention during the patient’s index MICU admission. We defined active physical therapy intervention as providing strengthening or mobility exercises both in bed and out of bed, and/or cycle ergometry exercises, based on physical therapist documentation. Although passive physical therapy interventions may have benefit, especially in preserving patients’ range of motion (53, 54), most ICU-based rehabilitation research has evaluated the benefits of active interventions in the ICU (13, 14, 20), which was the rationale for focusing on active intervention in this QI evaluation.
Secondary Outcome
Physiological abnormalities and potential safety events were not collected for the pre-QI period. These events were prospectively collected during the post-QI period and defined as any of the following occurring during physical therapy intervention: (1) removal, dislodgment, disruption, or dysfunction of airway, feeding tube, chest tube, vascular access, cardiac device, or wound dressing; (2) cardiopulmonary changes including arrhythmia, desaturation, and hyper- and hypotension; (3) falls; (4) cardiac arrest; and (5) death (37).
Covariates
Patient and ICU-related variables collected from the medical record were included in the analysis. Patient variables were age, sex, race, and body mass index (BMI). Baseline comorbidities were measured using the Charlson (55) and Functional (56) comorbidity indices. ICU-related variables were ICU admission diagnosis category and the Acute Physiology and Chronic Health Evaluation II (APACHE II) score within the first 24 hours of ICU admission (57). Daily organ failure status was measured via Sequential Organ Failure Assessment (SOFA) score (58). Daily mechanical ventilation status, FiO2, and positive end-expiratory pressure (PEEP) data were collected from morning ventilator settings. Daily benzodiazepine and opioid infusion status were measured in addition to daily morning sedation and delirium status using the RASS (59) and Confusion Assessment Method for the ICU (60), respectively. Patient sedation status was qualitatively classified as follows: awake (RASS ≥ −1), lightly sedated (RASS, −2 or −3), or deeply sedated/comatose (RASS, −4 or −5).
Statistical Analyses
Patient and ICU-related variables were compared between the pre-QI and post-QI groups using Pearson chi-square, Fisher exact, or Wilcoxon rank-sum tests as appropriate. Sedation and delirium assessments were not completed as part of routine care in the pre-QI period but completed by study staff. Hence, missing data (e.g., due to lack of staff coverage on weekends or during holidays) were imputed using multiple imputation (61) (with five datasets) as described elsewhere (62).
We compared time to initiation of active physical therapy intervention for patients admitted to the MICU during the post-QI versus pre-QI periods. We used locally weighted scatterplot smoothing to confirm linearity of the association between each continuous covariate and the outcome variable. Based on this analysis, all continuous covariates were entered into the model as linear predictors, with the exception of BMI, which was dichotomized as obese versus not obese. Bivariable associations of patient and ICU-related variables with time to initiation of active physical therapy intervention were evaluated using Fine and Gray proportional subdistribution hazards regression analysis. The Fine and Gray method was used to account for the competing risk of mortality while analyzing associations of patient and ICU characteristics with the time to first physical therapy intervention (63). To evaluate for violations of the proportionality assumption in the regression analysis, Schoenfeld residuals were plotted versus functions of time (64). Covariates with a bivariable association with time to first physical therapy intervention of P less than 0.150 were included in the multivariable Fine and Gray regression model. Finally, to evaluate the time to first active physical therapy intervention during each year after the post-QI period, the post-QI calendar year or portion thereafter (i.e., 2009, 2010, 2011, 2012) was included in the model as a categorical variable with the pre-QI period as the reference group.
Multicollinearity was assessed in the multivariable regression model using variance inflation factors (65). This analysis demonstrated that daily delirium status was strongly collinear with daily sedation status, and therefore delirium status was excluded from the final regression model. Cumulative incidence function curves were created by first fitting the final multivariable regression model to each of the five imputed sedation status datasets and obtaining the average cumulative incidence of initiation of active physical therapy intervention.
We assessed for statistical interaction between admission during the post-QI period and the following clinically relevant covariates based on a priori hypotheses: age, BMI, APACHE II score, SOFA score, mechanical ventilation status, FiO2, PEEP, and sedation status. Interaction terms were added individually to the model, with evaluation for statistical significance. Statistical analyses were performed using STATA 12.0 software (Stata Corporation, College Station, TX). Statistical significance was defined as a two-sided P less than 0.05. The institutional review board at Johns Hopkins University approved this evaluation.
Results
Patients in the pre-QI (n = 120) versus post-QI (n = 123) periods were similar in most demographic and ICU-related variables (Table 1). Across the entire patient cohort (n = 243), 52% were women, with a median (interquartile range [IQR]) age and APACHE II score of 49 (40, 59) years and 29 (24, 35), respectively. Moreover, among all patients, 92% had a PaO2/FiO2 less than 200 (46% had PaO2/FiO2 < 100) at ALI onset, and a median (IQR) daily average PEEP of 6.9 cm H2O (5.0, 9.3). ICU mortality was 45% (not significantly different in post-QI versus pre-QI: 41 vs. 48%, P = 0.282). In the post-QI versus pre-QI period, patients were more frequently admitted from an outside hospital (34 vs. 18%), and patients were less sedated with a higher median (IQR) daily average RASS score (−2.1 [−3.5, −1.0] vs. −3.2 [−4.3, −2.4], P < 0.001) and lower median (IQR) proportion of ICU days on a benzodiazepine infusion (50% [0, 100] vs. 72% [49, 100], P < 0.001) and with deep sedation/coma (33% [0, 70] vs. 60% [38, 88], P < 0.001).
Table 1.
Baseline characteristics
| All*,† (n = 243) | Pre-QI (n = 120) | Post-QI (n = 123) | P Value‡ | |
|---|---|---|---|---|
| Age, median (IQR), yr | 49 (40, 59) | 48 (40, 57) | 51 (37, 63) | 0.163 |
| Female, n (%) | 127 (52) | 57 (47) | 70 (57) | 0.142 |
| White race, n (%) | 106 (44) | 47 (39) | 59 (48) | 0.167 |
| BMI, median (IQR) | 26 (22, 32) | 27 (23, 32) | 26 (22, 32) | 0.601 |
| BMI ≥ 30, n (%) | 97 (40) | 43 (36) | 54 (44) | 0.238 |
| Charlson comorbidity index, median (IQR) | 2 (1, 4) | 3 (1, 5) | 2 (0, 4) | <0.001 |
| Functional comorbidity index, median (IQR) | 1 (1, 3) | 1 (1, 2) | 1 (1, 3) | 0.164 |
| ICU admission source, n (%) | 0.025 | |||
| Emergency room | 63 (26) | 33 (28) | 30 (24) | |
| Ward | 106 (44) | 61 (51) | 45 (37) | |
| Other ICU | 10 (4) | 4 (3) | 6 (5) | |
| Other hospital | 64 (26) | 22 (18) | 42 (34) | |
| ICU admission diagnosis, n (%) | 0.069 | |||
| Respiratory (including pneumonia) | 149 (61) | 80 (67) | 69 (56) | |
| Sepsis/infectious disease | 47 (19) | 18 (15) | 29 (24) | |
| Gastrointestinal | 25 (10) | 14 (12) | 11 (9) | |
| Cardiovascular | 6 (2) | 4 (3) | 2 (2) | |
| Central nervous system | 4 (2) | 2 (2) | 2 (2) | |
| Other | 12 (5) | 2 (2) | 10 (8) | |
| APACHE II at ICU admission, median (IQR) | 29 (24, 35) | 29 (23, 36) | 29 (25, 35) | 0.906 |
| SOFA-Respiratory score Day 1, median (IQR) | 3 (3, 4) | 4 (3, 4) | 3 (3, 4) | 0.079 |
| PaO2/FiO2 on Day 1, n (%) | 0.109 | |||
| ≥200 | 20 (8) | 10 (9) | 10 (9) | |
| ≥100 and <200 | 105 (46) | 46 (39) | 59 (52) | |
| <100 | 105 (46) | 61 (52) | 44 (39) | |
| Mean daily SOFA score, median (IQR) | 10 (6, 14) | 10 (6, 15) | 10 (7, 13) | 0.980 |
| Proportion of ICU days on mechanical ventilation, median (IQR) | 83 (67, 95) | 80 (67, 91) | 86 (67, 100) | 0.047 |
| Average FiO2, median (IQR)§ | 54 (44, 67) | 52 (43, 63) | 56 (47, 68) | 0.065 |
| Average PEEP, median (IQR)§ | 6.9 (5.0, 9.3) | 6.7 (5.0, 9.3) | 7.3 (5.0, 9.3) | 0.322 |
| Proportion of ICU days on benzodiazepine infusion, median (IQR) | 60 (29, 100) | 72 (49, 100) | 50 (0, 100) | <0.001 |
| Proportion of ICU days on opioid infusion, median (IQR) | 74 (39, 100) | 80 (50, 100) | 67 (14, 100) | 0.056 |
| Average RASS, median (IQR)§,|| | −2.9 (−3.9, −1.7) | −3.2 (−4.3, −2.4) | −2.1 (−3.5, −1.0) | 0.001 |
| Proportion of ICU days, median (IQR)|| | ||||
| Awake | 26 (0, 50) | 21 (2, 40) | 33 (0, 67) | 0.093 |
| Lightly sedated | 14 (0, 32) | 11 (0, 26) | 20 (0, 35) | 0.334 |
| Deeply sedated/comatose | 51 (17, 81) | 60 (38, 88) | 33 (0, 70) | <0.001 |
| Proportion of ICU days with delirium, median (IQR)§ | 32 (10, 56) | 25 (9, 42) | 43 (17, 75) | 0.008 |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; BMI = body mass index; ICU = intensive care unit; IQR = interquartile range; PEEP = positive end-expiratory pressure; QI = quality improvement; RASS = Richmond Agitation Sedation Scale; SOFA = Sequential Organ Failure Assessment.
Percentages may not add to 100 due to rounding.
All time-varying data, except when indicated as Day 1 of acute lung injury (ALI), are from date of ALI to date of first physical therapy or ICU discharge.
Missing data pre-QI/post-QI: BMI, 2 of 20; Day 1 Sofa Respiratory Score (P/F), 3 of 10; FiO2,1 of 6; PEEP, 1 of 9; RASS, 0 of 3.
Comparing pre-QI and post-QI groups and calculated using Pearson chi-square, Fisher exact, and Wilcoxon rank-sum tests, as appropriate.
Variable was averaged for each patient from the date of ALI to the date of first physical therapy, death, or ICU discharge, whichever came first, and then the median (IQR) of the averages across all patients was reported.
Patient sedation status was classified as follows: awake (RASS ≥ −1), lightly sedated (RASS, −2 or −3), or deeply sedated/comatose (RASS, −4 or −5). Five imputed datasets were used to address missing data for RASS and Confusion Assessment Method for the ICU in the pre-QI group; the data presented represents the mean value for the median (IQR) of the five datasets using a pooled Z score to obtain the P values (69).
Among all patients, 68% in post-QI and 16% in pre-QI group ever received physical therapy (Table 2). Moreover, those in the post-QI versus pre-QI group had a shorter median (IQR) time to first physical therapy intervention (4 [2, 6] vs. 11 [6, 29] days, P < 0.001), greater median (IQR) proportion of ICU days with physical therapy after its initiation (50% [33, 67] vs. 18% [4, 47], P = 0.003), and a greater proportion of patients achieving a highest daily activity level of standing, transferring, and/or ambulating during physical therapy treatment in the ICU (41% vs. 4%, P < 0.001) (Table 2). When restricting analyses to those surviving until ICU discharge, the median (IQR) time to first physical therapy intervention remained shorter in the post-QI versus pre-QI group (4 [2, 6] vs. 12 [6, 29], P < 0.001), and an even greater proportion of patients in the post-QI versus pre-QI group ever received physical therapy in the ICU (89% vs. 24%, P < 0.001) and achieved a highest daily activity level of standing, transferring, and/or ambulating in the ICU (64% vs. 7%, P < 0.001). There were no prospectively recorded physiological abnormalities or potential safety events in the post-QI group (Table 2).
Table 2.
Active physical therapy intervention in the medical intensive care unit, before and after a quality improvement project
| Pre-QI (n = 120) | Post-QI (n = 123) | P Value* | |
|---|---|---|---|
| Ever receiving active PT intervention, n (%)† | 19 (16) | 84 (68) | <0.001 |
| Days to first active PT intervention, median (IQR) | 11 (6, 29) | 4 (2, 6) | <0.001 |
| No. of PT sessions in index MICU admission, median (IQR) | 0 (0, 0) | 3 (0, 5) | <0.001 |
| % ICU days with PT session, median (IQR) | 0 (0, 0) | 23 (0, 43) | <0.001 |
| % ICU days with PT session after initiation, median (IQR) | 18 (4, 47) | 50 (33, 67) | 0.003 |
| Physiological abnormality or potential safety events, n (% of PT treatment sessions)‡ | — | 0 (0) | N/A |
| Highest daily activity during PT session during ICU stay, n (%) | <0.001 | ||
| Lying or sitting in bed | 113 (94) | 60 (49) | |
| Sitting at edge of bed | 3 (3) | 13 (11) | |
| Standing or transfer to chair | 3 (3) | 28 (23) | |
| Walking | 1 (1) | 22 (18) |
Definition of abbreviations: ICU = intensive care unit; IQR = interquartile range; N/A = not applicable; PT = physical therapy; QI = quality improvement.
Calculated using Pearson chi-square, Fisher exact, and Wilcoxon rank-sum tests, as appropriate.
The number (%) of patients receiving active PT, by year, during the post-QI period is as follows, 2009: 18 (67); 2010: 22 (61); 2011: 31 (78); and 2012: 13 (65).
Safety events were not collected for the pre-QI period. Safety events were prospectively collected during the post-QI period and defined as any of the following: (1) removal, dislodgment, disruption, or dysfunction of airway, feeding tube, chest tube, vascular access, cardiac device, or wound dressing; (2) cardiovascular/hemodynamic instability that includes hyper- and hypotension, desaturation, cardiac arrest, arrhythmia, and death; or (3) falls (37).
In regression analysis, being admitted during the post-QI period was independently associated with a shorter time to initiation of active physical therapy intervention (adjusted hazard ratio [HR] [95% confidence interval (CI)]: 8.38 [4.98, 14.11], P < 0.001) (Table 3; Figure 1). In a post hoc analysis, the pre-QI period was compared with each of the 5 calendar years (or portion thereof) within the 5-year post-QI period demonstrating a significantly shorter time to physical therapy across each of the 5 years without any temporal trends in the results (adjusted HR, [95% CI]: 2009: 7.08 [3.36, 14.95]; 2010: 8.44 [4.47, 15.96]; 2011: 10.96 [6.34, 18.96]; 2012: 6.53 [3.23, 13.19]; all P < 0.001). Among ICU-related variables (analyzed as time-varying daily exposures), the following were significantly associated with longer time to initiation of active physical therapy intervention (adjusted HR, [95% CI]): higher SOFA score (0.93 [0.89, 0.97], P < 0.001), higher FiO2 (0.86 [0.75, 0.99] for a 10% increase, P = 0.038), any opioid infusion (0.47 [0.25, 0.89], P = 0.021), and deep sedation/coma (0.24 [0.12, 0.46], P < 0.001). None of the a priori selected exposures (BMI, APACHE II score, SOFA score, mechanical ventilation status, FiO2, PEEP, and sedation status) had any significant statistical interaction with the post-QI (versus pre-QI) period and its association with timing of initiation of active physical therapy intervention in the ICU.
Table 3.
Factors associated with time to initiating physical therapy in the medical intensive care unit
| Unadjusted* |
Adjusted |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Age, yr | 1.00 | 0.99–1.02 | 0.523 | |||
| Male | 0.90 | 0.62–1.31 | 0.576 | |||
| White (vs. nonwhite) | 1.33 | 0.92–1.93 | 0.134 | 1.14 | 0.78–1.68 | 0.496 |
| BMI ≥ 30 kg/m2 | 0.68 | 0.46–1.02 | 0.059 | 0.71 | 0.48–1.06 | 0.096 |
| Charlson comorbidity index | 0.91 | 0.83–0.98 | 0.020 | 0.97 | 0.91–1.04 | 0.400 |
| Functional comorbidity index | 1.04 | 0.93–1.16 | 0.471 | |||
| Admission during early rehabilitation | 7.03 | 4.40–11.22 | <0.001 | 8.38 | 4.98–14.11 | <0.001 |
| Source of ICU admission | ||||||
| Emergency room | 1.00 | |||||
| Ward | 1.02 | 0.63–1.65 | 0.945 | |||
| Other ICU | 1.34 | 0.54–3.32 | 0.525 | |||
| Other hospital | 1.28 | 0.76–2.14 | 0.351 | |||
| ICU admission diagnosis | ||||||
| Respiratory (including pneumonia) | 1.00 | |||||
| Sepsis/infectious disease | 0.84 | 0.53–1.33 | 0.453 | |||
| Gastrointestinal | 0.56 | 0.28–1.11 | 0.096 | |||
| Cardiovascular | 0.37 | 0.05–2.56 | 0.311 | |||
| Central nervous system | 1.33 | 0.29–6.22 | 0.714 | |||
| Other | 1.64 | 0.70–3.83 | 0.256 | |||
| APACHE II at ICU admission | 0.98 | 0.96–1.00 | 0.088 | 0.99 | 0.97–1.02 | 0.595 |
| Daily SOFA score | 0.90 | 0.87–0.93 | <0.001 | 0.93 | 0.89–0.97 | 0.001 |
| Daily mechanical ventilation status | 0.34 | 0.20–0.58 | <0.001 | 0.83 | 0.41–1.71 | 0.614 |
| FiO2 received (every 10% increase) | 0.69 | 0.62–0.78 | <0.001 | 0.86 | 0.75–0.99 | 0.038 |
| PEEP | 0.86 | 0.82–0.90 | <0.001 | 0.99 | 0.91–1.08 | 0.812 |
| Benzodiazepine infusion | 0.23 | 0.15–0.35 | <0.001 | 0.90 | 0.44–1.86 | 0.778 |
| Opioid infusion | 0.25 | 0.16–0.37 | <0.001 | 0.47 | 0.25–0.89 | 0.021 |
| Sedation status† | ||||||
| Awake | 1.00 | 1.00 | ||||
| Light sedation | 0.56 | 0.27–1.16 | 0.109 | 0.66 | 0.41–1.04 | 0.077 |
| Deep sedation/comatose | 0.09 | 0.05–0.17 | <0.001 | 0.24 | 0.12–0.46 | <0.001 |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; BMI = body mass index; CI = confidence interval; HR = hazard ratio; ICU = intensive care unit; PEEP = positive end-expiratory pressure; SOFA = Sequential Organ Failure Assessment.
HRs greater than 1 are interpreted as the variable being associated with earlier initiation of active physical therapy in the MICU. HRs were calculated using Fine and Gray regression models (63), with daily time-varying exposures for SOFA score, mechanical ventilation status, FiO2, PEEP, benzodiazepine infusion, opioid infusion, and sedation status.
Patient sedation status was classified as follows: awake (RASS ≥ −1), lightly sedated (RASS, −2 or −3), or deeply sedated/comatose (RASS, −4 or −5).
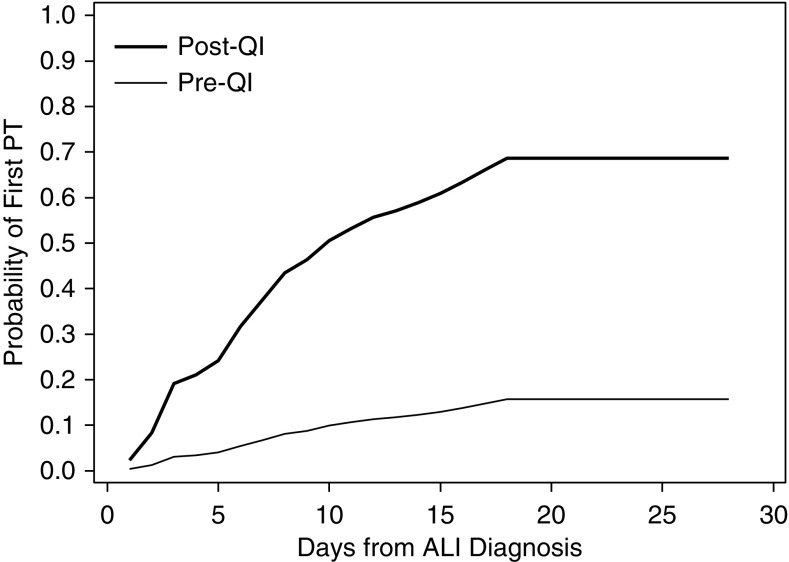
Figure 1.
Probability of active physical therapy (PT) intervention in the medical intensive care unit in the pre-quality improvement (QI) versus post-QI period. aEstimates are adjusted for all variables included in the multivariable regression model presented in Table 3. ALI = acute lung injury. Reprinted with permission from reference 43.
Discussion
In this single-site, pre–post evaluation of 243 patients with ALI, an early rehabilitation QI project was independently associated with a large and statistically significant decrease in the time to initiation of active physical therapy intervention, and this association was sustained for 5 years after completion of the QI project. There was no modification of the association between admission during the post-QI period and earlier initiation of active physical therapy intervention by any a priori selected patient- or ICU-related factors. Increased severity of illness and hypoxia, any opioid infusion, and deep sedation were independently associated with delayed initiation of active physical therapy intervention in the MICU.
To our knowledge, this is the first report of the sustainability of an ICU-based early rehabilitation QI project. Similar to prior publications on the implementation of successful QI projects, our MICU underwent a substantial culture change to engage the multidisciplinary team in promoting and executing the QI project (26). This culture change included education on the importance and success of early rehabilitation in the ICU, implementation of simple guidelines to ensure safe and timely referral to physical therapy, and improved multidisciplinary communication and clinical care. This report is novel because it demonstrates these changes can be incorporated into routine clinical practice across more than 20 physical therapists providing clinical care in the MICU, thereby contributing to successfully sustaining early rehabilitation long after completion of a QI project.
The time to initiation of active physical therapy intervention was significantly shorter in the post- versus pre-QI period (4 vs. 11 d) but longer than the 1.5 days reported in a randomized controlled trial (RCT) of 104 mechanically ventilated MICU patients (14). There are important distinctions between these two study designs that may account for this difference. The general aim of RCTs is to establish efficacy of an intervention, typically in the context of a resource-intensive research environment with a highly selected subset of consenting patients. These circumstances may not reflect routine clinical practice that is evaluated in QI projects (42, 66). For instance, in our evaluation versus the RCT, patients without baseline functional independence were not excluded and severity of illness was substantially higher as reflected by median APACHE II scores (29 vs. 20) and proportion of participants with ALI (100 vs. 55%).
Of those surviving until ICU discharge, 89% of patients in the post-QI group received physical therapy in the ICU, similar to the previously described RCT, in which 94% (46 of 49) of patients received physical therapy and occupational therapy in the ICU (14). Likewise, in another evaluation of an early mobility protocol including 116 mechanically ventilated MICU patients, 91% received physical therapy in the ICU (19). Among patients in the pre-QI control period in our evaluation, 16% ever received physical therapy in the ICU and 4% were mobilized out of bed. These findings are similar to other evaluations of routine ICU care. For instance, in a 1-day point prevalence study including 783 patients in 116 ICUs in Germany, 8% of patients with an endotracheal tube were mobilized out of bed (25). Moreover, in another point prevalence study of 514 patients in 38 New Zealand and Australian hospitals, no mechanically ventilated patients were mobilized out of bed (23). Finally, the control groups of ICU rehabilitation studies of mechanically ventilated patients (22) have demonstrated that 13% of mechanically ventilated patients received physical therapy in the ICU (19), and the median (IQR) duration of physical therapy and occupational therapy in the ICU was 0 (0, 0) minutes (14).
Two nonmodifiable risk factors, severity of organ failure and hypoxia, were associated with delayed initiation of physical therapy. In prior studies, increased organ failure (24, 67) and physiologic instability (25) were associated with delayed initiation of physical therapy, and respiratory instability also has been a barrier to early mobilization (21). Importantly, despite a high severity of illness in the post-QI patients, early physical therapy was initiated without the occurrence of any prospectively evaluated and defined major physiological abnormalities or potential safety events. These findings support existing literature demonstrating the safety of early rehabilitation in critically ill patients (12–14, 18, 19, 23, 37, 38).
In our analysis, there were two modifiable risk factors independently associated with delayed initiation of physical therapy: deep sedation and receiving an opioid infusion. Sedation is a known barrier to physical therapy in critically ill patients (16, 21, 25). In the current study, a sedation protocol was implemented coincident with the early rehabilitation program to reduce this modifiable barrier to physical therapy (46). Such pairing of an early rehabilitation program with a sedation protocol is common in other studies of ICU rehabilitation interventions (14, 28, 68). Despite adjusting for differences in sedation practice between pre- and post-QI periods, admission during the post-QI period remained significantly associated with earlier initiation of physical therapy.
There are possible limitations to this study. Because this was an observational pre–post evaluation, we cannot prove causation between the patient- and ICU-related variables evaluated and time to initiation of physical therapy, and residual confounding, particularly regarding change in practice over time, may influence the results. Nonetheless, our findings are consistent with prior RCTs evaluating early rehabilitation in critically ill patients (14, 18) and reports of barriers to mobilization (13, 16, 17, 21). Although we were able to demonstrate sustained earlier initiation of physical therapy interventions in the post-QI versus pre-QI periods, we are unable to provide detailed information comparing the pre-QI period to the QI period itself because the focus of the original QI project’s data collection was related to safety and feasibility issues, the number of physical therapy and occupational therapy consultations, and ICU length of stay, not the time to physical therapy intervention. We were unable to include average daily dose of neuromuscular blockers or sedatives in our analysis because these variables were not collected during the QI project. However, less than one-fourth of patients in the pre-QI period received neuromuscular blockade, and there was no significant difference in receipt of any sedative medications between patients in the pre- versus post-QI periods. Moreover, the study population consisted solely of patients with ALI (as defined by the American-European Consensus Conference criteria that were current at the time of the pre-QI period) in an adult MICU within a single academic hospital. Therefore, the findings may not be generalizable to other populations and settings. However, patients with ALI are the archetype of severely critically ill patients, thus supporting the generalizability of our findings to populations with a similar or lesser severity of illness (19, 29–36).
In conclusion, an early rehabilitation QI project was independently associated with a substantial decrease in the time to initiation of physical therapy intervention that was sustained for 5 years after project completion. The association between the post-QI rehabilitation program and earlier initiation of physical therapy was not modified by patient-related or ICU-specific factors. Modifiable factors independently associated with delayed initiation of physical therapy included opioid infusions and deep sedation, whereas severity of organ failure and hypoxia were nonmodifiable patient risk factors for delayed initiation. These findings may help inform new and existing rehabilitation programs regarding the possibility for sustainable benefits of structured quality improvement projects.
Footnotes
Supported by the National Institutes of Health Acute Lung Injury Specialized Centers of Clinically Oriented Research grant P050 HL 73994; and the National Institutes of Health grant 1KL2TR001077 (A.M.P.).
Author Contributions: All authors contributed to the study design, acquisition, and analysis and/or interpretation of data. V.D.D. and A.M.P. drafted the manuscript. All authors reviewed and provided final approval for the manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Spragg RG, Bernard GR, Checkley W, Curtis JR, Gajic O, Guyatt G, Hall J, Israel E, Jain M, Needham DM, et al. Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med. 2010;181:1121–1127. doi: 10.1164/rccm.201001-0024WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med. 2009;37:1574–1579. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moran JL, Bristow P, Solomon PJ, George C, Hart GK. Mortality and length-of-stay outcomes, 1993–2003, in the binational Australian and New Zealand intensive care adult patient database. Crit Care Med. 2008;36:46–61. doi: 10.1097/01.CCM.0000295313.08084.58. [DOI] [PubMed] [Google Scholar]
- 4.Esteban A, Frutos-Vivar F, Muriel A, Ferguson ND, Penuelas O, Abraira V, Raymondos K, Rios F, Nin N, Apezteguia C, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med. 2013;188:220–230. doi: 10.1164/rccm.201212-2169OC. [DOI] [PubMed] [Google Scholar]
- 5.Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, Himmelfarb CR, Desai SV, Ciesla N, Herridge MS, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014;42:849–859. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, et al. Groupe de Reflexion et d’Etude des Neuromyopathies en Réanimation. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher SN, Kennedy DD, Ghosh IR, Misra VP, Kiff K, Coakley JH, Hinds CJ. Persistent neuromuscular and neurophysiologic abnormalities in long-term survivors of prolonged critical illness. Crit Care Med. 2003;31:1012–1016. doi: 10.1097/01.CCM.0000053651.38421.D9. [DOI] [PubMed] [Google Scholar]
- 8.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, et al. Canadian Critical Care Trials G. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 9.Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 10.Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Shanholtz C, Husain N, Dennison CR, Herridge MS, Pronovost PJ, Needham DM. Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med. 2012;185:517–524. doi: 10.1164/rccm.201103-0503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Needham DM, Wozniak AW, Hough CL, Morris PE, Dinglas VD, Jackson JC, Mendez-Tellez PA, Shanholtz C, Ely EW, Colantuoni E, et al. Risk factors for physical impairment after acute lung injury in a national, multicenter study. Am J Respir Crit Care Med. 2014;189:1214–1224. doi: 10.1164/rccm.201401-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Peng X, Zhu B, Zhang Y, Xi X. Active mobilization for mechanically ventilated patients: a systematic review. Arch Phys Med Rehabil. 2013;94:551–561. doi: 10.1016/j.apmr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Adler J, Malone D. Early mobilization in the intensive care unit: a systematic review. Cardiopulm Phys Ther J. 2012;23:5–13. [PMC free article] [PubMed] [Google Scholar]
- 14.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey P, Thomsen GE, Spuhler VJ, Blair R, Jewkes J, Bezdjian L, Veale K, Rodriquez L, Hopkins RO. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145. doi: 10.1097/01.CCM.0000251130.69568.87. [DOI] [PubMed] [Google Scholar]
- 16.Thomsen GE, Snow GL, Rodriguez L, Hopkins RO. Patients with respiratory failure increase ambulation after transfer to an intensive care unit where early activity is a priority. Crit Care Med. 2008;36:1119–1124. doi: 10.1097/CCM.0b013e318168f986. [DOI] [PubMed] [Google Scholar]
- 17.Calvo-Ayala E, Khan BA, Farber MO, Ely EW, Boustani MA. Interventions to improve the physical function of ICU survivors: a systematic review. Chest. 2013;144:1469–1480. doi: 10.1378/chest.13-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burtin C, Clerckx B, Robbeets C, Ferdinande P, Langer D, Troosters T, Hermans G, Decramer M, Gosselink R. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37:2499–2505. doi: 10.1097/CCM.0b013e3181a38937. [DOI] [PubMed] [Google Scholar]
- 19.Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, Ross A, Anderson L, Baker S, Sanchez M, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 20.Kayambu G, Boots R, Paratz J. Physical therapy for the critically ill in the ICU: a systematic review and meta-analysis. Crit Care Med. 2013;41:1543–1554. doi: 10.1097/CCM.0b013e31827ca637. [DOI] [PubMed] [Google Scholar]
- 21.Leditschke IA, Green M, Irvine J, Bissett B, Mitchell IA. What are the barriers to mobilizing intensive care patients? Cardiopulm Phys Ther J. 2012;23:26–29. [PMC free article] [PubMed] [Google Scholar]
- 22.Parker A, Tehranchi KM, Needham DM. Critical care rehabilitation trials: the importance of ‘usual care’. Crit Care. 2013;17:R183. doi: 10.1186/cc12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berney SC, Harrold M, Webb SA, Seppelt I, Patman S, Thomas PJ, Denehy L. Intensive care unit mobility practices in Australia and New Zealand: a point prevalence study. Crit Care Resusc. 2013;15:260–265. [PubMed] [Google Scholar]
- 24.Mendez-Tellez PA, Dinglas VD, Colantuoni E, Ciesla N, Sevransky JE, Shanholtz C, Pronovost PJ, Needham DM. Factors associated with timing of initiation of physical therapy in patients with acute lung injury. J Crit Care. 2013;28:980–984. doi: 10.1016/j.jcrc.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nydahl P, Ruhl AP, Bartoszek G, Dubb R, Filipovic S, Flohr HJ, Kaltwasser A, Mende H, Rothaug O, Schuchhardt D, et al. Early mobilization of mechanically ventilated patients: a 1-day point-prevalence study in Germany. Crit Care Med. 2014;42:1178–1186. doi: 10.1097/CCM.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 26.Hopkins RO, Spuhler VJ, Thomsen GE. Transforming ICU culture to facilitate early mobility. Crit Care Clin. 2007;23:81–96. doi: 10.1016/j.ccc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Zanni JM, Korupolu R, Fan E, Pradhan P, Janjua K, Palmer JB, Brower RG, Needham DM. Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot project. J Crit Care. 2010;25:254–262. doi: 10.1016/j.jcrc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Needham DM, Korupolu R, Zanni JM, Pradhan P, Colantuoni E, Palmer JB, Brower RG, Fan E. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91:536–542. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Clark DE, Lowman JD, Griffin RL, Matthews HM, Reiff DA. Effectiveness of an early mobilization protocol in a trauma and burns intensive care unit: a retrospective cohort study. Phys Ther. 2013;93:186–196. doi: 10.2522/ptj.20110417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drolet A, Dejuilio P, Harkless S, Henricks S, Kamin E, Leddy EA, Lloyd JM, Waters C, Williams S. Move to improve: the feasibility of using an early mobility protocol to increase ambulation in the intensive and intermediate care settings. Phys Ther. 2013;93:197–207. doi: 10.2522/ptj.20110400. [DOI] [PubMed] [Google Scholar]
- 31.Olkowski BF, Devine MA, Slotnick LE, Veznedaroglu E, Liebman KM, Arcaro ML, Binning MJ. Safety and feasibility of an early mobilization program for patients with aneurysmal subarachnoid hemorrhage. Phys Ther. 2013;93:208–215. doi: 10.2522/ptj.20110334. [DOI] [PubMed] [Google Scholar]
- 32.Hildreth AN, Enniss T, Martin RS, Miller PR, Mitten-Long D, Gasaway J, Ebert F, Butcher W, Browder K, Chang MC, et al. Surgical intensive care unit mobility is increased after institution of a computerized mobility order set and intensive care unit mobility protocol: a prospective cohort analysis. Am Surg. 2010;76:818–822. [PubMed] [Google Scholar]
- 33.Engel HJ, Needham DM, Morris PE, Gropper MA. ICU early mobilization: from recommendation to implementation at three medical centers. Crit Care Med. 2013;41:S69–S80. doi: 10.1097/CCM.0b013e3182a240d5. [DOI] [PubMed] [Google Scholar]
- 34.Engel HJ, Tatebe S, Alonzo PB, Mustille RL, Rivera MJ. Physical therapist-established intensive care unit early mobilization program: quality improvement project for critical care at the University of California San Francisco Medical Center. Phys Ther. 2013;93:975–985. doi: 10.2522/ptj.20110420. [DOI] [PubMed] [Google Scholar]
- 35.Titsworth WL, Hester J, Correia T, Reed R, Guin P, Archibald L, Layon AJ, Mocco J. The effect of increased mobility on morbidity in the neurointensive care unit. J Neurosurg. 2012;116:1379–1388. doi: 10.3171/2012.2.JNS111881. [DOI] [PubMed] [Google Scholar]
- 36.Ohtake PJ, Strasser DC, Needham DM. Translating research into clinical practice: the role of quality improvement in providing rehabilitation for people with critical illness. Phys Ther. 2013;93:128–133. doi: 10.2522/ptj.2013.93.2.128. [DOI] [PubMed] [Google Scholar]
- 37.Sricharoenchai T, Parker AM, Zanni JM, Nelliot A, Dinglas VD, Needham DM. Safety of physical therapy interventions in critically ill patients: a single-center prospective evaluation of 1110 intensive care unit admissions. J Crit Care. 2014;29:395–400. doi: 10.1016/j.jcrc.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Stiller K, Phillips AC, Lambert P. The safety of mobilisation and its effects on haemodynamics and respiratory status of intensive care patients. Physiother Theory Pract. 2004;20:10. [Google Scholar]
- 39.Damluji A, Zanni JM, Mantheiy E, Colantuoni E, Kho ME, Needham DM.Safety and feasibility of femoral catheters during physical rehabilitation in the intensive care unit J Crit Care 201328535e9–15 [DOI] [PubMed] [Google Scholar]
- 40.Perme C, Nalty T, Winkelman C, Kenji Nawa R, Masud F. Safety and efficacy of mobility interventions in patients with femoral catheters in the ICU: a prospective observational study. Cardiopulm Phys Ther J. 2013;24:12–17. [PMC free article] [PubMed] [Google Scholar]
- 41.Talley CL, Wonnacott RO, Schuette JK, Jamieson J, Heung M. Extending the benefits of early mobility to critically ill patients undergoing continuous renal replacement therapy: the Michigan experience. Crit Care Nurs Q. 2013;36:89–100. doi: 10.1097/CNQ.0b013e3182753387. [DOI] [PubMed] [Google Scholar]
- 42.Fan E, Laupacis A, Pronovost PJ, Guyatt GH, Needham DM. How to use an article about quality improvement. JAMA. 2010;304:2279–2287. doi: 10.1001/jama.2010.1692. [DOI] [PubMed] [Google Scholar]
- 43.Parker A, Dinglas V, Reddy D, Colantuoni E, Zanni J, Turnbull A, Ciesla N, Needham D.A quality improvement intervention sustainably decreases time to first physical therapy intervention in acute respiratory distress syndrome patients [abstract] Am J Respir Crit Care Me 2014. A1633 [Google Scholar]
- 44.Needham DM, Korupolu R. Rehabilitation quality improvement in an intensive care unit setting: implementation of a quality improvement model. Top Stroke Rehabil. 2010;17:271–281. doi: 10.1310/tsr1704-271. [DOI] [PubMed] [Google Scholar]
- 45.Pronovost PJ, Berenholtz SM, Needham DM. Translating evidence into practice: a model for large scale knowledge translation. BMJ. 2008;337:a1714. doi: 10.1136/bmj.a1714. [DOI] [PubMed] [Google Scholar]
- 46.Hager DN, Dinglas VD, Subhas S, Rowden AM, Neufeld KJ, Bienvenu OJ, Touradji P, Colantuoni E, Reddy DR, Brower RG, et al. Reducing deep sedation and delirium in acute lung injury patients: a quality improvement project. Crit Care Med. 2013;41:1435–1442. doi: 10.1097/CCM.0b013e31827ca949. [DOI] [PubMed] [Google Scholar]
- 47.Kho ME, Damluji A, Zanni JM, Needham DM.Feasibility and observed safety of interactive video games for physical rehabilitation in the intensive care unit: a case series J Crit Care 201227219e1–6 [DOI] [PubMed] [Google Scholar]
- 48.Needham DM, Truong AD, Fan E. Technology to enhance physical rehabilitation of critically ill patients. Crit Care Med. 2009;37:S436–S441. doi: 10.1097/CCM.0b013e3181b6fa29. [DOI] [PubMed] [Google Scholar]
- 49.Rahimi RA, Skrzat J, Reddy DR, Zanni JM, Fan E, Stephens RS, Needham DM. Physical rehabilitation of patients in the intensive care unit requiring extracorporeal membrane oxygenation: a small case series. Phys Ther. 2013;93:248–255. doi: 10.2522/ptj.20120336. [DOI] [PubMed] [Google Scholar]
- 50.Lord RK, Mayhew CR, Korupolu R, Mantheiy EC, Friedman MA, Palmer JB, Needham DM. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41:717–724. doi: 10.1097/CCM.0b013e3182711de2. [DOI] [PubMed] [Google Scholar]
- 51.Needham DM, Dennison CR, Dowdy DW, Mendez-Tellez PA, Ciesla N, Desai SV, Sevransky J, Shanholtz C, Scharfstein D, Herridge MS, et al. Study protocol: the Improving Care of Acute Lung Injury Patients (ICAP) study. Crit Care. 2006;10:R9. doi: 10.1186/cc3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 53.Stockley RC, Morrison J, Rooney J, Hughes J. Move it or lose it? A survey of the aims of treatment when using passive movements in intensive care. Intensive Crit Care Nurs. 2012;28:82–87. doi: 10.1016/j.iccn.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 54.Griffiths RD, Palmer TE, Helliwell T, MacLennan P, MacMillan RR. Effect of passive stretching on the wasting of muscle in the critically ill. Nutrition. 1995;11:428–432. [PubMed] [Google Scholar]
- 55.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 56.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58:595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 57.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 58.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 59.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 60.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 61.Rubin DB.Multiple imputation for nonresponse in surveys. New York: Wiley & Sons; 1987 [Google Scholar]
- 62.Bienvenu OJ, Gellar JE, Althouse BM, Colantuoni E, Sricharoenchai T, Mendez-Tellez PA, Shanholtz C, Dennison CR, Pronovost PJ, Needham DM. Post-traumatic stress disorder symptoms after acute lung injury: a 2-year prospective longitudinal study. Psychol Med. 2013;43:2657–2671. doi: 10.1017/S0033291713000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fine JP, Gray RT. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 64.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- 65.Hamilton LC.Statistics With STATA - Update for Version 10. Belmont, CA: Brooks/Cole; 2009 [Google Scholar]
- 66.Nallamothu BK, Hayward RA, Bates ER. Beyond the randomized clinical trial: the role of effectiveness studies in evaluating cardiovascular therapies. Circulation. 2008;118:1294–1303. doi: 10.1161/CIRCULATIONAHA.107.703579. [DOI] [PubMed] [Google Scholar]
- 67.Dinglas VD, Colantuoni E, Ciesla N, Mendez-Tellez PA, Shanholtz C, Needham DM. Occupational therapy for patients with acute lung injury: factors associated with time to first intervention in the intensive care unit. Am J Occup Ther. 2013;67:355–362. doi: 10.5014/ajot.2013.007807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balas MC, Burke WJ, Gannon D, Cohen MZ, Colburn L, Bevil C, Franz D, Olsen KM, Ely EW, Vasilevskis EE. Implementing the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle into everyday care: opportunities, challenges, and lessons learned for implementing the ICU Pain, Agitation, and Delirium Guidelines. Crit Care Med. 2013;41:S116–S127. doi: 10.1097/CCM.0b013e3182a17064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei LJ, Lachin JM. Two-sample asymptotically distribution-free tests for incomplete multivariate observations. J Am Stat Assoc. 1984;79:653–661. [Google Scholar]