Abstract
Multidrug-resistant (MDR) Acinetobacter baumannii is one of the most difficult Gram-negative bacteria to treat and eradicate. In a cell-based screening of pleuromutilin derivatives against a drug sensitive A. baumannii strain, new molecules (2–4) exhibit bacteriostatic activity with 3.13 μg/mL concentration and 1 shows bactericidal activity with an MBC of 6.25 μg/mL. The pleuromutilin derivative 1 displays strong synergistic effects with doxycycline in a wide range of concentrations. A 35/1 ratio of 1 and doxycycline (1-Dox35/1) kills drug susceptible A. baumannii with the MBC of 2.0 μg/mL and an MDR A. baumannii with the MBC of 3.13 μg/mL. In vitro anti- Acinetobacter activity of 1-Dox35/1 is superior to that of clinical drugs such as tobramycin, tigecycline, and colistin. The efficacy of 1-Dox35/1 is evaluated in a mouse septicemia model; treatment of the infected C57BL/6 mice with 1-Dox35/1 protects from lethal infection of A. baumannii with an ED50 value of <2.0 mg/kg.
Keywords: Acinetobacter baumannii, Multidrug resistant, Gram-negative bacteria, Pleuromutilin derivatives, Tetracycline, Doxycycline, Synergistic effect, In vivo efficacy
TOC Image

INTRODUCTION
Acinetobacter baumannii is one of the most common healthcare-associated Gram-negative bacteria that causes a variety of diseases, ranging from pneumonia to serious blood infections, and affects people with compromised immune systems.1,2 A significant increase in the incidence of multidrug-resistant (MDR) strains has raised the profile of this emerging opportunistic pathogen.3,4 Due to its ability to survive on artificial surfaces and to resist desiccated conditions, A. baumannii survives in food, water, or soil, and it can be spread by direct contact.5 A. baumannii infections are commonly implicated in nosocomial infections, and in some countries high isolation rates of drug resistant A. baumannii have been reported from patients in the intensive care unit (ICU).6 Lately, high incidence of MDR A. baumannii infections have been reported in veterans in Iraq and Afghanistan.7,8 A. baumannii targets mucous membranes and injured skin area, causing necrotizing infections that lead to septicemia and death.1 Mortality rates from A. baumannii septicemia were reported to be 34.0–43.4% in the ICU and 16.3% outside the ICU.9 Clinically isolated A. baumannii strains exhibit a wide array of drug resistance mechanisms including a variety of efflux mechanisms that show resistance to all commonly prescribed antibacterial drugs.10 The therapeutic strategies for treating these highly resistant organisms are associated with significant toxicity with the limited number of antibacterial agents (e.g. sulbactam, imipenem-cillastatin, meropenem, doripenem, amikacin, tobramycin, polymyxins (or colistin), tigecycline, and minocycline).1 Although a significant number of studies has been devoted to optimizing the use of currently available agents or identifying any combination thereof, new chemical entities have not been developed for the treatment of MDR A. baumannii infections since the introduction of tigecyclin in 2005. Thus, the need for new agents to treat infections caused by A. baumannii is urgent.
Since the discovery of diverse structures of bacterial protein biosynthesis inhibitors (e.g. aminoglycosides, tetracyclines, chloramphenicol, linezolid, lincosamides, spectinomycin, streptogramins, mupirocin, and macrolides), rRNA is still an attractive antibacterial drug target.
Pleuromutilin, a strong binder of the 50S rRNA subunit, was first isolated in 1952 from two Basidiomycete spp. (Pleurotus mutilus and Pleurotus passeckerianus). Two pleuromutilin derivatives, tiamulin and valnemulin, were successfully developed as therapeutic agents for veterinary use.11–14 Tiamulin is a prophylactic and therapeutic agent for swine dysentery, and shows activity against anaerobic bacteria, intestinal spirochetes and Mycoplasma spp.15 Several other semisynthetic pleuromutilin derivatives have been designed for human use. Retapamulin is the first antibacterial agent of the pleuromutilin class, which shows excellent in vivo antibacterial activity. Retapamulin was approved in 2007 by FDA for the topical treatment of the impetigo and secondarily infected traumatic lesions of skin infections caused by Gram-positive bacteria such as S. aureus and S. pyogenes.13 Recently, significant advances were made in development of pleuromutilin to be systemic or orally bioavailable antibiotics for MRSA infections. BC-3781 (Nabriva Therapeutics) entered Phase II clinical studies, demonstrating a therapeutic potential for the treatment of bacterial skin and lung infections including community-acquired bacterial pneumonia.16,17 As represented by BC-3781 and other effective derivatives, thioether analogues of the C14-side chain promise the increase of antibacterial activity (Figure 1).18–28
Figure 1.

Pleuromutilin and Representative Pleuromutilin Analogues.
In our discovery program for effective protein biosynthesis inhibitors against Gram-negative bacteria, we identified that several pleuromutilin derivatives exhibited bacteriostatic and/or bactericidal activity against A. baumannii. Here, we wish to report that the analogue 1 showed significant synergistic effects with doxycycline against A. baumannii and a combination of 1 and doxycycline was effective in an in vivo lethal infection model.
RESULTS AND DISCUSSION
Chemistry and SAR
New drugs for drug resistant Gram-negative organisms are clearly a major unmet clinical need for new antibiotic agents. In order to identify new pleuromutilin derivatives that expand the spectrum of activity against Gram-negative organisms, we have generated a 50-membered library whose structure contained the methoxyamine-oxime (A), hydoxyamine-oxime (B), or 3-hydroxy (C) core structure; those structures were further diversified by reduction of the double bond (C16-position), amide-formations, and reductive aminations (see supporting information). The generated molecules were evaluated in the growth inhibitory assay against an A. baumannii strain (ATCC19606). We identified four anti-Acinetobacter pleuromutilin analogues (1–4) which exhibited MIC values less than 12.5 μg/mL (Figure 2).
Figure 2.

General Structures of Pleuromutilin Derivatives and Anti-Acinetobacter Molecules.
In order to confirm anti-Acinetobacter activity of 1–4, these molecules were resynthesized. Their syntheses are illustrated in Scheme 1. The C3-carbonyl group of pleuromutilin (5) was reduced with NaBH4 to form the secondary alcohol with R-configuration exclusively.29 The primary alcohol of the C14-side chain was tosylated with TsCl and DMAP to afford 6 in 90% overall yield. Thioether formation of 6 with 1-amino-2-methylpropane-2-thiol was accomplished according to a reported condition,19,20 yielding the free amine 7 in 90% yield. Amide-forming reaction of 7 with Boc-D-Val-OH under Glyceroacetonide-Oxyma, EDCI, and NaHCO3 in DMF-H2O followed by deprotection of the Boc group with 4N HCl afforded 1 in 85% overall yield.30–32 The dipeptide analogues 2 and 3 were synthesized by coupling with the corresponding Boc-protected amino acid and subsequent Boc-deprotection. Reductive amination of 1 with N-Boc-2-aminoacetoaldehyde followed by deprotection of the Boc group afforded 4 in 80% yield. The analogues synthesized in Scheme 1 were purified via reverse-phase HPLC, and their MIC values were determined against drug sensitive A. baumannii. The analogues 3–4 exhibited the MIC and MBC values of 3.13 and >12.5 μg/mL, respectively, suggesting that they are bacteriostatic molecules. On the other hand, analogue 1 displayed the MIC and MBC value of 3.13 and 6.25 μg/mL, respectively, indicating its bactericidal activity (Table 1). In vitro bacterial growth inhibitory activity of 1 was comparable to anti-acinetobacter drugs such as tobramycin and colistin (polymyxin E).
Scheme 1.

Syntheses of Pleuromutilin Analogues 1–4.
Table 1.
MICs and MBCs of 1–4, Representative Antibacterial Agents (Clinically Used), and Combinations of 1-Doxycyclin.
| Entry | Species and strain | MIC or MBC (μg/mL)a | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Valnemulin | Tobramycin | Colistin | |||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| 1 | A. baumannii ATCC 19606 | 1.75 | 6.25 | 3.13 | >12.5 | 3.13 | >12.5 | 3.13 | >12.5 | 3.13 | 12.5 | 6.25 | 12.5 | 3.13 | 6.25 |
| 2 | MDR-A. baumannii ATCC BAA-1800 | 6.25 | 12.5 | 3.13 | >12.5 | 3.13 | >12.5 | 3.15 | >12.5 | 6.25 | 25.0 | >25 | >25 | >12.5 | 25.0 |
| Entry | Compound(s) | MIC or MBC (μg/mL)a | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. baumannii ATCC 19606 | MDR-A. baumannii ATCC BAA-1800 | K. pneumoniae ATCC 8047 | E. coli ATCC 10798 | P. aeruginosa ATCC 27853 | S. aureus ATCC 25923 | M. tuberculosis H37Rv | |||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| 3 | 1-Dox 60/1 | 0.5 | 2.0 | 1.56 | 6.25 | 1.56 | 3.25 | 0.25 | 1.56 | 12.5 | 25.0 | 12.5 | 25.0 | – | 6.25 |
| 4 | 1-Dox 35/1 | 0.4 | 2.0 | 1.56 | 3.13 | 0.78 | 1.56 | 0.25 | 1.56 | 12.5 | 25.0 | 12.5 | 25.0 | – | 6.25 |
| 5 | 1-Dox 2/1 | 0.5 | 2.0 | 1.56 | 3.13 | 0.78 | 1.56 | 0.25 | 1.56 | 6.25 | 12.5 | 12.5 | 25.0 | – | 6.25 |
| 6 | Doxycycline (Dox) | 0.3 | 0.4 | >25 | >25 | 0.25 | 0.78 | 3.13 | 6.25 | 12.5 | 25.0 | 3.13 | 12.5 | – | – |
| 7 | 1 | 1.75 | 3.13 | 6.25 | 12.5 | <6.25 | 6.25 | 3.13 | 6.25 | >25 | >25 | 12.5 | 25.0 | – | 6.25 |
The broth dilution method was used.; MBC 2.00 μg/mL for 1-Dox 35/1.; A. baumannii: Acinetobacter baumannii.; K. pneumonia: Klebsiella pneumonia.; E. coli: Escherichia coli.; P. aeruginosa: Pseudomonas aeruginosa.; S. aureus: Staphylococcus aureus.; M. tuberculosis: Mycobacterium tuberculosis. MIC: the lowest concentration that inhibit bacterial growth.; MBC: the lowest concentration required to kill 99.9% of bacteria (in CFU/mL)
The identified molecules 1–4 are the C3-reduced analogues of valnemulin (Figure 1). Interestingly, 1 exhibited superior MIC and MBC levels to those of valnemulin. The analogue 1 killed an MDR strain, A. baumannii (ATCC BAA-1800) at 6.25–12.5 μg/mL concentrations, albeit tobramycin and colistin did not kill the same strain at the concentrations effective against a drug-susceptible strain (entry 2 in Table 1). Based on bacterial growth inhibitory assays of the pleuromutilin analogues against batteries of Gram-negative and -positive bacteria, the following structure-activity relationship (SAR) was realized. Alkylation or acylation of the D-valine amino group decreases bactericidal activity, although modification with a variety of functional groups (R in Figure 3) is possible to retain the bacteriostatic activity. Hydrogenation of the C12-vinyl group increases activity against Gram-positive bacteria. The C3-hydroxy group improves not only bactericidal activity against A. baumannii, but also biopharmaceutic and pharmacokinetic properties such as water-solubility (1.5 times greater than valnemulin)33 and metabolic stability (vide infra).
Figure 3.

SAR of Valnemulin Core Structure for Anti-Acinetobacter Agents.
Synergistic effect of 1 with doxycycline
The synergistic or antagonistic activities of 1 were assessed in vitro via micro dilution broth checkerboard technique.34–36 In the checkerboard analyses of a combination of 1 and anti-Acinetobacter drugs (tobramycin, gentamicin, tigecycline, minocycline, doxycycline, rifampicin, and polymyxin), 1 displayed strong synergistic effects with only doxycycline in a wide range of concentrations. Table 2 summarizes the results of FIC index analyses for a combination of 1 plus doxycycline and valnemulin plus doxycycline that showed synergistic combination (ΣFIC<0.5). The FIC index range of 0.16 to 0.50 was observed for 8 combinations of two molecules out of 96 different concentrations (entries 1–8 in Table 2). Compared to a wide range of synergistic effects observed for 1, valnemulin showed synergistic effect only at two combinations with doxycycline (entry 9). It was demonstrated that 60/1, 35/1, and 2/1 ratio of 1 and doxycycline (1-Dox 60/1, 1-Dox 35/1, and 1-Dox 2/1) killed a drug susceptible A. baumannii with the MBC of 0.78 μg/mL (entries 3–5 in Table 1). These combinations killed the rifampicin (32xMIC)- and 1 (16xMIC)-resistant strains at 0.78 and 3.13 μg/mL concentration in 24h. Significantly, 1-Dox 60/1, 1-Dox 35/1, and 1-Dox 2/1 killed MDR A. baumannii (ATCC BAA-1800) with much lower concentrations (MBC of 3.13–6.25 μg/mL) than the MBC values of the individual molecules (MBC >25 and 12.5 μg/mL for Dox and 1, respectively).
Table 2.
Fractional Inhibitory Concentration of a Combination of Doxycycline and 1 or Valnemulin.a
| Entry | Combination of A and Bb | MIC (μg/mL) CA and CBc |
ΣFICd |
|---|---|---|---|
| 1 | A: 1 | 0.20 | 0.16 |
| B: Doxycycline | 0.025 | ||
| 2 | A: 1 | 0.40 | 0.19 |
| B: Doxycycline | 0.025 | ||
| 3 | A: 1 | 0.78 | 0.25 |
| B: Doxycycline | 0.025 | ||
| 4 | A: 1 | 1.56 | 0.38 |
| B: Doxycycline | 0.025 | ||
| 5 | A: 1 | 0.20 | 0.28 |
| B: Doxycycline | 0.05 | ||
| 6 | A: 1 | 0.40 | 0.31 |
| B: Doxycycline | 0.05 | ||
| 7 | A: 1 | 0.78 | 0.37 |
| B: Doxycycline | 0.025 | ||
| 8 | A: 1 | 1.56 | 0.50 |
| B: Doxycycline | 0.025 | ||
| 9 | A: Valnemulin | 0.78 | 0.375 |
| B: Doxycycline | 0.025 | ||
| 10 | A: Valnemulin | 1.56 | 0.375 |
| B: Doxycycline | 0.05 |
ΣFIC index for the wells at growth–no growth interface.
The MIC values of molecule 1, valnemulin, and doxycycline against A. baumannii (ATCC 19606) are 6.25, 12.5, and 0.20 μg/mL, respectively.
CA and CB are concentrations of A and B.
ΣFIC is the sum of fractional inhibitory concentration calculated by the equation ΣFIC = FICA + FICB = CA/MICA + CB/MICB.
In vitro metabolic stability and toxicity of 1
Despite widespread use of valnemulin in veterinary fields, its metabolic profile has not been reported until recently. Several groups reported that the half-lives of valnemulin in plasma in vivo and ex-vivo are relatively short (1.3–2.9 h), suggesting challenges in its application for systemic antimicrobial therapy.37–39 In our in vitro metabolic stability testing,40 we observed a striking difference in half-life (t1/2) between 1 and valnemulin in rat liver microsomes; t1/2 of valnemulin was 0.58 min., on the other hand, t1/2 of 1 was >60 min. Thus, in vitro half-life was significantly extended by reduction of the C3-carbonyl group of valnemulin. In vitro cytotoxicity against mammalian cells (e.g. Vero cells) of 1 (IC50 45.3 μg/mL) was 2.85 times less toxic than that of valnemulin (IC50 14.9 μg/mL) (Figure 4).41–43
Figure 4.

Half-life and Cytotoxicity (1 vs. Valnemulin).
A. Half-life (t1/2) in the rat liver microsomesa
B. Cytotoxicity against Vero cellsa
IC50 45.3 μg/mL (for 1), 14.9 μg/mL (for valnemulin), 0.20 μg/mL (tunicamycin, SI), > 200 μg/mL (colistin, SI), > 200 μg/mL (tobramycin, SI).
aThe graphs were obtained via graph pad prism 7.0.
In vivo effect of 1-Dox 35/1
As summarized above, the favorable in vitro physicochemical properties of 1 over valnemulin were realized. In addition, it was determined that 1 binds to the rat plasma protein with 76.9% (PPB), providing insights into the in vivo efficacy of 1 in infected animal models.44 The in vivo efficacy of 1 and 1-Dox 35/1 was evaluated in a mouse septicemia model using the C57BL/6 mice and A. baumannii (ATCC19606) strain (at a dose that lead to 75% of death).45,46 One hour after the infection, the molecules (1, 1-Dox 35/1, or tobramycin) were administered intraperitoneally (IP) at a single-dose (from 2 to 60 mg/kg) (Figure 5). At the 10, 20, and 60 mg/kg 1-Dox 35/1 dose, 100% of the mice survived, while all mice in the control group succumbed to infection in two days. The reference molecule, tobramycin survived the mice at 6.0 mg/kg dose IP. 100% of the mice in the group administered 4.0 mg/kg 1-Dox 35/1 were rescued mortality of the A. baumannii infection. At the 2.0 mg/kg 1-Dox 35/1 dose, 60% of the mice survived. Similarly, 1 showed effectiveness in the same in vivo studies, but required higher dosage than that with 1-Dox35/1.
Figure 5.
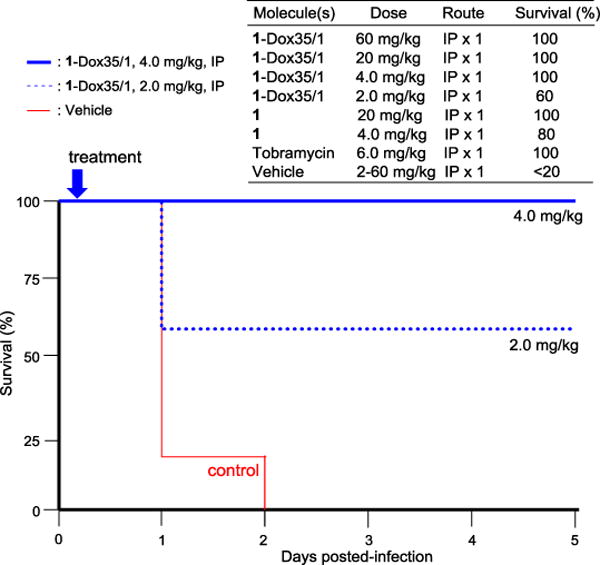
Effect of 1-Dox 35/1 and 1 on Survival Rate in the Mouse Infected with A. baumannii.a
aThe C57BL/6 mice (n = 5) were infected intraperitoneally with A. baumannii (ATCC19606) strain at a dose that lead to 100% of death in 2 day. 4 mice was used for the non-treatment control group. The test molecules were intraperitoneally administered once after 1 h of the infection. Mortality was monitored for 5 days for all groups. P < 0.05.
A resistance mechanism of A. baumannii against 1
It is well established that the pleuromutilin derivatives target the peptidyl transfer center of the 50S ribosomal protein L3 (rplC), inhibiting protein biosynthesis.47–54 Previously, other groups reported multiple mutations in rplC of S. aureus that can define a region of rplC capable of causing decreased susceptibility of the pleuromutilin derivative in S. aureus.48,49 In order to identify a potential mechanism of resistance to 1, we isolated the chromosomal DNA from the resistant mutant (1R, 16xMBC) and its parental wild-type control A. baumannii (ATCC19606). The rplC gene fragment was amplified using A. baumannii rplC specific primers and sequenced. 55,56 The DNA sequencing results were blasted against rplC DNA sequence of A. baumannii in the NIH genetic sequence database. The DNA sequence alignment revealed a C456A single nucleotide mutation, which corresponded to N152K mutation in the protein sequence of RplC (Figure 6). Interestingly, 1-Dox 35/1 and 1-Dox 2/1 effectively killed A. baumannii mutant 1R with the MBC value of 3.13–6.26 μg/mL (vide supra). Any other tetracyclines such as minocycline, tigecycline, and demeclocycline did not exhibit the same effect as observed with doxycycline (Dox) against A. baumannii mutant 1R.
Figure 6.

The Amino Acid Alignment of the 50S Ribosomal Protein L3 (RplC) from a 1-Resistant A. baumannii Strain (1R).
1R (Query) and wild-type control (Sbjct): the red color represents the site mutation in RplC.
Spontaneous mutation frequency
The frequency that an A. baumannii strain spontaneously developed resistance to 1-Dox 35/1 was evaluated by applying the culture of A. baumannii (ATCC19606) strain to agar media containing 1-Dox 35/1 at concentrations 4- and 8-fold the MIC on agar media. There was no colony on the plate containing 4xMIC after 48h incubation when 1×109 CFU bacteria were plated. However, two colonies were identified on the plate containing 8xMIC when applied 1×1010 CFU bacteria. Two strains isolated in these experiments did not grow on the agar plates containing 1-Dox35/1 at the 4x and 8xMIC concentrations. Thus, calculated spontaneous resistance mutation frequency of 1-Dox35/1 is less than 1×10−10 for the ATCC19606 strain. On the other hand, 1 alone showed spontaneous resistant mutants for the same strain with the mutation frequency of 1×10−8 at 4xMIC concentration.
CONCLUSION
This paper describes the identification of a new pleuromutilin derivative 1 that displays a strong synergistic effect with doxycycline (Dox). Combinations of 1 and Dox at a series of concentrations (i.e. 1-Dox 60/1, 1-Dox 35/1, and 1-Dox 2/1) exhibit bactericidal activity against drug-sensitive and -resistant A. baumannii at 1.56–6.25 μg/mL concentrations. In vitro bactericidal activity of 1-Dox35/1 against A. baumannii is superior to that of clinically utilized drugs such as tobramycin, tigecycline, and colistin. Structurally, 1 is a C3-hydroxy analogue of valnemulin. Despite such a simple chemical modification, in vitro half-life of 1 (t1/2 >60 min.) in rat microsomes is significantly extended compared to that of valnemulin (t1/2 0.58 min.), which promises in vivo efficacy of 1 and combinations of 1 and Dox in infected animal models. In vitro cytotoxicity of 1 was much lower than that of valnemulin. The in vivo efficacy of 1-Dox35/1 was demonstrated via a mouse septicemia model using the infected C57BL/6 mice with A. baumannii; the ED50 value of 1-Dox 35/1 was determined to be <2 mg/kg (a single-dose). The gene analyses of a 1-mutant A. baumannii strain revealed a single mutation in the rplC gene, which confers the binding site of 1 in bacterial 50S ribosome subunit. Although the mode of action of 1-Dox 35/1 is far from completely understood, 1-Dox 35/1 is effective against a resistant strain 1R. We will identify target and off-target mutations of resistant strain against 1-Dox 35/1 by whole-genome sequencing analyses.
The pleuromutilin analogue 1 exhibited permeability across Caco-2 epithelial monolayers (Papp rate coefficient>1, data not shown) with moderate levels of efflux (an efflux ratio of approximately 5). Pharmacokinetics and oral bioavailability of a combination of 1 and Dox will be evaluated using preclinical animal models, and these data including detailed evaluation on in vivo efficacy will be reported elsewhere.
EXPERIMENTAL SECTION
Chemistry. General Information
All chemicals were purchased from commercial sources and used without further purification unless otherwise noted. THF, CH2Cl2, and DMF were purified via Innovative Technology’s Pure-Solve System. All reactions were performed under an Argon atmosphere. All stirring was performed with an internal magnetic stirrer. Reactions were monitored by TLC using 0.25 mm coated commercial silica gel plates (EMD, Silica Gel 60F254). TLC spots were visualized by UV light at 254 nm, or developed with ceric ammonium molybdate or anisaldehyde or copper sulfate or ninhydrin solutions by heating on a hot plate. Reactions were also monitored by using SHIMADZU LCMS-2020 with solvents: A: 0.1% formic acid in water, B: acetonitrile. Flash chromatography was performed with SiliCycle silica gel (Purasil 60 Å, 230–400 Mesh). Proton magnetic resonance (1H-NMR) spectral data were recorded on 400, and 500 MHz instruments. Carbon magnetic resonance (13C-NMR) spectral data were recorded on 100 and 125 MHz instruments. For all NMR spectra, chemical shifts (δH, δC) were quoted in parts per million (ppm), and J values were quoted in Hz. 1H and 13C NMR spectra were calibrated with residual undeuterated solvent (CDCl3: δH = 7.26 ppm, δC = 77.16 ppm; CD3CN: δH = 1.94 ppm, δC = 1.32ppm; CD3OD: δH =3.31 ppm, δC =49.00 ppm; DMSO-d6: δH = 2.50 ppm, δC = 39.52 ppm; D2O: δH = 4.79 ppm) as an internal reference. The following abbreviations were used to designate the multiplicities: s = singlet, d = doublet, dd = double doublets, t = triplet, q = quartet, quin = quintet, hept = heptet, m = multiplet, br = broad. Infrared (IR) spectra were recorded on a Perkin-Elmer FT1600 spectrometer. HPLC analyses were performed with a Shimadzu LC-20AD HPLC system. All compounds were purified by reverse HPLC to be ≥95% purity.
(3R,3aR,4R,5R,7S,8S,9R,9aS,12R)-3,8-dihydroxy-4,7,9,12-tetramethyl-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-((R)-2-amino-3-methylbutanamido)-2-methylpropan-2-yl)thio)acetate (1)
To a stirred solution of 7 (1.65 g, 3.52 mmol), Boc-D-Val-OH (1.15 g, 5.28 mmol), NaHCO3 (2.96 g, 35.2 mmol) and Glyceroacetonide-Oxyma (1.20 g, 5.28 mmol) in DMF-H2O (9/1, 17.6 mL) was added EDCI (3.38 g, 17.6 mmol). The reaction mixture was stirred for 15h at rt, quenched with aq. sat. NaHCO3, and extracted with EtOAc. The combined organic extract was washed with 1N HCl, brine, dried over Na2SO4 and concentrated in vacuo. To a stirred solution of the crude product (0.30 g, 0.46 mmol) in dioxane (0.5 mL) was added a 4N solution of HCl/dioxane (1.14 mL). The reaction mixture was stirred for 1h at rt, and all volatiles were evaporated in vacuo. The crude mixture was purified by C18 reverse-phase HPLC [column: HYPERSIL GOLD™ (175 Å, 12 μm, 250 × 10 mm), solvents: 50:50 MeOH : H2O, flow rate: 2.0 mL/min, UV: 220 nm] to afford 1 (0.23 g, 0.40 mmol, 88%, retention time: 25 min): TLC (CHCl3/MeOH 90:10) Rf = 0.30; [α]22D −0.201 (c = 1.61, MeOH); IR (thin film) νmax = 3395 (br), 2957, 2875, 1716, 1658, 1525, 1463, 1370, 1285, 1144, 1020, 1005, 932 cm−1; 1H NMR (400 MHz, Methanol-d4) δ 6.40 – 6.31 (m, 1H), 5.62 (d, J = 9.2 Hz, 1H), 5.17 (q, J = 1.7 Hz, 1H), 5.15 – 5.12 (m, 1H), 4.49 (t, J = 5.3 Hz, 1H), 3.38 – 3.34 (m, 2H), 3.29 (d, J = 6.8 Hz, 2H), 3.26 – 3.21 (m, 2H), 2.36 (ddd, J = 11.8, 7.2, 4.2 Hz, 1H), 2.22 – 2.10 (m, 2H), 2.03 (quind, J = 6.9, 5.2 Hz, 1H), 1.92 (dddd, J = 14.7, 13.0, 10.5, 5.2 Hz, 2H), 1.72 – 1.57 (m, 4H), 1.50 – 1.35 (m, 3H), 1.33 (q, J = 3.8 Hz, 1H), 1.27 (s, 3H), 1.26 (s, 3H), 1.25 (s, 3H), 1.13 (s, 3H), 1.01 (d, J = 6.9 Hz, 3H), 0.95 (d, J = 6.8 Hz, 3H), 0.86 (d, J = 7.0 Hz, 3H), 0.70 (d, J = 7.2 Hz, 3H); 13C NMR (101 MHz, MeOD) δ 176.36, 171.35, 141.52, 116.22, 77.39, 76.08, 73.26, 61.58, 52.21, 47.65, 47.18, 46.72, 45.95, 42.56, 37.23, 37.13, 34.59, 33.32, 33.14, 32.90, 32.51, 28.98, 28.00, 26.97, 26.91, 19.87, 17.84, 17.68, 17.53, 12.51; HRMS (ESI+) m/z calcd for C31H55N2O5S [M + H] 567.3832, found: 567.3866.
(3R,3aR,4R,5R,7S,8S,9R,9aS,12R)-3,8-dihydroxy-4,7,9,12-tetramethyl-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-((R)-2-((S)-2,5-diaminopentanamido)-3-methylbutanamido)-2-methylpropan-2-yl)thio)acetate (2)
To a stirred solution of 1 (73.2 mg, 0.13 mmol), Boc-L-Orn(Boc)-OH (63.2 mg, 0.19 mmol), NaHCO3 (109 mg, 1.29 mmol) and Glyceroacetonide-Oxyma (59.3 mg, 0.26 mmol) in DMF-H2O (9/1, 0.65 mL) was added EDCI (124 mg, 0.65 mmol). The reaction mixture was stirred for 8h at rt, quenched with aq. sat. NaHCO3, and extracted with EtOAc. The combined organic extract was washed with 1N HCl, brine, dried over Na2SO4 and concentrated in vacuo. To a stirred solution of the crude product (45.6 mg, 0.051 mmol) in dioxane (0.2 mL) was added a 4N solution of HCl/dioxane (0.25 mL). The reaction mixture was stirred for 2h at rt, and all volatiles were evaporated in vacuo. The crude mixture was purified by C18 reverse-phase HPLC [column: HYPERSIL GOLD™ (175 Å, 12 μm, 250 × 10 mm), solvents: 50:50 MeOH:H2O, flow rate: 2.0 mL/min, UV: 220 nm] to afford 2 (33.0 mg, 0.047 mmol, 93%, retention time: 13 min): TLC (CHCl3/MeOH 90:10) Rf = 0.10; [α]22D +0.091 (c = 0.11, MeOH); IR (thin film) νmax = 3438 (br), 3202 (br), 2968, 1658, 1427, 1287, 1148, 1018, 724 cm−1; 1H NMR (400 MHz, Methanol-d4) δ 8.23 (d, J = 6.6 Hz, 1H), 6.35 (dd, J = 17.0, 11.3 Hz, 1H), 5.62 (d, J = 9.9 Hz, 1H), 5.19 – 5.14 (m, 1H), 5.13 (s, 1H), 4.49 (t, J = 5.7 Hz, 1H), 4.22 (d, J = 6.6 Hz, 1H), 4.01 (t, J = 6.1 Hz, 1H), 3.77 – 3.71 (m, 1H), 3.61 – 3.56 (m, 1H), 2.96 (t, J = 7.6 Hz, 2H), 2.41 – 2.29 (m, 1H), 2.21 – 2.05 (m, 3H), 1.99 – 1.85 (m, 4H), 1.76 – 1.57 (m, 6H), 1.56 – 1.38 (m, 5H), 1.35 – 1.29 (m, 2H), 1.26 (s, 3H), 1.26 (s, 3H), 1.25 (s, 3H), 1.13 (s, 3H), 1.05 (s, 3H), 1.03 (s, 3H), 0.86 (d, J = 6.4 Hz, 3H), 0.70 (d, J = 7.7 Hz, 3H); 13C NMR (101 MHz, MeOD) δ 173.61, 171.56, 169.98, 141.62, 116.18, 77.39, 76.02, 73.57, 73.31, 72.45, 62.18, 61.02, 53.82, 52.19, 47.78, 47.19, 45.94, 42.56, 40.29, 37.22, 37.16, 34.59, 32.91, 32.52, 32.18, 31.74, 28.06, 28.04, 27.02, 26.81, 22.54, 19.91, 18.88, 17.80, 17.54, 12.53; HRMS (ESI+) m/z calcd for C36H65N4O6S [M + H] 681.4625, found: 681.4602.
(3R,3aR,4R,5R,7S,8S,9R,9aS,12R)-3,8-dihydroxy-4,7,9,12-tetramethyl-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-((R)-2-(2-aminoacetamido)-3-methylbutanamido)-2-methylpropan-2-yl)thio)acetate (3)
To a stirred solution of 1 (73.2 mg, 0.13 mmol), Boc-Gly-OH (33.9 mg, 0.19 mmol), NaHCO3 (108 mg, 1.29 mmol) and Glyceroacetonide-Oxyma (43.3 mg, 0.19 mmol) in DMF-H2O (9/1, 0.65 mL) was added EDCI (124 mg, 0.65 mmol). The reaction mixture was stirred for 12h at rt, quenched with aq. sat. NaHCO3, and extracted with EtOAc. The combined organic extract was washed with 1N HCl, brine, dried over Na2SO4 and concentrated in vacuo. To a stirred solution of the crude product (39.7 mg, 0.055 mmol) in dioxane (0.2 mL) was added 4N HCl/dioxane (0.3 mL). The reaction mixture was stirred for 2h at rt, and all volatiles were evaporated in vacuo. The crude mixture was purified by C18 reverse-phase HPLC [column: HYPERSIL GOLD™ (175 Å, 12 μm, 250 × 10 mm), solvents: 80:20 MeOH : H2O, flow rate: 2.0 mL/min, UV: 220 nm] to afford 3 (32.6 mg, 0.052 mmol, 95%, retention time: 11 min): [α]22D +0.538 (c = 1.08, MeOH); IR (thin film) νmax = 3421 (br), 3320 (br), 3078 (br), 2961, 2877, 1715, 1661, 1541, 1463, 1456, 1388, 1370, 1279, 1146, 1020, 1004, 935 cm−1; 1H NMR (400 MHz, Methanol-d4) δ 6.35 (dd, J = 17.2, 11.4 Hz, 1H), 5.62 (d, J = 9.4 Hz, 1H), 5.17 (s, 1H), 5.16 – 5.12 (m, 1H), 4.49 (t, J = 5.5 Hz, 1H), 4.26 (d, J = 6.6 Hz, 1H), 3.66 (s, 1H), 3.35 (s, 1H), 3.30 – 3.25 (m, 2H), 2.40 – 2.31 (m, 1H), 2.20 – 2.09 (m, 3H), 1.96 (dd, J = 13.9, 4.3 Hz, 1H), 1.92 – 1.86 (m, 1H), 1.73 – 1.56 (m, 5H), 1.49 – 1.37 (m, 3H), 1.34 – 1.28 (m, 3H), 1.26 (s, 3H), 1.25 (s, 3H), 1.25 (s, 3H), 1.13 (s, 3H), 1.01 (d, J = 6.5 Hz, 3H), 0.99 (d, J = 6.5 Hz, 3H), 0.86 (d, J = 7.0 Hz, 3H), 0.70 (d, J = 7.2 Hz, 3H); 13C NMR (101 MHz, MeOD) δ 171.51, 141.55, 116.24, 77.41, 76.07, 73.31, 60.60, 52.22, 47.19, 45.96, 42.58, 37.23, 37.16, 34.59, 33.32, 32.91, 31.94, 29.11, 29.04, 28.99, 28.04, 26.96, 26.85, 19.89, 18.49, 17.82, 17.50, 12.52; HRMS (ESI+) m/z calcd for C33H58N3O6S [M + H] 624.4046, found: 624.4081.
(3R,3aR,4R,5R,7S,8S,9R,9aS,12R)-3,8-dihydroxy-4,7,9,12-tetramethyl-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-((R)-2-((2-aminoethyl)amino)-3-methylbutanamido)-2-methylpropan-2-yl)thio)acetate (4)
To a stirred solution of 1 (100 mg, 0.17 mmol) and N-Boc-2-aminoacetaldehyde (53.1 mg, 0.33 mmol) in MeOH (0.83 mL) was added sodium cyanoborohydride (21.0 mg, 0.33 mmol) at 0°C. After being stirred for 2h at rt, quenched with aq. sat. NaHCO3, and extracted with CHCl3. The combined organic extract was dried over Na2SO4 and concentrated in vacuo. To a stirred solution of the crude product (27.3 mg, 0.038 mmol) in dioxane (0.2 mL) was added a 4N HCl/dioxane (0.4 mL). The reaction mixture was stirred for 3h at rt, and all volatiles were evaporated in vacuo. The crude mixture was purified by C18 reverse-phase HPLC [column: HYPERSIL GOLD™ (175 Å, 12 μm, 250 × 10 mm), solvents: 50:50 MeOH : H2O, flow rate: 2.0 mL/min, UV: 220 nm] to afford 4 (21.6 mg, 0.035 mmol, 93%, retention time: 27 min): TLC (CHCl3/MeOH 90:10) Rf = 0.20; [α]22D 0.031 (c = 0.033, MeOH); IR (thin film) νmax = 3430 (br), 2959, 2931, 2878, 1716, 1676, 1563, 1555, 1542, 1463, 1457, 1287, 1130, 1020, 1005, 933 cm−1; 1H NMR (500 MHz, Methanol-d4) δ 6.37 – 6.31 (m, 1H), 5.62 (d, J = 9.3 Hz, 1H), 5.16 (d, J = 4.8 Hz, 1H), 5.13 (s, 1H), 4.49 (t, J = 5.5 Hz, 1H), 3.70 – 3.64 (m, 1H), 3.46 (d, J = 14.0 Hz, 1H), 3.34 (d, J = 2.9 Hz, 2H), 3.27 (d, J = 14.1 Hz, 3H), 3.23 – 3.16 (m, 1H), 2.40 – 2.33 (m, 1H), 2.29 – 2.21 (m, 1H), 2.19 – 2.11 (m, 2H), 1.98 – 1.87 (m, 2H), 1.72 – 1.61 (m, 3H), 1.61 – 1.57 (m, 1H), 1.48 – 1.36 (m, 2H), 1.35 – 1.32 (m, 1H), 1.29 (s, 6H), 1.24 (s, 3H), 1.15 (d, J = 6.7 Hz, 3H), 1.13 (s, 3H), 1.11 (d, J = 6.7 Hz, 3H), 0.86 (d, J = 7.0 Hz, 3H), 0.69 (d, J = 7.2 Hz, 3H); 13C NMR (101 MHz, MeOD) δ 171.49, 141.64, 116.18, 77.40, 76.02, 73.45, 68.59, 52.20, 47.80, 47.19, 46.71, 45.98, 45.83, 42.59, 37.21, 37.19, 34.60, 33.31, 32.90, 32.74, 31.99, 29.00, 28.05, 27.11, 19.07, 18.76, 17.80, 17.52, 12.51; HRMS (ESI+) m/z calcd for C33H60N3O5S [M + H] 610.4254, found 610.4245.
Pleuromutilin (5)
Pleuromutilin (5) (~85%) was purchased from Nanjing Pharmatechs Co., Ltd., and used after purification by silica gel column chromatography (hexanes/EtOAc 67:33 to 50:50): TLC (hexanes/EtOAc 50:50) Rf = 0.40; [α]21D +0.960 (c = 4.41, CHCl3); IR (thin film) νmax = 3448 (br), 2984, 2936, 2884, 2865, 1731, 1455, 1415, 1375, 1283, 1232, 1217, 1154, 1097, 1016, 978, 933, 916, 754 cm−1; 1H NMR (400 MHz, Chloroform-d) δ 6.49 (dd, J = 17.4, 11.0 Hz, 1H), 5.83 (d, J = 8.5 Hz, 1H), 5.36 (dd, J = 11.0, 1.5 Hz, 1H), 5.21 (dd, J = 17.4, 1.6 Hz, 1H), 4.07 (d, J = 17.1 Hz, 1H), 4.01 (d, J = 17.1 Hz, 1H), 3.36 (d, J = 6.5 Hz, 1H), 2.43 (brs, 1H), 2.34 (quin, J = 7.0 Hz, 1H), 2.25 (ddd, J = 10.3, 5.4, 2.2 Hz, 1H), 2.22 (quin, J = 9.2 Hz, 1H), 2.09 (quin, J = 8.7 Hz, 2H), 1.78 (dq, J = 14.3, 2.8 Hz, 1H), 1.72 – 1.61 (m, 2H), 1.55 (td, J = 13.8, 3.5 Hz, 1H), 1.49 (d, J = 3.3 Hz, 1H), 1.47 – 1.45 (m, 1H), 1.43 (s, 3H), 1.38 (dq, J = 14.4, 3.8 Hz, 1H), 1.32 (d, J = 16.1 Hz, 1H), 1.17 (s, 3H), 1.12 (dd, J = 13.9, 4.5 Hz, 1H), 0.89 (d, J = 7.0 Hz, 3H), 0.70 (d, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 216.88, 172.16, 138.79, 117.38, 74.54, 69.75, 61.29, 58.05, 45.40, 44.69, 43.98, 41.80, 36.57, 36.01, 34.40, 30.36, 26.80, 26.29, 24.81, 16.61, 14.75, 11.52; HRMS (ESI+) m/z calcd for C22H35O5 [M + H] 379.2484, found: 379.2438.
(3R,3aR,4R,5R,7S,8S,9R,9aS,12R)-3,8-dihydroxy-4,7,9,12-tetramethyl-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-(tosyloxy)acetate (6)
To a stirred solution of 5 (7.48 g, 19.8 mmol) in MeOH (80 mL) was added NaBH4 (1.50 g, 39.5 mmol) at 0°C. After 8h, the reaction mixture was quenched with aq. sat. NH4Cl. After 12h, the reaction mixture was extracted with EtOAc and the combined organic phase was dried over Na2SO4 and concentrated in vacuo. To a stirred solution of the crude product in CH2Cl2 (100 mL) was added TsCl (4.51 g, 23.7 mmol) and DMAP (3.61 g, 29.6 mmol) at 0°C. After 7h at 0°C, the reaction mixture was quenched with 1N HCl and extracted with EtOAc. The combined organic extract was washed with aq. sat. NaHCO3, dried over Na2SO4, and concentrated in vacuo. The crude product was purified by silica gel column chromatography (hexanes/EtOAc 60:40) to yield 6 (10.5 g, 19.7 mmol, 99%): TLC (hexanes/EtOAc 50:50) Rf = 0.50; [α]21D −0.124 (c = 0.66, CHCl3); IR (thin film) νmax = 3562 (br), 2946, 2878, 1755, 1734, 1453, 1370, 1293, 1224, 1190, 1176, 1118, 1096, 1042, 1020, 1005, 925, 839, 814, 765, 719, 663 cm−1; 1H NMR (400 MHz, Chloroform-d) δ 7.81 (d, J = 8.4 Hz, 2H), 7.36 – 7.33 (m, 2H), 6.45 (dd, J = 17.4, 11.0 Hz, 1H), 5.62 (d, J = 9.5 Hz, 1H), 5.31 (dd, J = 11.0, 1.6 Hz, 1H), 5.16 (dd, J = 17.4, 1.6 Hz, 1H), 4.55 (q, J = 5.1 Hz, 1H), 4.47 (s, 2H), 3.15 (dd, J = 11.1, 6.3 Hz, 1H), 2.45 (s, 3H), 2.26 – 2.19 (m, 1H), 2.14 – 2.05 (m, 2H), 1.99 – 1.90 (m, 1H), 1.84 (td, J = 13.4, 5.2 Hz, 1H), 1.73 – 1.69 (m, 1H), 1.63 (tdd, J = 16.1, 8.2, 4.1 Hz, 2H), 1.51 – 1.47 (m, 1H), 1.47 – 1.43 (m, 1H), 1.43 – 1.39 (m, 1H), 1.39 – 1.34 (m, 1H), 1.29 (d, J = 3.8 Hz, 1H), 1.19 (s, 3H), 1.13 (s, 3H), 0.80 (d, J = 7.0 Hz, 3H), 0.61 (d, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 216.73, 164.87, 145.23, 139.21, 132.62, 129.89 (2C), 128.11 (2C), 116.84, 74.81, 72.18, 65.16, 50.84, 45.93, 44.97, 44.86, 41.28, 36.34, 35.60, 34.27, 32.46, 31.71, 27.55, 26.23, 21.70, 17.47, 16.88, 12.16; HRMS (ESI+) m/z calcd for C29H43O7S [M + H] 535.2730, found: 535.2742.
(3R,3aR,4R,5R,7S,8S,9R,9aS,12R)-3,8-dihydroxy-4,7,9,12-tetramethyl-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-amino-2-methylpropan-2-yl)thio)acetate (7)
To a stirred solution of 6 (10.5 g, 19.7 mmol), 1-amino-2-methylpropane-2-thiol hydrochloride (5.58 g, 39.4 mmol) and nBu4NBr (0.64 g, 1.97 mmol) in THF (80 mL) was added 1N NaOH (79.2 mL). After 4h at 50°C, the reaction mixture was extracted with CHCl3. The combined organic extract was dried over Na2SO4, concentrated in vacuo. The crude product was purified by silica gel column chromatography (hexanes/EtOAc 50:50 to CHCl3/MeOH 75:25) to give 7 (8.75 g, 18.7 mmol, 95%): TLC (CHCl3/MeOH 90:10) Rf = 0.20; [α]21D −0.062 (c = 0.87, CHCl3); IR (thin film) νmax = 3421 (br), 2955, 2876, 1721, 1462, 1371, 1283, 1219, 1121, 1020, 1005, 932, 772 cm−1; 1H NMR (400 MHz, Chloroform-d) δ 6.51 (dd, J = 17.4, 11.0 Hz, 1H), 5.60 (d, J = 9.4 Hz, 1H), 5.32 (dd, J = 11.0, 1.7 Hz, 1H), 5.16 (dd, J = 17.4, 1.7 Hz, 1H), 4.55 (t, J = 5.5 Hz, 1H), 3.16 (d, J = 6.4 Hz, 1H), 3.13 (s, 2H), 2.61 (s, 2H), 2.26 – 2.18 (m, 1H), 2.15 (t, J = 6.8 Hz, 1H), 2.08 (dd, J = 15.8, 9.3 Hz, 1H), 2.00 – 1.91 (m, 1H), 1.85 (td, J = 13.7, 4.5 Hz, 1H), 1.74 – 1.58 (m, 4H), 1.51 (d, J = 5.2 Hz, 1H), 1.48 – 1.41 (m, 2H), 1.41 – 1.33 (m, 1H), 1.24 (s, 6H), 1.23 (s, 3H), 1.14 (s, 3H), 0.80 (d, J = 7.0 Hz, 3H), 0.71 (d, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 169.38, 139.56, 116.66, 74.93, 71.25, 51.68, 51.01, 48.44, 46.00, 45.20, 44.86, 41.24, 36.62, 35.61, 34.31, 32.63, 31.76, 31.33, 27.66, 26.30, 26.25 (2C), 17.64, 17.21, 12.16; HRMS (ESI+) m/z calcd for C26H46NO4S [M + H] 468.3148, found: 468.3181.
Minimum Inhibitory Concentration Assays
A single colony of A. baumannii (ATCC 19606) was grown on a Tryptic soy agar plate.57 Seed cultures and larger cultures were obtained using Tryptic soy agar broth. The flasks were incubated overnight in a shaking incubator at 37 °C with a shaking speed of 200 r.p.m. and cultured to mid-log phase (OD −0.5). The OD was monitored at 600 nm using a 96-well microplate reader. The inhibitors were dissolved in DMSO (a final concentration of 1 mg per 100 μL). This concentration was used as the stock solution for all studies. Bacterial cultures at 0.5 OD, was treated with serial dilutions of inhibitors and incubated at 37 °C for 48 h. 20 μL of resazurin (stock-0.02%) was added and incubated on a shaking incubator at 37 °C for 1 h. The lowest concentration at which the color of resazurin was completely retained as blue was read as the MIC (pink=growth, blue=no growth). The MBC defines that blue color retains over 4h. If necessary, viable bacteria in each well (96-well plate) were measured via colony-forming unit (CFU) on the agar plate. The absorbance measurements were also performed using a Biotek Synergy XT (Winooski, VT, USA), 96-well plate reader at 570 nm and 600 nm.
Synergistic Effect of Doxyclycine with 1 or Valnemulin
The synergistic or antagonistic activities of doxycycline with 1 or valnemulin were assessed in vitro via micro dilution broth checkerboard technique. The FIC index was calculated according to the following equation. ΣFIC=FICA+FICB=CA/MICA+CB/MICB where, MICA and MICB: MIC of drugs A and B, CA and CB=concentrations of drugs A and B used in combination. In these interaction studies, ΣFIC of less than 0.5 represents synergistic activity.
Cytotoxicity Assays
Cytotoxicity assays were performed using Vero monkey kidney (ATCC CCL-81) and HepG2 human hepatoblastoma cell (ATCC HB-8065) lines. Vero or HepG2 cells were cultured in 75 cm2 flasks and transferred to 96-well cell culture plates using ATCC-formulated Eagle’s minimum essential medium containing 10% FBS, and penicillin-streptomycin. Serially diluted aliquots of each test compound at concentrations ranging from 0.78–200 μg/mL were added to the cells. Control compounds with known toxicity such as tunicamycin, colistin or tobramycin were included on each plate. The plates were incubated and cytotoxic effects were determined via the MTT assay.
Microsomal Stability
Pooled Sprague-Dawley rat liver microsomes were purchased from Corning Life Sciences (Oneonta, NY, USA). Microsomes (20 mg/mL) were thawed on ice and diluted using phosphate buffer (100 mM, pH: 7.4), resulting in a protein concentration of 1 mg/mL. Stock solutions (10 mg/L) of 1, valnemulin and verapamil (positive control) was prepared in DMSO (50%). A final concentration of 500 ng/mL was used for incubation with microsomes. NADPH (final concentration: 1 mM) was used as a co-factor. All the above solutions except NADPH were added to individual wells (12-well) in triplicate and were allowed to equilibrate for 5 min at 37 °C. NADPH was then added. 50 μL aliquots in triplicate were drawn from the incubation mixture at 0, 5, 10, 20, 30, 45 and 60 min and immediately the reaction was quenched by addition of ice-cold methanol (4-volumes). Analysis was performed by LC-MS.
Protein Binding
Plasma protein binding of 1 and verapamil (reference) was determined by equilibrium dialysis. The ready to use red device inserts (MW cutoff 6000–8000 D, RED® device, Thermo Scientific, Rockford, USA) containing plasma and buffer chambers for dialysis were used. The inserts were placed in a base plate. High and low concentrations (5000 ng/mL and 500 ng/mL) of 1 and reference were prepared in rat plasma (Innovative Grade US Origin Sprague-Dawley Rat Plasma (anticoagulant: Lithium Heparin), catalog# IGRT-N)) and an aliquot of 300 μL was added in the plasma chamber in duplicate. A 500 μL aliquot of blank isotonic phosphate buffer, pH 7.4 was added to buffer chamber of dialysis device. The base plate was covered with sealing tape and incubated at 37°C at approximately 100 r.p.m on an orbital shaker for 4h to achieve equilibrium. At the end of incubation, 50 μL aliquots were withdrawn from plasma and buffer chambers. Four volumes of internal standard spiked methanol were vortexed for 30 seconds and centrifuged at 1000 rpm for 5 min. The supernatant was analyzed by LC-MS. The free fraction of the drug was calculated as ratio of the concentrations in the buffer and in plasma. The results were expressed in terms of % bound to plasma proteins.
In vivo Efficacy using a Mouse Model of Infection
16–18 gram female C57BL/6 mice (n=5) were used for the animal studies. The animal study protocol (protocol number: 1410–31903A) was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Minnesota on May 5, 2016. A. baumannii (ATCC19606) cultured at 37 °C were collected by centrifugation and infected into C57BL/6 mice to create a mouse septicemia model. One hour after the infection (at a dose that lead to 75% of death), the molecules (1, 1-Dox 35/1, or tobramycin) were administered intraperitoneally (IP) at single dose (from 2 to 60 mg/kg). Mice were monitored for 5 days and death was defined as the end point.
Gene Analyses of Resistant Strain of A. baumannii against 1
Spontaneous resistant mutants of A. baumannii (ATCC 19606) with decreased sensitivity towards 1 were isolated by sub-culturing bacteria on agar plates with concentrations of 1 at MIC and above. Resistant mutants were isolated at 16 × MIC of 1. Spontaneous resistant mutants with decreased sensitivity against rifampicin were isolated at 32 × MIC of rifampicin (control). The chromosomal DNAs from the resistant mutant (1R) and wild-type Acinetobacter baumannii ATCC19606 were isolated. The rplC gene fragment was amplified using A. baumannii rplC specific primers (AbrplCupPrimer 5′ CTTTGGGTTA AGGCTTTCGG 3′ and Abrplcdn 5′CAACAGCAGAGCCG GAAACAG 3′), purified, and DNA sequenced. The DNA sequencing result was used to blast against rplC DNA sequence of A. baumannii in NIH Genome database.
Supplementary Material
Acknowledgments
The National Institutes of Health is greatly acknowledged for financial support of this work (AI084411). We also thank University of Tennessee for generous financial support (CORNET award). NMR data were obtained on instruments supported by the NIH Shared Instrumentation Grant. The authors gratefully acknowledge Drs. Katsuhisa Yamazaki and Yoshimasa Ishizaki (The Institute of Microbial Chemistry) for useful discussions.
Funding Sources: NIH/NIAID AI084411
ABBREVIATIONS
- THF
tetrahydrofuran
- CH2Cl2
methylene chloride
- DMSO
Dimethyl sulfoxide
- DMF
N,N-dimethylformamide
- MeOH
methanol
- EtOAc
ethyl acetate
- CHCl3
chloroform
- HRMS
high resolution mass spectrometry
- HPLC
high performance liquid chromatography
- TLC
thin layer chromatography
- Bu
n-butyl
- Ts
p-toluenesulfonyl
- DMAP
N,N-dimethyl-4-aminopyridine
- Boc
tert-butoxycarbonyl
- EDCI
1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
- Val
Valine
- Orn
ornithine
- Gly
glycine
- rt
room temperatures
- ATCC
American type culture collection
- MIC
Minimum inhibitory concentration
- FIC
Fractional inhibitory concentration
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OD
Optical density
- FBS
Fetal bovine serum
- NADPH
Nico-tinamide adenine dinucleotide phosphate
Footnotes
Supporting Information. Some assay data, copies of NMR spectra, HPLC chromatogram of new compounds, and assay procedures. This material is available free of charge via the Internet at http://pubs.acs.org
REFERENCES AND NOTES
- 1.Maragakis LL, Perl TM. Acinetobacter baumannii: Epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46:1254–1263. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 2.Boucher HW, Talbot GH, Benjamin DK, Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D. 10 × 20 Progress-development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:1685–1694. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shrestha S, Tada T, Miyoshi-Akiyama T, Ohara H, Shimadab K, Satou K, Teruya K, Nakano K, Shiroma A, Sherchand JB, Rijal BP, Hirano T, Kirikae T, Pokhrel BM. Molecular epidemiology of multidrug-resistant Acinetobacter baumannii isolates in a university hospital in nepal reveals the emergence of a novel epidemic clonal lineage. Int J Antimicrob Agents. 2015;46:526–531. doi: 10.1016/j.ijantimicag.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Hindler JA, Humphries RM. Colistin MIC variability by method for contemporary clinical isolates of multidrug-resistant gram-negative bacilli. J Clin Microbiol. 2013;51:1678–1684. doi: 10.1128/JCM.03385-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaddy JA, Actis LA. Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol. 2009;4:273–278. doi: 10.2217/fmb.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362:1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Protic D, Pejovic A, Andjelkovic D, Djukanovic N, Savic D, Piperac P, Markovic DL, Zdravkovic M, Todorovic Z. Nosocomial infections caused by Acinetobacter baumannii: are we losing the battle? Surg Infect. 2016;17:236–242. doi: 10.1089/sur.2015.128. [DOI] [PubMed] [Google Scholar]
- 8.Guerrero DM, Perez F, Conger NG, Solomkin JS, Adams MD, Rather PN, Bonomo RA. Acinetobacter baumannii-associated skin and soft tissue infections: recognizing a broadening spectrum of disease. Surg Infect. 2010;11:49–57. doi: 10.1089/sur.2009.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard A, O’Donoghue M, Feeney A, Sleator RD. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence. 2012;3:243–250. doi: 10.4161/viru.19700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manchanda V, Sanchaita S, Singh NP. Multidrug resistant Acinetobacter. J Global Infect Dis. 2010;2:291–304. doi: 10.4103/0974-777X.68538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birch AJ, Holzapfel CW, Richards RW. Diterpenoid nature of pleuromutilin. Chem Ind. 1963;14:374–375. [Google Scholar]
- 12.Stipkovits L, Ripley P, Tenk M, Glávits R, Molnár T, Fodor L. The efficacy of valnemulin (Econor) in the control of disease caused by experimental infection of calves with Mycoplasma bovis. Res Vet Sci. 2005;78:207–215. doi: 10.1016/j.rvsc.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Jones RN, Fritsche TR, Sader HS, Ross JE. Activity of retapamulin (SB-275833), a novel pleuromutilin, against selected resistant gram-positive cocci. Antimicrob Agents Chemother. 2006;50:2583–2586. doi: 10.1128/AAC.01432-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novak R, Shlaes DM. The pleuromutilin antibiotics: a new class for human use. Curr Opin Invest Drugs. 2010;11:182–911. [PubMed] [Google Scholar]
- 15.Poulsen SM, Karlsson M, Johansson LB, Vester B. The pleuromutilin drugs tiamulin and valnemulin bind to the RNA at the peptidyl transferase center on the ribosome. Mol Microbiol. 2001;41:1091–1099. doi: 10.1046/j.1365-2958.2001.02595.x. [DOI] [PubMed] [Google Scholar]
- 16.Sader HS, Biedenbach DJ, Paukner S, Ivezic-Schoenfeld Z, Jonesa RN. Antimicrobial activity of the investigational pleuromutilin compound BC-3781 tested against gram-positive organisms commonly associated with acute bacterial skin and skin structure infections. Antimicrob Agents Chemother. 2012;56:1619–1623. doi: 10.1128/AAC.05789-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paukner S, Sader HS, Ivezic-Schoenfeld Z, Jonesb RN. Antimicrobial activity of the pleuromutilin antibiotic BC-3781 against bacterial pathogens isolated in the SENTRY antimicrobial surveillance program in 2010. Antimicrob Agents Chemother. 2013;57:4489–4495. doi: 10.1128/AAC.00358-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirokawa Y, Kinoshita H, Tanaka T, Nakata K, Kitadai N, Fujimoto K, Kashimoto S, Kojima T, Kato S. Water-soluble pleuromutilin derivative with excellent in vitro and in vivo antibacterial activity against gram-positive pathogens. J Med Chem. 2008;51:1991–1994. doi: 10.1021/jm8000136. [DOI] [PubMed] [Google Scholar]
- 18.Shang R, Wang G, Xu X, Liu S, Zhang C, Yi Y, Liang J, Liu Y. Synthesis and biological evaluation of new pleuromutilin derivatives as antibacterial agents. Molecules. 2014;19:19050–19065. doi: 10.3390/molecules191119050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Ling Y, Wang H, Yu J, Tang J, Zheng H, Zhao X, Wang D, Chen G, Qui W, Tao J. Novel Pleuromutilin derivatives as antibacterial agents: synthesis, biological evaluation and molecular docking studies. Bioorg Med Chem Lett. 2012;22:6166–6172. doi: 10.1016/j.bmcl.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Shang R, Pu X, Xu X, Xin Z, Zhang C, Guo W, Liu Y, Liang J. Synthesis and biological activities of novel pleuromutilin derivatives with a substituted thiadiazole moiety as potent drug-resistant bacteria inhibitors. J Med Chem. 2014;57:5664–5678. doi: 10.1021/jm500374c. [DOI] [PubMed] [Google Scholar]
- 21.Riedl K. Studies on pleuromutilin and some of its derivatives. J Antibiot. 1976;29:132–139. doi: 10.7164/antibiotics.29.132. [DOI] [PubMed] [Google Scholar]
- 22.Egger H, Reinshagen H. New pleuromutilin derivatives with enhanced antimicrobial activity. J Antibiot. 1976;29:915–922. doi: 10.7164/antibiotics.29.915. [DOI] [PubMed] [Google Scholar]
- 23.Shang R, Wang S, Xu X, Yi Y, Guo W, Liu Y, Liang J. Chemical synthesis and biological activities of novel pleuromutilin derivatives with substituted amino moiety. PLoS One. 2013;8:e82595. doi: 10.1371/journal.pone.0082595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu P, Z YY, Sun YX, Liu JH, Yang B, Wang YZ, Wang YL. Novel pleuromutilin derivatives with excellent antibacterial activity against Staphylococcus aureus. Chem Biol Drug Des. 2009;73:655–660. doi: 10.1111/j.1747-0285.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang YY, Xu KP, Ren D, Ge SR, Wang YL, Wang YZ. Synthesis and antibacterial activities of pleuromutilin derivatives. Chin Chem Lett. 2009;20:29–31. [Google Scholar]
- 26.Shang R, Liu Y, Xin Z, Guo W, Guo Z, Hao B, Jianping L. Synthesis and antibacterial evaluation of novel pleuromutilin derivatives. Eur J Med Chem. 2013;63:231–238. doi: 10.1016/j.ejmech.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 27.Ling CY, Tao YL, Chu WJ, Wang H, Wang HD, Yang YS. Design, synthesis and antibacterial activity of novel pleuromutilin derivatives with 4H-pyran-4-one and pyridin-4-one substitution in the C-14 side chain. Chin Chem Lett. 2016;27:235–240. [Google Scholar]
- 28.Tang YZ, Liu YH, Chen JX. Pleuromutilin and its derivatives-the lead compounds for novel antibiotics. Mini-Rev Med Chem. 2012;12:53–61. doi: 10.2174/138955712798868968. [DOI] [PubMed] [Google Scholar]
- 29.The stereochemistry of the C3-alcohol was determined via the NOESY experiment (see supporting information).
- 30.Wang Q, Wang Y, Kurosu M. A new oxyma derivative for nonracemizable amide-forming reactions in water. Org Lett. 2012;14:3372–3375. doi: 10.1021/ol3013556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Aleiwi BA, Wang Q, Kurosu M. Selective esterifications of primary alcohols in a water-containing solvent. Org Lett. 2012;14:4910–4913. doi: 10.1021/ol3022337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aleiwi BA, Mitachi K, Kurosu M. Mild and convenient N-formylation protocol in water-containing solvents. Tetrahedron Lett. 2013;54:2077–2081. doi: 10.1016/j.tetlet.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Water solubility of the molecules in this program was determined via the shake flask method. This experimental procedure was summarized in Supporting Information.
- 34.Hsieh MH, Chen MY, Victor LY, Chow JW. Synergy assessed by checkerboard. a critical analysis. Diagn Microbiol Infect Dis. 1993;16:343–349. doi: 10.1016/0732-8893(93)90087-n. [DOI] [PubMed] [Google Scholar]
- 35.Ohrt C, Willingmyre GD, Lee P, Knirsch C, Milhous W. Assessment of azithromycin in combination with other antimalarial drugs against Plasmodium falciparum in vitro. Antimicrob Agents Chemother. 2002;46:2518–2524. doi: 10.1128/AAC.46.8.2518-2524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siricilla S, Mitachi K, Wan B, Franzblau SG, Kurosu M. Discovery of a capuramycin analog that kills non-replicating Mycobacterium tuberculosis and its synergistic effects with translocase I inhibitors. J Antibiot. 2014;68:271–278. doi: 10.1038/ja.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou YF, Yu Y, Sun J, Tao MT, Zhou WJ, Li X, Liao XP, Liu YH. Ex vivo pharmacokinetic/pharmacodynamics relationship of valnemulin against Clostridium perfingens in plasma, the small intestinal and caecal contents of rabbits. Anaerobe. 2016;39:150–157. doi: 10.1016/j.anaerobe.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Huang Q, Li J, Xia L, Xia X, Duan P, Shen J, Ding S. Residue depletion of valnemulin in swine tissues after oral administration. Anal Chim Acta. 2010;664:62–67. doi: 10.1016/j.aca.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 39.Yang S, Shi W, Hu D, Zhang S, Zhang H, Wang Z, Cheng L, Sun F, Shen J, Cao X. In vitro and in vivo metabolite profiling of valnemulin using ultraperformance liquid chromatography–quadrupole/time-of-flight hybrid mass spectrometry. J Agric Food Chem. 2014;17:62, 9201–9210. doi: 10.1021/jf5012402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGinnity DF, Soars MG, Urbanowicz RA, Riley RJ. Evaluation of fresh and cryopreserved hepatocytes as in vitro drug metabolism tools for the prediction of metabolic clearance. Drug Metab Dispos. 2004;32:1247–1253. doi: 10.1124/dmd.104.000026. [DOI] [PubMed] [Google Scholar]
- 41.Stockert JC, Blázquez-Castro A, Cañete M, Horobin RW, Villanueva A. MTT assay for cell viability: intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012;114:785–96. doi: 10.1016/j.acthis.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Senthilraja P, Kathiresan K. In vitro cytotoxicity MTT assay in Vero, HepG2 and MCF -7 cell lines study of marine yeast. J Appl Pharm Sci. 2015;5:80–84. [Google Scholar]
- 43.Debnath J, Siricilla S, Wan B, Crick DC, Lenaerts AJ, Franzblau SG, Kurosu M. Discovery of selective menaquinone biosynthesis inhibitors against Mycobacterium tuberculosis. J Med Chem. 2012;55:3739–3755. doi: 10.1021/jm201608g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh JK, Solanki A, Maniyar RC, Banerjee D, Shirsath VS. Rapid equilibrium dialysis (RED): an in-vitro high-throughput screening technique for plasma protein binding using human and rat plasma. J Bioequivalence Bioavailability. 2012:S14. [Google Scholar]
- 45.Buras JA, Holzmann B, Sitkovsky M. Model organisms: animal models of sepsis: setting the stage. Nat Rev Drug Discovery. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 46.Bals R, Weiner DJ, Moscioni AD, Meegalla RL, Wilson JM. Augmentation of innate host defense by expression of a cathelicidin antimicrobial peptide. Infect Immun. 1999;67:6084–6089. doi: 10.1128/iai.67.11.6084-6089.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bøsling J, Poulsen SM, Vester B, Long KS. Resistance to the peptidyl transferase inhibitor tiamulin caused by mutation of ribosomal protein L3. Antimicrob Agents Chemother. 2003;47:2892–2896. doi: 10.1128/AAC.47.9.2892-2896.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pringle M, Poehlsgaard J, Vester B, Long KS. Mutations in ribosomal protein L3 and 23S ribosomal RNA at the peptidyl transferase center are associated with reduced susceptibility to tiamulin in Brachyspira spp. isolates. Mol Microbiol. 2004;54:1295–1306. doi: 10.1111/j.1365-2958.2004.04373.x. [DOI] [PubMed] [Google Scholar]
- 49.Schlunzen F, Pyetan E, Fucini P, Yonath A, Harms JM. Inhibition of peptide bond formation by pleuromutilins: the structure of the 50S ribosomal subunit from Deinococcus radiodurans in complex with tiamulin. Mol Microbiol. 2004;545:1287–1294. doi: 10.1111/j.1365-2958.2004.04346.x. [DOI] [PubMed] [Google Scholar]
- 50.Miller K, Dunsmore CJ, Fishwick CWG, Chopra I. Linezolid and tiamulin cross-resistance in Staphylococcus aureus mediated by point mutations in the peptidyl transferase center. Antimicrob Agents Chemother. 2008;52:1737–1742. doi: 10.1128/AAC.01015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gentry DR, Rittenhouse SF, McCloskey L, Holmes DJ. Stepwise exposure of Staphylococcus aureus to pleuromutilins is associated with stepwise acquisition of mutations in rplC and minimally affects susceptibility to retapamulin. Antimicrob Agents Chemother. 2007;51:2048–2052. doi: 10.1128/AAC.01066-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Long KS, Hansen LH, Jakobsen L, Vester B. Interaction of pleuromutilin derivatives with the ribosomal peptidyl transferase center Antimicrob. Agents Chemother. 2006;50:1458–1462. doi: 10.1128/AAC.50.4.1458-1462.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Long KS, Poehlsgaard J, Hansen LH, Hobbie SN, Bottger EC, Vester B. Single 23S rRNA mutations at the ribosomal peptidyl transferase centre confer resistance to valnemulin and other antibiotics in Mycobacterium smegmatis by perturbation of the drug binding pocket. Mol Microbiol. 2009;71:1218–1227. doi: 10.1111/j.1365-2958.2009.06596.x. [DOI] [PubMed] [Google Scholar]
- 54.Long KS, Poehlsgaard J, Kehrenberg C, Schwarz S, Vester B. The cfr rRNA methyltransferase confers resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A antibiotics Antimicrob. Agents Chemother. 2006;50:2500–2505. doi: 10.1128/AAC.00131-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peleg AY, de Breij A, Adams MD, Cerqueira GM, Mocali S, Galardini M, Nibbering PH, Earl AM, Ward DV, Paterson DL, Seifert H, Dijkshoorn L. The success of Acinetobacter species; genetic, metabolic and virulence attributes. PLoS One. 2012;7:e46984. doi: 10.1371/journal.pone.0046984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Zhang J, Ji Y. PCR-based approaches for the detection of clinical methicillin-resistant Staphylococcus aureus. Open Microbiol J. 2016;10:45–56. doi: 10.2174/1874285801610010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Growth rate of A. baumannii strains in tryptic soy broth is similar to that in Mueller-Hinton broth (CLSI conditions), and the MIC and MBC values obtained with both media are identical. Because tryptic soy broth is available from several venders at much lower-cost than Mueller-Hinton broth, we utilized tryptic soy broth or tryptic soy agar plate in this study.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


