Abstract
Objective
To describe the CT features of organizing pneumonia (OP) in an oncologic patient population and to also identify features associated with lung cancer and patients undergoing haemopoietic stem cell transplant (HSCT).
Methods
In retrospective CTs from 151 patients with pathologically confirmed OP between 01/09 and 12/14, number of lesions, location, size, margin type, and consistency, as well as volume of lymphadenopathy and the presence and size of pleural effusions, were recorded. Associated malignancy was noted.
Results
OP most commonly presented as a diffuse process (n = 62, 41%); frequently occupied both a central and peripheral location (n = 79, 53%); and commonly presented with a solid appearance (n = 67, 44%) or with ground glass opacity (n = 80, 53%). Pleural effusions were seen in 68 patients (45%). OP less frequently contained air bronchograms, cavitation, necrosis, surrounding GGO, or adjacent bronchiectasis. In lung cancer patients (n = 25, 17%), OP more likely presented as discrete lesions and occupied a peripheral location as compared to patients with other malignancies (p = 0.025, 0.002). In HSCT patients, (n = 29, 19%), a diffuse process was more commonly seen than non-HSCT patients (p = 0.038).
Conclusion
OP more commonly presents as discrete lesions with a peripheral location in lung cancer patients, and as a diffuse process in patients who had undergone HSCT.
Keywords: Organizing Pneumonia, Bronchiolitis Obliterans Organizing Pneumonia, Oncology, Lung Cancer, Hemopoietic Stem Cell Transplant
Introduction
Organizing Pneumonia (OP) is a pulmonary response to injury, demonstrating inflammatory intraalveolar infiltrates on pathology.1 OP can occur in association with a number of conditions, for example, infection, drug reactions, bone marrow/organ transplantation, and radiation therapy.2 Many of these conditions are commonly encountered in an oncologic population and so an awareness of the radiologic features of OP is important to avoid misdiagnosing OP as malignancy. When OP occurs as a primary entity with no known causation, it is then referred to as Cryptogenic Organizing Pneumonia. The histological evaluation of OP typically reveals polypoid plugs of granulation tissue filling the alveoli and small airways (Masson bodies).2,3 These inflammatory tissue plugs occlude the distal bronchioles, thus giving OP the previously used term, Bronchiolitis Obliterans Organizing Pneumonia.4
The radiographic appearance of OP is typically described as bilateral patchy airspace opacities–often in a subpleural or peribronchial distribution. In 1989, Cordier et al originally described three main imaging patterns of OP: multiple alveolar opacities, solitary opacity, and infiltrative opacities.5 Since then, others in the literature have described other imaging features such as ground glass opacities, consolidation with air bronchograms, small nodular or linear ill-defined opacities, bronchial wall thickening, and larger nodules or masses. Pleural effusions, although less commonly described in OP, have been reported in up to 22% of cases in one case series.6,7
These studies, done previously to evaluate the imaging appearances of OP, have often been limited by small numbers of patients. The largest series to date comprised of only forty-five patients.1 Moreover, no previous study has evaluated the CT appearances of OP in an oncologic patient population. The goal of this study then was to describe the CT features of OP in an oncologic patient population and to also identify any CT features associated with OP in lung cancer and undergoing Hemopoietic Stem Cell Transplant (HSCT).
Materials & Methods
Patient Cohort
This retrospective study received a waiver of consent from our Institutional Review Board. With the waiver in place, we searched the pathology patient database from January 2, 2009, to September 4, 2014, for “Organizing Pneumonia” and patients who had a chest CT performed within the prior 2 weeks of the pathology report. This yielded 250 unique consecutive patients, of which 99 patients were excluded for the following reasons: no history of malignancy (16 patients), no diagnosis of OP (14 patients), biopsy not performed within the 2 weeks of the pathology report (2 patients), OP found on autopsy reports (6 patients), and microscopic OP incidentally found without CT correlate (61 patients). 151 remaining patients were submitted for analysis.
Image Analysis
In order to ensure a baseline consensus on the lexicon used for data collection, a training session was held. Sample cases were reviewed by the two reading radiologists (N.L. and R.S), who were oncologic imaging fellows, and two dedicated chest radiologists (A.P. and M.G.), who had 2 and 19 years post-training experience respectively. After having established baseline consensus, each patient’s CT was reviewed by 1 of 2 reading radiologists (N.L. and R.S). The reading radiologist identified all patients who did not fit the terminology clearly; in such an event, the CT was subsequently reviewed in consensus with the senior radiologist (M.G). All images were reviewed on the institutional Picture Archiving and Communication System (PACS, GE Centricity RA100). The imaging protocol for each CT varied given that a number of patients had imaging from outside of the institution. Five millimeter slice images were available for review in all patients with additional thin slice images (1.25 mm) available in 26 patients (17%). IV contrast was administered in 67 patients (44%).
Characteristics of the lesion
The size of the lesion was measured on lung windows by selecting the longest measurement (length) and the greatest perpendicular distance (width), on the same slice. The location of the lesion was determined to be central if the lesion contacted a bronchus to the lobar level, and peripheral if the lesion contacted a segmental bronchus or smaller. A lesion bordering the two was classified as both peripheral and central in location. In the case of multiple lesions, the characteristics of the largest lesion were recorded.
The contour of the lesion was recorded as: round (sphere with clearly defined smooth margins), lobulated (not spherical but with clearly defined smooth margins), spiculated (solid lesion with linear extensions into the adjacent lung parenchyma), or ill defined (non-definable borders). The number of lesions was recorded as: solitary, 1–5, 6–10, or greater than 10. In the cases where definable lesion/lesions were not present but had an appearance similar to pneumonia, the number was recorded as diffuse. The consistency of the lesion was recorded as: solid (density obscures underlying pulmonary vessels and parenchyma), ground glass (increased attenuation of the lung parenchyma with preserved visualization of the underlying pulmonary vessels), or consisting of air bronchograms (persistent visualization of the bronchi with surrounding increased attenuation). Additional characteristics of surrounding ground glass opacity (GGO), cavitation (pockets of air within the lesion), and necrosis (central low attenuation with peripheral enhancement) were also recorded.
Ancillary features
Ancillary features of the lesion that were recorded were the presence of lymphadenopathy and volume (nodes with a short axis measurement of between ≥ 10mm and < 15mm, ≥ 15 and < 30mm, or ≥ 30mm), pleural effusion size (small, moderate, or large), and laterality.
Statistical Analysis
Fisher’s exact test was used to compare categorical features in patients with 1) lung cancer to other malignancies, and 2) patients post HSCT to those who did not receive HSCT. The Wilcoxon rank-sum test was used to compare age and baseline size between groups.
A test with p-value < 0.05 was considered statistically significant. All statistical analyses were performed in software packages SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
CT features of pathologically confirmed OP were examined in 151 patients, all of whom had a history of malignancy. Median patient age was 61 years (range 9–91) with a similar gender breakdown (female 48%, male 52%). The most common malignancies were leukemia (n = 36), lymphoma (n = 29), lung cancer (n = 25), and breast cancer (n = 13) (Table 1). There were 29 patients with history of HSCT. The lesions ranged in size from 4 mm to 79 mm (mean 25.1 mm, median 22 mm).
Table 1.
Breakdown of Underlying Malignancy
| Malignancy | |
|---|---|
| Leukemia | 36 (24%) |
| Lymphoma | 29 (19%) |
| Lung | 25 (17%) |
| Breast | 13 (9%) |
| Colorectal | 5 (3%) |
| Esophageal | 5 (3%) |
| Pancreas | 7 (5%) |
| Sarcoma | 7 (5%) |
| Melanoma | 6 (4%) |
| Head & Neck | 5 (3%) |
| Multiple Myeloma | 3 (2%) |
| Bladder & Renal | 5 (3%) |
| Ovarian | 1 (1%) |
| Prostate | 1 (1%) |
| Cholangiocarcinoma | 1 (1%) |
| Mesothelioma | 1 (1%) |
| Thymic | 1 (1%) |
Table 2 summarizes the imaging characteristics of OP. In all patients, 41% had diffuse presentation without definable lesions (Figure 1) which was more common than lesions presenting as either solitary or multiple lesions (Figure 2). OP presented as a solitary lesion in 21% of patients, 1–5 lesions in 21%, 6–10 lesions in 9%, and >10 lesions in 9%.
Table 2.
Organizing Pneumonia Characteristics
| Lesion Characteristics | Number of Patients | Percent (95%CI) |
|---|---|---|
| Lesion location | ||
| RUL | 92 | 61% (53%, 69%) |
| RML | 74 | 49% (41%, 57%) |
| RLL | 109 | 72% (64%, 79%) |
| LUL | 92 | 61% (53%, 69%) |
| LLL | 85 | 56% (48%, 64%) |
| Peripheral | 66 | 44% (36%, 52%) |
| Central | 5 | 3% (1%, 8%) |
| Both Peripheral and Central | 79 | 52% (44%, 60%) |
| No. of lesions | ||
| Solitary | 31 | 21% (14%, 28%) |
| 1–5 lesions | 31 | 21% (14%, 28%) |
| 6–10 lesions | 13 | 9% (5%, 14%) |
| >10 lesions | 14 | 9% (5%, 15%) |
| Diffuse | 62 | 41% (33%, 49%) |
| Margin of dominant leison | ||
| Smooth | 16 | 11% (6%, 17%) |
| Lobular | 21 | 14% (9%, 20%) |
| Spiculated | 35 | 23% (17%, 31%) |
| Ill-Defined | 78 | 52% (43%, 60%) |
| Lesion Consistency | ||
| Solid | 67 | 44% (36%, 53%) |
| Internal GGO | 80 | 53% (45%, 61%) |
| Air Bronchograms | 40 | 26% (20%, 34%) |
| Additional lesion Characteristics | ||
| Surrounding GGO | 33 | 22% (16%, 29%) |
| Cavitation | 9 | 6% (3%, 11%) |
| Necrosis | 1 | 1% (0%, 4%) |
| Adjacent Bronchiectasis | 4 | 3% (1%, 7%) |
| Ancillary features | ||
| Lymphadenopathy | 65 | 43% (35%, 51%) |
| Effusion | 68 | 45% (37%, 53%) |
| Associated Pathology Findings | ||
| None | 99 | 66% (57%, 73%) |
| Granulomas | 13 | 9% (5%, 14%) |
| Bacteria/Organism | 7 | 5% (2%, 9%) |
| Reactive Type II Pneumocytes | 8 | 5% (2%, 10%) |
| Chronic Inflammation | 9 | 6% (3%, 11%) |
| Acute Inflammation | 7 | 5% (2%, 9%) |
| Atypical Adenomatous Hyperplasia | 1 | 1% (0%, 4%) |
Figure 1.
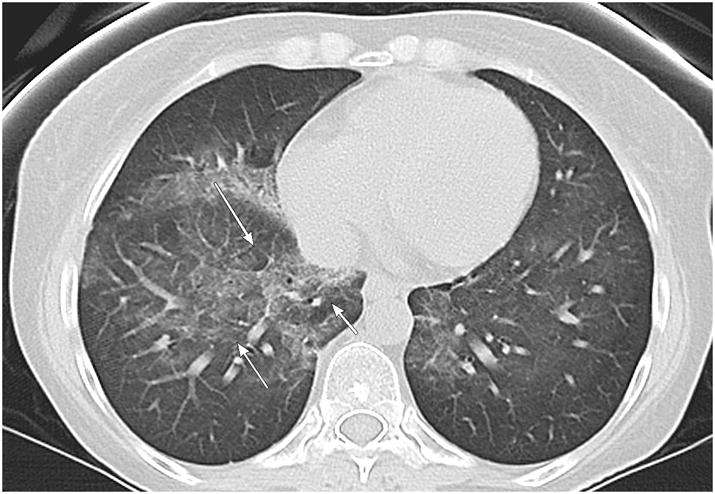
40 year old female with leukemia. Axial CT image demonstrates a diffuse pattern of OP predominately in the right lung lobe with both central and peripheral components (arrows).
Figure 2.
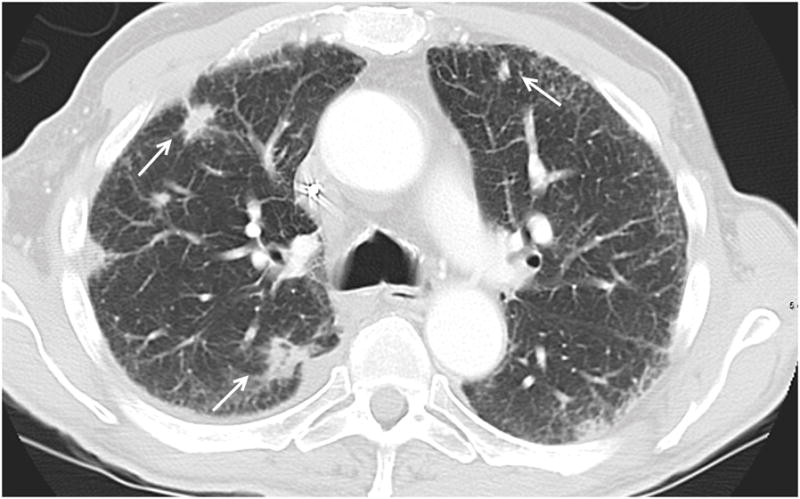
64 year old male patient with pancreatic carcinoma. Axial CT image shows multifocal OP as multiple solid spiculated lesions (arrows).
Lesions were most commonly seen in both peripheral and central in location (53%) (Figure 3). 44% of lesions were located peripherally with only 3% of lesions seen in a central location. The most common lobe involved was the right lower lobe (72%). Lesions of OP most commonly presented with an ill-defined margin (52%). A spiculated border was present in 23% and a lobular or smooth border was observed in 14% and 11% of patients respectively.
Figure 3.
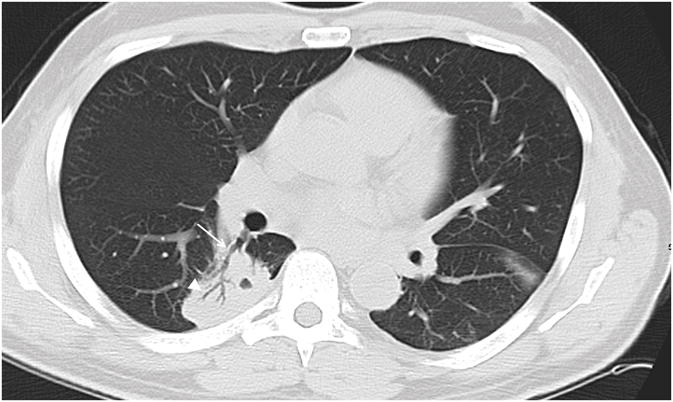
57 year old male patient with leukemia. Axial CT image demonstrates right lower lobe mass like consolidation contacting both central (arrow) and peripheral (arrowhead) bronchi.
The consistency of the lesion was predominantly solid (44%) or containing some internal ground glass attenuation (53%) (Figure 4). Air bronchograms were found in 27% of OP lesions and GGO in the surrounding parenchyma was seen with 22% of cases. Adjacent bronchiectasis, cavitation, and internal necrosis were rarely associated with lesions of OP (3%, 6%, and 1% respectively). Lymphadenopathy was observed in 43% of patients, most commonly mediastinal (27%). When lymphadenopathy was present, it was more commonly mild (42%) or moderate (39%), rather than severe (20%). OP lesions were presented with pleural effusions in 45% of patients (Figure 5). Pathological findings other than OP were uncommonly reported with granulomas being the most common associated finding seen in 9% of patients.
Figure 4.
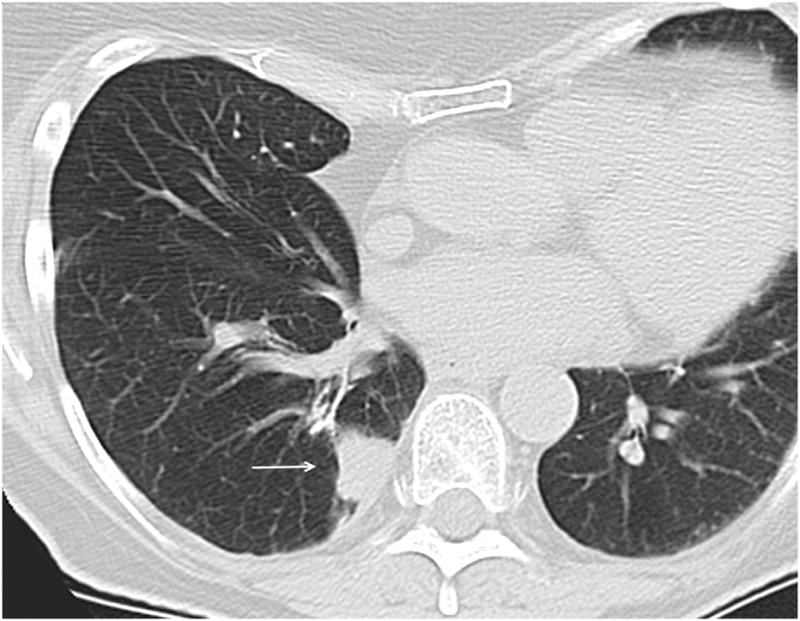
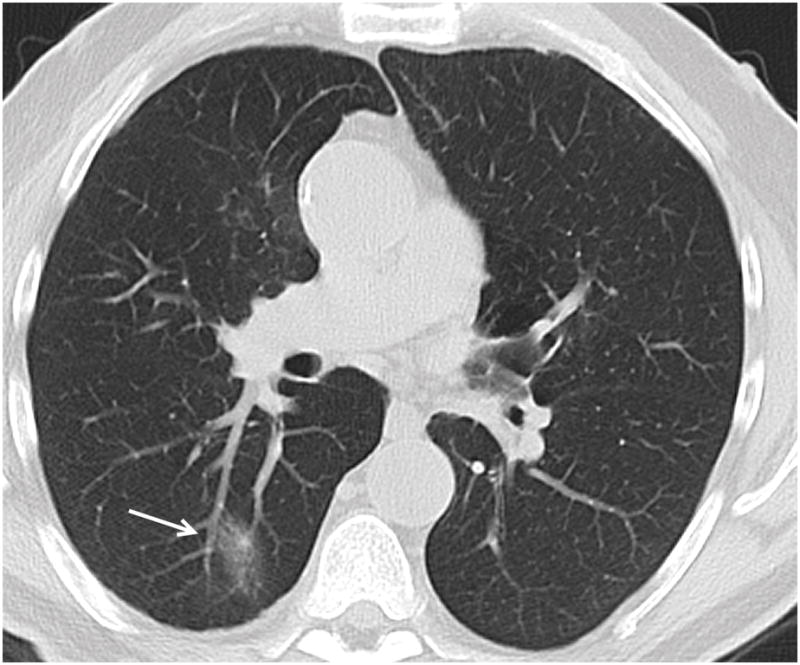
Figure 4a. 74 year old female patient with colon carcinoma. Axial CT image demonstrates OP presenting as a solid lesion in the right lower lobe (arrow).
Figure 4b. 66 year old male patient with head and neck carcinoma. Axial CT image demonstrates OP presenting as a predominantly ground glass nodule in the posterior right lower lobe (arrow).
Figure 5.
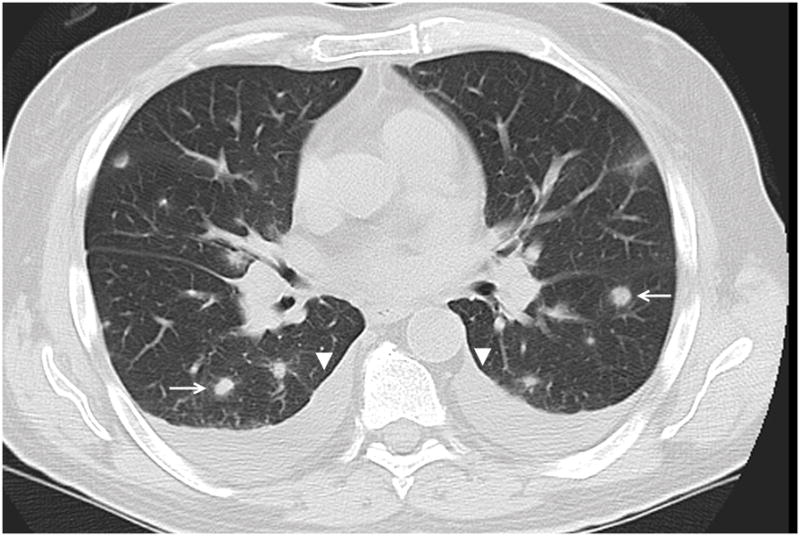
63 year old male patient with chronic lymphocytic leukemia. Axial CT image demonstrates multinodular appearance of OP (arrows) with bilateral pleural effusions (arrowheads).
OP in patients with lung malignancy was significantly more often found with single or multiple discrete lesions (80%, 95%CI: 59–93%) (Figure 6) rather than a diffuse process when compared to patients with other malignancies (55%, 95%CI: 46–64%, P = 0.025) (Table 3). In contrast, a diffuse process was significantly more often seen with HSCT (59%, 95%CI: 39–76%) (Figure 7) rather than single or multiple discrete lesions when compared to patients who had not received HSCT (37%, 95%CI: 28–46%, P = 0.038) (Table 4). OP presenting in a peripheral location was also more frequently seen in patients with lung cancer (76%, 95%CI: 55–91%) when compared to patients with other malignancies (38%, 95%CI: 29–47%, P = 0.002). A trend of more air bronchograms was also found in OP in the HSCT group (41%, 95%CI: 24–61%) when compared to patients without HSCT (23%, 95%CI: 16–31%) (P = 0.060).
Figure 6.
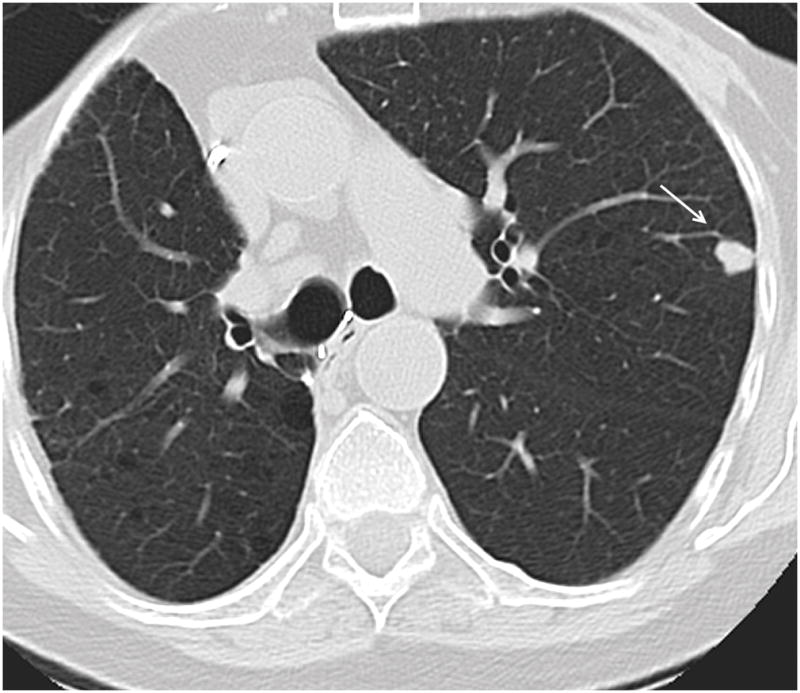
74 year old female patient with underlying diagnosis of right lower lobe squamous cell lung carcinoma. Axial CT image demonstrates OP presenting as a solitary nodule in the periphery of the left upper lobe (arrow).
Table 3.
Significant features of organizing pneumonia in patients with lung cancer
| Associated Lung Cancer | Associated with other cancers | P Value | |
|---|---|---|---|
| Age, mean±SD | 67.6± 12.9, | 55.8± 16.9 | 0.001 |
| Presenting as: | |||
| Diffuse | 5 (20%) | 57 (45%) | |
| Single/Multiple | 20 (80%) | 69 (55%) | 0.025 |
| Location: | |||
| Peripheral | 19 (76%) | 47 (38%) | 0.002 |
| Central | 0 (0%) | 5 ( 4%) | |
| Both Peripheral and Central | 6 (24%) | 73 (58%) | |
SD = standard deviation.
Figure 7.
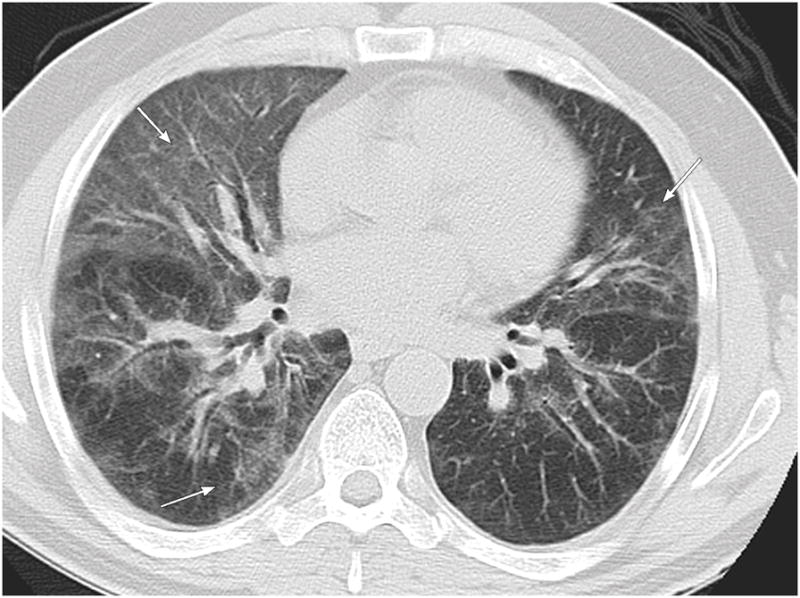
58 year old male patient with chronic lymphocytic leukemia presenting with cough and dyspnea 10 months following HSCT. Axial CT images demonstrate diffuse pattern of OP with bilateral ground glass opacities most pronounced in the lingula, right middle and right lower lobes (arrows).
Table 4.
Significant features of organizing pneumonia in patients with HSCT
| HSCT Group | Non-HSCT Group | P Value | |
|---|---|---|---|
| Age, mean±SD | 50.2± 16.1 | 59.5± 16.6 | 0.004 |
| Presenting as: | |||
| Diffuse | 17 (59%) | 45 (37%) | 0.038 |
| Single/Multiple | 12 (41%) | 77 (63%) | |
Discussion
A wide range of lung parenchymal findings are commonly encountered on CT imaging of oncologic patients, both incidentally during routine staging or during investigation of respiratory symptoms. Differential diagnosis for these findings includes tumor (primary or metastatic), infection, drug reactions, post radiation fibrosis, and OP. OP cannot be diagnosed on the basis of clinical presentation and is generally a diagnosis of exclusion.
In this study of 151 oncologic patients, OP most commonly presented as a process with an ill-defined margin. The presence of an irregular border has been reported in various studies. In a study correlating CT appearances of OP with pathological findings in 17 patients who underwent surgical resection, Yang et al attributed an irregular border to a desmoplastic response.8
Previous studies have demonstrated that OP uncommonly presents in a central location. In a review of 45 cases of focal organizing pneumonia by Zhao et al, OP never presented in a central location.1 Similarly, in a study of Focal OP in 18 patients by Kohno et al, all lesions were in a peripheral location.9 In our study, only 3% of patients demonstrated central lesions and OP was not found in a central location in any of the 25 lung cancer patients. Our findings suggest, as have previous studies, that OP is highly unlikely to present as a central lesion without peripheral lung findings.
Air bronchograms have been reported as a feature of OP in a number of studies,10,11 but were only identified it in 27% of cases in this study. Cavitation and necrosis, two features not typically associated with organizing pneumonia, were only identified in a very small number of cases (6% and 1%).
In all patients, a diffuse pattern was the most common presentation of OP; however, in patients with a history of lung cancer, OP most commonly presented as a single focal lesion (56%). In a lung cancer population, the presence of a new solitary lesion is most concerning for recurrent or metastatic disease and tissue biopsy is required to differentiate OP from recurrent tumor. In their study of 45 patients, Zhao et al sought to determine CT features which could differentiate OP from lung carcinoma; however, they concluded that small OP lesions may resemble lung carcinoma.1
The potential for misdiagnosis of OP as malignancy is well described in the literature. In an oncologic population, OP can occur as a complication of treatment, having been described following chemotherapy, radiation therapy, bone marrow, and solid organ transplant. In fact, pulmonary complications have been reported to occur in 40–60% of patients following HSCT.12 In a review of 5340 post HSCT patients with 49 cases of OP by Freudenberger et al, cases of OP were found as early as 5 days post transplant with a median occurrence of between 3 and 4 months after HSCT.13 A review of CT appearances of 43 patients with OP revealed greater ground-glass involvement (73% versus 56%) in post HSCT patients and other immunocompromised patients than in immunocompetent patient.10 This is in keeping with our study findings with ground glass opacities observed in 62% of HSCT patients versus 51% in the non-transplant group. In our study, 59% of patients who underwent HSCT demonstrated a diffuse process of alveolar infiltration versus 37% of the non-transplant group. A diffuse pattern of alveolar infiltration in an immunosuppressed population generally suggests infection; however, if features of OP are demonstrated, this should be considered as an alternate diagnosis.
Our study has a number of limitations. This was a retrospective study with potential for selection bias. Also, given that OP can occur as a complication of various oncologic treatments it would be interesting to correlate both the treatment and type of treatment received with the subsequent diagnosis of OP. This was outside the scope of the paper as our goal was to describe the CT features of OP in this unique patient population, however it could provide the basis for further study. To our knowledge, this is the largest group of OP patients evaluated in a radiology paper to date; however, the small numbers in the different patient limits the significance of the results. Lastly the image aquisition was not uniform for all patients. Due to the retrospective nature of the study, 1.25 mm slice images were only available for review in 26 of 151 patients.
In conclusion, the diagnosis of OP can prove quite challenging in an oncologic population where CT findings can mimic recurrent or metastatic tumor. A high index of suspicion is required to avoid misdiagnosis especially in patients who are unresponsive to conventional therapy. Our study demonstrated that OP commonly presented as diffuse lesions in both a central and peripheral location in the oncologic patient. When compared to OP in other malignancies, OP in lung cancer patients was significantly more likely to be associated with discrete lesions and in a peripheral location. In patients with HSCT, OP was significantly more often associated with a diffuse presentation when compared to patients without a history of HSCT.
Acknowledgments
We acknowledge the support of the NIH/NCI Cancer Center Support Grant P30 CA008748. The authors would like to thank Joanne Chin for assisting with the preparation of the manuscript.
Abbreviations List
- OP
Organizing Pneumonia
- HSCT
Hemopoietic Stem Cell Transplant
- GGO
Ground glass opacity
longn@mskcc.org
Telephone: 914-367-7105
Fax: 212 794 4010
Memorial Sloan Kettering Cancer Center
1275 York Avenue
New York, NY 10065
Conflict of interest: none
plodkowa@mskcc.org
Telephone: 315-383-7803
Fax: (646) 888-4912
Memorial Sloan Kettering Cancer Center
300 East 66th Street
New York, NY 10065
Conflict of interest: none
rachel.schor@sheba.health.gov.il
Memorial Sloan Kettering Cancer Center
1275 York Avenue
New York, NY 10065
Conflict of interest: none
geyera@mskcc.org
Telephone: 212-639-7588
Memorial Sloan Kettering Cancer Center
1275 York Avenue
New York, NY 10065
Conflict of interest: none
ZhengJ@mskcc.org
Telephone: 646-888-8287
Memorial Sloan Kettering Cancer Center
1275 York Avenue
New York, NY 10065
Conflict of interest: none
moskowc1@mskcc.org
Telephone: 646-888-8232
Memorial Sloan Kettering Cancer Center
1275 York Avenue
New York, NY 10065
Conflict of interest: none
ginsberm@mskcc.org
Telephone: 212-639-7292
Fax: 212 794 4010
Memorial Sloan Kettering Cancer Center
1275 York Avenue
New York, NY 10065
Conflict of interest: none
Footnotes
IRB: The institutional review board approved the study.
Conflict of interest: The authors have no conflict of interest to declare.
References
- 1.Zhao F, Yan SX, Wang GF, et al. CT features of focal organizing pneumonia: an analysis of consecutive histopathologically confirmed 45 cases. Eur J Radiol. 2014;83:73–8. doi: 10.1016/j.ejrad.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Cordier JF. Organising pneumonia. Thorax. 2000;55:318–28. doi: 10.1136/thorax.55.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miao L, Wang Y, Li Y, et al. Lesion with morphologic feature of organizing pneumonia (OP) in CT-guided lung biopsy samples for diagnosis of bronchiolitis obliterans organizing pneumonia (BOOP): a retrospective study of 134 cases in a single center. Journal of thoracic disease. 2014;6:1251–60. doi: 10.3978/j.issn.2072-1439.2014.08.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mueller-Mang C, Grosse C, Schmid K, et al. What every radiologist should know about idiopathic interstitial pneumonias. Radiographics : a review publication of the Radiological Society of North America, Inc. 2007;27:595–615. doi: 10.1148/rg.273065130. [DOI] [PubMed] [Google Scholar]
- 5.Cordier JF, Loire R, Brune J. Idiopathic bronchiolitis obliterans organizing pneumonia. Definition of characteristic clinical profiles in a series of 16 patients. Chest. 1989;96:999–1004. doi: 10.1378/chest.96.5.999. [DOI] [PubMed] [Google Scholar]
- 6.Cordier JF. Cryptogenic organising pneumonia. Eur Respir J. 2006;28:422–46. doi: 10.1183/09031936.06.00013505. [DOI] [PubMed] [Google Scholar]
- 7.Lohr RH, Boland BJ, Douglas WW, et al. Organizing pneumonia. Features and prognosis of cryptogenic, secondary, and focal variants. Archives of internal medicine. 1997;157:1323–9. doi: 10.1001/archinte.157.12.1323. [DOI] [PubMed] [Google Scholar]
- 8.Yang PS, Lee KS, Han J, et al. Focal organizing pneumonia: CT and pathologic findings. Journal of Korean medical science. 2001;16:573–8. doi: 10.3346/jkms.2001.16.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohno N, Ikezoe J, Johkoh T, et al. Focal organizing pneumonia: CT appearance. Radiology. 1993;189:119–23. doi: 10.1148/radiology.189.1.8372180. [DOI] [PubMed] [Google Scholar]
- 10.Lee KS, Kullnig P, Hartman TE, et al. Cryptogenic organizing pneumonia: CT findings in 43 patients. AJR American journal of roentgenology. 1994;162:543–6. doi: 10.2214/ajr.162.3.8109493. [DOI] [PubMed] [Google Scholar]
- 11.Lee JW, Lee KS, Lee HY, et al. Cryptogenic organizing pneumonia: serial high-resolution CT findings in 22 patients. AJR American journal of roentgenology. 2010;195:916–22. doi: 10.2214/AJR.09.3940. [DOI] [PubMed] [Google Scholar]
- 12.Yoshihara S, Yanik G, Cooke KR, et al. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13:749–59. doi: 10.1016/j.bbmt.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Freudenberger TD, Madtes DK, Curtis JR, et al. Association between acute and chronic graft-versus-host disease and bronchiolitis obliterans organizing pneumonia in recipients of hematopoietic stem cell transplants. Blood. 2003;102:3822–8. doi: 10.1182/blood-2002-06-1813. [DOI] [PubMed] [Google Scholar]


