Abstract
Neurological manifestations of Lyme disease in humans are attributed in part to penetration of the blood-brain barrier (BBB) and invasion of the central nervous system (CNS) by Borrelia burgdorferi. However, how the spirochetes cross the BBB remains an unresolved issue. We examined the traversal of B. burgdorferi across the human BBB and systemic endothelial cell barriers using in vitro model systems constructed of human brain microvascular endothelial cells (BMEC) and EA.hy 926, a human umbilical vein endothelial cell (HUVEC) line grown on Costar Transwell inserts. These studies showed that B. burgdorferi differentially crosses human BMEC and HUVEC and that the human BMEC form a barrier to traversal. During the transmigration by the spirochetes, it was found that the integrity of the endothelial cell monolayers was maintained, as assessed by transendothelial electrical resistance measurements at the end of the experimental period, and that B. burgdorferi appeared to bind human BMEC by their tips near or at cell borders, suggesting a paracellular route of transmigration. Importantly, traversal of B. burgdorferi across human BMEC induces the expression of plasminogen activators, plasminogen activator receptors, and matrix metalloproteinases. Thus, the fibrinolytic system linked by an activation cascade may lead to focal and transient degradation of tight junction proteins that allows B. burgdorferi to invade the CNS.
Lyme disease is a tick-borne bacterial infection caused by the spirochete Borrelia burgdorferi (82). The bacteria, which are transmitted to humans by the bite of infected ticks belonging to the Ixodes persulcatus complex, can infect a multitude of tissue sites, including the skin, heart, and joints, in addition to the peripheral nervous system and the central nervous system (CNS), including the eyes (30, 31, 39, 82). Some of the neurological manifestations of Lyme disease (or neuroborreliosis) that occur in 10 to 40% (depending on the study) of clinical infections (60, 75, 83, 93) include meningitis, cranial neuritis, and radiculoneuritis (82); if left untreated, these manifestations can lead to a variety of syndromes, such as musculoskeletal pain, cognitive impairment, radicular pain, paresthesias, dysesthesias, profound fatigue, polyradiculoneuropathy, and encephalopathy (23, 39, 48, 56, 64, 71, 76, 77). Lyme-associated parkinsonism (6) and hemorrhagic stroke (79) have also been reported to be manifestations of Lyme disease; however, the implications of these findings are still unclear. However, although there is evidence that B. burgdorferi may enter the CNS early after infection (22, 24), it is not clear how B. burgdorferi is able to invade the CNS.
To infect the brain, the circulating spirochetes must first cross the blood-brain barrier (BBB). This barrier is composed of tightly apposed brain microvascular endothelial cells (BMEC) held together by tight junctions. BMEC differ from systemic vascular endothelial cells in several important ways (3, 44, 45, 78). Besides being continuous and joined by tight junctions, BMEC display a high endothelial electrical resistance and have few endocytic vesicles. While the barrier contains transport systems for the passage of certain substances (e.g., glucose, essential amino acids, and certain macromolecules like transferrin), it denies access to many other compounds, including many drugs and pathogens. In contrast, systemic endothelial cells are either discontinuous or fenestrated (e.g., cells in the liver), display low electrical resistance, allow free exchange of proteins and nutrients, and are often easily penetrated by pathogens. Besides proteins normally associated with epithelial cell tight junctions, BMEC also express novel cell surface glycoproteins that are not expressed on other endothelial cells (72) and other unique molecules, such as the cerebral cell adhesion molecule, LK48 (an expressed sequence tag), BBB-specific anion transporter 1, and angiogenic factors like VEGF, follistatin, FGF-1, and FGF-5, as well as CXC chemokines with ELR motifs like ENA-78 and GRO-α (40, 52, 58, 59, 81).
Several bacteria express their own proteases that digest the extracellular matrix in order to invade tissues, but other bacteria, like B. burgdorferi, appear to utilize the fibrinolytic system of the host to disseminate (49). B. burgdorferi has been shown to bind and stabilize plasminogen (9, 19, 47). B. burgdorferi (8, 25) and Borrelia crocidurae (63) spirochetes use the fibrinolytic system to disseminate into host tissues, including the brain. At the same time B. burgdorferi induces the expression and secretion of the urokinase-type plasminogen activator (uPA) and expression of the uPA receptor (uPAR) by a variety of cell types, including monocytes (10, 12). As the fibrinolytic system can directly digest components of the extracellular matrix (11), it can also activate other proteases, including matrix metalloproteinases (MMPs). Not only do these enzymes naturally occur in the blood circulation, but they are also expressed as a result of the interaction between host cells and B. burgdorferi. There have been several reports showing that astrocytes (69), chondrocytes (33, 53), and monocytes (26, 27) express MMPs in response to B. burgdorferi. These reports have also shown that the interaction of B. burgdorferi with host cells leads to the activation of plasminogen that activates these MMPs, thus facilitating the degradation of the extracellular matrix.
In vitro models of the BBB have clearly become important tools for identifying the cellular and molecular elements that could be targets for intervention for the transmigration of many pathogens into the CNS (43, 45, 92, 96). Because in vivo experiments in humans are difficult or impossible, an in vitro model of the BBB is essential if we are to understand how B. burgdorferi crosses the human BMEC that comprise the functional unit of the BBB. Using an extensively tested in vitro model, we examined how Lyme disease spirochetes transverse the human BBB.
MATERIALS AND METHODS
Materials and biochemicals.
Transwell tissue culture inserts (diameter, 6.5 mm; pore size, 3.0 μm) and 24-well plates were purchased from Corning Costar Corp. (Corning, N.Y.). Spectrozyme PL (H-d-norleucylhexahydrotyrosyl-lysine-p-nitroanilide diacetate salt) and human urokinase were obtained from American Diagnostica (Greenwich, Conn.). PKH67 green fluorescent cell linker kits for general cell membrane labeling, plasminogen, rabbit serum, α2-antiplasmin, and ɛ-aminocaproic acid (EACA) were obtained from Sigma (St. Louis, Mo.). BB-94 (batimastat; British Biotech) was purchased from British Biotech, and BSK-II medium containing 10% rabbit serum was made as described by Barbour (1). Anti-MMP-1 antiserum was obtained from Calbiochem (La Jolla, Calif.), and medium 199, Dulbecco modified Eagle medium (DMEM), and Ham's F-12 medium were obtained from Invitrogen (Carlsbad, Calif.). Heat-inactivated fetal bovine serum was obtained from HyClone (Logan, Utah) or Omega (Irvine, Calif.). Mouse anti-human ZO-1 was obtained from Zymed (San Diego, Calif.), fluorescein isothiocyanate-conjugated mouse anti-human P-glycoprotein was obtained from BD PharMingen (San Diego, Calif.), and rabbit polyclonal antibody against factor VIII-Rag was purchased from Dako (Carpinteria, Calif.).
Human BBB model.
The human BMEC primary cell cultures that were used in this study have been described previously (70, 85, 86). After nonendothelial markers, such as glial fibrillary acidic protein for astrocytes, smooth muscle actin to identify pericytes, galactocerebroside C for oligoglia, cytokeratin for epithelial cells, and macrophage antigens for microglia, were studied, fluorescence-activated cell sorting-sorted human BMEC were found to be >99% pure (70, 85, 86).
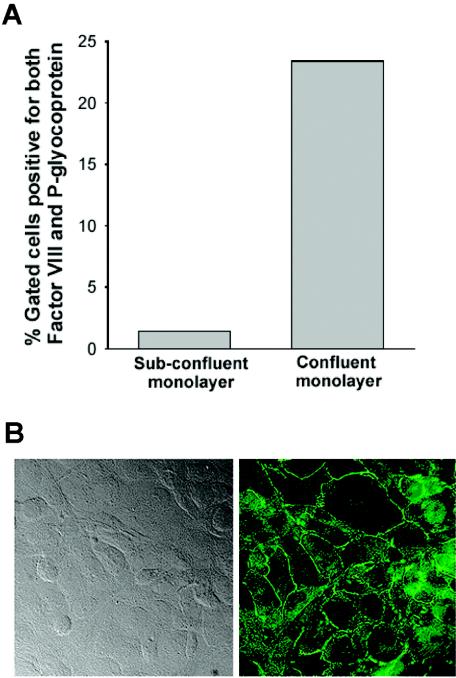
Because the morphological characteristics of human BMEC are known to be passage dependent, we used a cell line whose phenotypic expression was stabilized by immortalizing the cells with pSVT, a pBR322-based plasmid containing a DNA sequence encoding the simian virus 40 large-T antigen (86). Similar to the primary human BMEC cell line from which they were derived, the transfected human BMEC are positive for FVIII-Rag, carbonic anhydrase IV, and Ulex europaeus agglutinin I, take up acetylated low-density lipoprotein, and express gamma-glutamyl transpeptidase (85, 86). In response to tumor necrosis factor alpha, ICAM-1 expression on the apical side of the human BMEC indicates that our monolayers are polarized, in agreement with data from Dorovini-Zis and colleagues (14). As determined by fluorescence-activated cell sorting analysis, transfected human BMEC are also positive for the drug-transporting (or mdr1-type) P-glycoprotein (Fig. 1A) known to be present in endothelial cells that form the BBB (21, 80). In addition, protease-activated receptors 1, 2, 3, and 4 (46) and tight junctional proteins, such as ZO-1, are also expressed (Fig. 1B) (29). P-selectin expression but not E-selectin expression is induced by tumor necrosis factor alpha (7). The high transendothelial electrical resistance (TEER) of human BMEC relative to that of nonbrain endothelial cells is dependent on the clone and isolation (56, 62, 97). To create in vitro models of the human BBB, transfected human BMEC are seeded on top of Transwell tissue culture inserts that have been coated with type I collagen. After confluence is reached, this in vitro model of the BBB allows separate access to the upper compartment (blood side) and the lower compartment (brain side) (70).
FIG. 1.
Human BMEC in vitro express P-glycoprotein and tight junctional proteins. (A) Confluent and subconfluent human BMEC monolayers were lifted with EDTA. The cells were fixed with 2% paraformaldehyde in PBS for 30 min, permeabilized with PBS containing 0.01% Triton X-100, and quenched with 10 mM glycine-PBS. After blocking with 10% normal goat serum, the cells were incubated with fluorescein isothiocyanate-labeled mouse anti-P-glycoprotein monoclonal antibody and rabbit anti-factor VIII-Rag polyclonal antibody, followed by secondary antibody coupled to phycoerythrin. Analysis was done by using a FACScan and the CellQuest software (Becton Dickinson, San Jose, Calif.). The percentages of gated human BMEC positive for both factor VIII-Rag and P-glycoprotein are shown. (B) Human BMEC monolayers were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and stained with anti-human ZO-1 monoclonal antibody. The presence of ZO-1 was visualized with Alexa 488-conjugated secondary antibody. Differential interference contrast (left panel) and fluorescence (right panel) images of human BMEC showed that ZO-1 is expressed at the cell junctions. Magnification, ×400.
The limited number of other bacteria that are CNS-tropic (i.e., extracellular Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae, Escherichia coli K1, and group B Streptococcus and intracellular [within macrophages] Mycobacterium tuberculosis and Listeria monocytogenes) suggests that these bacteria have very specific attributes (61). Our in vitro model of the human BBB has been extensively used to examine how bacteria (E. coli, group B Streptococcus, S. pneumoniae, Citrobacter sp.), monocytes, viruses (human immunodeficiency virus), fungi (Candida albicans) (17, 28, 32, 36, 37, 38, 55, 62, 70, 74, 84, 87), and, more recently, African trypanosomes (Trypanosoma brucei gambiense and T. brucei brucei) (29) and Ehrlichia chaffeensis-infected monocytes (66) cross the BBB. A common feature of these studies is that one cannot extrapolate data concerning pathogen penetration of the BBB from experimental data based on nonbrain vascular endothelial cell models. For example, the E. coli K1 strain responsible for at least 80% of E. coli meningitis cases binds and invades BMEC but not endothelial cells of nonbrain origin, a process that requires the contribution of the cnf-1, ompA, ibeA, ibeB, and yijP genes (36, 45). This model has unique potential for exploring how Lyme disease spirochetes cross the BBB.
Nonbrain endothelial cells.
EA.hy926 cells, derived by fusion of A549 cells with human umbilical vein endothelial cells (HUVEC) and frequently used as a model of systemic endothelial cell barriers (2, 15), are grown in high-glucose (4.5 g/liter) DMEM (GIBCO) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1× HT supplement (GIBCO) and are seeded on top of Transwell tissue culture inserts as described above.
Spirochetes.
Low-passage (less than five in vitro passages) B. burgdorferi was routinely cultured at 34°C in BSK-II medium containing 10% rabbit serum as described by Barbour (1). In our studies we used B. burgdorferi 297, a strain which was originally isolated from human cerebrospinal fluid (54), and B. burgdorferi N40, a strain that has the ability to enter the CNS of the mouse (65) and nonhuman primates (5, 16). The bacteria were examined with a dark-field microscope to verify viability and that the organisms were thoroughly dispersed at the start of all assays. In some experiments the spirochetes were fluorescently tagged with the cell tracker dye PKH67 used according to the manufacturer's instructions (Sigma) (29).
Incubation of human BMEC and HUVEC with Lyme disease spirochetes.
Transfected human BMEC (isolate XIII) (86) were maintained in medium 199 supplemented with 10% heat-inactivated FBS. The cells were grown to confluence on 6.5-mm-diameter collagen-coated Costar Transwell inserts with a pore size of 3.0 μm. Prior to the experiments, the BMEC were equilibrated for several hours in experimental medium consisting of 1 part of medium 199 and 1 part of Ham's F-12 medium supplemented with 20% heat-inactivated FBS. To determine the ability of B. burgdorferi to cross our in vitro BBB model, spirochetes in the exponential phase of growth (approximately 107 spirochetes/ml) were centrifuged, washed, and resuspended in experimental medium. After this, 106 to 107 spirochetes were added to BMEC- or HUVEC-coated Transwell inserts (the upper compartment mimicking the blood side) in our in vitro human BBB model. As controls and to measure the ability of viable spirochetes to cross the inserts without the endothelial barriers, spirochetes were added to Transwell inserts without BMEC or HUVEC. The samples (containing 200 μl of medium in the inserts and 1 ml of medium in the wells) were incubated at 37°C in 5% CO2-95% air in a humidified environment. Aliquots were removed from the wells beneath the inserts (the bottom compartment mimicking the brain side) at selected times. The number of spirochetes that traversed the inserts, as determined by dark-field microscopy with a hemocytometer or by real-time PCR, was compared to the total number of bacteria present in the assay at the times studied. The spirochetes were counted in thin 10-sample disposable hemocytomter slides with nine independent counting areas per sample (Kova Glassitic Slide 10 with grids; Hycor Biomedical Ltd., Penicuik, United Kingdom) that were mounted so that the coverslip side was facing the dark-field condenser. Spirochete concentrations that were ≥5 × 103 cells/ml could be reliably counted in this manner. Each sample was assayed in triplicate, and the data were expressed as the means ± standard errors of the means. The viability of the spirochetes at the end of the experiment was unchanged, as determined by bacterial motility.
Real-time PCR.
In some experiments, we determined the quantity of the starting B. burgdorferi in the transmigrated medium by using a real-time PCR protocol based on the single-copy B. burgdorferi sensu stricto gene fla (51) and primers B.398f (GGGAAGCAGATTTGTTTGACA) and B.484r (ATAGAGCAACTTACAGACGAAATTAATAGA) with the fluorescent probe B.421p (6-carboxyfluorescein-ATGTGCATTTGGTTATATTGAGCTTGATCAGCAA-6-carboxytetramethylrhodamine). Analyses were conducted with a TaqMan 7700 instrument (ABI Biosystems, Foster City, Calif.).
Measurement of BMEC barrier function by TEER.
To assess the integrity of the human BMEC monolayers grown on the inserts, we determined the TEER of the BMEC monolayers using an Endohm Chamber coupled to an EVOM meter (World Precision Instruments Inc., Sarasota, Fla.). Although TEER is often expressed in ohms per square centimeter (e.g., for human BMEC, >300 Ω/cm2), because resistance is inversely proportional to unit area, the unit area resistance was obtained by multiplying the EVOM meter readings by the effective surface area of the Transwell filter membranes, a value that was independent of the area of the membrane used (as recommended in the World Precision Instruments manual [95]). Using this system with 6.5-mm-diameter inserts, we calculated that intact human BMEC monolayers had TEER values of >25 Ω × cm2 {[resistance (in ohms) of the inserts with human BMEC − resistance (in ohms) of the inserts alone] × 0.33 cm2}, which were comparable to the values for epithelial cells grown on inserts (58, 59). For comparison, the TEER values for nonbrain vascular endothelium (i.e., HUVEC) measured in the same way were much lower (<12 Ω × cm2 when cells were confluent).
Role of plasminogen and MMP in traversal of human BMEC by spirochetes.
To understand whether plasminogen can contribute to the traversal of human BMEC, we examined the ability of B. burgdorferi to cross our BBB model in the presence or absence of plasminogen (1 μg/ml) under serum-deprived conditions. Human BMEC were grown on Transwell inserts until they reached confluence, as judged by the increase in TEER (>25 Ω × cm2). The medium in the upper chamber was replaced with serum-free DMEM—F-12 medium, whereas the medium in the bottom chamber was replaced with DMEM—F-12 medium containing 10% FCS as a chemoattractant. After overnight incubation, 107 low-serum-concentration-adapted B. burgdorferi N40 cells were added to the top chamber, and the quantity of spirochetes crossing to the bottom chamber was determined by dark-field microscopy. In some experiments, cell culture supernatants were harvested and tested by immunoblot analysis with MMP-specific antibodies.
uPA expression in human BMEC.
To determine the ability of B. burgdorferi to induce uPA expression, 104 human BMEC were seeded into 96-well plates and cultured to confluence. Increasing amounts of B. burgdorferi N40 (103, 104, and 105 cells) in DMEM—F-12 medium were added to the human BMEC-coated wells, and the cocultures were incubated at 37°C for 24 h. The supernatants were harvested, and 1 μg of human plasminogen per ml was added along with 5 mM Spectrozyme PL, a plasmin substrate. Digestion of Spectrozyme PL by plasmin releases p-nitroanilide that absorbs at 405 nm. Absorbance readings were determined at the ambient temperature at zero time and 3 h.
Expression of uPAR by human BMEC.
To determine whether human BMEC express receptors for plasminogen activator, confluent cultures of BMEC grown in 96-well plates were incubated at 37°C for 24 h with 0 to 105 B. burgdorferi N40 cells in DMEM—F-12 medium. The human BMEC were then washed three times with DMEM—F-12 medium and incubated with 0 or 125 U of human urokinase per ml for 1 h at 37°C in 100 μl of DMEM—F-12 medium. Human plasminogen (1 μg/ml in 200 μl of Tris-buffered saline) and Spectrozyme PL were added after the wells were washed three times with 100 μl of DMEM—F-12 medium. Absorbance readings were determined at the ambient temperature at zero time and 3 h.
Visualization of the interaction of B. burgdorferi with human BMEC.
B. burgdorferi 297 prelabeled with PKH67 according to the manufacturer's instructions was incubated with human BMEC grown on collagen-coated glass coverslips. After 4 h of incubation, the samples were washed several times to remove unbound spirochetes prior to fixation with 4% paraformaldehyde in phosphate-buffered saline (PBS) (pH 7.2). After fixation, the samples were again washed with PBS, and the bound spirochetes were observed by phase-contrast and fluorescence microscopy.
Statistical analysis.
Statistical evaluations were conducted by using paired or unpaired one-tailed Student t tests; P values of <0.05 were considered significant.
RESULTS
B. burgdorferi crosses the human BMEC in vitro.
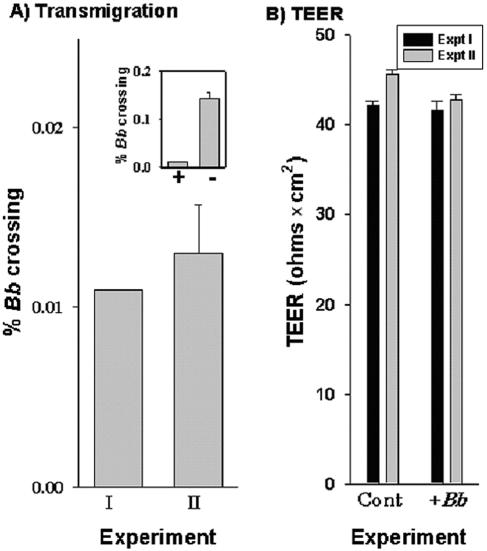
In two separate experiments, by 5 h approximately 0.012% of the spirochetes had crossed the human BMEC barrier (Fig. 2A). In the absence of any human BMEC, approximately 10 times more spirochetes (∼0.15% of the spirochetes added) migrated into the lower chamber of a Transwell insert (Fig. 2A, inset). To determine if the presence of B. burgdorferi compromised the integrity of the human BMEC monolayers, we determined the TEER of the BMEC monolayers. In the presence of B. burgdorferi 297 there were no major changes in the TEER (>40 Ω × cm2) compared to the controls, indicating that the integrity of the BMEC monolayer was essentially maintained at 5 h (Fig. 2B).
FIG. 2.
In vitro model for B. burgdodrferi 297 crossing the human BBB. A total of 107 B. burgdodrferi 297 (Bb) cells were incubated overnight with human BMEC grown to confluence in Transwell inserts. (A) Dark-field microscopy was used to determine the number of spirochetes that crossed the monolayers. The data from two independent experiments with triplicate determinations are expressed as the percentages of B. burgdorferi (means ± standard errors of the means) that crossed relative to the total number of spirochetes in control wells without inserts. (Inset) Comparison of B. burgdorferi crossing with human BMEC (bar +) or without human BMEC (bar −). (B) TEER expressed as means ± standard errors of the means for the experiments shown in panel A. Cont, control.
B. burgdorferi differentially crosses HUVEC and human BMEC.
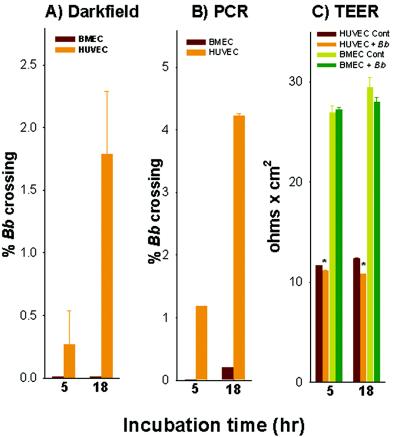
We also evaluated the ability of B. burgdorferi to cross both human BMEC and HUVEC barriers. Using 10-fold-fewer spirochetes than were used in the experiment shown in Fig. 2, we were unable to detect by dark-field microscopy spirochete transmigration across human BMEC (Fig. 3A). Interestingly, far more spirochetes crossed the HUVEC monolayers; 0.27 and 1.79% of the spirochetes had crossed HUVEC by 5 and 18 h, respectively. As observed in the experiment described above, in the presence of B. burgdorferi 297 there were no major changes in the TEER after 18 h of incubation for human BMEC cells compared to the uninfected controls (Fig. 3C). This finding indicates that the integrity of the BMEC monolayers was essentially maintained. However, there was a small but significant change in the TEER after incubation for 5 h (P = 0.019) and 18 h (P = 0.017) for the EA.hy926 cell line.
FIG. 3.
B. burgdorferi differentially crosses human BMEC and HUVEC (EA.hy926) monolayers. A total of 106 B. burgdodrferi 297 (Bb) cells were incubated overnight with human BMEC or HUVEC (EAhy.926) grown to confluence in Transwell inserts. (A) Dark-field microscopy was used to determine the number of spirochetes that crossed human BMEC or HUVEC monolayers. (B) Real-time PCR based on the single-copy B. burgdorferi sensu stricto fla gene (50) was used to determine the number of spirochetes that crossed human BMEC or HUVEC monolayers in the experiments whose results are shown in panel A. The data from triplicate determinations are expressed as the percentages of B. burgdorferi (means ± standard errors of the means) that crossed relative to the total number of spirochetes in control wells without inserts. (C) TEER measured as mean resistance (means ± standard errors of the means) for the experiments whose results are shown in panels A and B as described in Materials and Methods. The P values (as determined by a paired Student's t test) for the TEER changes in the 5- and 18-h HUVEC samples were 0.019 and 0.017, respectively (indicated by asterisks). Cont, control.
Because we were unable to detect spirochetes by dark-field microscopy in the bottom chamber for the human BMEC samples 5 and 18 h after the spirochetes were added to the top chamber, we reanalyzed the samples from the experiment shown in Fig. 3A by quantitative real-time PCR to determine the quantity of B. burgdorferi in the transmigrated medium (Fig. 3B). No B. burgdorferi was detected crossing human BMEC at 5 h, while 1.19% of the cells crossed HUVEC. In contrast, 21-fold more spirochetes (4.23 versus 0.20%) had crossed the HUVEC than had crossed the human BMEC after 18 h. We hypothesized that the fact that B. burgdorferi was able to cross HUVEC far more efficiently than it crossed human BMEC may have been due in part to the relative differences in the observed initial monolayer tightness, as reflected in the TEER; i.e., the spirochetes crossed the less tight EA.hy926 barriers (mean TEER, ∼11 Ω × cm2) more easily than they crossed the tighter barrier formed by the human BMEC (mean TEER, ∼28 Ω × cm2). It is of related interest that spirochete transendothelial migration across HUVEC is facilitated when the monolayer integrity is altered with EDTA (88).
B. burgdorferi traversal of human BMEC is facilitated by proteases.
It has been shown with monocytes that B. burgdorferi can induce the expression of uPA and its receptor (10, 19, 20). Furthermore, several Borrelia species may utilize the fibrinolytic system to cross vascular endothelium (9) and for dissemination (8, 25, 64). To understand whether plasminogen can contribute to invasion of the CNS, we examined the traversal of B. burgdorferi across our BBB model in the presence and absence of plasminogen under serum-deprived conditions. B. burgdorferi strain N40 was used for this part of the study, as this strain survives in low-serum culture conditions. The spirochetes (107 B. burgdorferi N40 cells) were incubated for 18 h with a confluent human BMEC monolayer grown in Transwell inserts. While only 0.4% ± 0.1% of the spirochetes traversed the BBB without added plasminogen, when 1 μg of plasminogen per ml was added in the top chamber, there was a dramatic increase in spirochete traversal of human BMEC (5.6% ± 0.7%).
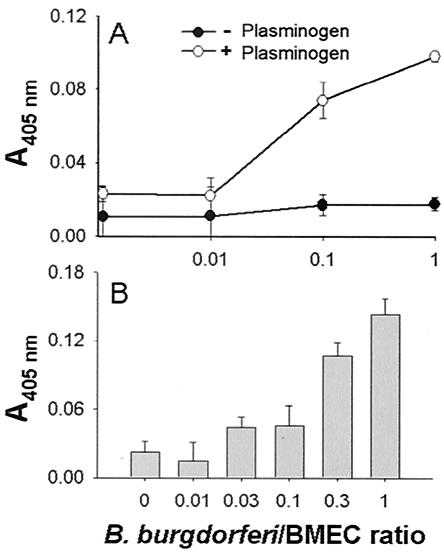
To determine whether the interaction of B. burgdorferi N40 with human BMEC can also lead to plasminogen activation, human BMEC monolayers were incubated with B. burgdorferi overnight at 37°C. The supernatant was collected, and its ability to activate human plasminogen was assessed with spectrozyme PL as a substrate. The supernatant of B. burgdorferi cultures alone was unable to activate plasminogen. However, the supernatants from the cocultures were able to activate plasminogen in a dose-dependent manner up to 3.3 times more efficiently than control human BMEC activated plasminogen (Fig. 4A). To determine whether the ability to activate plasminogen was present not only in the supernatant but also on the surface of the human BMEC, the cultures were washed, exogenous human uPA was added for 30 min, and the cultures were washed again. After adding exogenous human plasminogen and spectrozyme PL, we found that cells preincubated with B. burgdorferi were able to activate plasminogen 6.5-fold more efficiently than control human BMEC were able to activate plasminogen (Fig. 4B). These results suggest that B. burgdorferi not only induces the expression of a plasminogen activator but also induces the expression of a plasminogen activator receptor, presumably uPAR.
FIG. 4.
Plasminogen activation by cocultures of B. burgdorferi and human BMEC. Human BMEC were grown on 96-well plates until they reached confluence (approximately 105 cells). The medium was exchanged with DMEM—F-12 medium, and the next day increasing amounts of B. burgdorferi N40 (0, 103, 104, 105 cells) were added. (A) Plasminogen activation by human BMEC in response to spirochete addition. Twenty-four hours after spirochete addition the supernatants from the cultures were collected and incubated with 1 μg of human plasminogen per ml and 5 mM Spectrozyme PL. The increase in absorbance at 405 nm was directly proportional to the amount of plasmin in a given sample. The error bars indicate the standard deviations based on six experiments performed in duplicate. (B) Binding of exogenous urokinase-type plasminogen activator to human BMEC after spirochete addition. The cultures were washed three times with PBS prior to addition of 125 U of human recombinant uPA per ml. After a 60-min incubation, the cultures were again washed three times with PBS. After washing, 200 μl of PBS containing 1 μg of plasminogen per ml was added together with 5 mM Spectrozyme PL. The increase in the absorbance at 405 nm was determined after 3 h. Note the increased ability of B. burgdorferi N40 to stimulate human BMEC binding to uPA and activation of plasminogen compared to control cultures incubated with spirochetes. The error bars indicate the standard deviations based on three experiments performed in duplicate.
To ensure that this increase in spirochete crossing of the BBB was due to plasminogen, we examined the traversal of B. burgdorferi N40 across the BBB model in the presence of plasmin and MMP inhibitors. As shown in Fig. 5, traversal of spirochetes across the human BMEC was inhibited by 63% ± 5.6% in the presence of 50 nM BB-94, a broad-specificity matrix metalloproteinase inhibitor. Similarly, EACA (200 μM) and α2-antiplasmin (40 μg/ml), both of which are plasmin inhibitors, reduced traversal by approximately 60%. No further increase in inhibition was observed when both inhibitors were present together, indicating that BB-94 and EACA work through a common pathway. To ensure that this inhibition was not due to any adverse effect of EACA or BB-94 on spirochete viability (i.e., motility), we tested whether these two compounds could inhibit the traversal of B. burgdorferi across the inserts in the absence of human BMEC. The number of spirochetes incubated with BB-94 and/or EACA that crossed was identical to the number of spirochetes that crossed in the absence of these inhibitors and in the absence of human BMEC. These results suggest that the fibrinolytic system and matrix metalloproteinases participate in B. burgdorferi migration across the BBB model.
FIG. 5.
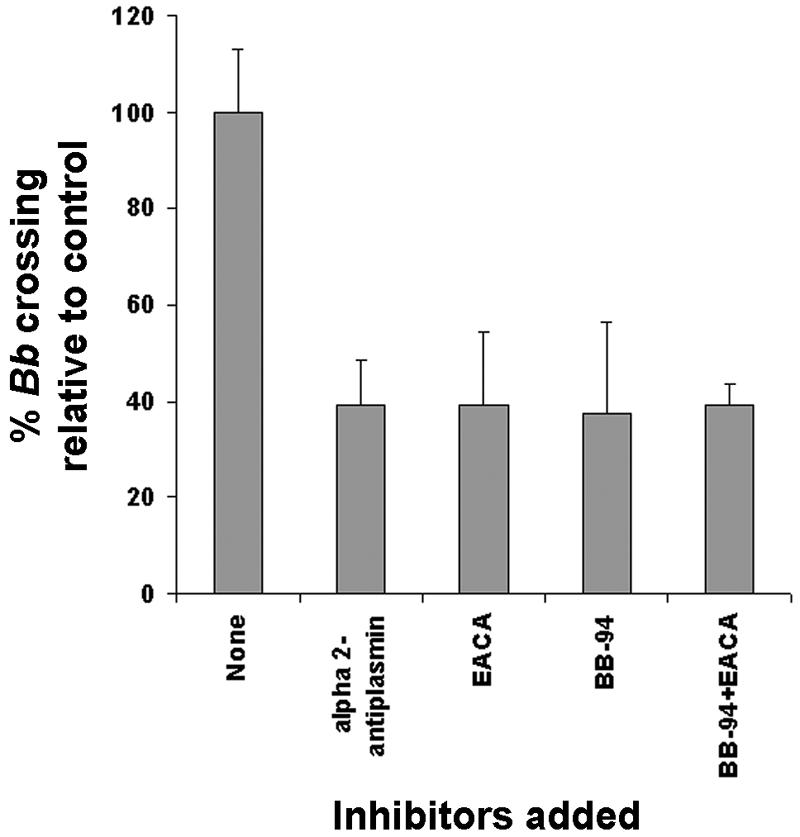
Role of proteases in B. burgdorferi (Bb) transmigration across human BMEC. Serum-reduced conditions were used to examine the role of host proteases in spirochete transmigration across human BMEC as described in Materials and Methods and the legend to Fig. 4. The traversal of human BMEC by B. burgdorferi N40 was examined in the presence of 1 μg of plasminogen per ml. The traversal of human BMEC by B. burgdorferi N40 with 50 nM BB-94 alone, with 200 μM EACA alone, with both BB-94 and EACA, or with 40 μg of α2-antiplasmin per ml is shown. The results are the averages of two experiments performed in duplicate. The error bars indicate the standard deviations.
Previous experiments with astrocytes (69), chondrocytes (33), and endothelial cells (Perides, unpublished data) have shown that these cells express various MMPs in response to interaction with B. burgdorferi. To determine whether some of the known matrix metalloproteinases (MMP-1, -2, or -9) are expressed in response to the interaction of human BMEC with B. burgdorferi, we performed zymography and immunoblot analysis with antibodies raised against MMPs. No induction or increased expression of MMP-2 or MMP-9 was detected in the supernatant of human BMEC cultures incubated with B. burgdorferi N40 (data not shown). However, when we used MMP-1 antibody, we found that there were significant levels of MMP-1 (interstitial collagenase 1) in the cocultures (Fig. 6). The induction appeared to be dependent on the spirochete/BMEC ratio.
FIG. 6.
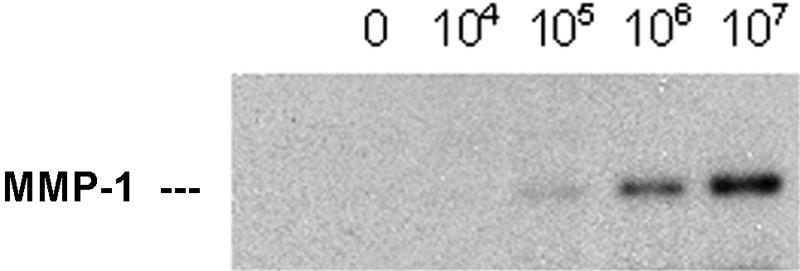
MMP-1 expression in response to B. burgdorferi. A total of 105 human BMEC were inoculated with increasing amounts of B. burgdorferi N40. After 24 h, cell culture supernatants were harvested and tested by immunoblot analysis with the antibody raised against MMP-1. Note the increased levels of MMP-1 with the increased numbers of B. burgdorferi cells.
DISCUSSION
The neurological manifestations of Lyme disease in humans caused by B. burgdorferi are attributed in part to penetration of the CNS by spirochetes, yet how the Lyme disease spirochetes cross the BBB remains an understudied and unresolved issue. B. burgdorferi freely crosses nonbrain vascular endothelium (13, 57, 88, 90). Our knowledge concerning how this occurs stems from in vitro studies that examined the ability of the spirochetes to bind to and cross confluent vascular endothelial cell monolayers in vitro (13, 57, 88, 90). After the initial binding event, how the spirochetes cross vascular endothelium (paracellular versus transcellular) remains controversial. By using electron microscopy, Comstock and Thomas (13) first demonstrated that B. burgdorferi spirochetes are able to enter and translocate across the cytoplasm of HUVEC grown on polycarbonate filters (Nuclepore inserts), a process that requires intact viable cells and bacteria (13, 57). The spirochetes were first observed to cross by dark-field microscopy as early as 2 h, and almost 8% of the added bacteria crossed by 4 h (13). Low-passage B. burgdorferi isolates adhere to HUVEC up to 30-fold more than spirochetes maintained continuously in culture adhere to HUVEC (88). While adherence to and transcellular crossing of endothelial cells is both time and inoculum dependent (13, 57, 90, 91), not all studies have supported a transcellular route of crossing. For example, Szczepanski et al. (88) cited the presence of B. burgdorferi in the intercellular junctions between endothelial cells, as well as beneath the monolayers, as evidence that spirochetes actually pass between the cells. More spirochetes crossed the barrier when the monolayers were pretreated with EDTA that was used to lower the TEER of the endothelial cell barrier (88). It is of related interest that Treponema pallidum, the causative agent of syphilis, also migrates across endothelial cell monolayers at intercellular junctions (89).
In spite of these investigations of B. burgdorferi-endothelial cell interactions, no study has been conducted to examine the interactions of these bacteria with brain microvascular endothelial cells (the functional unit of the BBB), an in vitro BBB model that has been used to study the transmigration of monocytes, neutrophils, bacteria, fungi, and African trypanosomes (17, 28, 29, 32, 36, 37, 38, 62, 66, 70, 74). Our data show that B. burgdorferi spirochetes differentially cross human BMEC and HUVEC and that the human BMEC form a barrier to traversal by B. burgdorferi. If spirochetes are able to cross human BMEC as easily as they cross systemic nonbrain endothelium, one might expect an earlier and/or far higher incidence of CNS involvement, observations not supported by the clinical findings that have been described. HUVEC lack the tight junctional complex that is key to BMEC's function as a barrier to pathogen entry into the brain. From a comparative viewpoint, it is also interesting that while Szczepanski et al. (88) observed that ≥22-fold more low-passage B. burgdorferi than high-passage spirochetes adhered and crossed HUVEC, we found that about 21-fold more low-passage Borrelia crossed HUVEC than crossed human BMEC. This finding also underscores the concept that one cannot extrapolate data concerning B. burgdorferi penetration of the BBB from experimental data based on nonbrain vascular endothelial cell models.
Many different types of cells produce MMPs, including stromal cells, glandular epithelial cells, and neutrophils, and most of these cells also express inhibitors of MMPs called tissue inhibitors of metalloproteinases (41, 67, 73, 94). All MMPs (except membrane-type MMPs) are secreted in proenzyme forms and require proteolytic cleavage at the N terminus for activation. The activation cascade for MMPs in the healthy host is closely tied to the fibrinolytic pathway. Plasmin can activate many MMPs, including MMP-1 (interstitial collagenase) and MMP-3 (stromelysin). Activated MMP-3 is believed to be the major physiological activator of most MMPs (49). While regulation of MMP production in normal cells is tightly controlled and occurs at many levels, dysregulation of MMPs (due to increased transcription of MMPs, up-regulation of plasminogen activators, and often down-regulation of inhibitors such as tissue inhibitors of metalloproteinases and plasminogen activator inhibitors [PAI-1 and PAI-2]) is often associated with disease.
It has been shown (68) that the enzymatic activity of human plasmin, which is highly unstable in solution, can be stabilized by the presence of B. burgdorferi. It has also been shown that plasminogen bound to the surface of a spirochete can be activated to plasmin by uPA (34), and binding to B. burgdorferi appears to protect the enzyme from autodigestion, as well as from inhibition by its natural inhibitor, α2-antiplasmin (9, 6, 8). The plasminogen binding protein has been isolated, sequenced, and expressed (35), and it stabilizes plasmin in animals and humans with Lyme disease (34). In fact, Benach and coworkers have shown that plasminogen plays a role in the in vitro migration of B. burgdorferi through HUVEC monolayers and that plasminogen facilitates infection of mice and ticks by B. burgdorferi (8, 9). Interestingly, under normal growth conditions, human BMEC express (i) serine or cysteine proteinase inhibitor clade E (nexin, plasminogen activator inhibitor type 1), (ii) plasminogen activator urokinase, (iii) urokinase-type plasminogen activator receptor, and (iv) soluble urokinase plasminogen activator receptor precursor genes (Francescopaolo Di Cello, Department of Pediatrics, Johns Hopkins University School of Medicine, personal communication).
B. burgdorferi can also induce MMP expression by neural cells, cartilage explants, and chondrocytes (33, 69). When such cells are incubated with B. burgdorferi, they express and secrete in a dose-dependent manner several MMPs, including MMP-1, MMP-3, and MMP-9, as well as proteolytic activity associated with ADAM-TS4 and ADAM-TS11 (33, 53, 69). Using actinomycin D to inhibit RNA transcription, we determined by reverse transcriptase PCR that MMP induction is transcriptionally regulated (69).
To understand whether proteases play a role in penetration of the BBB, we examined the roles of plasminogen and MMPs. To minimize the effects of natural inhibitors of proteolytic activity in serum, the addition of plasminogen under reduced serum conditions dramatically enhanced spirochete crossing of the human BMEC. This effect was significantly reduced in the presence of either MMP or plasmin inhibitors that appear to function through a common pathway. In addition, we showed that B. burgdorferi also induces the expression of a plasminogen activator, as well as the expression of a plasminogen activator receptor, presumably uPAR. Interestingly, while no induction or increased expression of MMP-2 or MMP-9 was detected in the supernatants of cultures of BMEC incubated with B. burgdorferi, there were significant levels of MMP-1 in these cocultures, and the induction appeared to be dependent on the spirochete/BMEC ratio. These results suggest that the fibrinolytic system and matrix metalloproteinases participate in B. burgdorferi migration through the BBB model.
In summary, B. burgdorferi depends heavily on matrixolytic enzymes secreted not by the spirochete itself but by its host (8, 33). These enzymes include the enzymes associated with the fibrinolytic system and metallopeptidases (e.g., MMPs) in particular. Thus, we hypothesized and showed that B. burgdorferi induces the expression of plasminogen activators and MMPs. These enzymes linked by an activation cascade may lead to the focal and transient degradation of tight junction proteins that allows B. burgdorferi to invade the CNS, yet our preliminary experiments indicated that B. burgdorferi cells could bind via their tips prior to crossing the in vitro human BBB model (data not shown) and that they did so without evidence of breakdown of the BBB integrity based on endpoint Endohm TEER measurements. The TEER and permeability data are consistent with what has been observed in vivo; i.e., unlike what is seen in purulent bacterial meningitis, B. burgdorferi infection usually causes aseptic meningitis in which the permeability of the BBB is not substantially altered (18).
While the failure to see a generalized loss of tight junctional integrity could indicate that B. burgdorferi enters the brain via transcytosis across endothelial cells, tight junctions are maintained after paracellular transendothelial migration of large cells, such as monocytes (28) and neutrophils (4). A critical investigation of the phenomenon linking morphological methods (i.e., immunoelectron microscopy) with sensitive real-time TEER measurements (i.e., electric cell-substrate impedance sensing [42]) during the course of spirochete interaction with human BMEC is required before a concrete determination concerning the mechanism of spirochete BBB traversal can be made.
Our in vitro model of the human BBB mimics many of the important features of in vivo B. burgdorferi interactions with the BBB. Hence, this model should be an important tool for identifying the cellular and molecular elements implicated in B. burgdorferi interactions with the BMEC, as well as for helping characterize the biochemical mechanisms by which the bacteria cross the BBB. It may also help identify possible targets for intervening in the transmigration of the Lyme disease spirochetes into the CNS.
Acknowledgments
EA.hy926 cells were kindly supplied by C. Edgell, University of North Carolina. We are grateful for the contributions of Clara Lema to preparation of BSK-II medium for B. burgdorferi cultivation, and we are grateful to Peggy Coulter for the quantitative real-time PCR. We also thank Barbara Johnson (Centers for Disease Control and Prevention, Fort Collins, Colo.) and Toshiyuki Mazuzawa (University of Shizuoka, Shizuoka, Japan) for providing low-passage B. burgdorferi strain 297. We are also grateful to Donna Pearce for technical assistance and the flow cytometry analysis.
This work was supported by a grant to D.J.G. from the Thomas Wilson Sanitarium for the Children of Baltimore and by grants from the National Institutes of Health to D.J.G. (grant 1 RO AI1464-01), to J.S.D. (grant R01-AI44102), and to K.S.K. (grants 1-RO-AI47225, NS26310, HL61951, and AA13858).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521-525. [PMC free article] [PubMed] [Google Scholar]
- 2.Bouis, D., G. A. Hospers, C. Meijer, G. Molema, and N. H. Mulder. 2001. Endothelium in vitro: a review of human vascular endothelial cell lines for blood vessel-related research. Angiogenesis 4:91-102. [DOI] [PubMed] [Google Scholar]
- 3.Broadwell, R. D., B. J. Baker-Cairns, P. M. Friden, C. Oliver, and J. C. Villegas. 1996. Transcytosis of protein through the mammalian cerebral epithelium and endothelium. III. Receptor-mediated transcytosis through the blood-brain barrier of blood-borne transferring and antibody against the transferring-receptor. Exp. Neurol. 142:47-65. [DOI] [PubMed] [Google Scholar]
- 4.Burns, A. R., R. A. Bowden, S. D. MacDonell, D. C. Walker, T. O. Odebunmi, E. M. Donnachie, S. I. Simon, M. L. Entman, and C. W. Smith. 2000. Analysis of tight junctions during neutrophil transendothelial migration. J. Cell Sci. 113:45-57. [DOI] [PubMed] [Google Scholar]
- 5.Cadavid, D., T. O'Neill, H. Schaefer, and A. R. Pachner. 2000. Localization of Borrelia burgdorferi in the nervous system and other organs in a nonhuman primate model of Lyme disease. Lab. Investig. 80:1043-1054. [DOI] [PubMed] [Google Scholar]
- 6.Cassarino, D. S., M. M. Quezado, N. R. Ghatak, and P. H. Duray. 2003. Lyme-associated parkinsonism: a neuropathologic case study and review of the literature. Arch. Pathol. Lab. Med. 127:1204-1206. [DOI] [PubMed] [Google Scholar]
- 7.Choi, K. S., J. Garyu, J. Park, and J. S. Dumler. 2003. Diminished adhesion of Anaplasma phagocytophilum-infected neutrophils to endothelial cells is associated with reduced expression of leukocyte surface selectin. Infect. Immun. 71:4586-4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman, J. L., J. A. Gebbia, J. Piesman, J. L. Degen, T. H. Bugge, and J. L. Benach. 1997. Plasminogen is required for efficient dissemination of B. burdorferi in ticks and for enhancement of spirochetemia in mice. Cell 89:1111-1119. [DOI] [PubMed] [Google Scholar]
- 9.Coleman, J. L., T. J. Sellati, J. E. Testa, R. R. Kew, M. B. Furie, and J. L. Benach. 1995. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect. Immun. 63:2478-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman, J. L., J. A. Gebbia, and J. L. Benach. 2001. Borrelia burgdorferi and other bacterial products induce expression and release of the urokinase receptor (CD87). J. Immunol. 166:473-480. [DOI] [PubMed] [Google Scholar]
- 11.Coleman, J. L., E. J. Roemer, and J. L. Benach. 1999. Plasmin-coated Borrelia burgdorferi degrades soluble and insoluble components of the mammalian extracellular matrix. Infect. Immun. 67:3929-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman, J. L., and J. L. Benach. 2003. The urokinase receptor can be induced by Borrelia burgdorferi through receptors of the innate immune system. Infect. Immun. 71:5556-5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comstock, L. E., and D. D. Thomas. 1989. Penetration of endothelial cell monolayers by Borrelia burgdorferi. Infect. Immun. 57:1626-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorovini-Zis, K., P. D. Bowman, and R. Prameya. 1992. Adhesion and migration of human polymorphonuclear leukocytes across cultured bovine brain microvessel endothelial cells. J. Neuropathol. Exp. Neurol. 51:194-205. [DOI] [PubMed] [Google Scholar]
- 15.Edgell, C. J., C. C. McDonald, and J. B. Graham. 1983. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. USA 80:3734-3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.England, J. D., R. P. Bohm, Jr., E. D. Roberts, and M. T. Philipp. 1997. Lyme neuroborreliosis in the rhesus monkey. Semin. Neurol. 17:53-56. [DOI] [PubMed] [Google Scholar]
- 17.Fiala, M., D. J. Looney, M. Stins, D. D. Way, L. Zhang, X. Gan, F. Chiappelli, E. S. Schweitzer, P. Shapshak, M. Weinand, M. C. Graves, M. Witte, and K. S. Kim. 1997. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol. Med. 3:553-564. [PMC free article] [PubMed] [Google Scholar]
- 18.Fikrig, E., P. K. Coyle, S. E. Schutzer, M. Chen, Z. Deng, and R. A. Flavell. 2004. Preferential presence of decorin-binding protein B (BBA25) and BBA50 antibodies in cerebrospinal fluid of patients with neurologic Lyme disease. J. Clin. Microbiol. 42:1243-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs, H., R. Wallich, M. M. Simon, and M. D. Kramer. 1994. The outer surface protein A of the spirochete Borrelia burgdorferi is a plasmin(ogen) receptor. Proc. Natl. Acad. Sci. USA 91:12594-12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuchs, H., M. M. Simon, R. Wallich, M. Bechtel, and M. D. Kramer. 1996. Borrelia burgdorferi induces secretion of pro-urokinase-type plasminogen activator by human monocytes. Infect. Immun. 64:4307-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaillard, P. J., L. H. Voorwinden, J. L. Nielsen, A. Ivanov, R. Atsumi, H. Engman, C. Ringbom, A. G. de Boer, and D. D. Breimer. 2001. Establishment and functional characterization of an in vitro model of the blood-brain barrier, comprising a co-culture of brain capillary endothelial cells and astrocytes. Eur. J. Pharm. Sci. 12:215-222. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Monco, J. C., B. F. Villar, J. C., Alen, and J. L. Benach. 1990. Borrelia burgdorferi in the central nervous system: experimental and clinical evidence for early invasion. J. Infect. Dis. 161:1187-1193. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Monco, J. C., and J. L. Benach. 1995. Lyme neuroborreliosis. Ann. Neurol. 37:691-702. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Monco, J. C., N. S. Miller, P. B. Backenson, P. Anda, and J. L. Benach. 1997. A mouse model for Borrelia meningitis after intradermal injection. J. Infect. Dis. 175:1243-1245. [DOI] [PubMed] [Google Scholar]
- 25.Gebbia, J. A., J. C. Monco, J. L. Degen, T. H. Bugge, and J. L. Benach. 1999. The plasminogen activation system enhances brain and heart invasion in murine relapsing fever borreliosis. J. Clin. Investig. 103:81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gebbia, J. A., J. L. Coleman, and J. L. Benach. 2001. Borrelia spirochetes upregulate release and activation of matrix metalloproteinase gelatinase B (MMP-9) and collagenase 1 (MMP-1) in human cells. Infect. Immun. 69:456-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gebbia, J. A., J. L. Coleman, and J. L. Benach. 2004. Selective induction of matrix metalloproteinases by Borrelia burgdorferi via Toll-like receptor 2 in monocytes. J. Infect. Dis. 189:113-119. [DOI] [PubMed] [Google Scholar]
- 28.Giri, R., Y. Shen, M. Stins, S. Du Yan, A. M. Schmidt, D. Stern, K. S. Kim, B. Zlokovic, and V. K. Kalra. 2000. Beta-amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am. J. Physiol. Cell Physiol. 279:C1772—C1781. [DOI] [PubMed] [Google Scholar]
- 29.Grab, D. J., O. Nikolskaia, Y. V. Kim, J. D. Lonsdale-Eccles, S. Ito, T. Hara, T. Fukuma, E. Nyarko, K. J. Kim, M. Stins, M. J. Delannoy, J. Rodgers, and K. S. Kim. 2004. African trypanosome interactions with an in vitro model of the human blood-brain barrier. J. Parasitol. 90:970-979. [DOI] [PubMed]
- 30.Halperin, J. J. 1995. Neuroborreliosis. Am. J. Med. 98:52S-56S. [DOI] [PubMed] [Google Scholar]
- 31.Halperin, J. J. 2003. Lyme disease and the peripheral nervous system. Muscle Nerve 28:133-143. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman, J. A., J. L. Badger, Y. Zhang, S. H. Huang, and K. S. Kim. 2000. Escherichia coli K1 aslA contributes to invasion of brain microvascular endothelial cells in vitro and in vivo. Infect. Immun. 68:5062-5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu, L. T., M. A. Eskildsen, C. Masgala, A. C. Steere, E. C. Arner, M. A. Pratta, A. J. Grodzinsky, A. Loening, and G. Perides. 2001. Host metalloproteinases in Lyme arthritis. Arthritis Rheum. 44:1401-1410. [DOI] [PubMed] [Google Scholar]
- 34.Hu, L. T., G. Perides, R. Noring, and M. S. Klempner. 1995. Binding of human plasminogen to Borrelia burgdorferi. Infect. Immun. 63:3491-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu, L. T., S. D. Pratt, G. Perides, L. Katz, R. A. Rogers, and M. S. Klempner. 1997. Isolation, cloning, and expression of a 70-kilodalton plasminogen binding protein of Borrelia burgdorferi. Infect. Immun. 65:4989-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang, S.-H., M. F. Stins, and K. S. Kim. 2000. Bacterial penetration across the blood-brain barrier during the development of neonatal meningitis. Microbes Infect. 2:1237-1244. [DOI] [PubMed] [Google Scholar]
- 37.Huang, S.-H., C. Wass, Q. Fu, N. V. Prasadarao, M. Stins, and K. S. Kim. 1995. Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of invasion gene ibe10. Infect. Immun. 63:4470-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jong, A. Y., M. F. Stins, S. H. Huang, S. H. M. Chen, and K. S. Kim. 2001. Traversal of Candida albicans across human blood-brain barrier in vitro. Infect. Immun. 69:4536-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalish, R. A. 1993. Lyme disease. Rheum. Dis. Clin. N. Am. 19:399-426. [PubMed] [Google Scholar]
- 40.Kallmann, B. A., S. Wagner, V. Hummel, M. Buttmann, A. Bayas, J. C. Tonn, and P. Rieckmann. 2002. Characteristic gene expression profile of primary human cerebral endothelial cells. FASEB J. 16:589-591. [DOI] [PubMed] [Google Scholar]
- 41.Keck, T., J. H. Balcom IV, C. Fernandez-Del Castillo, B. A. Antoniu, and A. L. Warshaw. 2002. Matrix metalloproteinase-9 promotes neutrophil migration and alveolar capillary leakage in pancreatitis-associated lung injury in the rat. Gastroenterology 122:188-201. [DOI] [PubMed] [Google Scholar]
- 42.Keese, C. 2001. Monitoring of cells during signal transduction. Genet. Eng. News 21:51. [Google Scholar]
- 43.Kim, K. S. 2000. E. coli invasion of brain microvascular endothelial cells as a pathogenetic basis of meningitis. Subcell. Biochem. 33:47-59. [DOI] [PubMed] [Google Scholar]
- 44.Kim, K. S. 2001. Escherichia coli translocation at the blood-brain barrier. Infect. Immun. 69:5217-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim, K. S. 2003. Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury. Nat. Rev. Neurosci. 4:376-385. [DOI] [PubMed] [Google Scholar]
- 46.Kim, Y. V., F. P. Di Cello, C. S. Hillaire, and K. S. Kim. 2004. Protease-activated receptors of human brain microvascular endothelial cells: expression and differential Ca2+ signaling induced by thrombin and protease activated receptor-1 activating peptide. Am. J. Physiol. Cell Physiol. 286:C31-C42. [DOI] [PubMed] [Google Scholar]
- 47.Klempner, M. S., R. Noring, M. P. Epstein, B. McCloud, R. Hu, S. A. Limentani, and R. A. Rogers. 1995. Binding of human plasminogen and urokinase-type plasminogen activator to the Lyme disease spirochete, Borrelia burgdorferi. J. Infect. Dis. 171:1258-1265. [DOI] [PubMed] [Google Scholar]
- 48.Klempner, M. S., L. T. Hu, J. Evans, C. H. Schmid, G. M. Johnson, R. P. Trevino, D. Norton, L. Levy, D. Wall, J. McCall, M. Kosinski, and A. Weinstein. 2001. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N. Engl. J. Med. 345:85-92. [DOI] [PubMed] [Google Scholar]
- 49.Knäuper, V., and G. Murphy. 1998. Membrane-type matrix metalloproteinases and cell-surface associated activation cascades from matrix metalloproteinases, p. 199-218. In W. C. Parks and R. P. Mecham (ed.), Matrix metalloproteinases. Academic Press, San Diego, Calif.
- 50.Lahteenmaki, K., P. Kuusela, and T. K. Korhonen. 2001. Bacterial plasminogen activators and receptors. FEMS Microbiol. Rev. 25:531-552. [DOI] [PubMed] [Google Scholar]
- 51.Leutenegger, C. M., N. Pusterla, C. N. Mislin, R. Weber, and H. Lutz. 1999. Molecular evidence of coinfection of ticks with Borrelia burgdorferi sensu lato and the human granulocytic ehrlichiosis agent in Switzerland. J. Clin. Microbiol. 37:3390-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li, J. Y., R. J. Boado, and W. M. Pardridge. 2001. Blood-brain barrier genomics. J. Cereb. Blood Flow Metab. 21:61-68. [DOI] [PubMed] [Google Scholar]
- 53.Lin, B., J. M. Kidder, R. Noring, A. C. Steere, M. S. Klempner, and L. T. Hu. 2001. Differences in synovial fluid levels of matrix metalloproteinases suggest separate mechanisms of pathogenesis in Lyme arthritis before and after antibiotic treatment. J. Infect. Dis. 184:174-180. [DOI] [PubMed] [Google Scholar]
- 54.Liveris, D., A. Gazumyan, and I. Schwartz. 1995. Molecular typing of Borrelia burgdorferi sensu lato by PCR-restriction fragment length polymorphism analysis. J. Clin. Microbiol. 33:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lo, H. J., J. R. Kohler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 56.Logigian, E. L., R. F. Kaplan, and A. C. Steere. 1990. Chronic neurologic manifestations of Lyme disease. N. Engl. J. Med. 323:1438-1444. [DOI] [PubMed] [Google Scholar]
- 57.Ma, Y., A. Sturrock, and J. J. Weis. 1991. Intracellular localization of Borrelia burgdorferi within human endothelial cells. Infect. Immun. 59:671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matter, K., and M. S. Balda. 2003. Functional analysis of tight junctions. Methods 30:228-234. [DOI] [PubMed] [Google Scholar]
- 59.Matter, K., and M. S. Balba. 2003. Signalling to and from tight junctions. Nat. Rev. Mol. Biol. 4:225-236. [DOI] [PubMed] [Google Scholar]
- 60.Nadelman, R. B., and G. P. Wormser. 1998. Lyme borreliosis. Lancet 352:557-565. [DOI] [PubMed] [Google Scholar]
- 61.Nassif, X., S. Bourdoulous, E. Eugene, and P.-O. Couraud. 2002. How do extracellular pathogens cross the blood-brain barrier? Trends Microbiol. 10:227-231. [DOI] [PubMed] [Google Scholar]
- 62.Nizet, V., K. S. Kim, M. Stins, M. Jonas, E. Y. Chi, D. Nguyen, and C. E. Rubens. 1997. Invasion of brain microvascular endothelial cells by group B streptococci. Infect. Immun. 65:5074-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nordstrand, A., A. Shamaei-Tousi, A. Ny, and S. Bergstrom. 2001. Delayed invasion of the kidney and brain by Borrelia crocidurae in plasminogen-deficient mice. Infect. Immun. 69:5832-5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pachner, A. R. 1995. Early disseminated Lyme disease: Lyme meningitis. Am. J. Med. 98:30S-37S. [DOI] [PubMed] [Google Scholar]
- 65.Pachner, A. R., A. Itano. 1990. Borrelia burgdorferi infection of the brain: characterization of the organism and response to antibiotics and immune sera in the mouse model. Neurology 40:1535-1540. [DOI] [PubMed] [Google Scholar]
- 66.Park, J., K. S. Choi, D. J. Grab, and J. S. Dumler. 2003. Divergent interactions of Ehrlichia chaffeensis- and Anaplasma phagocytophilum-infected leukocytes with endothelial cell barriers. Infect. Immun. 71:6728-6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parks, W. C., and R. P. Mecham (ed.). 1998. Matrix metalloproteinases. Biology of extracellular matrix series. Academic Press, San Diego, Calif.
- 68.Perides, G., R. Noring, and M. S. Klempner. 1996. Inhibition of Borrelia burgdorferi-bound fibrinolytic enzymes by alpha(2)-antiplasmin, PAI-1 and PAI-2. Biochem. Biophys. Res. Commun. 219:690-695. [DOI] [PubMed] [Google Scholar]
- 69.Perides, G., L. M. Tanner-Brown, M. A. Eskildsen, and M. S. Klempner. 1999. Borrelia burgdorferi induces matrix metalloproteinases by neural cultures. J. Neurosci. Res. 58:779-790. [DOI] [PubMed] [Google Scholar]
- 70.Persidsky, Y., M. Stins, D. Way, M. H. Witte, M. Weinand, K. S. Kim, P. Bock, H. E. Gendelman, and M. Fiala. 1997. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J. Immunol. 158:3499-3510. [PubMed] [Google Scholar]
- 71.Philipp, M. T., and B. J. Johnson. 1994. Animal models of Lyme disease: pathogenesis and immunoprophylaxis. Trends Microbiol. 2:431-437. [DOI] [PubMed] [Google Scholar]
- 72.Prasadarao, N. V., C. A. Wass, J. N. Weiser, M. F. Stins, S.-H. Huang, and K. S. Kim. 1996. Endothelial cell GlcNAcβ1-4GlcNAc epitopes for outer membrane protein A enhance traversal of Escherichia coli across the blood-brain barrier. Infect. Immun. 64:154-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rawlings, N. D., and A. J. Barret. 1995. Evolutionary families of metalloproteinases. Methods Enzymol. 248:183-228. [DOI] [PubMed] [Google Scholar]
- 74.Reddy, M. A., C. A. Wass, K. S. Kim, D. D. Schlaepfer, and N. V. Prasadarao. 2000. Involvement of focal adhesion kinase in Escherichia coli invasion of human brain microvascular endothelial cells. Infect. Immun. 68:6423-6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reik, L., Jr. 1991. Lyme disease and the nervous system. Theime Medical Publishers, New York, N.Y.
- 76.Roberts, E. D., R. P. Bohm, Jr., R. C. Lowie, Jr., G. Habicht, L. Katona, J. Piesman, and M. T. Philipp. 1998. Pathogenesis of Lyme neuroborreliosis in the rhesus monkey: the early disseminated and chronic phases of disease in the peripheral nervous system. J. Infect. Dis. 178:722-732. [DOI] [PubMed] [Google Scholar]
- 77.Roberts, R. D., R. P. Bohm, Jr., F. B. Cogswell, H. N. Lanners, R. C. Lowrie, L. Povinelli, J. Piesman, and M. T. Philipp. 1995. Chronic Lyme disease in the rhesus monkey. Lab. Investig. 72:146-160. [PubMed] [Google Scholar]
- 78.Rubin, L. L., and J. M. Staddon. 1999. The cell biology of the blood-brain barrier. Annu. Rev. Neurosci. 22:11-28. [DOI] [PubMed] [Google Scholar]
- 79.Scheid, R., M. Hund-Georgiadis, and D. V. von Cramon. 2003. Intracerebral haemorrhage as a manifestation of Lyme neuroborreliosis? Eur. J. Neurol. 10:99-101. [DOI] [PubMed] [Google Scholar]
- 80.Schinkel, A. H. 1999. P-glycoprotein, a gatekeeper in the blood-brain barrier. Adv. Drug Delivery Rev. 36:179-194. [DOI] [PubMed] [Google Scholar]
- 81.Starzyk, R. M., C. Rosenow, J. Fyre, M. Leismann, E. Rodzinski, S. Putney, and E. I. Tuomanen. 2000. Cerebral cell adhesion molecule: a novel leukocyte adhesion determinant on blood-brain barrier capillary endothelium. J. Infect. Dis. 181:181-187. [DOI] [PubMed] [Google Scholar]
- 82.Steere, A. C. 1989. Lyme disease. N. Engl. J. Med. 321:586-596. [DOI] [PubMed] [Google Scholar]
- 83.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 84.Stins, M. F., J. Badger, and K. S. Kim. 2001. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb. Pathog. 30:19-28. [DOI] [PubMed] [Google Scholar]
- 85.Stins, M. F., F. Gilles, and K. S. Kim. 1997. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J. Neuroimmunol. 76:81-90. [DOI] [PubMed] [Google Scholar]
- 86.Stins, M. F., N. V. Prasadarao, J. Zhou, M. Arditi, and K. S. Kim. 1997. Bovine brain microvascular endothelial cells transfected with SV40-large T antigen: development of an immortalized cell line to study pathophysiology of CNS disease. In Vitro Cell Dev. Biol. Anim. 33:243-247. [DOI] [PubMed] [Google Scholar]
- 87.Stins, M. F., N. V. Prasadarao, C. Wass, and K. S. Kim. 1999. Escherichia coli binding to and invasion of brain microvascular endothelial cells derived from humans and rats of different ages. Infect. Immun. 67:5522-5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Szczepanski, A., M. B. Furie, J. L. Benach, B. P. Lane, and H. B. Fleit. 1990. Interaction between Borrelia burgdorferi and endothelium in vitro. J. Clin. Investig. 85:1637-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thomas, D. D., M. Navab, D. A. Haake, A. M. Fogelman, J. N. Miller, and M. A. Lovett. 1988. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc. Natl. Acad. Sci. USA 85:3608-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thomas, D. D., and L. E. Comstock. 1989. Interaction of Lyme disease spirochetes with cultured eucaryotic cells. Infect. Immun. 57:1324-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomas, D. D., D. Cadavid, and A. G. Barbour. 1993. Differential association of Borrelia species with cultured neural cells. J. Infect. Dis. 169:445-448. [DOI] [PubMed] [Google Scholar]
- 92.Tuomanen, E. I. 1996. Entry of pathogens into the central nervous system. FEMS Microbiol. Rev. 18:289-299. [DOI] [PubMed] [Google Scholar]
- 93.Wilke, M., H. Eiffert, H. J. Christen, and F. Hanefeld. 2000. Primarily chronic and cerebrovascular course of Lyme neuroborreliosis: case reports and literature review. Arch. Dis. Child. 83:67-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woessner, F. J. 1998. The matrix metalloproteinase family, p. 1-14. In W. C. Parks and R. P. Mecham (ed.), Matrix metalloproteinases. Academic Press, San Diego, Calif.
- 95.World Precision Instruments, Inc. 2001. EVOM & EVOMX epithelial voltohmmeters. World Precision Instruments Instruction manual. World Precision Instruments, Inc., Sarasota, Fla.
- 96.Zhang, J. R., and E. I. Tuomanen. 1999. Molecular and cellular mechanisms for microbial entry into the CNS. J. Neurovirol. 5:591-603. [DOI] [PubMed] [Google Scholar]
- 97.Zink, S., T. Nass, P. Rosen, and J. F. Ernst. 1996. Migration of the fungal pathogen Candida albicans across endothelial monolayers. Infect. Immun. 64:5085-5091. [DOI] [PMC free article] [PubMed] [Google Scholar]