Abstract
RNA interference has been used to study insects’ gene function and regulation. Glycogen synthase (GS) and glycogen phosphorylase (GP) are two key enzymes in carbohydrates’ conversion in insects. Glycogen content and GP and GS gene expression in several tissues and developmental stages of the Brown planthopper Nilaparvata lugens Stål (Hemiptera: Delphacidae) were analyzed in the present study, using quantitative reverse-transcription polymerase chain reaction to determine their response to double-stranded trehalases (dsTREs), trehalose-6-phosphate synthases (dsTPSs), and validamycin injection. The highest expression of both genes was detected in the wing bud, followed by leg and head tissues, and different expression patterns were shown across the developmental stages analyzed. Glycogen content significantly decreased 48 and 72 h after dsTPSs injection and 48 h after dsTREs injection. GP expression increased 48 h after dsTREs and dsTPSs injection and significantly decreased 72 h after dsTPSs, dsTRE1-1, and dsTRE1-2 injection. GS expression significantly decreased 48 h after dsTPS2 and dsTRE2 injection and 72 h after dsTRE1-1 and dsTRE1-2 injection. GP and GS expression and glycogen content significantly decreased 48 h after validamycin injection. The GP activity significantly decreased 48 h after validamycin injection, while GS activities of dsTPS1 and dsTRE2 injection groups were significantly higher than that of double-stranded GFP (dsGFP) 48 h after injection, respectively. Thus, glycogen is synthesized, released, and degraded across several insect tissues according to the need to maintain stable trehalose levels.
Keywords: Nilaparvata lugens, RNA interference, glycogen, glycogen synthase, glycogen phosphorylase
Rice is one of the most important food crops worldwide, and it is mostly produced in China and other Asian countries. High and stable rice yields are important to guarantee food production but their safety is affected by 800 insect species, both in the field and during storage (Barrion and Litsinger 1994). In recent years, biological disasters, including pests and plant pathogens, have become a significant factor affecting rice output. Investigation showed that, from 2000 to 2010–2011, rice pests caused a loss of up to 48 million ha in China (Zhao et al. 2014). The hemimetabolous Brown planthopper Nilaparvata lugens Stål (Hemiptera: Delphacidae), which causes huge yield losses directly, is one of the most destructive insect pests, as they can only feed and breed on rice or wild rice (Xi et al. 2014, 2015a,b). This pest damages rice plants by directly sucking the phloem sap and transmits plant viruses (Ghaffar et al. 2011, Yang et al. 2014). Originally, insecticide control was an important and very convenient way to control and decrease pest populations, but its improper use has led to pest resurgence and resistance, and to the accumulation of chemical residues (Xi et al. 2014). In N. lugens, gene-function studies have also shown that RNAi of target genes could be used a pest-control strategy (Wang et al. 2012, 2015; Xi et al. 2014, 2015a,b; Liu et al. 2015; Zhao et al. 2016; Yang et al. 2017).
Glycogen is an important metabolic and energy substance in insects (Tolmasky et al. 2001, Liu et al. 2009), and insects must accumulate glycogen before they enter diapause (Pullin 1996, Liu et al. 2009). For example, the ladybird Coccinella septempunctata Linnaeus accumulates a substantial amount of glycogen before entering diapauses (Ren et al. 2015) and Zygaena trifolii (Esper) (Lepidoptera: Zygaenidae) larvae that enter diapause store two times more glycogen than larvae that do not (Wipking et al. 1995). Glycogen is also one of the major carbohydrates found in insects, and it is mostly synthesized and stored in the fat body (Tang et al. 2012a). Here, it can be rapidly converted into dextrose or trehalose and then transported to other tissues when needed (Tang et al. 2012a). The synthesis and degradation of glycogen molecules require the concerted action of a set of enzymes, and are primarily regulated by glycogen synthase (GS) and glycogen phosphorylase (GP), respectively (Prats et al. 2005). When insects need energy for flying, trehalose is transported to the flight muscles leading to a decrease in the content of trehalose in the blood; the glycogen stored in the fat body is then converted to trehalose to maintain its concentration in the blood (Yu et al. 2008). Thus, in insects, glycogen usually acts in a supporting role rather than being the protagonist, as in mammals. However, under stress conditions, such as low temperature or diapause, glycogen is the essential sugar for some insects (Ren et al. 2015).
Trehalose, which is formed by two glucose units, is the “blood sugar” of insects (Tang et al. 2012b), while glucose is the “blood sugar” of mammals. Hemolymph sugars are composed of myo-inositol and trehalose (Moriwaki et al. 2003), and myo-inositol is the main sugar in the hemolymph of N. lugens (Kikuta et al. 2012); however, trehalose plays a key role in all developmental stages, including larvae, pupae, and adults (Becker et al. 1996, Elbein et al. 2003, Tang et al. 2014b, Zhao et al. 2016). In addition, trehalose acts as an energy reserve under low temperature, starvation, drought, and other environmental stresses (Tang et al. 2014a, Shi et al. 2016). This sugar is mainly synthesized by trehalose-6-phosphate synthase (TPS) and trehalose-6-phosphate phosphatase and released in the hemolymph (Bell et al. 1992, Tang et al. 2016, Yang et al. 2017), reaching several tissues where it is degraded to glucose by trehalase (TRE or Treh). Insects have two kinds of trehalase (Shukla et al. 2015, Zhao et al. 2016, Tang et al. 2016), which participate and regulate the chitin biosynthesis pathway (Tang et al. 2012b). In addition, glucose-6-phosphate and uridine diphosphate (UDP)-glycogen are substrates for trehalose synthesis, and UDP-glycogen is the sole substrate for glycogen synthesis under the action of GS (Tang et al. 2012a). Glucose-1-phosphate, when degraded by GP, can be converted into glucose-6-phosphate and enter the trehalose synthesis pathway in the presence of phosphoglucomutase. Thus, trehalose and glycogen are two important and closely related sugars involved in insect physiological activities, which can be transformed into each other according to insects’ physiological needs (Tang et al. 2012a).
Given its importance in insects as an energy source, many studies focused on glycogen function under stress pressures like starvation and cold hardness (Koštál et al. 2014; Heydari and Izadi 2014; Keshan et al. 2016; Wang et al. 2016a,b). The rate-limiting enzyme of glycogen degradation, GP, was first identified from rabbit skeletal muscle in 1936 (Cori and Cori 1936). In mammals, GP is classified into muscular, liver, and cerebral GP, and it degrades glycogen into glucose-1-phosphate. In 1977, the amino acid sequence of rabbit GP was reported (Titani et al. 1977), and the complete cDNA sequence of rabbit muscle GP was cloned in 1986 (Nakano and Fukui 1986). In insects, the complete cDNA sequence of GP was cloned and first reported from Drosophila melanogaster Meigen (Gabriella et al. 1999). In Lepidoptera, GP and GS genes have been reported and cloned from Bombyx mori Linnaeus, Danaus plexippus (Linnaeus), Spodoptera exigua (Hübner), and Ostrinia furnacalis (Guenée) (Tang et al. 2012a, Guo et al. 2016).
RNA interference (RNAi) has been widely used to investigate gene function in insects, especially for silencing important genes in N. lugens and other insects, by injecting double-stranded RNA (dsRNA) or single-stranded RNA to suppress gene expression (Belles 2010, Liu et al. 2010, Wang et al. 2012). Our previous studies showed that RNAi inhibited the expression of trehalase (TRE or Treh) or TPS in the chitin synthesis pathway, leading to molting deformities in most tested N. lugens and to their death (Zhao et al. 2016, Yang et al. 2017). These results suggested that inhibiting the expression of TPS and TRE might also affect the metabolism and the utilization of carbohydrates. Therefore, the present study aimed to further investigate the functions of GS and GP in N. lugens, by evaluating their expression patterns when trehalose synthesis and degradation pathways are inhibited.
Materials and Methods
N. lugens Rearing and Dissection
The N. lugens used in this study were collected from the rice fields located at China National Rice Research Institute, Hangzhou, Zhejiang, China, and were kindly provided by Professor Qiang Fu. Insects were fed fresh rice seedlings (Oryza sativa L. var.TN1), and kept in a stable environment at 25 ± 1 °C, 60–70% RH, and a photoperiod of 16/8 (L:D) h.
Brown planthopper individuals (three sets of 10 individuals) used in the gene expression analyses were obtained from several populations at the same developmental stage and growth rate. Tissues were sampled from the whole body of fifth instar nymphs in their first day (0 h) at this stage (5L-0), and every 12 h after that until adults were three days old (72 h). The head, leg, wing bud, cuticle, and fat body of fourth and fifth larval instars were dissected in a saline solution (0.75% NaCl) under an EZ4 microscope (Leica, Germany). Three biological replicates were used for each developmental stage and tissue/whole body sample. All samples were kept at −80 °C until RNA extraction.
Total RNA Extraction and cDNA Reverse Transcription
Total RNA was extracted from the tissues or whole bodies of N. lugens using the TRIzol reagent (Life Tech, Carlsbad, CA) as instructed by the manufacturer. RNA concentration was determined by measuring samples’ absorbance at 260 nm in a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA) (Yang et al. 2017), and purified RNA was stored at −80 °C before use. First-stand cDNA was synthesized using the PrimeScript RT reagent Kit with gDNA Eraser (TaKaRa, Dalian, China) following the manufacturer’s instructions.
Cloning GP and GS Genes
Primers were designed in Vector NTI Suite 7 software using cDNA as the template and according to the sequences of GP and GS obtained from transcriptome sequencing (Zhao et al. 2016, Yang et al. 2017). Full-length cDNAs of NLGP and NLGS genes were cloned using the primer pairs presented in Table 1. Amplification reactions were performed in a 25 µl final volume, containing polymerase chain reaction (PCR) buffer, 0.1 mM dNTPs, 0.2 µM each primer, and 0.5 U of HiFi-Taq DNA polymerase (Transgene, Beijing, China). The cycling conditions were as follows: 10 min of initial denaturation at 94 °C, 30 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 180 s, and 10 min for a final extension at 72 °C. The PCR products were then analyzed by agarose gel electrophoresis, and those with the expected size (approximately 2,500 and 2,200 bp) were excised from the gel and purified using a DNA gel-extraction kit. The purified DNA was ligated into the pMD18-T vector (TaKaRa, Dalian, China) and Sanger sequenced.
Table 1.
Primers used for the cloning and qRT-PCR of the GS and GP genes in N. lugens (NL)
| Gene | Forward (5′–3′) | Reverse (5′–3′) | Function |
|---|---|---|---|
| NLGS | ATGTCTCGAGAACGTGCCAATA | TTATGTTACCTCTTTTTCATCATCC | Gene cloning |
| NLGP | ATGGCTACGCCACAATCAGATG | TTAAGCTTCACGAGGCTCATGTG | |
| NLGS | GCTCCAAAGCCTATGTTTCTACTG | TGGTAACCCCTGTCCCTCA | qRT-PCR |
| NLGP | GCTGCCTATGGCTATGGTATTC | TCTGAGTGTTGACCCACTTCTTG | |
| NL18S | CGCTACTACCGATTGAA | GGAAACCTTGTTACGACTT |
Sequence Analysis
The amino acid sequences of NLGP and NLGS were translated from cDNA sequences using the tool available at the ExPASy Proteomics website (http://expasy.org/tools/dna.html). The NLGP and NLGS putative sequences obtained were compared to protein sequences deposited in GenBank, using the BLAST-N or BLAST-X tools available at the National Center for Biotechnology Information (NCBI) website (http://www.ncbi.nlm.nih.gov/).
NLGP and NLGS Expression in Several Tissues and Developmental Stages Using Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
Complementary DNA synthesis and qRT-PCR were performed to analyze the distribution of NLGP and NLGS using gene-specific primers (Table 1). Based on the previously cloned NLGP and NLGS cDNAs, two pairs of specific primers were designed in Vector NTI Suite 7 software to amplify the unique regions found in the cDNA alignment. Using 1 µg total RNA as template, and a specifically designed NL18S primer pair (Table 1) the stability of 18S RNA was demonstrated in a PCR performed under the following conditions: 95 °C for 5 min, 28 cycles at 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s, followed by a final extension at 72 °C for 10 min.
The expression of NLGP and NLGS in the several tissues and developmental stages was estimated by qRT-PCR using a Bio-Rad CFX96 system and the SsoFast EvaGreen Supermix (both from Bio-Rad Laboratories, Hercules, CA). Each reaction was performed in a 20 µl final volume, containing 1 µl cDNA (or standard), 1 µl (10 µM) each primer, 7 µl RNAase- and DNAase-free water, and 10 µl SsoFast EvaGreen Supermix. The reactions were performed the following conditions: 3 min at 95 °C, followed by 40 cycles of 5 sat 95 °C and annealing at 55–62.5 °C for 20 s, with a melting curve at 65–95 °C, as instructed by the manufacturer.
dsRNA Synthesis and Injections
Using the N. lugens cDNA template and specific primers containing the T7 promoter sequence at their 5’ ends (Table 2), regions of three NLTRE and two NLTPS genes were amplified by qRT-PCR. The profile used in the reactions included 40 cycles at 95 °C for 30 s, 58 °C for 30s, 72 °C for 45 s, and a last extension at 72 °C for 10 min. Purified TRE and TPS amplicons were transcribed in vitro to synthesize dsRNA using T7 RiboMax Express RNAi System (Promega Corporation, Madison, WI) (Zhao et al. 2016). A green fluorescence protein (GFP) amplicon was used as a control. Sense and antisense strands were first produced in two separate transcriptive procedures and then mixed for annealing. Reactions were incubated for 10 min at 70 °C and then placed on an ice bath for 20 min. Finally, dsRNAs were precipitated with 95% ethanol and 3 M sodium acetate (pH 5.2), washed with 70% ethanol, air dried, and resuspended. The integrity and quantity of dsRNAs were evaluated by spectroscopy analysis with Nanodrop 2000 (Thermo Fisher Scientific) and by agarose gel electrophoresis.
Table 2.
Primers used in dsRNA synthesis
| Gene | Application | Primer name | Primer sequence (5′–3′) |
|---|---|---|---|
| NlTPS1 | RNAi | DSNLTPS1-F | ACCAGGAGTTGAAGGAGGAG |
| DSNLTPS1-R | GATCAGGGTGCCCATAGC | ||
| DSNLTPS1-FT | T7-ACCAGGAGTTGAAGGAGGAG | ||
| DSNLTPS1-RT | T7-GATCAGGGTGCCCATAGC | ||
| NlTPS2 | RNAi | DSNLTPS2-F | CACCAAAGGTCTAAGGCACA |
| DSNLTPS2-R | CATCGTTGATCTCGTAGGGA | ||
| DSNLTPS2-FT | T7-CACCAAAGGTCTAAGGCACA | ||
| DSNLTPS2-RT | T7-CATCGTTGATCTCGTAGGGA | ||
| NlTRE1-1 | RNAi | DSNLTRE1-1-F | GATGCAATCAAGGAGGTGTTATGGC |
| DSNLTRE1-1-R | CGTATTCACCTCCACCTCCGT | ||
| DSNLTRE1-1-FT | T7-GATGCAATCAAGGAGGTGTTATGGC | ||
| DSNLTRE1-1-RT | T7-CGTATTCACCTCCACCTCCGT | ||
| NlTRE1-2 | RNAi | DSNLTRE1-2-F | AGATGAAGGCATGTGGTTCG |
| DSNLTRE1-2-R | CATCGATTCGCCAACTGGTAAGC | ||
| DSNLTRE1-2-FT | T7-AGATGAAGGCATGTGGTTCG | ||
| DSNLTRE1-2-RT | T7-CATCGATTCGCCAACTGGTAAGC | ||
| NlTRE2 | RNAi | DSNLTRE2-F | CCAACTGCTATGACACCGACAAG |
| DSNLTRE2-R | GGGTTCAGATCCTGCCGTCGCT | ||
| DSNLTRE2-FT | T7-CCAACTGCTATGACACCGACAAG | ||
| DSNLTRE2-RT | T7-GGGTTCAGATCCTGCCGTCGCT | ||
| NlGFP | RNAi | DSNLGFP-F | AAGGGCGAGGAGCTGTTCACCG |
| DSNLGFP-R | CAGCAGGACCATGTGATCGCGC | ||
| DSNLGFP-FT | T7- AAGGGCGAGGAGCTGTTCACCG | ||
| DSNLGFP-RT | T7- CAGCAGGACCATGTGATCGCGC |
T7 sequence: GGATCCTAATACGACTCACTATAGG.
Using an IM-31 microinjector (NARISHIGE, Tokyo, Japan), dsTRE1-1, dsTRE1-2, dsTRE2, dsTPS1, and dsTPS2 (200 ng of each) were injected into the abdomen of N. lugens nymphs. Control groups were injected with dsGFP or with 0.1 to 10 µg/µl of validamycin, which is a specific trehalase inhibitor. The efficiency of gene knockdown resulting from RNAi was calculated as the ratio of gene expression between insects injected with target dsRNAs and GFP dsRNA, determined at 48 and 72 h after injection. Data for the validamycin-inhibited groups were collected 48 h after injection.
Measurement of Glycogen Content
Glycogen content (mg glucose/g total protein) was measured as described by Santos et al. (2008), using 100 μl of the supernatant . These were incubated for 4 h at 37 °C in the presence of 20 μl (1 U) amyloglucosidase (EC 3.2.1.3, Sigma, Darmstadt, Germany) diluted in 100 mM sodium acetate (pH 5.5) to hydrolyze glycogen. The amount of glucose generated from glycogen was determined using a Glucose Assay Kit (GAGO20-1KT, Sigma Jurong Town, Singapore), following the manufacturer’s instructions. Controls were prepared in the absence of the enzyme, and their amount of glycogen was calculated by excluding endogenous glucose.
Quantification of GP and GS mRNA Expression Levels
The effects of RNAi on the transcript expression of GP and GS genes were analyzed by qRT-PCR. Total RNA (1 µg) from each sample was reverse-transcribed to generate first-strand cDNA using the PrimeScript RT reagent kit with gDNA Eraser (TaKaRa, Dalian, China). Through qRT-PCR, relative gene expressions were detected using the Bio-Rad CFX96 system and the SsoFast EvaGreen Supermix (both from Bio-Rad Laboratories), as described in section before. Ten-fold serial dilutions of pooled total RNA were used in standard curves.
GP and GS Enzyme Activities Assays
Protein content in the enzyme source was measured using BCA Protein Assay Kit (Sangon Biotech, Shanghai, China), according to Chutipongtanate (2012), BSA was using as standard protein. The kits used to measure GS and GP activities were purchased from Genmed Scientifics, Inc. (USA), and the manufacturer’s instructions were followed. In the continuous circulatory system consisting of GS, pyruvate kinase, and lactate dehydrogenase (LDH), GS activity was quantified by measuring the change in absorbance values after reduced nicotinamide adenine dinucleotide (NADH) was oxidized at 340 nm. One unit of GS activity was defined as causing the oxidation of 1 μmol NADH/min per gram of protein at 30°C pH 8.2. In the continuous reaction system consisting of GP, phosphoglucomutase, and glucose-6 phosphate dehydrogenase, GP activity was assayed by measuring the reduction of oxidized nicotinamide adenine dinucleotide phosphate (NADP) during the process of glycogen breakdown at 340 nm and was expressed in micromole NADP/min per gram of protein. One unit of GP activity was defined by the catalysis of 1 μmol glycogen and orthophosphate into glucose-1 phosphate per minute at 30°C and pH 6.8 (Hao et al. 2013).
Statistical Analyses
The mRNA expression levels in the noninjected and dsGFP-injected groups were the designated controls. All data obtained in the present study were analyzed through one-way analysis of variance and presented as means ±SEs of three to six biological replicates. In Duncan’s new multiple range tests, a P < 0.01 or 0.05 was considered as extremely significant or significant, respectively.
Results
GP and GS cDNA Sequence Analyses
The molecular weight and isoelectric point of complete cDNA and amino acid sequences of GP and GS in N. lugens found using ExPaSy were 97.28 kDa and 6.10 for GP (Fig. 1A) and 84.14 kDa and 6.20 for GS (Fig. 1B), respectively.
Fig. 1.
Complementary DNA and putative amino acid sequences of NLGP and NLGS, as submitted to GenBank. (A) NLGP, accession number FJ809918; (B) NLGS, accession number FJ754277. Initiation and termination codons, as well as the termination codon before the first Methionine are indicated in bold-italics.
Tissue Expression of GP and GS
According to the qRT-PCR results, GP and GS had similar trends in mRNA expression but different levels of gene expression among the five tissues: head, leg, wing bud, cuticle, and fat body. As evidenced in Fig. 2, the highest expression of both genes was registered in wing bud tissues, followed by leg and head tissues, and the lowest in cuticle and fat body tissues. The quantitative analysis of GP and GS expression in the different tissues used the expression obtained in head tissues as the control levels for both genes. The expression of GP in the wing bud was significantly different (P < 0.05) from that in other tissues, and its expression in leg tissues was significantly higher than in head, cuticle, and fat body tissues (Fig. 2A). An identical pattern was found for GS expression, with levels in the wing bud being extremely different (P < 0.01) from that in other tissues (Fig. 2B). Overall, results showed that GP and GS were differentially expressed across the several tissues analyzed, and that their expression was significantly higher in the wing bud of the Brown planthopper, followed by leg and head tissues.
Fig. 2.
Expression of GP (A) and GS (B) in the five different tissues of Nilaparvata lugens analyzed. Total RNA was extracted from head, leg, wing bud, cuticle, and fat body tissues and the expression of both genes was obtained by quantitative real-time PCR, using 18S RNA as the internal control. Values are means ± SEs from three independent measurements. The relative expression of each gene was determined in relation to that obtained in the head of N. lugens adults. Different letters indicate significant differences according to Duncan’s test (P < 0.05).
Expression of GP and GS in Several Developmental Stages
We analyzed the expression of GP and GS in different developmental stages, from fifth-instar nymph to adult. The expression of GP was highest in 12- to 48-h fifth-instar nymphs and lowest in 60-h fifth-instar nymphs to 72-h adults (Fig. 3). A different pattern was obtained for GS: its expression was highest in 12-, 36-, 48-, and 60-h fifth-instar nymphs and in 24-, 60-, and 72-h adults and lowest in 0-, 72-, and 84-h fifth-instar nymphs and 0- and 36-h adults.
Fig. 3.

Relative expression of GP and GS in the several developmental stages of Nilaparvata lugens, as measured by quantitative real-time PCR and using 18S RNA as the internal control. Values are means± SEs from three independent measurements. The age of Brown planthopperwas defined as follows: 5L-0, 0-h fifth-instar nymph; 5L-12, 12-h fifth-instar nymph; 5L-24, 24-h fifth-instar nymph; 5L-36, 36-h fifth-instar nymph; 5L-48, 48-h fifth-instar nymph; 5L-60, 60-h fifth-instar nymph; 5L-72, 72-h fifth-instar nymph; 5L-84, 84-h fifth-instar nymph; A-0, 0-h adults; A-12, 12-h adults; A-24, 24-h adults; A-36, 36-h adults; A-48, 48-h adults; A-60, 60-h adults; A-72, 72-h adults.
Changes in Glycogen During TPS and TRE Genes Knockdown
After the successful RNAi targeting of NLTPS, a significant decrease in the expression of the two TPS genes was observed at 48 and 72 h after dsTPSs injections (Yang et al. 2017); RNAi targeting of NLTRE produced similar results (Zhao et al. 2016). In the present study, glycogen contents were detected at 48 and 72 h after dsTPSs, dsTREs, and dsGFP RNA injections, as well as at 48 h after validamycin injection at different concentrations. A highly significant decrease in glycogen content was detected at 48 and 72 h after injection in the dsTPS RNAi treatment, compared to the control treatment (Fig. 4A). In the dsTRE-injected groups, glycogen content decreased significantly at 48 h after dsTRE1-2 and dsTRE2 injection and increased significantly at 72 h after dsTRE1-1 and dsTRE2 injection, compared to the control treatment (Fig. 4B).
Fig. 4.
Glycogen content after RNAi targeting of two dsTPS and three dsTRE. Nilaparvata lugens nymphs were divided into six groups and each was injected with dsGFP, dsNlTPS1, dsNlTPS2, dsTRE1-1, dsTRE1-2, or dsTRE2. Insects were collected 48 (A) and 72 h (B) after dsRNA injection and their glycogen content was determined, in triplicate. Values are means ± SEs from three independent measurements. *Indicates significant differences at P <0.05 and ** indicates significant differences at P <0.01. GFP was used as control.
Effects of NLTPS and NLTRE RNAi on the Expression of GP and GS Genes
Results evidenced an increase in GP gene expression 48 h after dsTPS and dsTRE injections although this increase differed between treatments (Fig. 5A and B). An extreme decrease in GP expression was registered 72 h after dsTPS2 and dsTRE1-1 injections (P < 0.01), and it significantly decreased 72 h after dsTRE1-2 injection (P < 0.05). The expression of GS increased 48 h after dsTPS1, dsTRE1-1, and dsTRE1-2 injections and significantly decreased 48 h after dsTPS2 and dsTRE2 injections (P < 0.01) (Fig. 5C and D). The expression of GS decreased 72 h after the two TPS genes were knocked down and decreased significantly 72 h after the two dsTRE1s were injected (Fig. 5D).
Fig. 5.
Relative expression of GP and GS after RNAi targeting of two dsTPS and three dsTRE. The expressions of GP (A,B) and GS (C,D) were measured 48 and 72 h after dsTPS1, dsTPS2, dsTRE1-1, dsTRE1-1, dsTRE2, and dsGFP injection, quantitative real-time PCR and using 18S RNA as the internal control. Values are means ± SEs from three independent measurements. *Significant differences at P <0.05, and **indicates significant differences at P <0.01. Green fluorescence protein (GFP) was used as control.
Effects of Validamycin on the Expression of GP and GS Genes and on Glycogen Content
Results showed that GP expression decreased significantly (P < 0.05 or P < 0.01) 48 h after the injection of validamycin at several concentrations. Whereas GP expression decreased with increasing levels of validamycin (Fig. 6A), GS expression increased significantly 48 h after 0.1 μg/μl validamycin injection, but presented an extremely significant decrease 48 h after 0.5 to 10 μg/μl validamycin injections, showing a weak increasing trend with increasing concentrations of validamycin (Fig. 6B). Glycogen content significantly decreased (P < 0.01) 48 h after validamycin was injected, irrespective of its concentration (Fig. 6C).
Fig.6.
Contents of GP, GS, and glycogen after the injection of validamycin at several concentrations. The six groups of Nilaparvata lugens nymphs were injected with a solution of validamycin or deionized water. Insects were collected 48 h after injection of validamycin and the expression of GP (A) and GS (B) in relation to that of 18S mRNA was determined by quantitative real-time PCR. Glycogen content (C) was also measured 48 h after validamycin injections. Every measurement was performed in triplicate. Values are means ± SEs from three independent measurements. *Indicates significant differences at P <0.05 and **indicates significant differences at P <0.01. Deionized water was injected in the control group (CK).
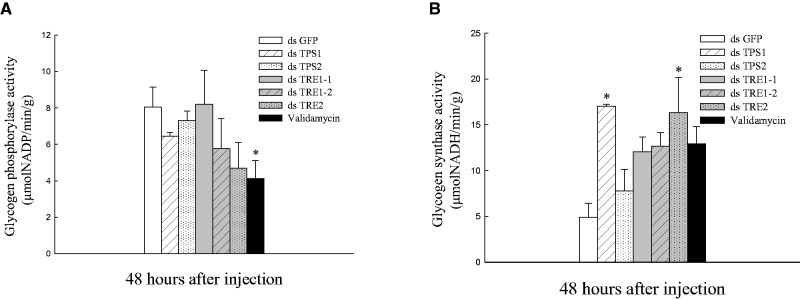
Enzyme Activity of GP and GS After RNAi and Validamycin Injection
There was no significant difference in GP activity among the first six groups. The lowest GP activity was the group of validamycin injected and it significantly (P < 0.05) decreased compared with dsGFP injection group (Fig. 7A). GS activities of dsTPS1 and dsTRE2 groups were significantly higher than that of dsGFP group after 48-h injection. Furthermore, GS activities were increased compared with the dsGFP injection group, but there was no significant (P < 0.05) difference among dsTPS2, two dsTRE1 and validamycin injection groups (Fig. 7B).
Fig. 7.
Enzyme activity of GP and GS after RNAi targeting of two dsTPS, three dsTRE and the injection of validamycin. Enzyme activity of GP (A) and GS (B) were measured 48 h after dsTPS1, dsTPS2, dsTRE1-1, dsTRE1-1, dsTRE2, dsGFP and validamycin injection. Values are means ± SEs from three independent measurements. *Significant differences at P <0.05. GFP was used as control.
Discussion
GS cDNA has been cloned from many organisms, including the yeast Saccharomyces cerevisiae Meyen ex E.C. Hansen (Farkas et al. 1990, 1991), rat liver (Bai et al. 1990), the amoeba Dictyostelium discoideum Raper (Williamson et al. 1996), the mold Neurospora crassa Shear & B. O. Dodge (de Paula et al. 2002) and S. exigua (Tang et al. 2012a). GP cDNA was first cloned from human brain (Newgard et al. 1988), D. discoideum (Rutherford et al. 1988), Escherichia coli (Migula) Castellani & Chalmers (Choi et al. 1989), S. cerevisiae (Hwang et al. 1989), Dictyostelium (Rutherford et al. 1992), D. melanogaster (Gabriella et al. 1999), S. exigua (Tang et al. 2012a), and O. furnacalis (Guo et al. 2016); it was first cloned from human muscle in 1989 and in insects, GP was cloned for the fruit fly D. melanogaster (Tick et al. 1999). The analysis of N. lugens GS and GP protein sequences and of the catalytic mechanisms of GS and GP enzymes revealed these were conserved (Fig. 1). Amino acid sequence alignment revealed a high degree of conservation between invertebrate and vertebrate orthologs (Bacca et al. 2005, Tang et al. 2012a). Two GS isoforms, GS1 and GS2, have been found in S. cerevisiae (Farkas et al. 1990, 1991), but not in insects, where only one GS and one GP have been cloned or reported from genomic analyses.
In insects, glycogen synthesized by the fat body (or by other tissues) is released in the hemolymph and then transported to several tissues where it is used as a major energy source (Tang et al. 2012a). Insect tissues contain GS, which catalyzes glycogen synthesis from UDP-glucose, and GP, which catalyzes the hydrolysis of glycogen to glucose-1-phosphate. Thus, in insects, GP is an essential enzyme for the uptake or utilization of glycogen (Tick et al. 1999). Unlike trehalose and TPS genes that were only expressed in the fat body and in trehalose-synthesis tissues (Tang et al. 2010), GS and GP transcripts were detected in the brain, fat body, mid-gut, Malpighian tubules, spermary, and tracheae of S. exigua larvae, suggesting they were expressed in different tissues and organs (Tang et al. 2012a). Similarly, in our study, GS and GP transcripts were also expressed in the head, leg, wing bud, cuticle, and fat body of N. lugens (Fig. 2). In addition, GP was not expressed in S. exigua epidermis (Tang et al. 2014b) and it presented the lowest levels in the cuticle of N. lugens, whereas the highest expression of this gene was found in wing bud tissues (Fig. 2). Overall, the widespread expression of GS and GP in insect tissues suggested they synthesize and degrade glycogen, and that flying insects such as N. lugens might need to store more glycogen than non-flying insects. In the present study, the expression of GS increased from 0- to 72-h adults, whereas GP was expressed at relatively lower levels (Fig. 3). Expression levels of GS also increased with pupa development in S. exigua, whereas GP was expressed at a lower level (Tang et al. 2014b). Thus, glycogen metabolism differs among insect species and more studies are needed to examine it in several kinds of insects, or among populations of the same insect species such as the long- and short-winged populations of N. lugens.
Glycogen is hydrolyzed under carbon starvation and accumulated during the diauxic growth phase or in response to carbon, nitrogen, sulfur, or phosphorus limitations (Johnston et al. 1992). When the ladybug C. septempunctata enters diapause during winter, lipids, total sugars, and glycogen contents increase significantly, while trehalose content is similar to that of insects that are not in diapauses (Ren et al. 2015). In the beetle Pityogenes chalcographus Linnaeus, trehalose and glycogen contents were higher in November than in other months (except March in the case of glycogen) while the content of glycerol was high all months (Koštál et al. 2014). Trehalose can be accumulated during late autumn and early winter (Kostál et al. 2007), and sugars can be inter-converted when insects are under cold hardness, starvation, or other stress conditions: molecular trehalose can be hydrolyzed to two glucose molecules by TRE and glycogen can be transformed into trehalose by the trehalose and glycogen metabolism pathway (Tang et al. 2012a). In the present study, glycogen content changed after TPS or TRE knockdown, following trehalose changes similar to those observed under stress conditions. Our results showed that glycogen decreased 48 h after dsTPS, dsTRE, and validamycin injection (Figs. 4 and 6C), whereas trehalose content increased 48 and 72 h after dsTPSs, dsTRE1-1, and validamycin injection (Zhao et al. 2016, Tang et al. 2016, Yang et al. 2017). Similarly, glycogen content increased 72 h after dsTRE1-1 and dsTRE2 were injected (Fig. 4B), whereas trehalose decreased significantly. Thus, as insect trehalose content seems to require some balance, glycogen content decreased due to its conversion into trehalose when trehalose synthesis or degradation pathways were inhibited. Overall, although trehalose is the “blood sugar” of insects, other sugars, including glycogen, also play an important role in insect physiological activity, especially when TPS or TRE RNAi inhibit trehalose pathways or when validamycin inhibits trehalase.
Many previous studies reported that the trehalose metabolism regulating insect’s chitin synthesis and degradation was seriously affected when TPS or TRE were inhibited, preventing insects from completing their developmental and molting process (Chen et al. 2010a,b; Zhao et al. 2016; Yang et al. 2017). Our results showed that the chitin synthesis pathway and chitinase genes expression were regulated by trehalose metabolism. Although GP and GS expressions were correlated with TPS and TRE, GP expression increased 48 h after dsTPSs injection whereas GS expression decreased after dsTPS2 injection (Fig. 5A and C). The expression of GP increased 48 h after dsTREs injection, whereas GS was maintained at a similar level or decreased (Fig. 5B and D). Glycogen was also affected by dsTPSs and dsTREs, generally decreasing significantly 48 h after their injection (Fig. 4). Glycogen contents changed differently 72 h post dsRNAs injection, following the changes observed in GP and GS expression. Glycogen content and GP and GS expression also decreased significantly when validamycin was injected, except for GS when 0.1 μg/μl of validamycin was injected (Fig. 6).
Synthesis and degradation of glycogen molecules involves the concerted action of a set of enzymes, with GS and GP activity primarily controlling this process, respectively (Prats et al. 2005). When GS activity is high and GP activity is low, glycogenesis exceeds glycogenolysis, resulting in glycogen accumulation. However, when GP activity is high and GS activity is low, glycogenolysis exceeds glycogenesis, resulting in glycogen reduction (Hao et al. 2013). The activity of GP decreased significantly at 48 h when validamycin were injected. The activity of the two kinds of trehalase also decreased significantly at 48 h and 72 h when validamycin were injected (Tang et al. 2016), and it decreased at 48 h after the three dsTREs were injected, while TRE expression increased (Zhao et al. 2016). In comparison, there was no significant difference in GP activity among the dsTRE and dsTPS injection groups (Fig. 7A). However, in the process of glycogen metabolism, the degradation of glycogen requires the action of 3 enzymes, and it can be regulated by strict and complex structure and hormone (Buschiazzo et al. 2004). In this atudy, there was a significant difference in GS activity for dsTPS1 or dsTRE2 compared with dsGFP, respectively. And there were no significant difference in GS activity among dsTPS2, two dsTRE1 and Validamycin injection groups. (Fig. 7B) Glycogen content has been detected under different stress conditions, including starvation, cold hardness, over-winter or seasonal acclimation, and diapauses in previous studies (Koštál et al. 2014; Heydari and Izadi 2014; Ren et al. 2015; Keshan et al. 2016; Wang et al. 2016a,b), but few studies reported GP and GS expression levels and enzyme activity. Thus, more studies are needed to understand their function in insect physiology, including GP and GS RNAi to study the specific function of these two genes in glycogen and trehalose metabolism regulation, as well as in energy and chitin metabolism regulation in insects.
Acknowledgments
This work was supported National Natural Science Foundation of China (grant nos. 31672081, 31371996, and 31672022) and the Program for Excellent Young Teachers at Hangzhou Normal University (grant no. JTAS 2011-01-031).
References Cited
- Bacca H., Huvet A., Fabioux C., Daniel J. Y., Delaporte M., Pouvreau S., Van Wormhoudt A., Moal J. 2005. Molecular cloning and seasonal expression of oyster glycogen phosphorylase and glycogen synthase genes. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 140: 635–646. [DOI] [PubMed] [Google Scholar]
- Bai G., Hang Z. J., Werner R., Nuttall F. Q., Tan A. W., Lee E. Y. 1990. The primary structure of rat liver glycogen synthase deduced by cDNA cloning. Absence of phosphorylation sites 1a and 1b. J. Biol. Chem. 265: 7843–7848. [PubMed] [Google Scholar]
- Barrion A. T., Litsinger J. A. 1994. Taxonomy of rice insect pests and their arthropod parasites and predators, pp. 13–362. InHeinrichs E. A. (ed) Biology and management of rice insects. Wiley Eastern Ltd. India and IRRI; Manila, Philippines. [Google Scholar]
- Becker A., Schlöder P., Steele J. E., Wegener G. 1996. The regulation of trehalose metabolism in insects. Experientia. 52: 433–439. [DOI] [PubMed] [Google Scholar]
- Belles X. 2010. Beyond Drosophila: RNAi in vivo and functional genomics in insects. Annu. Rev. Entomol. 55: 111–128. [DOI] [PubMed] [Google Scholar]
- Bell W., Klaassen P., Ohnacker M., Boller T., Herweijer M., Schoppink P., Van der Zee P., Wiemken A. 1992. Characterization of the 56-kDa subunit of yeast trehalose-6-phosphate synthase and cloning of its gene reveal its identity with the product of CIF1, a regulator of carbon catabolite inactivation. Eur. J. Biochem. 209: 951–959. [DOI] [PubMed] [Google Scholar]
- Browner M. F., Nakano, Bang A. G., Fletterick R. J. 1989. Human muscle glycogen synthase cDNA sequence: a negatively charged protein with an asymmetric charge distribution. Proc. Natl. Acad. Sci. USA. 86: 1443–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschiazzo A., Ugalde J. E., Guerin M. E., Shepard W., Ugalde R. A., Alzari P. M. 2004. Crystal structure of glycogen synthase: homologous enzymes catalyze glycogen synthesis and degradation. Embo. J. 23: 3196–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Tang B., Chen H. X., Yao Q., Huang X. F., Chen J., Zhang D. W., Zhang W. Q. 2010a. Differential functions of the soluble and membrane-bound trehalase genes of a lepidopteran pest Spodoptera exigua for chitin biosynthesis revealed by RNA interference. PLoS One. 5: e10133.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Zhang D., Yao Q., Zhang J., Dong X., Tian H., Zhang W. 2010b. Feeding‐based RNA interference of a trehalose phosphate synthase gene in the brown planthopper, Nilaparvata lugens. Insect. Mol. Biol. 19: 777–786. [DOI] [PubMed] [Google Scholar]
- Choi Y. L., Kawamukai, Utsumi R., Sakai H., Komano T. 1989. Molecular cloning and sequencing of the glycogen phosphorylase gene from Escherichia coli. FEBS. Lett. 243: 193–198. [DOI] [PubMed] [Google Scholar]
- Chutipongtanate S., Watcharatanyatip K., Homvises T., Jaturongkakul K., Thongboonkerd V. 2012. Systematic comparisons of various spectrophotometric and colorimetric methods to measure concentrations of protein, peptide and amino acid: Detectable limits, linear dynamicranges, interferences, practicality and unit costs. Talanta. 98: 123–129. [DOI] [PubMed] [Google Scholar]
- Cori C. F., Cori G. T. 1936. Mechanism and formation of hexosemonophosphate in muscle and isolation of a new phosphateester. P. Soc. Exp. Biol. Med. 34: 702–705. [Google Scholar]
- de Paula R., Azzariti de Pinho C., Terenzi H. F., Bertolini M. C. 2002. Molecular and biochemical characterization of the Neurospora crassa glycogen synthase encoded by the gsn cDNA. Mol. Genet. Genomics. 267: 241–253. [DOI] [PubMed] [Google Scholar]
- Elbein A. D., Pan T., Pastuszak I., Carroll D. 2003. New insights on trehalose: a multifunctional molecule. Glycobiology. 13: 17R–27R. [DOI] [PubMed] [Google Scholar]
- Farkas I., Hardy T. A., DePaoli-Roach A. A., Roach P. J. 1990. Isolation of the GSY1 gene encoding yeast glycogen synthase and evidence for the existence of a second gene. J. Biol. Chem. 265: 20879–20886. [PubMed] [Google Scholar]
- Farkas I., Hardy T. A., Goebl M. G., Roach P. J. 1991. Two glycogen synthase isoforms in Saccharomyces cerevisiae are coded by distinct genes that are differentially controlled. J. Biol. Chem. 266: 15602–15607. [PubMed] [Google Scholar]
- Gabriella T., Cserpan I., Dombradi V., Mechler B. M., Torok I., Kiss I. 1999. Structural and functional characterization of the Drosophila glycogen phosphorylase gene. Biochem. Bioph. Res. C. 257: 34–43. [DOI] [PubMed] [Google Scholar]
- Ghaffar M. B., Pritchard, Ford-Lloyd B. 2011. Brown planthopper (N. lugens Stål) feeding behavior on rice germplasm as an indicator of resistance. PLoS One. 6: e22137.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Zhang, Edwards M., Wang Z., Bai S., He K. 2016. Expression patterns of the glycogen phosphorylas gene related to larval diapauses in Ostrinia furnacalis. Arch. Insect. Biochem. Physiol. 91: 210–220. [DOI] [PubMed] [Google Scholar]
- Hao Z. P., Tang, Chen C., He Y. P., Shi Z. H. 2013. Maternal effects of photoperiods on glycogen metabolism related to induction of diapause in Cotesia vestalis (Hymenoptera: Braconidae) from Jilin, China. Appl. Entomol. Zool. 48: 47–56. [Google Scholar]
- Heydari M., Izadi H. 2014. Effects of seasonal acclimation on cold tolerance and biochemical status of the carob moth, Ectomyelois ceratoniae Zeller, last instar larvae. Bull. Entomol. Res. 104: 592–600. [DOI] [PubMed] [Google Scholar]
- Hwang P. K., Tugendreich, Fletterick R. J. 1989. Molecular analysis of GPH1, the gene encoding glycogen phosphorylase in Saccharomyces cerevisiae. Mol. Cell. Biol. 9: 1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M., Carlson M. 1992. Regulation of carbon and phosphate utilization, pp. 193–281. InEW Jones, JR Pringle, JR Broach. (eds.) The molecular and cellular biology of the yeast Saccharomyces Cold Spring Harbor, NY. Cold Spring Harbor Laboratory Press: New York. [Google Scholar]
- Keshan B., Thounaojam B., Kh S. D. 2016. Insulinand 20-hydroxyecdysone action in Bombyx mori: Glycogen content and expression pattern of insulinand ecdysone receptors in fat body. Gen. Comp. Endocrinol. 241: 108–117. [DOI] [PubMed] [Google Scholar]
- Kikuta S., Yuka H. K., Hiroaki N., Takahiro K. 2012. A novel member of the trehalose transporter family functions as an H+-dependent trehalose transporter in the reabsorption of trehalose in malpighian tubules. Front. Physiol. 3: 290.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostál V., Zahradnícková H., Simek P., Zelený J. 2007. Multiple component system of sugars and polyols in the overwintering spruce bark beetle, Ips typographus. J. Insect. Physiol. 53: 580–586. [DOI] [PubMed] [Google Scholar]
- Koštál V., Miklas B., Doležal P., Rozsypal J., Zahradníčková H. 2014. Physiology of cold tolerance in the bark beetle, Pityogenes chalcographus and its overwintering in spruce stands. J. Insect. Physiol. 63: 62–70. [DOI] [PubMed] [Google Scholar]
- Liu S., Ding Z., Zhang C., Yang B., Liu Z. 2010. Gene knockdown by intro-thoracic injection of double-stranded RNA in the brown planthopper, Nilaparvata lugens. Insect. Biochem. Mol. Biol. 40: 666–671. [DOI] [PubMed] [Google Scholar]
- Liu S., Liang Q. M., Zhou W. W., Jiang Y. D., Zhu Q. Z., Yu H., Zhang C. X., Gurr G. M., Zhu Z. R. 2015. RNA interference of NADPH-cytochrome P450 reductase of the rice brown planthopper, Nilaparvata lugens, increases susceptibility to insecticides. Pest. Manag. Sci. 71: 32–39. [DOI] [PubMed] [Google Scholar]
- Liu Z., Gong, Heckel D. G., Wei W., Sun J., Li D. 2009. Effects of larval host plants on over-wintering physiological dynamics and survival of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). J. Insect. Physiol. 55: 1–9. [DOI] [PubMed] [Google Scholar]
- Moriwaki N., Kazuhiro M., Masami N., Kazuhiro M., Yoshiaki K. 2003. High myo-inositol concentration in the hemolymph of planthoppers. Appl. Entomol. Zool. 38: 359–364. [Google Scholar]
- Nakano K., Fukui T. 1986. The complete amino acid sequence of potato alpha-glucan phosphorylase. J. Biol. Chem. 261: 8230–8236. [PubMed] [Google Scholar]
- Newgard C. B., Littman R., van Genderen C., Smith M., Fletterick R. J. 1988. Human brain glycogen phosphorylase. Cloning, sequence analysis, chromosomal mapping, tissue expression, and comparison with the human liver and muscle isozymes. J. Biol. Chem. 263: 3850–3857. [PubMed] [Google Scholar]
- Nuttall F. Q., Gannon C., Bai G., Lee E. Y. 1994. Primary structure of human liver glycogen synthase deduced by cDNA cloning. Arch. Biochem. Biophys. 311: 443–449. [DOI] [PubMed] [Google Scholar]
- Prats C., Cadefau J. A., Cusso R., Qvortrup K., Nielsen J. N., Wojtaszewki J. F. P., Hardie D. G., Stewart G., Hansen B. F., Ploug T. 2005. Phosphorylation-dependent translocation of glycogen synthase to a novel structure during glycogen resynthesis. J. Biol. Chem. 280: 23165–23172. [DOI] [PubMed] [Google Scholar]
- Pullin A. S. 1996. Physiological relationships between insect diapauses and cold tolerance: coevolution or coincidence? Eur. J. Entomol. 93: 121–129. [Google Scholar]
- Ren X. Y., Zhang L. S., Qi X. Y., An T., Han Y. H., Chen H. Y. 2015. Metabolic adaption and evaluation of cold hardiness on diapausing ladybird, Coccinella septempunctata L. J. Environ. Entomol. 37: 1195–1202. [Google Scholar]
- Rutherford C. L., Naranan, Brickey D. A., Sucic J. F., Rogers P. V., Selmin O. 1988. Glycogen phosphorylase in Dictyostelium discoideum: demonstration of two developmentally regulated forms, purification to homogeneity, immunochemical analysis, cAMP induction, in vitro translation, and molecular cloning. Dev. Genet. 9: 469–481. [DOI] [PubMed] [Google Scholar]
- Rutherford C. L., Peery B., Sucic J. F., Yin Y. Z., Rogers P. V., Luo S., Selmin O. 1992. Cloning, structural analysis, and expression of the glycogen phosphorylase-2 gene in Dictyostelium. J. Biol. Chem. 267: 2294–2302. [PubMed] [Google Scholar]
- Santos R., Mariano, Rosas A. C. R., Oliveira, Pascarelli B., Machado E. A., Meyer‐Fernandes J. R., Gondim K. C. 2008. Carbohydrate accumulation and utilization by oocytes of Rhodnius prolixus. Arch. Insect. Biochem. Physiol. 67: 55–62. [DOI] [PubMed] [Google Scholar]
- Shi Z., Liu X., Xu Q., Qin Z., Wang S., Zhang F., Wang S. G., Tang B. 2016. Two novel soluble trehalase genes cloned from Harmonia axyridis and regulation of the enzyme in a rapid changing temperature. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 198: 10–18. [DOI] [PubMed] [Google Scholar]
- Shukla E., Thorat L. J., Nath B. B., Gaikwad S. M. 2015. Insect trehalase: physiological significance and potential applications. Glycobiology. 25: 357–367. [DOI] [PubMed] [Google Scholar]
- Tang B., Chen, Yao Q., Pan Z. Q., Xu W. H., Wang S. G., Zhang W. Q. 2010. Characterization of a trehalose-6-phosphate synthase gene from Spodoptera exigua and its function identification through RNA interference. J. Insect. Physiol. 56: 813–821. [DOI] [PubMed] [Google Scholar]
- Tang B., Wei, Chen J., Wang S. G., Zhang W. Q. 2012a. Progress in gene features and functions of insect trehalases. Acta. Entomol. Sin. 55: 1315–1321. [Google Scholar]
- Tang B., Xu Q., Zou Q., Fang Q., Wang S. G., Ye G. Y. 2012b. Sequencing and characterization of glycogen synthase and glycogen phosphorylase genes from Spodoptera exigua and analysis of their function in starvation and excessive sugar intake. Arch. Insect. Biochem. Physiol. 80: 42–62. [DOI] [PubMed] [Google Scholar]
- Tang B., Qin Z., Shi Z. K., Wang S., Guo X. J., Wang S. G., Zhang F. 2014a. Trehalase in Harmonia axyridis (Coleoptera: Coccinellidae): effects on beetle locomotory activity and the correlation with trehalose metabolism under starvation conditions. Appl. Entomol. Zool. 49: 255–264. [Google Scholar]
- Tang B., Xu Y., Zhao L. N., Wang S. G., Zhang F. 2014b. Progress in research on the characteristics and functions of trehalose and the TPS gene in insect. Chin. J. Appl. Entomol. 51: 1397–1405. [Google Scholar]
- Tang B., Wei P., Zhao L. N., Shi Z. K., Shen Q. D., Yang M. M., Xie G. Q., Wang S. G. 2016. Knockdown of five trehalase genes using RNA interference regulates the gene expression of the chitin biosynthesis pathways in Tribolium castaneum. BMC Biotechnology. 16: 67.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tick G., Cserpán I., Dombrádi V., Mechler B. M., Török I., Kiss I. 1999. Structural and functional characterization of the Drosophila glycogen phosphorylase gene. Biochem. Biophys. Res. Commun. 257: 34–43. [DOI] [PubMed] [Google Scholar]
- Titani K., Koide A., Hermann J., Ericsson L. H., Kumar S., Wade R. D., Walsh K. A., Neurath H., Fischer E. H. 1977. Complete amino acid sequence of rabbit muscle glycogen phosphorylase. Proc. Natl. Acad. Sci. USA. 74: 4762–4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmasky D. S., Rabossi, Quesada-Allue L. A. 2001. Synthesis and mobilization of glycogen during metamorphosis of the medfly Ceratitis capitata. Arch. Biochem. Biophys. 392: 38–47. [DOI] [PubMed] [Google Scholar]
- Wang Y., Fan H. W., Huang H. J., Xue J., Wu W. J., Bao Y. Y., Xu H. J., Zhu Z. R., Cheng J. A., Zhang C. X. 2012. Chitin synthase 1 gene and its two alternative splicing variants from two sap-sucking insects, Nilaparvata lugens and Laodelphax striatellus (Hemiptera: Delphacidae). Insect. Biochem. Mol. Biol. 42: 637–646. [DOI] [PubMed] [Google Scholar]
- Wang W. X., Li L., Chen Y., Lai F. X., Fu Q. 2015. Identification and Function Analysis of enolase Gene NlEno1 from Nilaparvata lugens (Stål) (Hemiptera:Delphacidae). J. Insect. Sci. 15: 66.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Campbell J. B., Kaftanoglu O., Page R. E., Jr., Amdam G. V., Harrison J. F. 2016a. Larval starvation improves metabolic response to adultstarvationin honey bees (Apis mellifera L.). J. Exp. Biol. 219: 960–968. [DOI] [PubMed] [Google Scholar]
- Wang Y., Kaftanoglu O., Brent C. S., Page R. E., Jr., Amdam G. V. 2016b. Starvationstress during larval development facilitates an adaptive response in adult worker honey bees (Apis mellifera L.). J. Exp. Biol. 219: 949–959. [DOI] [PubMed] [Google Scholar]
- Williamson B. D., Favis, Brickey D. A., Rutherford C. L. 1996. Isolation and characterization of glycogen synthase in Dictyostelium discoideum. Dev. Genet. 19: 350–364. [DOI] [PubMed] [Google Scholar]
- Wipking W., Viebahn M., Neumann D. 1995. Oxygen consumption, water, lipid and glycogen content of early and late diapause and non-diapause larvae of the burnet moth Zygaena trifolii. J. Insect. Physiol. 41: 47–56. [Google Scholar]
- Xi Y., Pan P. L., Ye Y. X., Yu B., Zhang C. X. 2014. Chitindeacetylase family genes in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Insect. Mol. Biol. 23: 695–705. [DOI] [PubMed] [Google Scholar]
- Xi Y., Pan P. L., Ye Y. X., Yu B., Xu H. J., Zhang C. X. 2015a. Chitinase-like gene family in the brown planthopper, Nilaparvata lugens. Insect. Mol. Biol. 24: 29–40. [DOI] [PubMed] [Google Scholar]
- Xi Y., Pan P. L., Zhang C. X. 2015b. The β-N-acetylhexosaminidase gene family in the brown planthopper, Nilaparvata lugens. Insect. Mol. Biol. 24: 601–610. [DOI] [PubMed] [Google Scholar]
- Yang K., He, Dong S. L. 2014. Different expression profiles suggest functional differentiation among chemosensory proteins in Nilaparvata lugens (Hemiptera: Delphacidae). J. Insect. Sci. 14: 270.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M. M., Zhao N., Shen Q. D., Xie G. Q., Wang S. G., Tang B. 2017. Knockdown of two trehalose-6-phosphate synthases severely affects/decrease the chitinases’ expression of the rice brown planthopper, Nilaparvata lugens. Pest. Manag. Sci. 73: 206–216. [DOI] [PubMed] [Google Scholar]
- Yu C. H., Lu, Lin R. H., Wang X. J., Jiang H., Zhao F. 2008. Trehalose-Insect blood sugar. Chin. Bull. Entomol. 45: 832–837. [Google Scholar]
- Zhao M., Fang O. Y., Zhang Y. S., Li W., Cao J., Ge F. 2014. Analysis on occurrence and damage of the rice diseases caused by insect pests in China during 2000-2010. Bio. Disast. Sci. 4: 275–280. [Google Scholar]
- Zhao L. N., Yang M., Shen Q. D., Liu X., Shi Z. K., Wang S. G., Tang B. 2016. Functional characterization of three trehalase genes regulating the chitin metabolism pathway in rice brown planthopper using RNA interference. Sci. Repts. 6: 27841.. [DOI] [PMC free article] [PubMed] [Google Scholar]