Abstract
Temporal niche partitioning may result from interference competition if animals shift their activity patterns to avoid aggressive competitors. If doing so also shifts food sources, it is difficult to distinguish the effects of interference and consumptive competition in selecting for temporal niche shift. Bees compete for pollen and nectar from flowers through both interference and consumptive competition, and some species of bees have evolved nocturnality. Here, we use tropical forest canopy towers to observe bees (the night-flying sweat bees Megalopta genalis and M. centralis [Halictidae], honey bees, and stingless bees [Apidae]) visiting flowers of the balsa tree (Ochroma pyramalidae, Malvaceae). Because Ochroma flowers are open in the late afternoon through the night we can test the relative influence of each competition type on temporal nice. Niche shift due to consumptive competition predicts that Megalopta forage when resources are available: from afternoon into the night. Niche shift due to interference competition predicts that Megalopta forage only in the absence of diurnal bees. We found no overlap between diurnal bees and Megalopta in the evening, and only one instance of overlap in the morning, despite the abundance of pollen and nectar in the late afternoon and evening. This supports the hypothesis that Megalopta are avoiding interference competition, but not the hypothesis that they are limited by consumptive competition. We propose that the release from interference competition enables Megalopta to provision cells quickly, and spend most of their time investing in nest defense. Thus, increases in foraging efficiency directly resulting from temporal shifts to escape interference competition may indirectly lead to reduced predation and parasitism.
Keywords: competition, temporal niche, resource partitioning, niche overlap
Competition for floral nectar and pollen is typically treated as consumptive competition (competition to consume resources before others do). Consumers who arrive first find abundant resources, while later arrivals are relegated to the dregs (Heinrich 1976, Inouye 1978, Pleasants 1983, Abrol 2012). However, foragers are also subject to interference competition (driving away or otherwise preventing competitors from accessing resources). Because flowers renew nectar and pollen, they are often monopolized by aggressive foragers to deter access to potential competitors (reviewed by Roubik 1992, Kronfeld-Schor and Dayan 2003, Willmer 2011, Abrol 2012). Avoiding this interference competition may result in novel temporal niches when one species shifts its activity patterns to avoid aggressive interactions from another (Morse 1974, Case and Gilpin 1974, Carothers and Jaksić 1984). For example, subordinate hummingbird species forage at flowers early in the day when dominants are away hunting insects (Lara et al. 2009), and less aggressive stingless bee species forage earlier or later than the dominant species that control flowers during peak resource availability (Nagamitsu and Inoue 1997). Disentangling the effects of each competition type can be difficult. For instance, some bee species have shifted temporal activity even further, into the night. Consumptive competition may have driven this niche shift, as nocturnal species have new sources of food (nocturnal flowers) and first access to early opening diurnal flowers (Wcislo and Tierney 2009). However, such a shift also eliminates interference competition with diurnal bees. Here we hypothesize that avoiding interference competition from aggressive diurnal bees may be an important reason for nocturnal foraging.
We used observations of the Neotropical, night-flying bees Megalopta genalis and M. centralis on flowers of the balsa tree, Ochroma pyrmalidae, to test if escaping interference competition structures nocturnal foraging. Megalopta (Halictidae) are solitary or weakly social (typically 2–3 colony members) bees that forage from both diurnal flowers that remain open past sunset or open before sunrise, and flowers with nocturnal anthesis (Janzen 1968, Roulston 1997, Hopkins et al. 2000, Wcislo et al. 2004, Kelber et al. 2005, Franco and Gimenes 2011, Smith et al. 2012, Krug et al. 2015, Oliveria et al. 2016, Cordeiro et al. 2016). Megalopta are generalist foragers, with 64 species of pollen recorded from nests at a single site, an active nesting season of ∼9 months and opportunistic shifts among pollen sources based on availability (Smith et al. 2012). Megalopta have numerous morphological and neurobiological adaptations that allow them to fly in near-darkness, during which time most other sympatric bees are in their nests. However, because they cannot navigate beyond astronomical twilight, they stop foraging about one hour after sunset, and do not resume again until about an hour before sunrise. As a result, they rarely venture from their nests for >2 h per day, and usually much less (Kelber et al. 2005, Theobald et al. 2007, Warrant 2008). Flying during the approximately one hour after sunset and before sunrise offers access to night blooming flowers with little competition from other bees, and may also offer an escape from diurnal predators and parasites (reviewed in Wcislo et al. 2004, Wcislo and Tierney 2009, Smith et al. 2012).
Low light levels constrain Megalopta foraging times at night, but why not extend foraging into the late afternoon and early morning? Because their foraging period is so short, even a modest expansion would translate into a substantial increase in the number of provisioning trips and thus reproductive output, all other things being equal (Neff 2008). We hypothesize that interference competition from behaviorally aggressive eusocial honey bees and stingless bees (Apis mellifera and Melliponini) may reduce the rewards of foraging when other bees are active. These bees can displace other bees from flowers, often aggressively (Johnson and Hubbell 1974, Roubik 1992, Nagamitsu and Inoue 1997, Gross and Mackay 1998, Paini 2004, Lichtenberg et al. 2010, Abrol 2012). Megalopta can nest solitarily or in small social groups with a queen and worker(s), but foragers work alone and do not recruit to flowers like honey bees and stingless bees do (Wcislo et al. 2004; Smith et al. 2003, 2007). Thus, interference competition from eusocial bees may cause Megalopta to refrain from foraging even when floral resources are abundant. To test this hypothesis, we observed bees foraging on canopy flowers of the balsa tree (Ochroma pyramalidae, Malvaceae) in Central Panama. Ochroma flowers open about an hour before sunset and remain open for a single night. Each flower produces ∼ 25 ml of nectar, enough that bees sometimes swim in it (Fig. 1B), so consumptive competition is not an issue: pollen and nectar are still abundant after both diurnal bees and Megalopta stop foraging when the trees’ known pollinators, kinkajous and bats, begin visiting (Kays et al. 2012). The timing of Ochroma floral resource availability offers the chance to disentangle the relative effects of consumptive and interference competition.
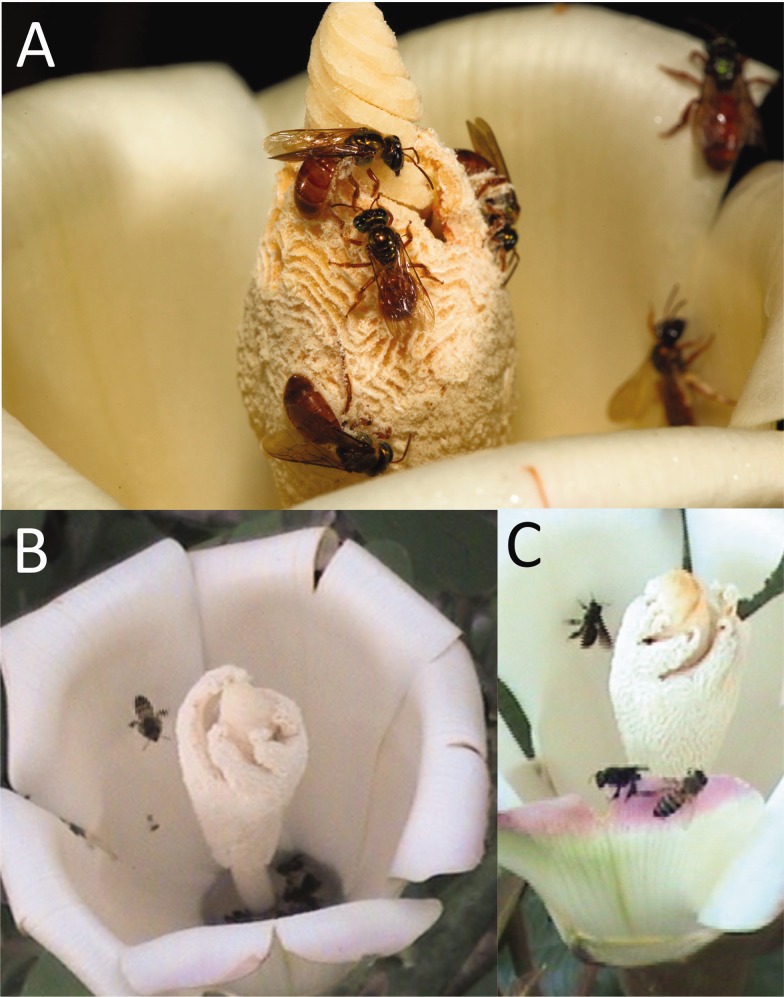
Fig. 1.
(A) Megalopta foraging for pollen on the anther of an Ochroma flower, Photo by CZ. (B) Diurnal honey bee, flying, and stingless bees, drowned in copious nectar in bottom of the flower. (C) Honey bee (right) and stingless bees foraging. B and C are screenshots from videotape.
If Megalopta foraging time is determined by resource availability, we predict that bees will begin foraging when the flowers open in order to maximize floral resource acquisition before it becomes too dark to forage.
If Megalopta foraging time is influenced by interference competition, we predict that the cessation of diurnal bee foraging, rather than flower opening, will determine when Megalopta begin foraging. Alternatively, if Megalopta do not wait for diurnal bees to stop foraging, we predict that they will suffer aggressive displacement from flowers.
Methods
All observations were conducted from the platforms of scaffolding towers built into the canopies of three Ochroma trees for a photography project (Angier and Ziegler 2011). All trees were located in second growth tropical moist forest in central Panama. One was near the town of Gamboa (9°7′40.8″, −79°41′48.8394″), one was near Pipeline road in Parque Nacional Soberania (9°7′57″, −79°43′34.32″), and the third was on Barro Colorado Island field station (BCI) near the lab clearing (9°9′59.0394″, −79°50′12.8394″). All were ≥ 10 m crown diameter (for more details of tree sites see Kays et al. 2012).
We conducted observations between 2 January and 23 February, 2010. We chose flowers opportunistically based on the ∼2 m range of our video equipment from the scaffolds. We recorded one flower at a time with a Sony (Tokyo, Japan) MiniDV camera and infrared light, and recorded presence or absence data from the resulting video tapes. To standardize for slight differences in start and finish times, we only recorded data beginning 10 min before sunset to 50 min after sunset in the evening, as this is when Megalopta typically stop foraging due to their inability to navigate back to their nests in low-light conditions; both species of Megalopta have similar foraging times and behavior (Kelber et al. 2005, Theobald et al. 2007, Smith et al. 2012). The data used from morning recordings began 65 min before sunrise and continued until 25 min after. We include data from 27.5 h of observations, including 9 morning (810 min total) and 14 evening (840 min total) filming sessions. We have 8 sessions from the Pipeline road tree, 7 from Gamboa, and 8 from BCI.
We recorded the number and type of insect visitors seen in ten-second scans taken at one minute intervals (Ochroma also receive many vertebrate visitors; Angier and Ziegler 2011, Kays et al. 2012). We recorded the presence of Megalopta, honey bees (Apis mellifera), stingless bees (Meliponini), moths, and the nocturnal vespid wasp Apoica pallens. We could not identify species of Megalopta, moths, or stingless bees from the videos, although nearly all of the stingless bees appeared to be Trigona sp. We analyzed foraging time relative to sunrise or sunset to standardize for change in day length across the study: negative values reflect minutes before sunrise or sunset, and positive values minutes after sunrise or sunset. Because our data were not normally distributed, we use a Kruskal–Wallace test followed by Conover post-hoc comparisons to test for differences between groups. Sunrise, sunset, moonrise and moonset times, and moon phase data were downloaded from the US Naval observatory (http://aa.usno.navy.mil/data/docs/RS_OneDay.php). For analysis of moon visibility, days in which the moon had not yet risen before foraging were counted as “no moon”. Days were counted as “visible” if the moon was up and 60–100% full. Our recordings included no days with risen moons at <60% visibility or cloudy days. Due to our narrow field of view, which focused on one flower, as well as our scan methodology and unmarked animals, we could not distinguish between repeat visits of a single forager and visits of two separate foragers (Fig. 1). Since we could not differentiate between individual foragers, we did not attempt to classify visits as nectar versus pollen collecting. For instance, a bee that flew from the anther of the flower out of the field of view and then quickly returned to drink nectar would appear the same as two different bees arriving in sequence to collect pollen and nectar, making such a classification difficult.
We recorded potentially aggressive interactions between bees. These included “push”, when a bee moved toward another bee and pushed it away, either forwards or backwards, “bite”, when a bee closed its mandibles on another bee, “no land” when a bee occupied the space another bee was attempting to land in, preventing the flying bee from landing, “delay land”, which is similar to “no land” above, but the flying bee was able to land nearby, and “land and displace” in which the flying bee forced the bee on the flower to move so as not to be landed on. For “push” and “bite” the pushing or biting bee was classified as the aggressor. For “no land” and “delay land” the stationary bee was classified as the aggressor. For “land and displace” the landing bee was defined as the aggressor.
Results
Observations of Flower Visitors
We recorded 967 visits across all Ochroma flowers (Table 1). Visitor species composition varied between sites (χ2 = 31.05, df = 2, P < 0.001) but there was no significant effect of site on foraging time for Megalopta, honey bees or stingless bees. No honey bees were observed at the Pipeline Road tree, and only a single Megalopta was observed at the BCI tree even though the species is common on BCI; stingless bees were common at all three trees.
Table 1.
All scans of floral visitors included in our study
| Evening | Morning | Total | |
|---|---|---|---|
| Honey bee | 254 | 17 | 271 |
| Stingless bee | 457 | 70 | 527 |
| Megalopta | 108 | 16 | 124 |
| Apoica pallens | 18 | 0 | 18 |
| Moth | 14 | 13 | 27 |
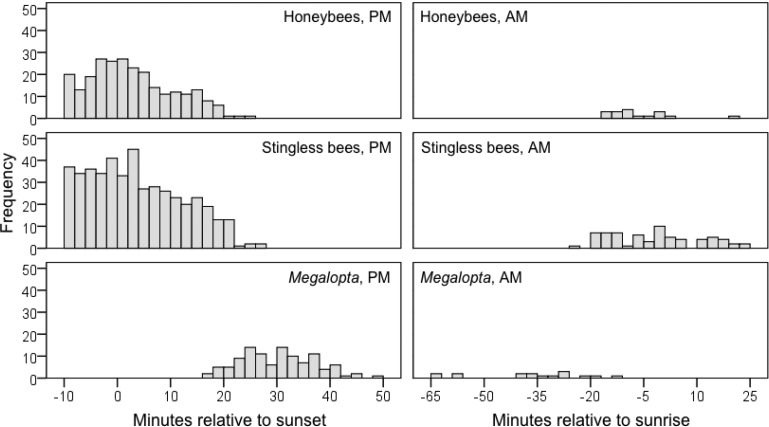
All flowers were open when evening filming began, and the evening observations typically had diurnal bees already present at the flower. Mean Megalopta foraging time was significantly later than both diurnal bees, but there was no significant difference between stingless bees and honey bees (Kruskal–Wallis χ2 = 288.84, df = 2, P < 0.001. Conover post hoc tests for Megalopta versus. stingless and honey bees both P < 0.001, stingless versus. honey bee P = 0.57). We observed honey bees in 10 of our 14 evening foraging observation periods. The latest we observed honey bees foraging was 24 min after sunset (mean latest visit ± SD = 11 ± 10 min after sunset; Fig. 2). We saw stingless bees in 13 of 14 observations. The latest stingless bee was 27 min after sunset (mean = 13 ± 10 min). Megalopta visited during only 8 of our 14 evening foraging observation periods. The earliest Megalopta visit we recorded was 16 min after sunset (mean first visit = 26 ± 10 min).
Fig. 2.
Frequency histogram of bee foraging patterns. The X-axis origin on the left and right panels represents time of sunset and sunrise, respectively. Bars in the left panels represent 2 min intervals, bars in the right panels represent 3 min intervals.
We recorded fewer visits during morning foraging observation periods than evening periods (Table 1). Mean Megalopta foraging time was significantly earlier than both diurnal bees, but there was no significant difference between stingless and honey bees (Kruskal–Wallis χ2 = 38.18, df = 2, P < 0.001). Conover post hoc tests for Megalopta versus stingless and honey bees both (P < 0.001), stingless versus honey bee (P = 0.18). We observed honey bees in 3 of our 9 morning foraging observation periods. The earliest we observed honey bees foraging was 16 min before sunrise (mean earliest visit ± SD = 14 ± 2 min before sunrise; Fig. 2). We saw stingless bees in 5 of 9 observations. The earliest stingless bee was 24 min before sunrise (mean = 6 ± 13 min). Megalopta visited during 6 of our 9 morning foraging observation periods. The latest Megalopta visit we recorded was 14 min before sunrise (mean last visit = 32 ± 15 min before sunrise).
We recorded few visits of moths or the nocturnal paper wasp Apoica pallens during our observation periods (Table 1), but both groups likely continued foraging past the end of our evening observation periods (Hunt et al. 1995, Nascimento and Tannure-Nascimento 2005, Camargo et al. 2016).
Foraging Overlap
Across all evening samples pooled together, Megalopta foraging overlapped with diurnal bees. However, within each foraging period, Megalopta always began foraging after the final diurnal bee visit. The mean time between the last diurnal bee visit and first Megalopta visit was 14 ± 15 min (range: 2–40, n = 7). There was slight overlap of Megalopta foraging with diurnal bees in the morning samples. In one session, we observed one instance of a Megalopta foraging 1 min after the first stingless bee arrived. In the every other sample, the last Megalopta visit occurred before the first diurnal bee visit. The mean time between the last Megalopta visit and the first diurnal bee visit was 23 ± 18 min (range: −1 to 52, n = 5).
Moonlight affected diurnal bee foraging, but in opposite directions. Honey bees were more likely than expected to forage until or after 16 min post-sunset (the minimum for overlap with Megalopta) on nights when the moon was visible (11 to 17 instances of foraging past 16 min occurred on moonlight nights, with expected probability of 0.4 because moon was visible two of the five nights, exact binomial test two-tailed P = 0.047). However, stingless bees were less likely to forage until or after 16 min after sunset on nights when the moon was visible (16 of 50 instances occurred on moonlight nights, expected probability of 0.5 because moon was visible three of the six nights with foraging past 16 min, binomal P = 0.015). There was no correlation between the latest diurnal bee visit and first Megalopta visit for each day (Spearman’s Rho = 0.26, P = 0.57, n = 7). There were also no significant correlations between the number of diurnal bees and Megalopta foraging on a given day, nor between stingless bees and honey bees on a given day, nor were there any significant partial correlations between the same variables after controlling for tree site.
Aggression
We observed 101 instances of potentially aggressive behavior (45.5% push, 1% bite, 6.9% no land, 23.8% delay land, 22.8% land and displace). No instances of aggression were observed between Megalopta, even though 24.5% of the scans with Megalopta (N = 106) contained >1 individual. Of the potentially aggressive interactions that we observed, 30.7% were from honey bees directed toward stingless bees, 28.7% were between honey bees, 11.9% were from stingless bees directed toward honey bees, and 28.7% were between stingless bees. The single instance of biting was between honey bees.
General Observations
Pooled nectar was still present at the end of all the evening observation periods in which the bottom of the flower was visible (in four cases it was not visible), and was present at the beginning of 4 of the 6 morning observation flowers in which the bottom of the flower was visible. The anther had at least some pollen available during all observed foraging periods. Many flowers contained dead, apparently drowned, honey bees or stingless bees floating in the nectar (Fig. 1b, Kays et al. 2012, Brighenti and Brighenti 2010). We never found a dead Megalopta floating in the nectar, although we did occasionally see one fall into the nectar and then climb back out. Because the flowers are so large, bees could not gather pollen and nectar at the same time. They either landed on the anther to collect pollen, or climbed down the petals to drink nectar (Fig. 1). Although we could not distinguish nectar versus pollen visits because bees were unmarked and often flew out of the field of view (see Methods), Megalopta occasionally switched between nectar and pollen collecting in the same visit without leaving the camera field of view. We never observed males or mating in any of the observed bee species.
Discussion
Megalopta foraging behavior was consistent with the hypothesis that they avoid interference competition from diurnal bees. Megalopta did not overlap with diurnal bees on Ochroma flowers. Ours is the first study of Megalopta to analyze data minute by minute and separate observations by flower to explicitly test for overlap, although studies of other Megalopta species show results similar to our Fig. 2 (Franco and Gimenes 2011, Krug et al. 2015, Oliveria et al. 2016, Cordeiro et al. 2016).
Moonlight may extend the foraging time of diurnal bees past sunset (reviewed in Kelber et al. 2005, Somanathan et al. 2009, Wcislo and Tierney 2009). However, our data show only a modest and inconsistent effect of moonlight on extending foraging time. Megalopta do not extend their foraging with moonlight because they are limited by their ability to navigate back to their nest below the canopy, where moonlight does not penetrate (Kelber et al. 2005), although in a study of other Megalopta species in a Brazilian agricultural setting, bees did begin foraging earlier in the morning when moonlight was present (Krug et al. 2015).
As assumed by previous authors (Wcislo et al. 2004), Megalopta face little competition for Ochroma nectar and pollen after sunset. We saw few Apoica and moths. Kays et al. (2012) report that two species of bats and five species of arboreal nocturnal mammals visited Ochroma and often left flowers empty, but this typically occurred after the Megalopta foraging period; in our observations, we saw one opossum visit and no other vertebrates. Because flowers produced little nectar after midnight (Kays et al. 2012), it is unsurprising that overall visitation rates were low in the morning. Other species of flowers that open late at night are visited by Megalopta more frequently in the pre-dawn foraging period (Roulston 1997, Franco and Gimenes 2011, Smith et al. 2012, Krug et al. 2015, Oliveria et al. 2016, Cordeiro et al. 2016).
Our data do not support the hypothesis that Megalopta are trying to maximize resource acquisition: flowers were open with copious nectar and pollen well before Megalopta began foraging. Floral resources were not limited—there was still enough nectar to literally swim in when Megalopta stopped foraging at night—but time was restricted. Megalopta typically forage for 20–45 min in the evening, and again in the morning (foraging bouts over an hour are rare; Kelber et al. 2005, A.R.S. pers. obs.). Nevertheless, Megalopta did not extend their foraging into daylight to take advantage of available resources—why not?
We can rule out an alternative hypothesis, that the optical adaptations that enable nocturnal vision render Megalopta blind in bright light. Megalopta that flee their nest upon disturbance during the day return a few minutes later, and, if the nest was removed, orient to the now-empty space where their nest used to be, before starting to search for the nest in expanding circles (A.R.S. pers. obs.). This demonstrates functioning visual navigation during daylight.
Would the eusocial bees displace Megalopta if their foraging periods overlapped? Among tropical species, the literature on aggressive monopolization of floral resources by bees focuses on competition between species of stingless bees and honey bees (Johnson and Hubbell 1974, Roubik 1992, Nagamitsu and Inoue 1997, Lichtenberg et al. 2010). Our study found 101 instances of potential aggression, although it is difficult to tell to what extent the interference we observed is incidental or aggressive, with the exception of the single bite that we observed. It seems likely that bee species intolerant of foragers from other social nests would be equally intolerant of solitary foragers, but this hypothesis is untested.
We have anecdotal evidence that Megalopta may lose in competition with stingless bees. Two of us (A.R.S and C.Z.) cut a bud of the canopy tree Pseudobombax septenatum from a tree and allowed it to open overnight before taking it to a canopy tower on BCI before dawn. P. septenatum opens at night, and is gathered by Megalopta during morning foraging flights on BCI when available. At sunrise we placed chilled Megalopta foragers on the flower. In <10 min, the flower, which was not near a P. septenatum tree, was discovered by a Trigona sp. forager, who proceeded to grab the slow-moving Megalopta and throw her off the flower. The same result occurred with a second Megalopta placed on the flower. While this interaction was contrived, and an ambient temperature Megalopta likely would have flown away, stingless bees are commonly seen foraging on P. septenatum flowers shortly after sunrise (A.R.S pers. obs). This suggests that pollen and nectar are still available, but that extending foraging time into the daylight would not be productive for Megalopta due to interference competition. Unfortunately, because Megalopta avoid interactions with diurnal bees, it is difficult to test this prediction in nature, although observations of interactions between stingless bees and diurnal solitary bees may be fruitful.
Perhaps Megalopta are not lazy, but efficient. Nocturnal foraging allows Megalopta to be such efficient foragers that they can afford to remain nestbound for ≥22 h per day guarding their offspring and still be as productive as day-active bees. Megalopta nests contain an average of 3.12–7.30 active brood cells, depending on the season and sample (Smith et al. 2007, 2012). These values are similar to other solitary and facultatively social species in the tribe Augochlorini, of which Megalopta is a member (see reviews by Sakagami and Michener 1962; Michener 1974; Eickwort and Sakagami 1979; also Packer 1990; Zillikens et al. 2001; Coelho 2002; Wcislo et al. 2003; Brosi et al. 2006; Tierney et al. 2008; Dalmazzo and Roig-Alsina 2012, 2015; Pietsch et al. 2016). Adult presence is required to defend the brood against predatory ants (Smith et al. 2003, 2007). Megalopta suffer much lower rates of brood parasitism than most solitary bees (Wcislo et al. 2004). Reducing foraging time, shifting it out of the activity time of parasites, and leaving a guard at the nest can reduce brood parasitism (Goodell 2003, Lienhard et al. 2010, Rehan et al. 2011). It is not clear how important shifting activity time is for brood parasites. Of the two brood parasites that attack the nest, one is a mutillid wasp with unknown temporal niche, and the other is a parasitic species of Megalopta that is also nocturnal (Cambra et al. 2005, Biani and Wcislo 2007). However, reducing the time a nest is left unattended likely reduces exposure to enemies (Smith et al. 2003). The ability to gather provisions in a short amount of time because foragers are unmolested may thus indirectly reduce Megalopta predation and brood parasitism. Megalopta are abundant on BCI (the only site where they have been systematically studied), widespread throughout the Neotropics, and have diversified into ∼27 species, suggesting that their narrow temporal niche is successful (Wolda and Roubik 1986, Wcislo et al. 2004, Wcislo and Tierney 2009).
Ecologists generally appreciate that both escaping predation pressure and gaining access to new resources can lead to the evolution of new temporal niches (Rydell and Speakman 1995, McCauley et al. 2012, Monterroso et al. 2013, Lima et al. 1999), but interference competition is less often considered (but see Ziv et al. 1993, Kronfeld-Schor and Dayan 2003, Valeix et al. 2007, Harrington et al. 2009, Stuble et al. 2013, Camargo et al. 2016). Here we propose that escaping interference competition may also be important in selecting for a temporal niche shift. By foraging at night, Megalopta gain access to nocturnal flowers, but also to competitor-free space (Wcislo et al. 2004, Wcislo and Tierney 2009). Our observations on Ochroma flowers, where abundant resources are available but unused before sunset, suggest that interference competition may influence the evolution of nocturnality in Megalopta. We hypothesize that efficient forging without interference, rather than just access to nocturnal flowers, may ultimately benefit Megalopta by allowing investment in nest defense and reduced predation and parasitism. This perspective suggests that the quality of foraging rather than just the quantity of resources available may be a selective benefit of nocturnal foraging.
Acknowledgments
A.R.S. was supported by a fellowship from the Smithsonian Institution. Elizabeth Rodríguez and Lina María Valencia helped film flowers; Hannah Jeffress assisted with data entry. National Geographic Magazine provided the funds to build the observation towers, and the Smithsonian Tropical Research Institute (STRI), the Gamboa Resort, and the Foundation Eisenmann allowed the temporary towers on their properties. The manuscript was improved by the comments of two anonymous reviewers.
References Cited
- Abrol D. P. 2012. Consequences of introduced honeybees upon native bee communities, pp. 635–67. In: Pollination Biology. Springer, London. [Google Scholar]
- Angier N., Ziegler C.. 2011. Open all night. Natl. Geogr. May:130–143. [Google Scholar]
- Biani N. B., Wcislo W. T.. 2007. Notes on the reproductive morphology of the parasitic bee Megalopta byroni (Hymenoptera: Halictidae), and a tentative new host record. J. Kans. Entomol. Soc. 80: 392–394. [Google Scholar]
- Brighenti D. M., Brighenti C. R. G.. 2010. Bees (Hymenoptera: Apidae) present in the flowers of the balsa wood Ochroma lagopus Swartz, 1788. Acta Sci. Biol. Sci. 32: 343–348. [Google Scholar]
- Brosi B. J., Smith-Pardo H., Gonzalez V. H.. 2006. A new wood-nesting Neocorynura (Hymenoptera: Halictidae: Augochlorini) from Costa Rica, with notes on its biology. Zootaxa. 1189: 55–68. [Google Scholar]
- Camargo N. F., de Camargo R., do D., Corrêa C. V., de Camargo A. J., Vieira E. M.. 2016. Adult feeding moths (Sphingidae) differ from non-adult feeding ones (Saturniidae) in activity-timing overlap and temporal niche width. Oecologia. 180: 313–324. [DOI] [PubMed] [Google Scholar]
- Cambra R. A., Gonzalez H., Wcislo W. T.. 2005. Description of the male, host associations, and new distribution records for Lophostigma cincta (du Buysson)(Hymenoptera: Mutillidae). Proc. Entomol. Soc. Wash. 107: 229–234. [Google Scholar]
- Carothers J. H., Jaksić F. M.. 1984. Time as a niche difference: the role of interference competition. Oikos. 42: 403–406. [Google Scholar]
- Case T. J., Gilpin M. E.. 1974. Interference competition and niche theory. Proc. Natl. Acad. Sci. U S A. 71: 3073–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho B. W. T. 2002. The biology of the primitively eusocial Augochloropsis iris (Schrottky, 1902)(Hymenoptera, Halictidae). Insectes Sociaux. 49: 181–190. [Google Scholar]
- Cordeiro G. D., Pinheiro, Dötterl S., Alves-dos-Santos I.. 2016. Pollination of Campomanesia phaea (Myrtaceae) by night-active bees: a new nocturnal pollination system mediated by floral scent. Plant Biol. 19: 132–139. [DOI] [PubMed] [Google Scholar]
- Dalmazzo M., Roig-Alsina A.. 2012. Nest structure and notes on the social behavior of Augochlora amphitrite (Schrottky) (Hymenoptera, Halictidae). J. Hymenoptera Res. 26: 17–29. [Google Scholar]
- Dalmazzo M., Roig-Alsina A.. 2015. Social biology of Augochlora (Augochlora) phoemonoe (Hymenoptera, Halictidae) reared in laboratory nests. Insectes Sociaux. 3: 315–323. [Google Scholar]
- Eickwort G. C., Sakagami S. F.. 1979. A classification of nest architecture of bees in the tribe Augochlorini (Hymenoptera: Halictidae; Halictinae), with description of a Brazilian nest of Rhinocorynura inflaticeps. Biotropica. 28–37. [Google Scholar]
- Franco E. L., Gimenes M.. 2011. Pollination of Cambessedesia wurdackii in Brazilian campo rupestre vegetation, with special reference to crepuscular bees. J. Insect Sci. 11: 97.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell K. 2003. Food availability affects Osmia pumila (Hymenoptera: Megachilidae) foraging, reproduction, and brood parasitism. Oecologia. 134: 518–527. [DOI] [PubMed] [Google Scholar]
- Gross C. L., Mackay D.. 1998. Honeybees reduce fitness in the pioneer shrub Melastoma affine (Melastomataceae). Biol. Conserv. 86: 169–178. [Google Scholar]
- Harrington L. A., Harrington L., Yamaguchi N., Thom M. D., Ferreras P., Windham T. R., Macdonald D. W.. 2009. The impact of native competitors on an alien invasive: temporal niche shifts to avoid interspecific aggression. Ecology. 90: 1207–1216. [DOI] [PubMed] [Google Scholar]
- Heinrich B. 1976. The foraging specializations of individual bumblebees. Ecol. Monogr. 46: 105–128. [Google Scholar]
- Hopkins M. J. G., Hopkins F., Sothers C. A.. 2000. Nocturnal pollination of Parkia velutina by Megalopta bees in Amazonia and its possible significance in the evolution of chiropterophily. J. Trop. Ecol. 16: 733–746. [Google Scholar]
- Hunt J. H., Jeanne L., Keeping M. G.. 1995. Observations on Apoica pallens, a nocturnal neotropical social wasp (Hymenoptera: Vespidae, Polistinae, Epiponini). Insectes Sociaux. 42: 223–236. [Google Scholar]
- Inouye D. W. 1978. Resource partitioning in bumblebees: experimental studies of foraging behavior. Ecology. 59: 672–678. [Google Scholar]
- Janzen D. H. 1968. Notes on nesting and foraging behavior of Megalopta (Hymenoptera: Halictidae) in Costa Rica. J. Kans. Entomol. Soc. 342–350. [Google Scholar]
- Johnson L. K., Hubbell S. P.. 1974. Aggression and competition among stingless bees: field studies. Ecology. 55: 120–127. [Google Scholar]
- Kays R., Rodríguez M. E., Valencia L. M., Horan R., Smith A. R., Ziegler C.. 2012. Animal visitation and pollination of flowering balsa trees (Ochroma pyramidale) in Panama. Mesoamericana. 16: 56–70. [Google Scholar]
- Kelber A., Warrant E. J., Pfaff M., Wallén R., Theobald J. C., Wcislo W. T., Raguso R. A.. 2005. Light intensity limits foraging activity in nocturnal and crepuscular bees. Behav. Ecol. 17: 63–72. [Google Scholar]
- Kronfeld-Schor N., Dayan T.. 2003. Partitioning of time as an ecological resource. Annu. Rev. Ecol. Evol. Syst. 34: 153–181. [Google Scholar]
- Krug C., Garcia M. V. B., Gomes F. B.. 2015. A scientific note on new insights in the pollination of guarana (Paullinia cupana var. sorbilis). Apidologie. 46: 164–166. [Google Scholar]
- Lara C., Lumbreras K., González M.. 2009. Niche partitioning among hummingbirds foraging on Penstemon roseus (Plantaginaceae) in central Mexico. Ornitol. Neotropical. 20: 73–83. [Google Scholar]
- Lichtenberg E. M., Imperatriz-Fonseca L., Nieh J. C.. 2010. Behavioral suites mediate group-level foraging dynamics in communities of tropical stingless bees. Insectes Sociaux. 57: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienhard A., L. Mirwald, T. Hötzl, I. Kranner, G Kastberger (2010). Trade-off between foraging activity and infestation by nest parasites in the primitively eusocial bee Halictus scabiosae. Psyche: A Journal of Entomology, 2010. [Google Scholar]
- Lima S. L., Bednekoff A., Sih A. E. A.. 1999. Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am. Nat. 153: 649–659. [DOI] [PubMed] [Google Scholar]
- McCauley D. J., Hoffmann, Young H. S., Micheli F.. 2012. Night shift: expansion of temporal niche use following reductions in predator density. PLoS One. 7: e38871.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michener C. D. 1974. The social behavior of the bees: a comparative study. Harvard University Press, Cambridge, MA. [Google Scholar]
- Monterroso P., Alves P. C., Ferreras P.. 2013. Catch me if you can: diel activity patterns of mammalian prey and predators. Ethology. 119: 1044–1056. [Google Scholar]
- Morse D. H. 1974. Niche breadth as a function of social dominance. Am. Nat. 818–830. [Google Scholar]
- Nagamitsu T., Inoue T.. 1997. Aggressive foraging of social bees as a mechanism of floral resource partitioning in an Asian tropical rainforest. Oecologia. 110: 432–439. [DOI] [PubMed] [Google Scholar]
- Nascimento F. S., Tannure-Nascimento I. C.. 2005. Foraging patterns in a nocturnal swarm-founding wasp, Apoica flavissima van der Vecht (Hymenoptera: Vespidae). Neotrop. Entomol. 34: 177–181. [Google Scholar]
- Neff J. L. 2008. Components of nest provisioning behavior in solitary bees (Hymenoptera: Apoidea). Apidologie. 39: 30–45. [Google Scholar]
- Oliveria F. D. S., Ribeiro H. M., Nunez C. V., Albuquerque P. M. C.. 2016. Flowering phenology of Mouriri guianensis (Melastomataceae) and its interaction with the crepuscular bee Megalopta amoena (Halictidae) in the restinga of Lençóis Maranhenses National Park, Brazil. Acta Amazonica. 46: 281–290. [Google Scholar]
- Packer L. 1990. Solitary and eusocial nests in a population of Augochlorella striata (Provaneher) (Hymenoptera; Halictidae) at the northern edge of its range. Behav. Ecol. Sociobiol. 27: 339–344. [Google Scholar]
- Paini D. R. 2004. Impact of the introduced honey bee (Apis mellifera)(Hymenoptera: Apidae) on native bees: a review. Aust. Ecol. 29: 399–407. [Google Scholar]
- Pietsch C., Köhler A., Zillikens A., Engels W.. 2016. Nests of the soil dwelling sweat bee Augochloropsis caerulans (Hymenoptera: Halictinae) in a waterlogged environment in southern Brazil. Stud. Neotropical Fauna Environ. 51: 1–6. [Google Scholar]
- Pleasants J. M. 1983. Structure of plant and pollinator communities, pp. 375–393. InJones C.E. (ed.), Handbook of Experimental Pollination Biology. Van Nostrand Reinhold Company Inc, New York. [Google Scholar]
- Rehan S. M., Schwarz P., Richards M. H.. 2011. Fitness consequences of ecological constraints and implications for the evolution of sociality in an incipiently social bee. Biol. J. Linn. Soc. 103: 57–67. [Google Scholar]
- Roubik D. W. 1992. Ecology and natural history of tropical bees. Cambridge University Press, Cambridge. [DOI] [PubMed] [Google Scholar]
- Roulston T. H. 1997. Hourly capture of two species of Megalopta (Hymenoptera: Apoidea; Halictidae) at black lights in Panama with notes on nocturnal foraging by bees. J. Kans. Entomol. Soc. 70: 189–196. [Google Scholar]
- Rydell J., Speakman J. R.. 1995. Evolution of nocturnality in bats: potential competitors and predators during their early history. Biol. J. Linn. Soc. 54: 183–191. [Google Scholar]
- Sakagami S., Michener C. D.. 1962. Nest architecture of the sweat bees (Halictinae). The University of Kansas Press, Lawerence. [Google Scholar]
- Smith A. R., López Quintero J., Moreno Patiño J. E., Roubik D. W., Wcislo W. T.. 2012. Pollen use by Megalopta sweat bees in relation to resource availability in a tropical forest. Ecol. Entomol. 37: 309–317. [Google Scholar]
- Smith A. R., Wcislo T., O’donnell S.. 2003. Assured fitness returns favor sociality in a mass-provisioning sweat bee, Megalopta genalis (Hymenoptera: Halictidae). Behav. Ecol. Sociobiol. 54: 14–21. [Google Scholar]
- Smith A. R., Wcislo T., O’Donnell S.. 2007. Survival and productivity benefits to social nesting in the sweat bee Megalopta genalis (Hymenoptera: Halictidae). Behav. Ecol. Sociobiol. 61: 1111–1120. [Google Scholar]
- Somanathan H., A. Kelber, R. M. Borges, R. Wallén, E. J Warrant (2009). Visual ecology of Indian carpenter bees II: adaptations of eyes and ocelli to nocturnal and diurnal lifestyles. Journal of Comparative Physiology A. 196(6): 571–583. [DOI] [PubMed] [Google Scholar]
- Stuble K. L., Rodriguez-Cabal A., McCormick G. L., Jurić I., Dunn R. R., Sanders N. J.. 2013. Tradeoffs, competition, and coexistence in eastern deciduous forest ant communities. Oecologia. 171: 981–992. [DOI] [PubMed] [Google Scholar]
- Theobald J. C., Coates M., Wcislo W. T., Warrant E. J.. 2007. Flight performance in night-flying sweat bees suffers at low light levels. J. Exp. Biol. 210: 4034–4042. [DOI] [PubMed] [Google Scholar]
- Tierney S. M., Gonzales-Ojeda, Wcislo W. T.. 2008. Nesting biology and social behavior of Xenochlora bees (Hymenoptera: Halictidae: Augochlorini) from Perú. J. Kansas Entomol. Soc. 81: 61–72. [Google Scholar]
- Valeix M., Chamaillé-Jammes S., Fritz H.. 2007. Interference competition and temporal niche shifts: elephants and herbivore communities at waterholes. Oecologia. 153: 739–748. [DOI] [PubMed] [Google Scholar]
- Warrant E. J. 2008. Seeing in the dark: vision and visual behaviour in nocturnal bees and wasps. J. Exp. Biol. 211: 1737–1746. [DOI] [PubMed] [Google Scholar]
- Wcislo W. T., Arneson, Roesch K., Gonzalez V., Smith A., Fernández H.. 2004. The evolution of nocturnal behaviour in sweat bees, Megalopta genalis and M. ecuadoria (Hymenoptera: Halictidae): an escape from competitors and enemies? Biol. J. Linn. Soc. 83: 377–387. [Google Scholar]
- Wcislo W. T., Gonzalez H., Engel M. S.. 2003. Nesting and social behavior of a wood-dwelling neotropical bee, Augochlora isthmii (Schwarz), and notes on a new species, A. alexanderi Engel (Hymenoptera: Halictidae). J. Kansas Entomol. Soc. 76: 588–602. [Google Scholar]
- Wcislo W. T., Tierney S. M.. 2009. Behavioural environments and niche construction: the evolution of dim-light foraging in bees. Biol. Rev. 84: 19–37. [DOI] [PubMed] [Google Scholar]
- Willmer P. 2011. Pollination and floral ecology. Princeton University Press, Princeton, NJ. [Google Scholar]
- Wolda H., Roubik D. W.. 1986. Nocturnal bee abundance and seasonal bee activity in a Panamanian forest. Ecology. 67: 426–433. [Google Scholar]
- Zillikens A., Steiner J., Mihalkó Z.. 2001. Nests of Augochlora (A.) esox in bromeliads, a previously unknown site for sweat bees (Hymenoptera: Halictidae). Stud. Neotropical Fauna Environ. 36: 137–142. [Google Scholar]
- Ziv Y., Abramsky Z., Kotler B. P., Subach A.. 1993. Interference competition and temporal and habitat partitioning in two gerbil species. Oikos. 237–246. [Google Scholar]