Abstract
Purpose
The foveal avascular zone (FAZ) is altered in numerous diseases. We assessed factors (axial length, segmentation method, age, sex) impacting FAZ measurements from optical coherence tomography (OCT) angiography images.
Methods
We recruited 116 Caucasian subjects without ocular disease, and acquired two 3 × 3 mm AngioVue scans per each right eye (232 total scans). In images of the superficial plexus, the FAZ was segmented using the AngioVue semiautomatic nonflow measurement tool and ImageJ manual segmentation. In images from the full retinal thickness, the FAZ was segmented using the AngioAnalytics automatic FAZ tool. Repeatability, reliability, and reproducibility were calculated for FAZ measurements (acircularity, area).
Results
FAZ area (mean ± SD) for manual segmentation was 0.257 ± 0.104 mm2, greater than both semiautomatic (0.231 ± 0.0939 mm2) and automatic (0.234 ± 0.0933 mm2) segmentation (P < 0.05). Not correcting for axial length introduced errors up to 31% in FAZ area. Manual area segmentation had better repeatability (0.022 mm2) than semiautomatic (0.046 mm2) or automatic (0.060 mm2). FAZ acircularity had better repeatability with automatic than manual segmentation (0.086 vs. 0.114). Reliability of all area measurements was excellent (intraclass correlation coefficient [ICC] = 0.994 manual, 0.969 semiautomatic, 0.948 automatic). Reliability of acircularity measurements was 0.879 for manual and 0.606 for automatic.
Conclusion
We identified numerous factors affecting FAZ measurements. These errors confound comparisons across studies and studies examining factors that may correlate with FAZ measures.
Translational Relevance
Using FAZ measurements as biomarkers for disease progression requires assessing and controlling for different sources of error. Not correcting for ocular magnification can result in significant inaccuracy in FAZ measurements, while choice of segmentation method affects both repeatability and accuracy.
Keywords: acircularity, foveal avascular zone, imaging, ocular magnification, optical coherence tomography angiography, repeatability
Introduction
The foveal avascular zone (FAZ) is an area within the central macula that is devoid of retinal capillaries. The size of the FAZ varies dramatically among normal individuals and is correlated with the size of the foveal pit.1,2 The FAZ is known to be reduced in size or altogether absent in individuals born prematurely,3,4 along with patients with albinism,5 idiopathic foveal hypoplasia,6,7 and aniridia.8 The FAZ is enlarged in patients with sickle cell disease.9 In addition, retinal vascular changes around the FAZ also occur early in diabetes mellitus with capillary dropout occurring even before the onset of retinopathy,10,11 leading to a significant increase in the size of the FAZ. Furthermore, FAZ size is correlated with visual acuity in patients with diabetic retinopathy,12 suggesting that assessment of the FAZ could be a useful biomarker for studying diabetic patients.
The variability of the FAZ in health and disease has made it an often-studied structure using a variety of techniques, including fluorescein angiography (FA), retinal function imager, and adaptive optics (AO)–based methods.13 While these approaches have facilitated a diverse array of studies of the FAZ, the recent advent of optical coherence tomography-angiography (OCT-A) has made noninvasive FAZ visualization mainstream. With this has come a plethora of studies examining FAZ size and shape in a myriad of diseases. Across a number of studies, mean area of the superficial FAZ using OCT-A ranges from 0.24 to 0.30 mm2 in healthy eyes.11,14,15 However, as with other OCT-based measurements (such as retinal nerve fiber layer [RNFL] thickness, foveal morphology, and retinal thickness), it is critical to evaluate factors affecting the accuracy and reliability of FAZ measurements.
One of the major factors affecting FAZ measurements is the segmentation or marking of the FAZ boundary. Commercial systems have algorithms that provide estimates of the “nonflow” area, defining the FAZ. Various studies have demonstrated excellent repeatability and reliability of such measurements.16–19 However, as new devices and different segmentation algorithms become available, it is important to continually reassess the reliability and repeatability of FAZ measurements. Furthermore, there are conflicting data on whether semiautomatic measurements agree with measurements obtained using manual segmentation. La Spina et al.20 reported that semiautomatic measurements were larger on average than manual measurements, while Magrath et al.21 reported no difference between semiautomatic and manual measurements when using the AngioVue OCT-A system.
Beyond the specific segmentation method, correct scaling of the retinal image also could impact the accuracy of FAZ measurements. While scans typically are 3 × 3 or 6 × 6 mm in size, individual differences in axial length (and, thus, ocular magnification) affect the absolute dimensions of the scan, meaning that measurements of the FAZ obtained from uncorrected scans will be inaccurate. Recently, FAZ area was shown to be correlated negatively with axial length, yet the investigators did not correct their images for ocular magnification.15 As such, the relationship could be due, at least in part, to the differences in ocular magnification across eyes. Thus, the purpose of our study was to assess the effect of axial length on FAZ measurements as well as to compare our previously published manual FAZ segmentation method22 to semiautomatic and automatic methods. We also explored the effect of sex and age on FAZ measurements in this relatively large Caucasian cohort, working towards the long-term goal of establishing robust normative databases for open dissemination.
Methods
Subjects
This study was approved by the Institutional Review Board (IRB) of the Medical College of Wisconsin and was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained for all subjects once the nature and risks of the study were explained. Exclusion criteria included any prior history or clinical evidence of retinal or systemic vascular disease. We imaged 116 Caucasian subjects (51 male, 65 female). The mean (± standard deviation) age was 30.5 ± 14.5 years (range, 8–77 years). Axial length measurements were obtained from all subjects using an IOL Master (Carl Zeiss, Meditec, Dublin, CA).
Assessing the Foveal Avascular Zone
Subjects were imaged with the AngioVue OCT-A system (Optovue, Inc., Fremont, CA). Two scans (one horizontal and one vertical, each consisting of 304 B-scans at 304 A-scans per B-scan) were acquired at the fovea of the right eye with a nominal scan size of 3 × 3 mm. These two scans then were coregistered by the device to minimize motion artifact and create a single volume from which an image of the superficial plexus and an image of the full retinal thickness angiogram was extracted.23,24 Two such volumes were obtained for each subject. The superficial plexus image was created by integrating motion contrast data from 3 μm below the internal limiting membrane (ILM) to 16 μm above the inner plexiform layer (IPL), while the full retinal thickness angiogram was created by integrating motion contrast data from the ILM to 75 μm above the retinal pigment epithelium (RPE). The 232 superficial plexus images were manually segmented by a single masked observer (R.L.) using ImageJ (National Institutes of Health [NIH], Bethesda, MD). The coordinates from the manual segmentation along with the image dimensions were entered into a previously described custom Matlab script (Mathworks, Natick, MA) using the function poly2mask to produce a mask defining the area of the FAZ.2,22 The nominal area of the FAZ (in mm2) was calculated according to the formula:
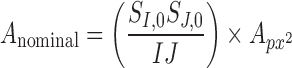 |
where 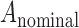 is the nominal area of the FAZ in mm2,
is the nominal area of the FAZ in mm2, 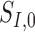 and
and 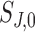 are the nominal scan dimensions in mm,
are the nominal scan dimensions in mm, 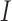 and
and 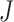 are the number of samples in each dimension in pixels, and
are the number of samples in each dimension in pixels, and 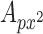 is the nominal area of the FAZ in in pixels2, which was calculated using the Matlab function regionprops. The actual area of the FAZ (in mm2) was then calculated as follows:
is the nominal area of the FAZ in in pixels2, which was calculated using the Matlab function regionprops. The actual area of the FAZ (in mm2) was then calculated as follows:
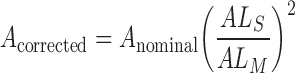 |
where 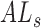 is the axial length of the subject in mm, and
is the axial length of the subject in mm, and 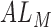 is the axial length assumed for the model eye by the manufacturer (23.95 mm).
is the axial length assumed for the model eye by the manufacturer (23.95 mm).
Nominal FAZ perimeter in pixels (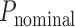 ) was also obtained using the Matlab function regionprops and adjusted for ocular magnification to obtain the FAZ perimeter in mm (
) was also obtained using the Matlab function regionprops and adjusted for ocular magnification to obtain the FAZ perimeter in mm (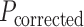 ):
):
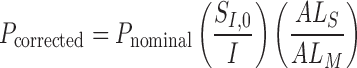 |
FAZ acircularity then was calculated as the ratio of the FAZ perimeter to the circumference of a circle with an area equivalent to that of the FAZ.25
Using the AngioVue review software (Optovue, Inc.; ver. 2016.2.0.16), the FAZ area for each superficial plexus image was found using the built-in nonflow measurement tool using a single seed point (i.e., semiautomatic). The FAZ acircularity was not calculated using this algorithm due to the software not reporting perimeter values. Finally, the FAZ area and FAZ acircularity for each full retinal thickness angiogram were found using the AngioVue review software (clinical ver. 2016.200.0.37) with “AngioAnalytics” enabled (i.e., automatic).
Statistics
Intrasession repeatability and reliability of the FAZ area and FAZ acircularity (where possible) for the three segmentation methods were assessed.26 Repeatability was calculated as 2.77σw, where σw represents the average within-subject standard deviation, and measurement error was calculated as 1.96σw. The 95% confidence intervals were calculated using the formula 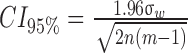 where
where 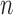 is the number of subjects (116) and
is the number of subjects (116) and 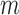 is the number of measurements (2). Reliability was assessed by finding the intraclass correlation coefficient (ICC) using the R statistical package (The R Foundation for Statistical Computing, Vienna, Austria). Sex differences were assessed using Mann-Whitney U tests, while linear regressions were used to assess for a relationship between FAZ area or FAZ acircularity and age using R statistical package. Reproducibility among the three methods was assessed using the Friedman test with post-test and further analyzed using Wilcoxon signed-rank tests and Bland-Altman plots.26
is the number of measurements (2). Reliability was assessed by finding the intraclass correlation coefficient (ICC) using the R statistical package (The R Foundation for Statistical Computing, Vienna, Austria). Sex differences were assessed using Mann-Whitney U tests, while linear regressions were used to assess for a relationship between FAZ area or FAZ acircularity and age using R statistical package. Reproducibility among the three methods was assessed using the Friedman test with post-test and further analyzed using Wilcoxon signed-rank tests and Bland-Altman plots.26
Results
Effect of Axial Length on FAZ Measurements
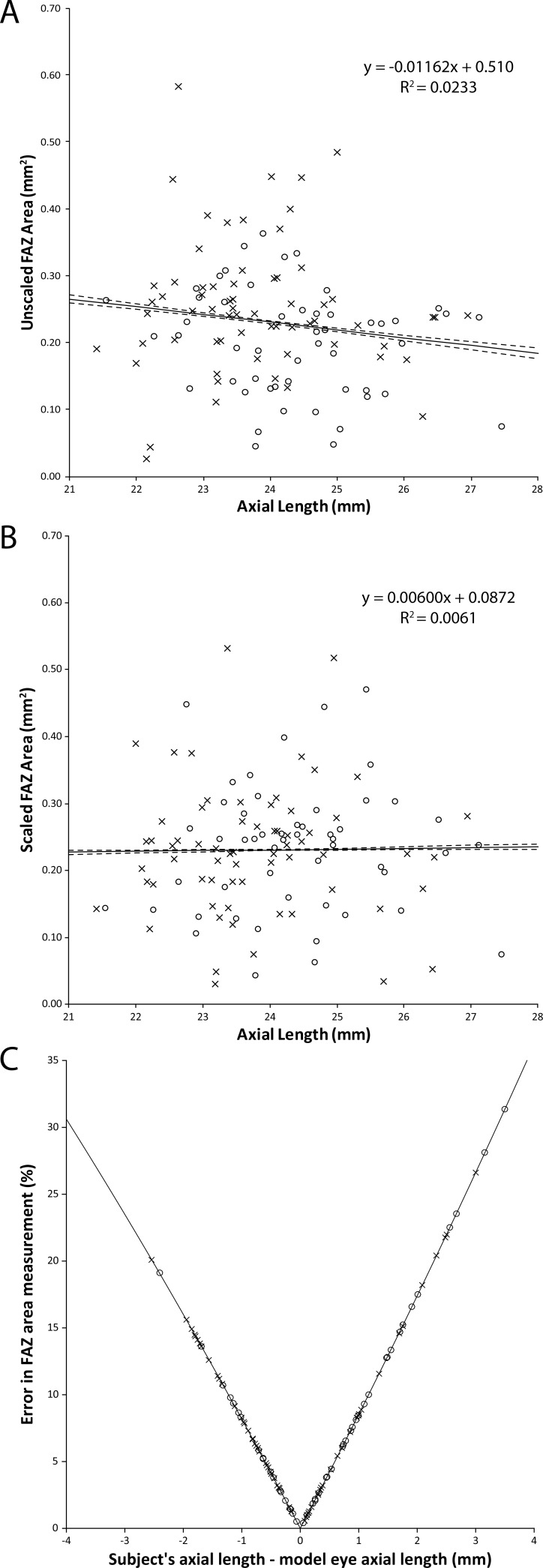
The average axial length was 24.04 ± 1.25 mm (mean ± SD; range, 21.45–27.45 mm). Assuming a nominal 3 × 3 mm scan area, we observed a slight negative trend between FAZ area and axial length computed using the semiautomatic method, though this did not reach statistical significance (R2 = 0.023, P = 0.10, Fig. 1A). When correcting the scan size for differences in ocular magnification, the trend is abolished (R2 = 0.0061, P = 0.87, Fig. 1B). The error in FAZ area estimates as a function of axial length is shown in Figure 1C; the average error was 8.29% with a maximum error of 31.36%. The absolute maximum error was 0.07 mm2. Similar trends were seen for the manual and automatic segmentation methods (data not shown); thus, for subsequent FAZ area analyses, we used data that were corrected for axial length. Differences in ocular magnification would not affect FAZ acircularity measurements, owing to the method by which it is calculated.
Figure 1.
The effect of axial length on semiautomatic FAZ area measurements. (A) FAZ area assuming a nominal 3 × 3 mm scan area. There is a slight negative trend as axial length increases (solid line is trendline, dashed lines are 95% confidence intervals), though not significant (P = 0.10). (B) FAZ area when accounting for axial length. The downward trend disappears when axial length is considered (P = 0.87). (C) The error in FAZ area measurement resulting from a failure to account for axial length. As the deviation in axial length increases, so does the error in the FAZ area. (Error model, E = [1 − (ALS/ALM)2] ∗ 100%; solid line.) Data plotted separately for males (open circles) and females (crosses).
Effect of Different Segmentation Methods
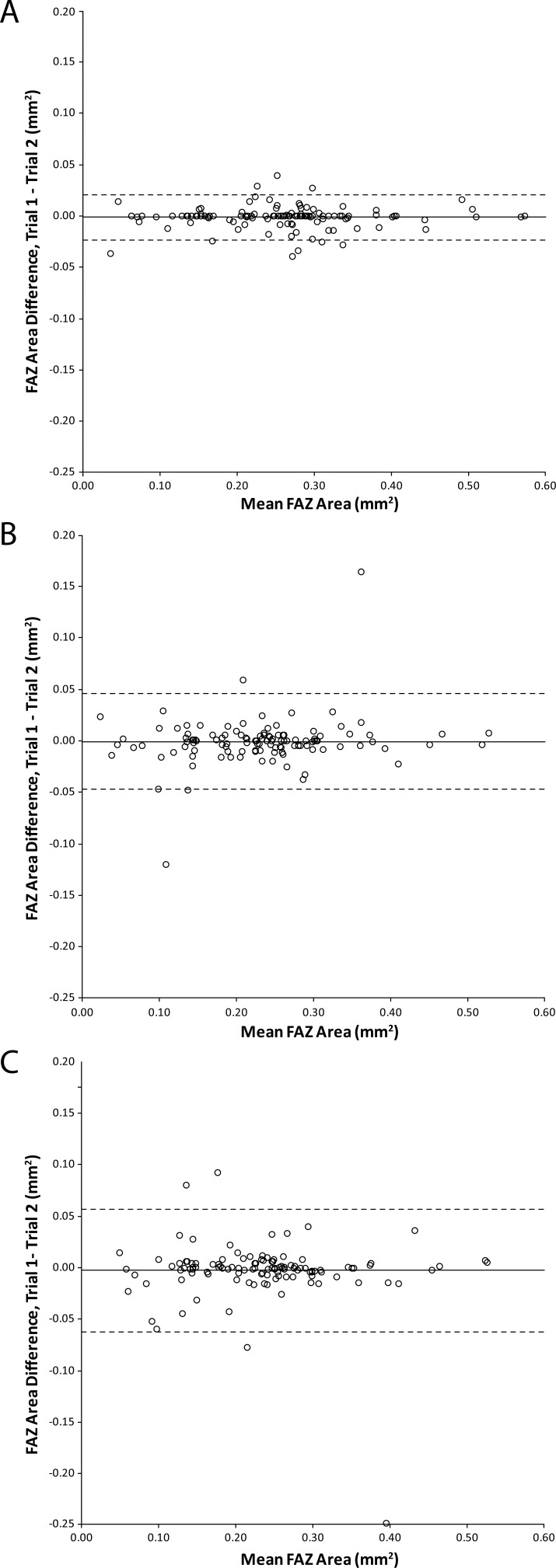
When comparing different segmentation methods, the manual segmentation performed better than the semiautomatic or the automatic segmentation, though all methods showed excellent repeatability and reliability (Fig. 2, Table 1) for FAZ area.
Figure 2.
Effect of segmentation method on intrasession repeatability. Data are expressed in Bland-Altman plots to show the repeatability of FAZ area measurements using either (A) manual segmentation, (B) semiautomatic segmentation software (version 2016.2.0.16), or (C) automatic segmentation software (version 2016.200.0.37). Solid lines represent the average difference between the two trials, while dashed lines represent 95% confidence intervals.
Table 1.
A Comparison between Manual, Semi-Automatic and Automatic Segmentation Methods
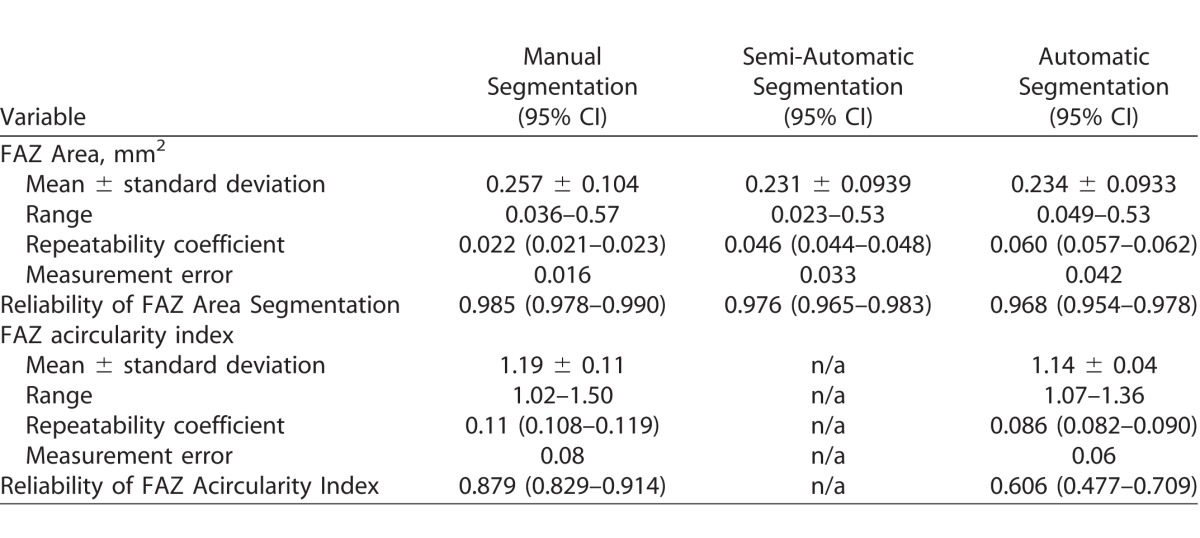
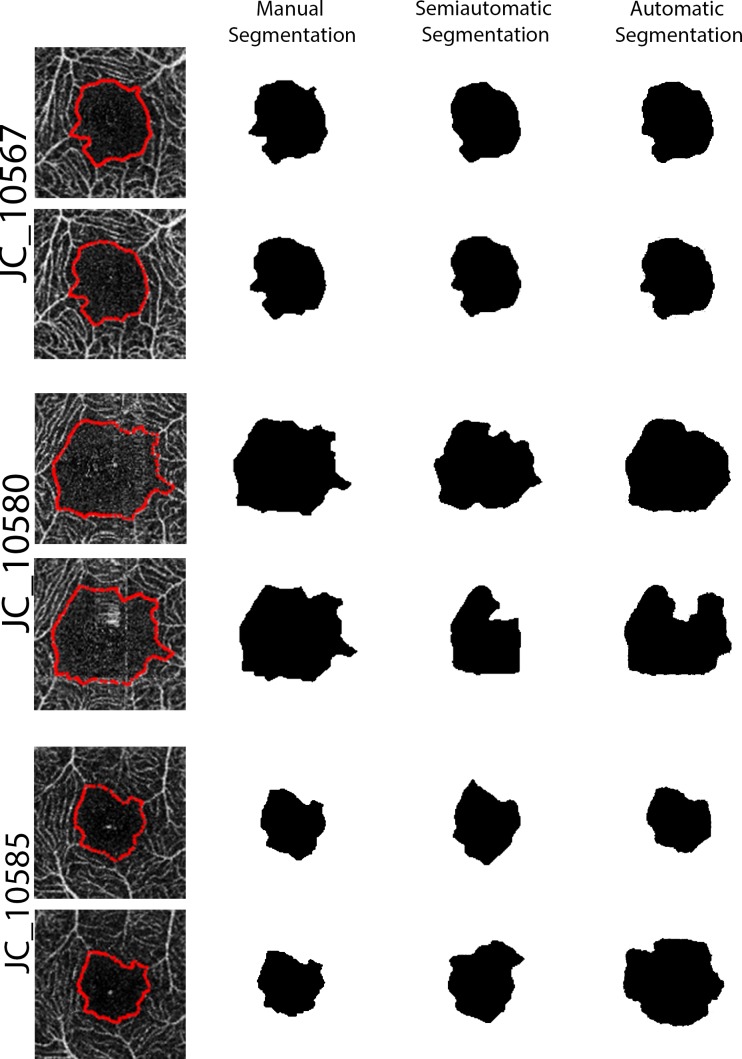
This difference is likely due to reduced image quality (from motion artifacts) and low vessel contrast affecting the algorithm-driven methods more than the human observer (Fig. 3). We used average values for each method to compare the FAZ area measurements and observed a significant difference between the manual segmentation and both automatic segmentations (Friedman test with post-test, P < 0.0001) but not between the two automatic segmentation methods (P > 0.05). When comparing the automatic to the manual segmentation methods, the mean difference (± SD) was 0.0240 ± 0.0259 mm2 (Wilcoxon signed-rank test, P < 0.0001) with manual being, on average, larger than automatic segmentation for FAZ area. As a percentage of the automatic FAZ area, the difference ranged from 2.2% to 54.4% (the absolute maximum error was 0.145 mm2). The difference between the two methods increased as a function of FAZ area (y = −0.874x + 0.0086; P = 0.0001). A similar difference was observed between the manual and semiautomatic methods, while no difference was observed between the semiautomatic and automatic methods (data not shown).
Figure 3.
The effect of image quality on manual, semiautomatic, and automatic measurements. Manual segmentation is shown on the OCT-A images of the superficial plexus marked with red dots. Subject JC_10567 had similar image quality between images and had a corresponding similarity between all methods. A large motion artifact is present in the second image from JC_10580, which has a greater effect on the semiautomatic segmentation than the manual or automatic segmentation. The second image from JC_10585 exhibited decreased contrast that affected all methods, though the automatic segmentation appeared most severely affected.
When comparing FAZ acircularity between automatic and manual segmentations, the mean difference (± SD) was 0.045 ± 0.0975 with the manual, on average, being larger than the automatic segmentation. As a percentage of the automatic FAZ acircularity, the difference ranged from 0.1% to 32.0% (the absolute maximum error was 0.36). The difference between the two methods increased as a function of FAZ acircularity (y = −0.230x + 0.2574; P < 0.0001). Across all subjects, we observed good repeatability and reliability of the FAZ acircularity index (Table 1), though it was worse than that observed for FAZ area measurements, indicating it is more sensitive to small errors in segmentation.
Other Biological Variables Affecting FAZ Measurements
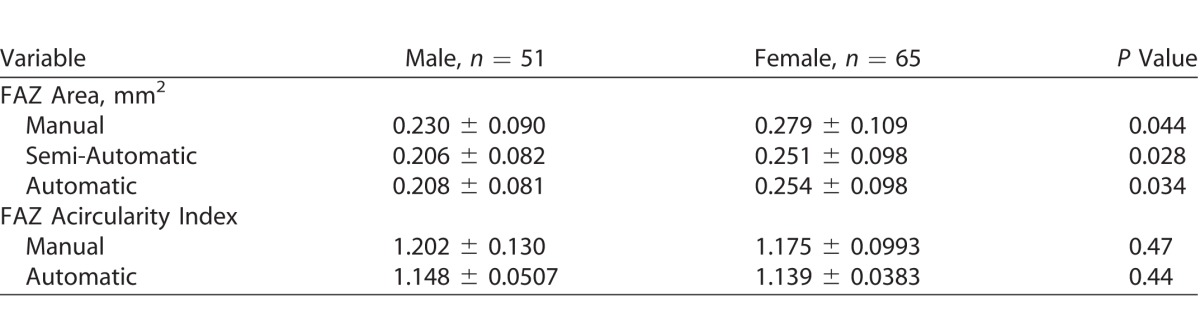
Females had larger FAZ area than males for all segmentation methods, though no difference in FAZ acircularity was observed between the groups (Table 2). No correlation between age and FAZ area was observed (manual, P = 0.920; semiautomatic, P = 0.996; automatic, P = 0.920), nor was there any correlation between age and FAZ acircularity (manual, P = 0.912; automatic, P = 0.337).
Table 2.
The Effect of Gender on Measurements of the FAZ
Discussion
We examined various factors impacting FAZ measurements, including axial length, sex, age, and segmentation method. Not correcting for axial length represents a significant source of error for measuring FAZ area. While this may not be critical for longitudinal studies comparing multiple scans from one person, these errors limit the ability to compare or combine FAZ area measurements across studies. In addition, our analysis suggests that a previous report15 of a negative correlation between axial length and FAZ area is likely due to the differences in ocular magnification across eyes. These errors are likely even more significant when studying populations with high refractive error.
Our estimates of FAZ area obtained with the manual segmentation method generally agreed with previous reports.13–15,17–19,22 As has been reported previously,15,27 we found increased FAZ area in females, though this is in contrast with data from Samara et al.14 Similarly, our observation of no relationship between FAZ size and age is in agreement with that of Tan et al.15 and Samara et al.14 but conflicts with other studies.27–29 These differences could be due to different sex, age, and/or racial distribution. Further, none of the aforementioned studies corrected for axial length.14,15,28 As such, prior conclusions drawn from studies are in many cases confounded by the lack of correction for ocular magnification, and revisiting these data may be worthwhile.
Despite excellent repeatability across all three segmentation methods, we observed a significant difference in FAZ area when comparing methods, with the algorithm-driven methods tending to produce smaller FAZ areas on average. This is in contrast with Magrath et al.,21 who found no difference between semiautomatic and manual measurements when using the Optovue system, and La Spina et al.,20 who found an increase in FAZ area when using the semiautomatic algorithm.20 It is important to note that these studies used different versions of the semiautomatic algorithm (La Spina et al.20 used version 2014.4.0.68; Magrath et al.21 used version 2014.4.0.13; this study used versions 2016.2.0.16 [semiautomatic] and clinical version 2016.200.0.37 [automatic]). Reporting the version of analysis software used is critical to facilitate comparisons across studies. FAZ area obtained from the automatic or semiautomatic segmentation algorithms used here can result in errors of nearly 60%. Different algorithms may produce different results, thus similar validation studies would need to be performed on one's segmentation algorithm of interest.
It is important to note that all subjects in this study are Caucasian. This could limit the observed range of FAZ areas due to African Americans having significantly larger foveal pits (and, thus, FAZs).30 With the difference between the segmentation methods increasing as the FAZ area becomes larger, it is important to further examine the performance of the automatic algorithm in subjects with larger pits. Similarly, normative databases comprising data from Caucasians should not be used to assess FAZ measurements from non-Caucasian subjects. Also, we had a significant number of subjects (52%) between 20 and 29 years old. More work must occur to increase the number of younger and older individuals to better understand the true variability across all age groups. In addition, all subjects had normal vision resulting in subjectively good image quality and minimal motion artifacts. While this led to excellent repeatability for FAZ area and FAZ acircularity, the introduction of unsteady fixation will lead to an increase in motion artifacts and a corresponding decreased image quality.31 Thus, the differences observed here between the different segmentation approaches may not hold for different patient populations for whom fixation is known to be unstable.
In conclusion, the two different Optovue FAZ measurement algorithms assessed herein have similar repeatability when compared to manual segmentation. However, accurate measurements require correction for axial length and careful review of automatic segmentation results (possibly with manual correction). Other metrics, like vessel density, may be affected similarly, especially when reported over Early Treatment of Diabetic Retinopathy Study (ETDRS)-like retinal areas of some fixed distance. The fact that FAZ acircularity and axis ratio are not impacted by ocular magnification make them attractive metrics to explore further, considering ocular magnification adjustments currently are not available in clinical devices.32
Acknowledgments
The authors thank Erin Curran, Mara Goldberg, Phyllis Summerfelt, and Vesper Williams for help recruiting and imaging subjects, and Tina Hsiao and Zhou Qienyuan for help using the Optovue machine and software, as well as helpful comments on the manuscript.
Supported in part by the National Eye Institute of the NIH under award numbers R01EY024969 and P30EY001931, and by the National Institute of Aging of the NIH under award number T35AG029793. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Rachel Linderman is the recipient of a Fight for Sight – Nick Cacciola Summer Student Fellowship Award.
References
- 1. Chui TYP,, Zhong Z,, Song H,, Burns SA. Foveal avascular zone and its relationship to foveal pit shape. Optom Vis Sci. 2012; 89: 602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dubis AM,, Hansen BR,, Cooper RF,, Beringer J,, Dubra A,, Carroll J. Relationship between the foveal avascular zone and foveal pit morphology. Invest Ophthalmol Vis Sci. 2012; 53: 1628–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falavarjani KG,, Iafe NA,, Velez FG,, et al. Optical coherence tomography angiography of the fovea in children born preterm [published online ahead of print January 16, 2017]. Retina. [DOI] [PubMed] [Google Scholar]
- 4. Mintz-Hittner HA,, Knight-Nanan DM,, Satriano DR,, Kretzer FL. A small foveal avascular zone may be an historic mark of prematurity. Ophthalmology. 1999; 106: 1409–1413. [DOI] [PubMed] [Google Scholar]
- 5. Wilk MA,, McAllister JT,, Cooper RF,, et al. Relationship between foveal cone specialization and pit morphology in albinism. Invest Ophthalmol Vis Sci. 2014; 55: 4186–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bazvand F,, Karkhaneh R,, Roohipoor R,, et al. Optical coherence tomography angiography in foveal hypoplasia. Ophthalmic Surg Lasers Imaging Retina. 2016; 47: 1127–1131. [DOI] [PubMed] [Google Scholar]
- 7. Pakzad-Vaezi K,, Keane PA,, Cardoso JN,, Egan C,, Tufail A. Optical coherence tomography angiography of foveal hypoplasia [published online ahead of print November 29, 2016]. Br J Ophthalmol. [DOI] [PubMed] [Google Scholar]
- 8. Nelson LB,, Spaeth GL,, Nowinski TS,, Margo CE,, Jackson, L. Aniridia. A review. Surv Ophthalmol. 1984; 28: 621–642. [DOI] [PubMed] [Google Scholar]
- 9. Sanders RJ,, Brown GC,, Rosenstein RB,, Margargal L. Foveal avascular zone diameter and sickle cell disease. Arch Ophthalmol. 1991; 109: 812–815. [DOI] [PubMed] [Google Scholar]
- 10. Tam J,, Dhamdhere KP,, Tiruveedhula P,, et al. Subclinical capillary changes in non-proliferative diabetic retinopathy. Optom Vis Sci. 2012; 89: E692–E703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takase N,, Nozaki M,, Kato A,, Ozeki H,, Yoshida M,, Ogura Y. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina. 2015; 35: 2377–2383. [DOI] [PubMed] [Google Scholar]
- 12. Balaratnasingam C,, Inoue M,, Ahn S,, et al. Visual acuity is correlated with the area of the foveal avascular zone in diabetic retinopathy and retinal vein occlusion. Ophthalmology. 2016; 123: 2352–2367. [DOI] [PubMed] [Google Scholar]
- 13. Shahlaee A,, Pefkianaki M,, Hsu J,, Ho AC. Measurement of foveal avascular zone dimensions and its reliability in healthy eyes using optical coherence tomography angiography. Am J Ophthalmol. 2016; 161: 50–55. [DOI] [PubMed] [Google Scholar]
- 14. Samara WA,, Say EA,, Khoo CT,, et al. Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina. 2015; 35: 2188–2195. [DOI] [PubMed] [Google Scholar]
- 15. Tan CS,, Lim LW,, Chow VS,, et al. Optical coherence tomography angiography evaluation of the parafoveal vasculature and its relationship with ocular factors. Invest Ophthalmol Vis Sci. 2016; 57: OCT224–OCT234. [DOI] [PubMed] [Google Scholar]
- 16. Hwang TS,, Gao SS,, Liu L,, et al. Automated quantification of capillary nonperfusion using optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmol. 2016; 134: 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lupidi M,, Coscas F,, Cagini C,, et al. Automated quantitative analysis of retinal microvasculature in normal eyes on optical coherence tomography angiography. Am J Ophthalmol. 2016; 169: 9–23. [DOI] [PubMed] [Google Scholar]
- 18. Coscas F,, Sellam A,, Glacet-Bernard A,, et al. Normative data for vascular density in superficial and deep capillary plexuses of healthy adults assessed by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016; 57: OCT211–OCT223. [DOI] [PubMed] [Google Scholar]
- 19. Carpineto P,, Mastropasqua R,, Marchini G,, Toto L,, Di Nicola M,, Di Antonio L. Reproducibility and repeatability of foveal avascular zone measurements in healthy subjects by optical coherence tomography angiography. Br J Ophthalmol. 2016; 100: 671–676. [DOI] [PubMed] [Google Scholar]
- 20. La Spina C,, Carnevali A,, Marchese A,, Querques G,, Bandello F. Reproducibility and reliability of optical coherence tomography angiography for foveal avascular zone evaluation and measurement in different settings [published online ahead of print December 20, 2016]. Retina. [DOI] [PubMed] [Google Scholar]
- 21. Magrath GN,, Say EA,, Sioufi K,, Ferenczy S,, Samara WA,, Shields CL. Variability in foveal avascular zone and capillary density using optical coherence tomography machines in healthy eyes [published online ahead of print December 16, 2016]. Retina. [DOI] [PubMed] [Google Scholar]
- 22. Wilk MA,, Dubis AM,, Cooper RF,, Summerfelt P,, Dubra A,, Carroll J. Assessing the spatial relationships between fixation and foveal specializations. Vision Res. 2017; 132: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kraus MF,, Potsaid B,, Mayer MA,, et al. Motion correction in optical coherence tomography volumes on a per A-scan basis using orthogonal scan patterns. Biomed Opt Express. 2012; 3: 1182–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kraus MF,, Liu JJ,, Schottenhamml J,, et al. Quantitative 3D-OCT motion correction with tilt and illumination correction, robust similarity measure and regularization. Biomed Opt Express. 2014; 5: 2591–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tam J,, Dhamdhere KP,, Tiruveedhula P,, et al. Disruption of the retinal parafoveal capillary network in type 2 diabetes before the onset of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011; 52: 9257–9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bland JM,, Altman DG. Statistics notes: Measurement error. Br Med J (Clin Res Ed). 1996; 313: 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu J,, Jiang C,, Wang X,, et al. Macular perfusion in healthy Chinese: an optical coherence tomography angiogram study. Invest Ophthalmol Vis Sci. 2015; 56: 3212–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iafe NA,, Phasukkijwatana N,, Chen X,, Sarraf D. Retinal capillary density and foveal avascular zone area are age-dependent: Quantitative analysis using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016; 57: 5780–5787. [DOI] [PubMed] [Google Scholar]
- 29. Gong D,, Zou X,, Zhang X,, Yu W,, Qu Y,, Dong F. The influence of age and central foveal thickness on foveal zone size in healthy people. Ophthal Surg Lasers Imag Retina. 2016; 47: 142–148. [DOI] [PubMed] [Google Scholar]
- 30. Wagner-Schuman M,, Dubis AM,, Nordgren RN,, et al. Race- and sex-related differences in retinal thickness and foveal pit morphology. Invest Ophthalmol Vis Sci. 2011; 52: 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ghasemi Falavarjani K,, Al-Sheikh M,, Akil H,, Sadda SR. Image artefacts in swept-sources optical coherence tomography angiography. Br J Ophthalmol. 2016; 101: 564–568. [DOI] [PubMed] [Google Scholar]
- 32. Krawitz BD,, Mo S,, Geyman LS,, et al. Acircularity index and axis ratio of the foveal avascular zone in diabetic eyes and healthy controls measured by optical coherence tomography angiography [published online ahead of print February 26, 2017]. Vision Res. [DOI] [PubMed] [Google Scholar]