Highlights
-
•
C. daubneyi, F. hepatica and H. cylindracea all commonly infect UK G. truncatula.
-
•
C. daubneyi may be less adept at infecting and developing in UK G. truncatula.
-
•
Paramphistomosis risk in the UK may increase if C. daubneyi can adapt to this host.
-
•
Evidence of interactions between digenean species infecting G. truncatula.
Keywords: Fasciola hepatica, Generalized estimation equation (GEE), UK, Calicophoron daubneyi, Haplometra cylindracea, Galba truncatula
Abstract
During the past decade, rumen fluke (Calicophoron daubneyi) has established as a prominent parasite of livestock within numerous European countries. Its development and spread is enabled by the presence of its intermediate snail host G. truncatula. However, the dynamics of this stage of the C. daubneyi lifecycle is yet to be recorded in numerous northern European countries including the UK. Here, the prevalence of C. daubneyi along with F. hepatica, H. cylindracea and other parasites infecting G. truncatula snails on 10 Welsh farms was recorded using morphological and PCR techniques.
A total of 892 G. truncatula snails were collected between May and October 2016. The prevalence of C. daubneyi in sampled G. truncatula snails (4%) was lower compared to F. hepatica (5.6%). No association in prevalence between these species was recorded. Haplometra cylindracea was found infecting 8.2% of G. truncatula snails, with its prevalence within G. truncatula populations negatively associated with F. hepatica cercariae prevalence (P = 0.004). Generalized estimation equation (GEE) linear regression models identified the levels of respective fluke eggs shed onto pasture as the main significant association between prevalence levels of both C. daubneyi and F. hepatica within G. truncatula populations (P < 0.001). However, equivalent prevalence levels of C. daubneyi and F. hepatica within G. truncatula populations were associated with higher C. daubneyi egg outputs and lower F. hepatica egg outputs from livestock grazing the G. truncatula habitats. Only one of 36C. daubneyi infected G. truncatula snails was found harbouring its cercarial stages, a significantly lower proportion compared to the 29 of 50 F. hepatica infected G. truncatula harbouring its respective cercariae (P < 0.05).
These results signify that C. daubneyi may be less adept at infecting and developing in the UK’s native G. truncatula populations in comparison with F. hepatica. However, C. daubneyi has previously demonstrated its ability to progressively adapt to an intermediate host in a new environment. If C. daubneyi were to adapt to infect and develop more efficiently in UK G. truncatula populations, paramphistomosis risk would significantly increase leading to increased livestock losses. Questions are also raised regarding potential interaction between digenean species at intermediate snail host level, which could impact subsequent livestock trematodosis risk.
1. Introduction
In recent years, the UK (Jones et al., 2017) and numerous other European countries (Malrait et al., 2015, Martinez-Ibeas et al., 2016) have witnessed the establishment of rumen fluke infections within their livestock. This is of concern to livestock producers due to rumen fluke’s potential to cause disease and mortality in both cattle and sheep (Mason et al., 2012, Millar et al., 2012).
As a digenean trematode, rumen fluke requires an intermediate snail host to complete its lifecycle. During this stage, the parasites’ miracidia will penetrate and infect a mollusc and use this interior environment to develop, multiply and mature efficiently. The numerous genera and species of rumen fluke within the paramphistome family have differing preferences for intermediate snail host, a factor which mainly determines the potential geographical range of each species. Historically, Paramphistomum spp. were regarded as the prominent rumen fluke species in the UK, a genus which uses aquatic planorbid snails as their intermediate hosts (Sey, 1980). However, increasing reports of rumen fluke occurrence during the past decade has coincided with the detection of Calicophoron daubneyi as the prominent rumen fluke in UK livestock (Gordon et al., 2013). This finding is significant due to the fact that the predominant intermediate snail host of C. daubneyi is Galba truncatula (Dinnik, 1962), a snail which is also the predominant host of liver fluke (Fasciola hepatica). G. truncatula snails are widespread in the UK, especially on pastures grazed by livestock and thus the potential geographical spread of C. daubneyi along with its subsequent degree of contact with livestock in the country is substantial. In Wales, C. daubneyi has been shown to be very common in livestock, especially in western areas (Jones et al., 2017) where climate modelling of F. hepatica has shown it to be one of the UK’s most prone regions for fasciolosis occurrence due to its climatic suitability for G. truncatula (Fox et al., 2011). However, despite PCR confirmation of C. daubneyi infecting G. truncatula in the UK (Jones et al., 2015), it remains unclear how susceptible the UK’s indigenous G. truncatula populations are to C. daubneyi infection. Due to the nature of paramphistomosis, where heavy juvenile infections seem to be the prominent cause of disease (Millar et al., 2012), the susceptibility of the intermediate host snail population will be a major factor in determining disease risk due to the necessity for large quantities of metacercariae to be present on pasture to lead to heavy infections.
Potential competition between C. daubneyi and F. hepatica to infect their common prospective intermediate host may also ultimately influence respective digenean diseases. These parasites have been shown to protect their positions in the snails’ digestive gland, the prime location for development, by predating on subsequent infecting species (Rondelaud et al., 2007). This, along with indirect antagonism, which may be caused by increased snail mortality or reduced nutrient availability associated with dual infections, can fuel population-wide digenean antagonism, where the presence of one digenean parasite within a snail population diminishes the presence of another (Lim and Heyneman, 1972, Combes, 1982). Moreover, G. truncatula are also infected by numerous other parasites known to antagonise F. hepatica, including trematodes Echinostoma spp. (Gordon and Boray, 1970) and Haplometra cylindracea (Whitelaw and Fawcett, 1982), and non-trematodes protostrongylid spp. (Hourdin et al., 1993) and oligochaete worm, Chaetogaster limnaei (Rajasekariah, 1978). However, detailed information on the potential relationship between all the potential parasites infecting G. truncatula remains scarce, and it is unclear if potential competition or antagonism can affect subsequent infection levels in their respective final hosts.
In this study, the prevalence of C. daubneyi, F. hepatica and other parasites within G. truncatula populations on Welsh farms were recorded. Data for C. daubneyi and F. hepatica prevalence was compared with faecal egg counts from livestock grazing on the pasture surrounding collection sites. This was performed with the aim of measuring the establishment and development of C. daubneyi within Welsh G. truncatula populations, and to record any potential interaction between the various other parasites infecting these snails.
2. Materials and methods
2.1. Snail collection
Between May and October 2016, a subset of farms (n = 10) identified as being positive for both C. daubneyi and F. hepatica during a previous study (Jones et al., 2017) were re-visited to study the intermediate host of both parasites. Farms were located in the Welsh counties of Ceredigion (n = 7) and Gwynedd (n = 3), and were visited on four separate occasions during late spring (May – early June), mid-summer (July – early August), September and October. During the first visit, multiple G. truncatula habitats in grazed fields were identified. These habitats were searched for adult G. truncatula (>4 mm in length) for approximately 20 min during each visit with the snails collected transported to the laboratory for further analyses. Other snail species were found and collected on the study’s farms. However, this paper will only focus on data surrounding the primary intermediate host of C. daubneyi, G. truncatula.
Faecal samples from ruminants grazing each G. truncatula containing field were also collected. The aim was to gauge the approximate levels of F. hepatica and C. daubneyi eggs shed onto pasture, and subsequently the level of C. daubneyi and F. hepatica infective stages that G. truncatula snails may be exposed to. Approximately 20 ml of fresh faeces was collected from 20 fresh individual faecal pats from each snail sampled field. When cattle and sheep were grazing in the same field, faeces from both species were collected and kept separate for the following analysis. On return to the laboratory, samples were kept at 4 °C prior to homogenisation and the submission of approximately 20 g of a pooled sample for sedimentation faecal egg count (FEC). For a detailed account of the FEC protocol, see Jones et al. (2017). The numbers of each fluke’s eggs counted during the FEC protocol were recorded as eggs per gram of faeces (EPG).
2.2. Detection of snail infection
Within 24 h of collection, each snail was morphologically identified as G. truncatula (Macan, 1977) prior to being crushed between microscope slides and viewed under a light microscope. Larval stages of C. daubneyi, F. hepatica or other digenean species infecting each snail were morphologically identified following Frandsen and Christensen (1984). The tissue of the snail was transferred into a 0.5 ml centrifuge tube, before DNA was extracted using the Chelex® method of Caron et al. (2011) adapted for the inclusion of 3 μl of proteinase K (20 mg/ml, Fisher Scientific, Waltham, USA) prior to initial incubation. Following DNA extraction, the supernatant was diluted 10 times with nuclease free water, with samples negative for fluke by visual inspection pooled in groups of six snails.
A multiplex polymerase chain reaction (PCR) assay was designed and used to confirm the identity of species identified morphologically as C. daubneyi or F. hepatica and to detect immature infections of each or both flukes in each snail. This PCR was optimised to detect species specific F. hepatica and C. daubneyi DNA. Primers, F: 5′-GTTTGTGTGGTTTGCCACGG-3′; R: 5′-CTACCCCAAGCAGCCACTAC-3′ (Jones et al., 2017) were used to amplify a region of C. daubneyi cytochrome c oxidase subunit 1 (cox1) gene [GenBank JQ815200], with primers, F: 5′-GCCGGGTCCTCAACATAATA-3′; R: 5′-AGCACAAAATCCTGATCTTACCA-3′ (Martinez-Ibeas et al., 2013) used to amplify a region of F. hepatica cox1 gene [GenBank AF216697]. Generic mollusc primers based on G. truncatula 18S gene [GenBank: Y09019.1] were designed (F: 5′-GGAAAGAGCGCTTTTATTAGTTCAA-3′; R: 5′-CAGAGTCATCGAAGCAACTCCT-3′) using Geneious software (Kearse et al., 2012) to amplify snail DNA as a positive control. For each sample, a 25 μl master mix was created containing 12.5 μl of MyTaq™ red mix (Bioline, London, UK), 80 μM of each parasite primer set, 40 μM of the snail primer set, one μl of the extracted DNA and nuclease-free-water. Each sample was subjected to PCR amplification consisting of an initial denaturation at 95 °C for 2 min followed by 38 cycles consisting of stages of denaturation (30 s at 95 °C) annealing (30 s at 63 °C) and extension (45 s at 72 °C), before a final 10 min extension phase at 72 °C. PCR products were visualised in 1.5% agarose gel stained with GelRed (Biotium, Hayward, USA) along with positive and negative controls. Amplification of a 912 bp 18S band of snail DNA confirmed the DNA isolation and PCR reaction were successful, with 169 bp and 425 bp bands signifying positive species identification for C. daubneyi and F. hepatica, respectively. In order to test the sensitivity of this newly created PCR assay, 100 ng/μl of DNA extracted directly from both adult C. daubneyi and F. hepatica was serially diluted ×10 with 100 ng/μl fluke free G. truncatula DNA, with each diluted DNA sample submitted for PCR via the newly designed multiplex protocol. The first dilution where the multiplex PCR would not amplify DNA from each parasite would indicate its limit of detection.
A subset of DNA amplicons of the cercariae identified morphologically as H. cylindracea (n = 8) were sequenced (ABI 3100, Applied Biosystems, Waltham, USA) to confirm species identity. Primers were designed (F: 5′-ACAGCCGTCAGGCTGCTT-3′, R: 5′-AAATCGGTCCGAAAACAACTG-3′) using Geneious software (Kearse et al., 2012) to amplify a 542 bp region of H. cylindracea 28S gene [GenBank: AF151933.1]. The sequenced subset of these H. cylindracea amplicons incorporated samples from each farm where the parasite was detected. Sequences were aligned to the amplicon’s reference sequence using Geneious software (Kearse et al., 2012) to confirm species identity.
2.3. Statistical analysis
A linear regression model with a generalized estimating equation (GEE) was used to identify factors associated with the prevalence of C. daubneyi, F. hepatica and H. cylindracea within G. truncatula populations of sampled habitats nested within farms. Generalized estimation equation models account for potential correlations within subject and missing data points; both of which were relevant to this data set due to data collection occurring across sequential time points and the variable nature of G. truncatula visible presence. The working correlation matrix for data inputted into each GEE model was set as independent, as the lowest Corrected Quasi Likelihood under Independence Model Criterion (QICC) values were associated with models created when this working correlation matrix was specified (Cui, 2007). Candidate models were built using a stepwise backward elimination procedure, where the variables with the highest P values were sequentially removed. Both main effect variables (number of fluke eggs shed onto pasture, number of snails collected, habitat type, pasture type, altitude, farm ID, sampling period and infection prevalence of other fluke species in G. truncatula populations) and interaction effect variables (created using two main effect variables) were offered during model creation. The final models were selected via their QICC values (Pan, 2001, Burnham and Anderson, 2002), with the models with the smallest QICC regarded as having the best fit.
To analyse the development of fluke species in G. truncatula snails, the proportions of C. daubneyi and F. hepatica infected snails harbouring the respective cercariae of each species were compared using a Fisher exact test. This analysis was performed on data from each sampling period.
3. Results
3.1. Prevalence of parasite infection within G. truncatula
A total 892 G. truncatula snails were collected from 22 habitats within studied farms. Infection prevalence data for these snails are presented in Table 1. Serial dilutions revealed the PCR assay was capable of detecting 0.001 ng of both C. daubneyi and F. hepatica DNA, a similar amount of DNA as other PCR assays designed to detect fluke infections in G. truncatula (Caron et al., 2011, Martinez-Ibeas et al., 2013). However, this PCR assay may have lacked the capability of identifying very low levels of DNA potentially associated with early miracidial infections (Kaplan et al., 1997). The identity of H. cylindracea cercariae was confirmed via DNA sequencing, with all amplicons sequenced showing >99% similarity with the 28S reference sequence. Six G. truncatula snails were infected with non-digenean parasites, all of which were morphologically identified as C. limnaei (Brinkhurst, 1971).
Table 1.
Prevalence of F. hepatica, C. daubneyi and H. cylindracea in G. truncatula snails and livestock (where applicable) across study period.
| May/Jun | Jul/Aug | Sep | Oct | All | |
|---|---|---|---|---|---|
| G. truncatula collected (n) | 217 | 392 | 203 | 80 | 892 |
| C. daubneyi GT prevalence% | 2.3 | 4.1 | 5.9 | 3.8 | 4.0 |
| C. daubneyi GT cercariae prevalence% | 0.0 | 0.0 | 0.5 | 0.0 | 0.1 |
| F. hepatica GT prevalence% | 4.6 | 5.6 | 8.4 | 1.3 | 5.6 |
| F. hepatica GT cercariae prevalence% | 1.4 | 2.8 | 6.9 | 1.3 | 3.3 |
| H. cylindracea GT cercariae prevalence% | 9.2 | 7.7 | 6.9 | 11.3 | 8.2 |
| Ruminant C. daubneyi mean EPG | 20 | 26 | 19 | 23 | 20 |
| Ruminant F. hepatica mean EPG | 1 | 1 | 7 | 1 | 2 |
GT = G. truncatula, EPG = eggs per gram of faeces shed onto pasture.
A total of twenty-two snails (1.88%) were co-infected with two digenean parasites, although none of these were shown to be harbouring cercarial stages of more than one parasite. Two snails were co-infected with C. daubneyi and F. hepatica (one F. hepatica cercariae + C. daubneyi DNA, and one C. daubneyi + F. hepatica DNA), with seven and thirteen snails infected with H. cylindracea cercariae and F. hepatica or C. daubneyi, respectively. Infections of two or more digenean parasites within G. truncatula populations were seen in 50% of studied habitats. Twenty seven percent of habitats sampled harboured two parasites within the snail population and 23% harboured three parasites. C. limnaei was seen co-infecting with F. hepatica cercariae in two G. truncatula snails.
3.2. GEE models
Details on selected GEE models can be seen in Table 2. Final C. daubneyi and F. hepatica models identified the levels of their respective eggs shed onto snail containing pastures as the only variable significantly associated with the parasites prevalence within G. truncatula populations. Both final models had QICC values at least two points lower than the QICC of other candidate models including models where interaction terms were included. Two final H. cylindracea models were selected as their calculated QICC values were separated by less than two and thus the superior model could not be identified (Burnham and Anderson, 2002). The first model identified the prevalence of C. daubneyi within G. truncatula populations as a significant positively associated variable, while the second model identified the prevalence of F. hepatica cercariae within G. truncatula populations as a significant negatively associated variable.
Table 2.
GEE linear regression model of factors associated with the prevalence of C. daubneyi, F. hepatica and H. cylindracea within populations of G. truncatula on Welsh farms.
| Model | QICC | Variable | β | S.E | 95% CI | Wald χ 2 | Sig. |
|---|---|---|---|---|---|---|---|
| C. daubneyi prevalence% | 3.383 | Intercept | 0.0121 | 0.0082 | −0.004: 0.028 | 2.17 | 0.14 |
| C. daubneyi EPG | 0.0015 | 0.0002 | 0.001: 0.002 | 42.57 | 0.000 | ||
| F. hepatica prevalence% | 4.538 | Intercept | 0.0292 | 0.0131 | 0.003: 0.055 | 4.95 | 0.026 |
| F. hepatica EPG | 0.0115 | 0.0006 | 0.010: 0.013 | 418.8 | 0.000 | ||
| H. cylindracea prevalence% 1 | 5.607 | Intercept | 0.068 | 0.0344 | 0.000: 0.135 | 3.89 | 0.049 |
| C. daubneyi snail prevalence% | 0.69 | 0.1438 | 0.408: 0.972 | 23.07 | 0.000 | ||
| H. cylindracea prevalence% 2 | 5.802 | Intercept | 0.109 | 0.0316 | 0.047: 0.171 | 11.97 | 0.001 |
| F. hepatica cercariae snail prevalence% | −0.297 | 0.1034 | −0.50: −0.095 | 8.26 | 0.004 | ||
EPG = eggs per gram of faeces shed onto pasture.
3.3. Galba truncatula infectivity
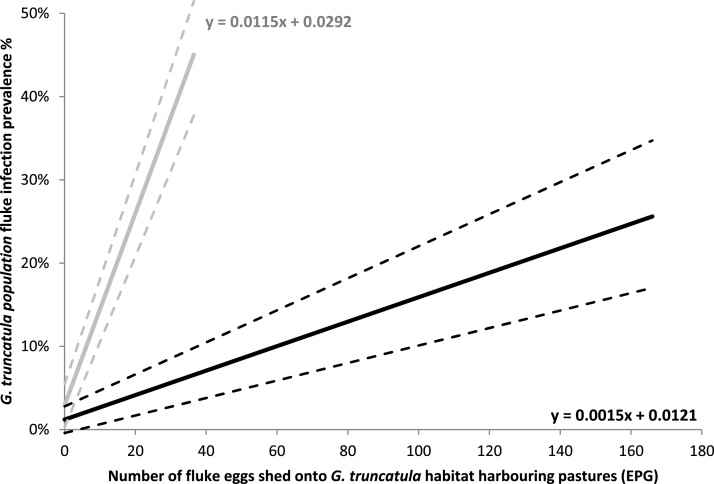
Discrepancies between F. hepatica and C. daubneyi levels in G. truncatula snails (where overall F. hepatica prevalence was higher) and in grazing livestock (where overall C. daubneyi egg counts were higher) were observed and can be seen in Table 1. These discrepancies were highlighted in the regression equations calculated in the final F. hepatica and C. daubneyi models, and are visualized in Fig. 1.
Fig. 1.
Fitted GEE linear regression equations of C. daubneyi (black) and F. hepatica (grey) along with their respective 95% confidence intervals (- - -).
The proportions of C. daubneyi and F. hepatica infected snails harbouring their respective cercariae during the sampling periods of July/August and September were significantly higher for F. hepatica compared to C. daubneyi (Table 3). Higher proportions of F. hepatica infected snails harbouring cercariae were also seen in the sampling periods of May/June and October (Table 3), however, these differences were not significant, likely due to the small sample size of infected snails collected during those periods.
Table 3.
Fisher exact tests for the proportion of C. daubneyi and F. hepatica infected G. truncatula snails harbouring cercarial stages of each respective parasite.
| May/Jun | Jul/Aug | Sep | Oct | ||
|---|---|---|---|---|---|
| C. daubneyi | Cercariae harbouring snails | 0 | 0 | 1 | 0 |
| Total infected snails | 5 | 16 | 12 | 3 | |
| Percentage of infected snails harbouring cercariae | 0 | 0 | 8.33 | 0 | |
| F. hepatica | Cercariae harbouring snails | 3 | 11 | 14 | 1 |
| Total infected snails | 10 | 22 | 17 | 1 | |
| Percentage of infected snails harbouring cercariae | 30 | 50 | 82.35 | 100 | |
| χ 2 | 1.39 | 6.877 | 5.723 | 1.875 | |
| Sig. | 0.520 | 0.009 | 0.034 | 0.400 | |
4. Discussion
This study is the first to record C. daubneyi prevalence within G. truncatula snails in the UK. The recorded prevalence levels of C. daubneyi and F. hepatica infecting G. truncatula snails were within the range of prevalences recorded in studies in France (Abrous et al., 1999, Mage et al., 2002) and Spain (Manga-González et al., 2009, Iglesias-Piñeiro et al., 2016). The GEE models identified the number of respective fluke eggs shed onto pasture as the main association between prevalence levels of both C. daubneyi and F. hepatica within snail populations. However, equivalent increases in the prevalence levels of C. daubneyi and F. hepatica within G. truncatula populations were associated with larger increases in the numbers of C. daubneyi eggs, and smaller increases in numbers of F. hepatica eggs shed by livestock onto pastures containing G. truncatula habitats. This may indicate that C. daubneyi was less effective at infecting sampled snails compared to F. hepatica, which may be indicative of C. daubneyi snail infection prevalence which remained low in comparison to F. hepatica despite C. daubneyi egg output being on average 10-fold greater than that of F. hepatica. This higher burden for C. daubneyi in livestock is likely to have occurred due to Welsh farmers’ lack of awareness of, and reluctance to treat rumen fluke infections (Jones et al., 2017); with only one farm in the study indicating that they specifically treat against the parasite. The models also highlight the importance of reducing fluke egg contamination of pasture to reduce the future infective snail population, a practice which is commonly ignored by farmers within their liver fluke treatment regimens (as cited by Claxton, 2015). Yet, it remains to be seen how effective a C. daubneyi treatment regimen can be in the UK, with treatment options limited to Oxyclozanide, an anthelmintic that is unlicensed for treatment against rumen fluke in Europe (Malrait et al., 2015) and is known to have variable efficacy against paramphistomes in the absence of multiple administration of the drug (Rolfe and Boray, 1987). Differences in the proportions of C. daubneyi and F. hepatica infected snails harbouring infective cercarial stages of each respective parasite were also recorded which raise questions regarding the effectiveness of C. daubneyi development within sampled snails. Indeed, only one C. daubneyi cercariae harbouring G. truncatula was identified throughout the study.
Overall, these findings indicate that C. daubneyi may be less suited to infecting and developing in the UK’s native G. truncatula populations compared to F. hepatica. This suggests that at present, the potential for C. daubneyi to become a highly problematic parasite in the UK may be limited. As a preferential selfer, G. truncatula snails have a high genetic differentiation between populations (Trouvé et al., 2003), a trait which could explain why G. truncatula susceptibility to F. hepatica can vary significantly between populations, with snails sourced from habitats devoid of natural contact with livestock shown to be more resistant to F. hepatica infection and its subsequent development (Rondelaud, 1993). The latter finding may be caused by incomplete adaptation between snail and parasite due to lack of contact (Rondelaud, 1993, Vignoles et al., 2002, Dreyfuss et al., 2012), with digenean/snail adaptation processes reliant on persistent contact (Boray, 1966, Rondelaud et al., 2014, Rondelaud et al., 2015). It remains unclear whether C. daubneyi is a new parasite in the UK, or has been present in the country at undetectable levels prior to its recent apparent emergence (Jones et al., 2017). However, even in the latter instance, its historical exposure to the majority of G. truncatula populations would have been negligible. With numerous G. truncatula populations across the UK now being newly exposed to C. daubneyi, it is feasible that the parasite could be in the process of progressively adapting to infect and develop within these snail populations. Ominously, C. daubneyi has already demonstrated its ability to progressively adapt to an intermediate host in a new environment. Retrospective analysis of over eighteen thousand G. truncatula snails collected in Corrèze, France, revealed prevalence of C. daubneyi within these snails had progressively increased between 1989 and 2000 (Mage et al., 2002). Furthermore, free redial burdens within individual infected snails had also increased over the same period, with levels rising to become similar to consistently observed F. hepatica redial burdens in the same populations by the final year of the study (Mage et al., 2002). Similar findings have also been observed in two other regions of France regarding C. daubneyi prevalence and its infection intensity in G. truncatula snails, with the number of G. truncatula snails found harbouring three or four sporocyst progressively increasing during a twelve year period from 1994 (Dreyfuss et al., 2008).
If this progressive adaptation was to occur in the UK as well as in other potentially novel geographical areas for C. daubneyi, it could have major implications for future paramphistomosis risk. At present, all reports of paramphistomosis in the UK have been attributed to heavy juvenile infections (Mason et al., 2012, Millar et al., 2012, SAC, 2016, Anon, 2016). For this to occur, a ruminant would have to simultaneously ingest large numbers of C. daubneyi metacercariae, which in turn would only be present on pastures inhabited by a large population of C. daubneyi shedding snails. Currently, high levels of C. daubneyi eggs are being shed onto pastures leading to, presumably, a high level of miracidial challenge for G. truncatula populations. If C. daubneyi was to adapt to the UK’s G. truncatula snails, in a similar manner as witnessed in France, conditions may become ideal for paramphistomosis risk to significantly increase. Worryingly, western areas of the UK along with Ireland are regarded as areas within Europe with the best conditions for G. truncatula activity, and are inhabited with the highest densities of grazing ruminants (Caminade et al., 2015). Thus, future paramphistomosis losses in these areas could potentially be greater than previously recorded in countries with historic C. daubneyi issues.
One possible positive effect of this potential progressive adaptation would be increased competition for F. hepatica to infect and develop within G. truncatula populations, potentially leading to reduced burdens of its metacercariae on pastures. No significant associations between C. daubneyi and F. hepatica were recorded during GEE model analysis in this study. However, with C. daubneyi still potentially in the process of adapting to its intermediate host in the UK, any possible interactions negating F. hepatica may only be prospective. It has been suggested that C. daubneyi spread in France was partly fuelled by an increasing efficacy of F. hepatica control and the subsequent reduction in F. hepatica infected G. truncatula snails (Rieu et al., 2007). Moreover, the intensity of C. daubneyi infections in cattle herds was shown to be negatively correlated with F. hepatica infection intensity in a recent study in Wales (Jones et al., 2017). This latter finding was partly attributed to varying F. hepatica treatment efficacy, with C. daubneyi hypothesised to have difficulty in establishing and infecting in high intensities on farms where levels of F. hepatica were consistently high. This theory would be supported further if C. daubneyi is indeed less adept at infecting and developing in the UK’s G. truncatula populations compared to F. hepatica, especially considering F. hepatica is regarded as having dominance over C. daubneyi in dually infected snails (Chipev et al., 1985). Co-infections between C. daubneyi and F. hepatica within G. truncatula were recorded, however, these were rare as was seen in comprehensive studies in France (Rondelaud et al., 2004, Rondelaud et al., 2016). This lack of co-infections could be an indication of competition occurring in the field, with secondary infecting fluke species failing to establish in the snail, or its successful infection leading to snail death due to internal pressures (Goumghar et al., 2000). Competition between C. daubneyi and F. hepatica has already been demonstrated in laboratory studies, with interspecies predation, competition for nutrients and changing biochemical composition of snail tissue all antagonising factors observed when co-infections of both species were initiated (Chipev et al., 1985, Rondelaud et al., 2007).
The most common parasite found infecting G. truncatula during the study was H. cylindracea, and thus the F. hepatica lifecycle may already be affected due to competition on some farms. Information on H. cylindracea in the UK is scarce, and thus it is unclear if the relatively high levels observed in these Welsh farms differ from its prevalence both historically and across various regions of the UK. In France, overall prevalence has been shown to be low (Mage et al., 2002, Rondelaud et al., 2016), although levels within individual habitats have been recorded as high as 31.5% (Goumghar et al., 2000). In the latter study, F. hepatica prevalence within G. truncatula populations was shown to be lower in habitats where H. cylindracea was present, while Whitelaw and Fawcett (1982) suggested that high levels of H. cylindracea within a Scottish farm’s G. truncatula population might have been the cause of the absence of F. hepatica in those snails. The negative association recorded in the prevalence of both H. cylindracea and F. hepatica cercariae within G. truncatula populations in this study could be an indication of antagonism. On the contrary, C. daubneyi prevalence was positively associated with H. cylindracea which potentially indicates a facilitation effect between two parasites, a phenomenon whereby the presence of one infecting parasite may increase a snail’s susceptibility to another infecting species (Combes, 1982). This potential facilitation effect could be an important factor in C. daubneyi establishment if indeed UK G. truncatula are currently not fully susceptible to C. daubneyi infection. However, no snails harbouring cercariae of more than one species were recorded. Laboratory studies have shown that H. cylindracea infected G. truncatula snails are capable of being infected and fully sustaining a F. hepatica or C. daubneyi infection through to shedding, however, cercerial output of both subsequent infecting parasites was very low due to indirect antagonistic effects (Moukrim et al., 1993).
C. limnaei, a parasite known to predate on (Michelson, 1964, Rajasekariah, 1978) and potentially regulate trematode larvae (Ibrahim, 2007), was also recorded infecting G. truncatula snails. Considering its rarity and its presence co-infecting G. truncatula with F. hepatica cercariae, it is unlikely that C. limnaei had any major negating effects on F. hepatica prevalence within sampled snails, although this could differ if G. truncatula populations commonly infected with C. limnaei exist. Indeed, it remains to be seen whether the presence of any parasite including H. cylindracea, or a fully adapted C. daubneyi can have any major negating effects on subsequent livestock F. hepatica infections, with no known research on this aspect found in the literature. Spatial modelling of F. hepatica at herd level has been shown to be less accurate compared to regional models, with numerous unknown localised variations believed to cause a prevalence disparity in small regions exposed to the same climate variables (Howell et al., 2015). If H. cylindracea does indeed have an antagonistic effect on F. hepatica, it could be one of many factors causing these localised variations, with H. cylindracea prevalence shown to be capable of varying significantly within G. truncatula habitats in neighbouring fields in this study. However, populations of the definitive hosts of H. cylindracea, toads (Bufo bufo) and frogs (Rana temporaria), have both been shown to be in decline in the UK (Teacher et al., 2010, Petrovan and Schmidt, 2016), which subsequently may cause a reduction in H. cylindracea prevalence within G. truncatula populations. This, on some farms at least, may indirectly heighten F. hepatica prevalence within its G. truncatula populations and subsequent fasciolosis risk.
5. Conclusion
This study demonstrates that C. daubneyi is infecting G. truncatula snails in the UK, although questions remain regarding its capabilities to infect and develop within these populations efficiently. However, evidence suggests that C. daubneyi could adapt to become more suitable to this intermediate host. If so, the risk of paramphistomosis could increase significantly in the future, and thus further research on C. daubneyi epidemiology is imperative in the eventuality of this scenario. The study also recorded high levels of F. hepatica and H. cylindracea infecting G. truncatula snails on Welsh farms. This raises numerous questions regarding potential interactions at intermediate host level between the three digenean parasites studied and potentially other parasite groups as well. These interactions may involve an antagonistic effect which in theory could be a novel method of negating livestock losses due to trematodosis.
Acknowledgements
Rhys Aled Jones gratefully acknowledges the Owen Price scholarship for funding his Ph.D. Ayodeji Sinmidele and Rowan K Thomas gratefully acknowledge funding from Aberystwyth University’s Aber Forward scheme. The project was funded by the Coleg Cymraeg Cenedlaethol (to H. W. W.) and BBSRC Innovate UK (102108) to P.M.B. The authors would like to thank all participating farmers; the staff at YFC Wales; Dr Martin Vickers for assistance in creating the project website; and Dr Basil T. Wolf for statistical advice.
References
- Abrous M., Rondelaud D., Dreyfuss G., Cabaret J. Infection of Lymnaea truncatula and Lymnaea glabra by Fasciola hepatica and Paramphistomum daubneyi in farms of central France. Vet. Res. 1999;30:113–118. [PubMed] [Google Scholar]
- Anon Northern Ireland disease surveillance report, july to september 2016. Vet. Rec. 2016;179:482–485. doi: 10.1136/vr.i5856. [DOI] [PubMed] [Google Scholar]
- Boray J.C. Studies on the relative susceptibility of some lymnaeids to infection with Fasciola hepatica and F. gigantica and on the adaptation of Fasciola spp. Ann. Trop. Med. Parasitol. 1966;60:114–124. doi: 10.1080/00034983.1966.11686394. [DOI] [PubMed] [Google Scholar]
- Brinkhurst R.O. 2nd ed. Freshwater Biological Association; Ambleside, UK: 1971. A Guide for the Identification of British Aquatic Oligochaete. [Google Scholar]
- Burnham K.P., Anderson D.R. 2nd ed. Springer New York; NY, USA: 2002. Model Selection and Multimodel Inference a Practical Information-theoretic Approach. [Google Scholar]
- Caminade C., van Dijk J., Baylis M., Williams D. Modelling recent and future climatic suitability for fasciolosis in Europe. Geospat. Health. 2015;9:301–308. doi: 10.4081/gh.2015.352. [DOI] [PubMed] [Google Scholar]
- Caron Y., Righi S., Lempereur L., Saegerman C., Losson B. An optimized DNA extraction and multiplex PCR for the detection of Fasciola sp. in lymnaeid snails. Vet. Parasitol. 2011;178:93–99. doi: 10.1016/j.vetpar.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Chipev N., Vassilev I., Samnaliev P. Interactions between Paramphistomum daubneyi Dinnik, 1962 and Fasciola hepatica in successive cross-invasions of Lymnaea (Galba) truncatula. Helminthologia. 1985;20:80–88. [Google Scholar]
- Claxton G. Farmers Weekly. 2015. Fluke survey reveals only 8% of farmers are treating correctly. http://www.fwi.co.uk/livestock/fluke-survey-reveals-only-8-percent-of-farmers-are-treating-correctly.htm. (Accessed 07.02.2017) [Google Scholar]
- Combes C. Trematodes: antagonism between species and sterilizing effects on snails in biological control. Parasitology. 1982;84:151–175. [Google Scholar]
- Cui J. QIC program and model selection in GEE. Stata J. 2007;7:209–220. [Google Scholar]
- Dinnik J.A. Paramphistomum daubneyi sp. nov. from cattle and its snail host in the Kenya Highlands. Parasitology. 1962;52:143–151. [Google Scholar]
- Dreyfuss G., Vignoles P., Rondelaud D. Paramphistomum daubneyi: the number of sporocysts developing in experimentally and naturally infected Galba truncatula. Parasitol. Res. 2008;103:345–349. doi: 10.1007/s00436-008-0978-4. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Vignoles P., Rondelaud D. Local adaptation of the trematode Fasciola hepatica to the snail Galba truncatula. Parasite. 2012;19:271–275. doi: 10.1051/parasite/2012193271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox N.J., White P.C.L., McClean C.J., Marion G., Evans A., Hutchings M.R. Predicting impacts of climate change on Fasciola hepatica risk. PLoS One. 2011;6:e16126. doi: 10.1371/journal.pone.0016126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen F., Christensen N.O. An introductory guide to the identification of cercariae from African freshwater snails with special reference to cercariae of trematode species of medical and veterinary importance. Acta Trop. 1984;41:181–202. [PubMed] [Google Scholar]
- Gordon H.M., Boray J.C. Controlling liver-fluke: a case for wildlife conservation? Vet. Rec. 1970;86:288–289. doi: 10.1136/vr.86.10.288. [DOI] [PubMed] [Google Scholar]
- Gordon D.K., Roberts L.C.P., Lean N., Zadoks R.N., Sargison N.D., Skuce P.J. Identification of the rumen fluke, Calicophoron daubneyi, in GB livestock: possible implications for liver fluke diagnosis. Vet. Parasitol. 2013;195:65–71. doi: 10.1016/j.vetpar.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Goumghar M.D., Abrous M., Ferdonnet D., Dreyfuss G., Rondelaud D. Prevalence of Haplometra cylindracea infection in three species of Lymnaea snails in central France. Parasitol. Res. 2000;86:337–339. doi: 10.1007/s004360050054. [DOI] [PubMed] [Google Scholar]
- Hourdin P., Rondelaud D., Cabaret J. The development of Fasciola hepatica parthenitae in Lymnaea truncatula by modification of Muellerius capillaris infection. Int. J. Parasitol. 1993;23:235–243. doi: 10.1016/0020-7519(93)90146-p. [DOI] [PubMed] [Google Scholar]
- Howell A., Baylis M., Smith R., Pinchbeck G., Williams D. Epidemiology and impact of Fasciola hepatica exposure in high-yielding dairy herds. Prev. Vet. Med. 2015;121:41–48. doi: 10.1016/j.prevetmed.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M.M. Population dynamics of Chaetogaster limnaei (Oligochaeta: Naididae) in the field populations of freshwater snails and its implications as a potential regulator of trematode larvae community. Parasitol. Res. 2007;101:25–33. doi: 10.1007/s00436-006-0436-0. [DOI] [PubMed] [Google Scholar]
- Iglesias-Piñeiro J., González-Warleta M., Castro-Hermida J.A., Córdoba M., González-Lanza C., Manga-González Y., Mezo M. Transmission of Calicophoron daubneyi and Fasciola hepatica in Galicia (Spain): temporal follow-up in the intermediate and definitive hosts. Parasites Vectors. 2016;9:610. doi: 10.1186/s13071-016-1892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.A., Williams H.W., Dalesman S., Brophy P.M. Confirmation of Galba truncatula as an intermediate host snail for Calicophoron daubneyi in Great Britain, with evidence of alternative snail species hosting Fasciola hepatica. Parasites Vectors. 2015;8:656. doi: 10.1186/s13071-015-1271-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.A., Brophy P.M., Mitchell E.S., Williams H.W. Rumen fluke (Calicophoron daubneyi) on Welsh farms: prevalence, risk factors and observations on co-infection with Fasciola hepatica. Parasitology. 2017;144:237–247. doi: 10.1017/S0031182016001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R.M., Dame J.B., Reddy G.R., Courtney C.H. The prevalence of Fasciola hepatica in its snail intermediate host determined by DNA probe assay. Int. J. Parasitol. 1997;27:1585–1593. doi: 10.1016/s0020-7519(97)00139-2. [DOI] [PubMed] [Google Scholar]
- Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., Thierer T., Ashton B., Mentjies P., Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H.K., Heyneman D. Intramolluscan inter-trematode antagonism: a review of factors influencing the host-parasite system and its possible role in biological control. Adv. Parasitol. 1972;10:191–268. doi: 10.1016/s0065-308x(08)60175-x. [DOI] [PubMed] [Google Scholar]
- Macan T.T. 4th ed. Freshwater Biological Association; Ambleside, UK: 1977. A Key to the British Fresh- and Brackish-water Gastropods: with Notes on Their Ecology. [Google Scholar]
- Mage C., Bourgne H., Toullieu J.M., Rondelaud D., Dreyfuss G. Fasciola hepatica and Paramphistomum daubneyi: changes in prevalences of natural infections in cattle and in Lymnaea truncatula from central France over the past 12 years. Vet. Res. 2002;33:439–447. doi: 10.1051/vetres:2002030. [DOI] [PubMed] [Google Scholar]
- Malrait K., Verschave S., Skuce P., Loo H., Vercruysse J., Charlier J. Novel insights into the pathogenic importance, diagnosis and treatment of the rumen fluke (Calicophoron daubneyi) in cattle. Vet. Parasitol. 2015;207:134–139. doi: 10.1016/j.vetpar.2014.10.033. [DOI] [PubMed] [Google Scholar]
- Manga-González M.Y., González Lanza C., González-Warleta M., Miñambres Rodríguez B., Carro-Corral J.I., Castillejo J., Mezo M. Infection kinetic of Galba truncatula (Mollusca, Basommatophora) by Fasciola hepatica and Calicophoron daubneyi (Trematoda, Digenea) in Galicia (Spain) Acta Parasit. Portuguesa. 2009;16:118–119. [Google Scholar]
- Martinez-Ibeas A.M., Gonzalez-Warleta M., Martinez-Valladares M., Castro-Hermida J.A., Gonzalez-Lanza C., Minambres B., Ferreras C., Mezo M., Manga-Gonzalez M.Y. Development and validation of a mtDNA multiplex PCR for identification and discrimination of Calicophoron daubneyi and Fasciola hepatica in the Galba truncatula snail. Vet. Parasitol. 2013;195:57–64. doi: 10.1016/j.vetpar.2012.12.048. [DOI] [PubMed] [Google Scholar]
- Martinez-Ibeas A.M., Munita M.P., Lawlor K., Sekiya M., Mulcahy G., Sayers R. Rumen fluke in Irish sheep: prevalence, risk factors and molecular identification of two paramphistome species. BMC Vet. Res. 2016;12:143. doi: 10.1186/s12917-016-0770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason C., Stevenson H., Cox A., Dick I., Rodger C. Disease associated with immature paramphistome infection in sheep. Vet. Rec. 2012;170:343–344. doi: 10.1136/vr.e2368. [DOI] [PubMed] [Google Scholar]
- Michelson E.H. The protective action of Chaetogaster limnaei on snails exposed to Schistosoma mansoni. J. Parasitol. 1964;50:441–444. [PubMed] [Google Scholar]
- Millar M., Colloff A., Scholes S. Disease associated with immature paramphistome infection. Vet. Rec. 2012;171:509–510. doi: 10.1136/vr.e7738. [DOI] [PubMed] [Google Scholar]
- Moukrim A., Vareille-Morel C., Rondelaud D., Mas Coma S. Cercarial sheddings of Haplometra cylindracea: about several observations in Lymnaea truncatula during single or dual infections. Res. Rev. Parasitol. 1993;53:57–61. [Google Scholar]
- Pan W. Akaike's information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- Petrovan S.O., Schmidt B.R. Volunteer conservation action data reveals large-scale and long-term negative population trends of a widespread amphibian, the common toad (Bufo bufo) PLoS One. 2016;11:e0161943. doi: 10.1371/journal.pone.0161943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekariah G.R. Chaetogaster limnaei K von Baer 1872 on Lymnaea tomentosa: ingestion of Fasciola hepatica cercariae. Experientia. 1978;34:1458–1459. doi: 10.1007/BF01932351. [DOI] [PubMed] [Google Scholar]
- Rieu E., Recca A., Bénet J.J., Saana M., Dorchies P., Guillot J. Reliability of coprological diagnosis of Paramphistomum sp. infection in cows. Vet. Parasitol. 2007;146:249–253. doi: 10.1016/j.vetpar.2007.02.033. [DOI] [PubMed] [Google Scholar]
- Rolfe P.F., Boray J.C. Chemotherapy of paramphistomosis in cattle. Aust. Vet. J. 1987;64:328–332. doi: 10.1111/j.1751-0813.1987.tb06060.x. [DOI] [PubMed] [Google Scholar]
- Rondelaud D., Vignoles P., Dreyfuss G. Fasciola hepatica: the developmental patterns of redial generations in naturally infected Galba truncatula. Parasitol. Res. 2004;94:183–187. doi: 10.1007/s00436-004-1191-8. [DOI] [PubMed] [Google Scholar]
- Rondelaud D., Vignoles P., Dreyfuss G. Parasite development and visceral pathology in Galba truncatula co-infected with Fasciola hepatica and Paramphistomum daubneyi. J. Helminthol. 2007;81:317–322. doi: 10.1017/S0022149X07818542. [DOI] [PubMed] [Google Scholar]
- Rondelaud D., Titi A., Vignoles P., Mekroud A., Dreyfuss G. Adaptation of Lymnaea fuscus and Radix balthica to Fasciola hepatica through the experimental infection of several successive snail generations. Parasites Vectors. 2014;7:296. doi: 10.1186/1756-3305-7-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondelaud D., Teukeng F.F.D., Vignoles P., Dreyfuss G. Lymnaea glabra: progressive increase in susceptibility to Fasciola hepatica through successive generations of experimentally infected snails. J. Helminthol. 2015;89:398–403. doi: 10.1017/S0022149X14000169. [DOI] [PubMed] [Google Scholar]
- Rondelaud D., Vignoles P., Dreyfuss G. Larval trematode infections in Galba truncatula (Gastropoda, Lymnaeidae) from the Brenne Regional Natural Park, central France. J. Helminthol. 2016;90:256–261. doi: 10.1017/S0022149X15000073. [DOI] [PubMed] [Google Scholar]
- Rondelaud D. Variabilité interpopulationelle de l'infestation fasciolienne chez le mollusque Lymnaea truncatula Müller. Influence du contact préalable de la population avec le parasite. Bull. Soc. Zool. Fr. 1993;118:185–193. [Google Scholar]
- SAC Immature rumen fluke cause deaths of ewes. Vet. Rec. 2016;179:538–542. doi: 10.1136/vr.i6343. [DOI] [PubMed] [Google Scholar]
- Sey O. Revision of the amphistomes of European ruminants. J. Helminthol. 1980;13:13–25. [Google Scholar]
- Teacher A.G.F., Cunningham A.A., Garner T.W.J. Assessing the long-term impact of Ranavirus infection in wild common frog populations. Anim. Conserv. 2010;13:514–522. [Google Scholar]
- Trouvé S., Degen L., Renaud F., Goudet J. Evolutionary implications of a high selfing rate in the freshwater snail Lymnaea truncatula. Evolution. 2003;57:2303–2314. [PubMed] [Google Scholar]
- Vignoles P., Dreyfuss G., Rondelaud D. Larval development of Fasciola hepatica in experimental infections: variations with populations of Lymnaea truncatula. J. Helminthol. 2002;76:179–183. doi: 10.1079/JOH2002112. [DOI] [PubMed] [Google Scholar]
- Whitelaw A., Fawcett A.R. Biological control of liver fluke. Vet. Rec. 1982;110:500–501. doi: 10.1136/vr.110.21.500. [DOI] [PubMed] [Google Scholar]